Abstract
Patient: Female, 28
Final Diagnosis: Gastrinoma
Symptoms: Vomiting • diarrhea • epigastric soreness • heartburn • nausea • significant weight loss
Medication: —
Clinical Procedure: Esophagogastroduodenoscopy • blood tests • abdomen CT scan • surgery
Specialty: Gastroenterology and Hepatology
Objective:
Rare co-existance of disease or pathology
Background:
Pancreatic endocrine tumors (PETs) are rare and can occur as part of neurofibromatosis type 1 (NF1). Gastrinomas are functional PETs that are rarely associated with NF1. Only two cases of their occurrence have been reported in the literature.
Case Report:
A 28-year-old woman was admitted for further evaluation of epigastric soreness, heartburn, nausea, vomiting, diarrhea, and a significant weight loss. Physical examination was remarkable for cutaneous findings (axillary freckling and multiple café-au-lait spots) as well as neurofibromas (dermal, plexiform). A diagnosis of NF1 was confirmed.
Esophagogastroduodenoscopy (EGD) revealed multiple ulcers in the duodenum and the upper jejunum. A fasting gastrin level exceeded ten times the normal limit. An abdominal multi-slice 128 computed tomography (CT) scan revealed an oval mass of 26 mm in diameter adjacent to the second segment of the duodenum. The patient was examined carefully to rule out multiple endocrine neoplasia type 1 (MEN1). Surgical resection was performed and a gastrinoma, causing Zollinger-Ellison syndrome (ZES), was diagnosed by histological examinations of the extirpated mass. The serum gastrin level decreased to normal limits shortly after surgery. Continuous follow-up revealed that the symptoms and the EGD findings completely resolved without recurrences.
Conclusions:
Although NF1 has common skeletal, visual, neurological, and cardiovascular complications, it also has a rare association with duodenal or pancreatic gastrinomas. Vigilance for this possible association is important to promote timely and careful management to help eliminate serious and potentially life-threatening complications.
MeSH Keywords: Gastrinoma, Neurofibromatosis 1, Zollinger-Ellison Syndrome
Background
Pancreatic endocrine tumors (PETs) are a constellation of relatively rare malignancies that arise from the neuroendocrine cells of the pancreas. Generally, these tumors have sporadic patterns of occurrence, but they can also occur in association with familial inherited syndromes such as multiple endocrine neoplasia type 1 (MEN1), von Hippel-Lindau disease (VHL), neurofibromatosis type 1 (NF1), also called von Recklinghausen disease, and tuberous sclerosis complex (TSC). PETs can be functional or non-functional tumors, depending on the tumor’s ability to secrete biologically active peptides into the blood stream, which can lead to interesting clinical features [1]. Gastrinomas are the most common functional PETs observed in patients with MEN1, and they are rarely associated with NF1 [1].
Neuroendocrine tumors (NETs) of the small intestine are common in NF1. An interesting association between von Recklinghausen disease and carcinoid tumors of the duodenum has been reported in the literature [2–10]. However, only two reports have described gastrinoma associated with NF1, the first case was reported by Chagnon et al. in 1985 [11] and the second by Lee et al. in 2005 [12].
Case Report
A 28-year-old woman was admitted in 2011 to the general internal medicine department in Aleppo University Hospital (AUH). The patient was suffering from intractable and agonizing pain in the epigastrium. Her complaint was accompanied by heartburn, acid reflux, nausea, and vomiting. Due to pain, the patient was avoiding meals, so she had lost 9 kg of weight over the last three months. The patient stated that she had recently developed frequent episodes of watery diarrhea.
Clinically, she was pale with an ill appearance, had moderate hypotension (blood pressure 100/55 mmHg) and tachycardia (105 beats/minute). Her extremities were cold and her pulse rate was fast and thready.
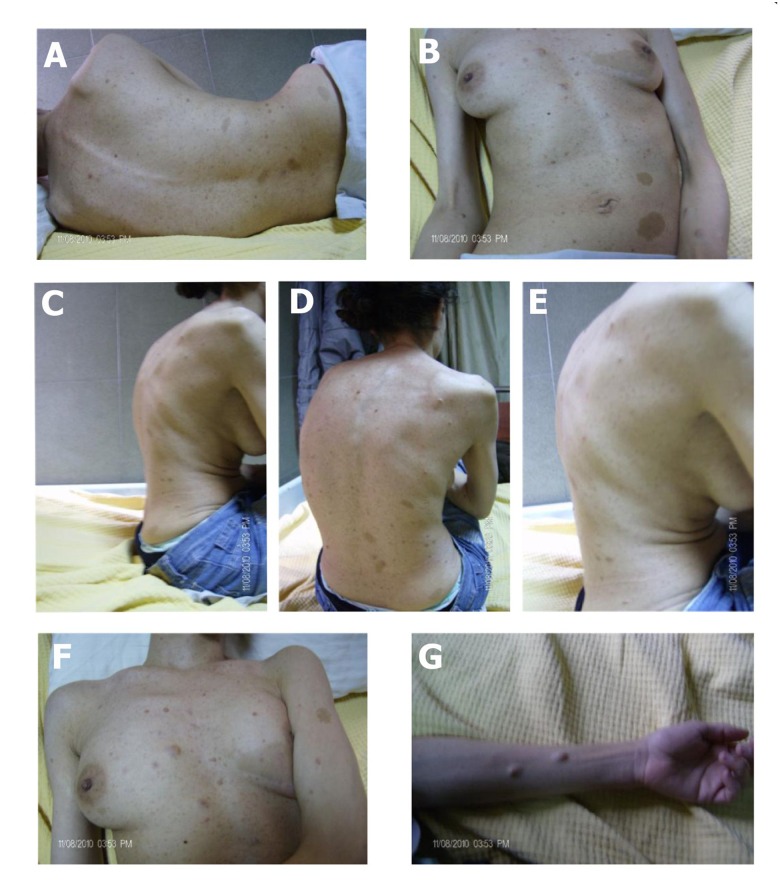
The physical examination revealed body skeletal deformities, scoliosis, kyphosis, and skin nodules on the forearms, chest wall, and sub-armpit area. The nodules were accompanied by axillary freckles and café au lait spots (Figure 1). Further investigation revealed that her father, brother, and sister share some of these physical anomalies. These findings raised the suspicion of NF1, and a diagnosis was established by pertinent investigative testing.
Figure 1.
The clinical manifestations of neurofibromatosis type 1 in our patient. Scoliosis (A, B); kyphosis (C–E); café au lait spots and freckles (F); nodules (G).
The patient was living in a rural area, and due to family traditions she did not request any medical advice. She had no past medical history of note, and was not on any regular medications. She had tried multiple traditional herbal remedies to relieve her symptoms.
On admission, the patient was managed with an IV fluid. A day later, her vital signs stabilized; her pain was controlled with an IV pain medication. A gastroenterologist investigated her upper gastrointestinal tract by EGD. This examination revealed multiple ulcers distributed throughout the pylorus, duodenum, and upper jejunum. There were more than ten ulcers spread in the duodenum with a bloody surface, and there was a distinct distortion in the duodenal bulb. During the endoscopy session, a rapid urease test was performed on a sample biopsy obtained from the second section of the duodenum. The test was negative for any infection with Helicobacter pylori.
The constellation of clinical symptoms supported by the EGD findings strongly suggested a diagnosis of gastrinoma causing Zollinger-Ellison syndrome (ZES). A secretin stimulation test was unavailable; therefore, we further investigated the gastrinoma by measuring serum fasting gastrin level. The test was repeated twice, thirty days apart, and showed a high level of serum gastrin that exceeded ten times the normal limit. The first test was 1,250 pg/mL and the second test, after one month, was 1,432 pg/mL. The patient was not on any proton pump inhibitor (PPI) or H2-blocker regimens during the time between the two tests that could have lead to a false positive in the measured serum gastrin level. The general aim of medical management in ZES is to reduce clinical manifestations and complications of acid hypersecretion. The optimal treatment is to locate and isolate the causative tumor. However, in this case, to avoid any false positive findings related to treatments with proton pump inhibitors (PPI) or H2-blockers, octreotide was administered temporarily instead of these medications.
Endoscopic ultrasonography and somatostatin receptor scintigraphy (SRS) are currently the gold standard for localization of gastrinomas [13]. However, these valuable investigations were regrettably unavailable. Therefore, CT scan was the method of choice to locate the tumor.
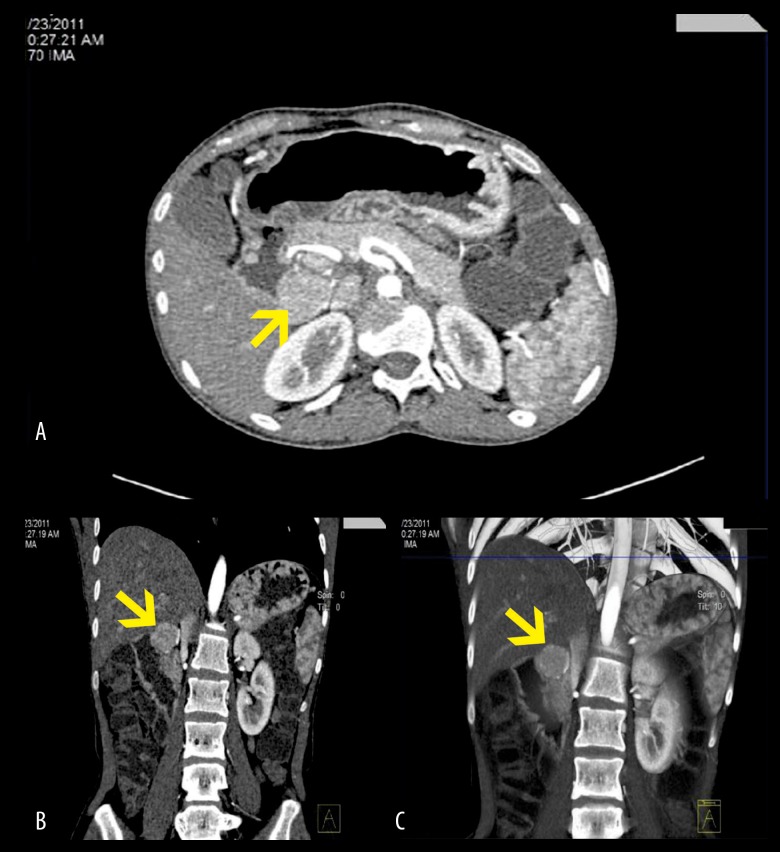
An abdominal multi-slice 128 CT scan was performed before and after contrast infusion. The CT scan showed a 26-mm oval mass residing in the sub-hepatic space with an intimate connection to the second segment of the duodenum and supplied by two branches of the mesenteric artery (Figure 2). The isolated mass was contrast-enhanced and showed very well-defined borders. No metastatic lesions were observed in the liver or regional lymph nodes.
Figure 2.
Abdominal multi-slice 128 computed tomography (CT) scan of the abdomen showing a 26 mm oval mass (yellow arrow) adjacent to the duodenal loop in the sub-hepatic space and supplied by two branches of the mesenteric artery. Axial section (A); coronal section (B); 3D coronal section (C).
MEN1 is an important differential diagnosis for both NF1 and ZES. Skin manifestations are also a clinical feature of both NF1 and MEN1. In addition, gastrinomas can be identified in MEN1 and ZES. Hence, careful investigation for MEN1 was done with comprehensive laboratory and radiology testing. All these investigations were negative for any involvement of MEN1.
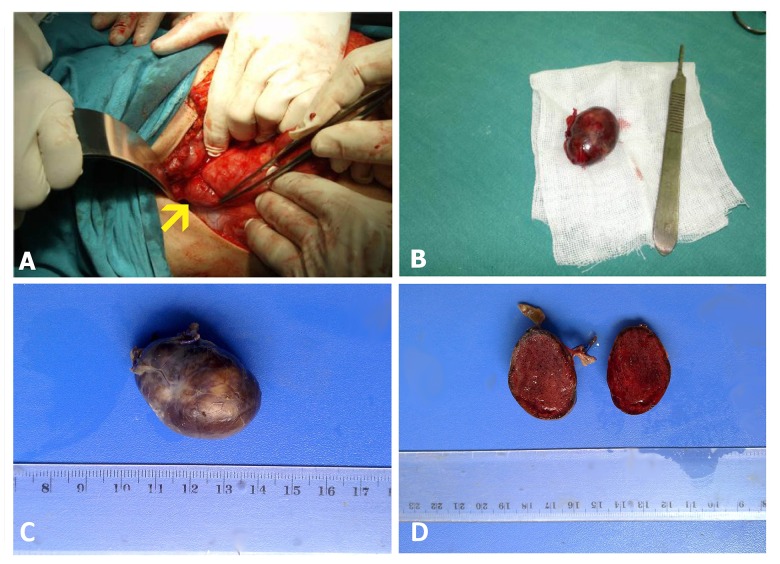
Finally, our surgical investigation revealed an intra-abdominal encapsulated oval mass. The mass was soft in consistency and brown in color with smooth outer surfaces and sharp outlines. It measured 2.5×3.5×2.5 cm and weighed 11 g (Figure 3). The mass was found in the right upper quadrant of the abdomen near the descending duodenum, exactly in the duodenal triangle without any connection with adjacent structures. The mass was completely resected and fully isolated, and the regional lymph nodes were removed. Gastrinoma was definitively diagnosed by histological examination and immunohistochemical staining. The regional lymph nodes were negative for any metastatic spread. The patient was followed for a period of 18 months after the operation. Her gastrin level dropped to near normal limits within two weeks of the surgery. She was free of symptoms by one month of the operation. Two EGDs were later performed; the last one, 14 months after the surgery, showed no signs of ulcers and was only significant for scar formations and a disfigured duodenal bulb.
Figure 3.
The surgical operation and the excised mass: (A) intraoperative photograph showing the gastrinoma (yellow arrow); (B–D) the excised mass, oval in shape, dark red in color with smooth outer surfaces. It measured 2.5×3.5×2.5 cm and weighed 11 g.
Discussion
A literature search found only two reported cases of gastrinoma associated with von Recklinghausen disease. A summary of the literature review for endocrine tumors reported in neurofibromatosis type 1 is presented in Table 1.
Table 1.
Literature review of endocrine tumors in neurofibromatosis type 1.
| Tumors | Origin | Risk* | Cases# | Year | Author |
|---|---|---|---|---|---|
| Gastrointestinal Stromal Tumor (GIST) | Interstitial cells of Cajal inthe small intestine | 1–6% | 8 | 2000 | Karatzas et al. |
| 2004 | suzuki et al. | ||||
| 2005 | Kramer et al. | ||||
| 2006 | Lisewski et al. | ||||
| 2006 | Juergens et al. | ||||
| 2007 | Bettini et al. | ||||
| 2010 | Relles et al. | ||||
| 2013 | Salvi | ||||
| Pheochromocytoma | Paraganglionic chromaffin cells | 1–5% | 152 | 1910 | Suzuki et al. |
| 1999 | Walther et al. | ||||
| 1968 | Galera et al. | ||||
| 2010 | Zografos et al. | ||||
| 2011 | Kumar et al. | ||||
| Somatistonoma | Duodenal carcinoids or pancreatic somatistonoma | 1% | 50 | 2004 | Cappelli et al. |
| 2006 | Caiazzo et al. | ||||
| Pancreatic neuroendocrine tumors (PETs) | Gastrinomas | – | 2 | 1985 | Chagnon et al. |
| 2005 | Lee et al. | ||||
| Insulinomas | – | 2 | 1964 | Coskey et al. | |
| 2006 | Perren et al. | ||||
| Paragangliomas | Extra adrenal paraganglia | – | 1 | 1987 | De Angelis et al. |
Number of a reported case reports or the total number of reported case reports;
life time risk for malignancy.
In 1985, Chagnon et al. [11] reported a case of a 49-year-old man who had NF1; their investigation used exploratory laparotomy and failed to detect the tumor mass or its location. This was probably due to the small size of the gastrinoma or its distribution in the intestinal wall or stomach. It is well known that as many as 70% of gastrinomas form in the duodenum, 20% arise in the pancreas, and approximately 10% in the lymph nodes adjacent to the pancreas. Duodenal wall gastrinomas are frequently small, occult, multiple, and very hard to locate [14,15]. Sporadic tumors occurring in the pancreas tend to be solitary, larger, and have greater malignant potential compared to duodenal gastrinomas [16].
Chagnon et al. established the patient’s diagnosis by finding an elevated level of gastric basal acid secretion and a positive secretin test. These measures, integrated with the patient’s clinical history, were highly suggestive of ZES in the context of NF1.
In the second report, Lee et al. [12] described a case of a 41-year-old woman with NF1 and ZES. The gastrinoma was large in size. A CT scan easily located it near the duodenum. The final diagnosis was obtained by a histological examination of the excised mass.
Patients with NF1 develop both benign and malignant tumors at increased frequency throughout their life [17,18]. In our case, a family history of NF1 in first-degree relatives and a pathological proof of neurofibromas for three skin nodule biopsies, two from the patient and one from the father, as well as characteristic dermatological features, were enough to eliminate the need for genetic studies.
Because MEN1 is an important differential diagnosis that can present in up to 20–30% of patients with ZES, and it can share some clinical features with NF1, we carefully investigated the parathyroid, pituitary, pancreas, and adrenal glands, of which all proved to be negative for MEN1. Moreover, an MRI of the brain and an ophthalmology consult were performed and both were negative for any lesions.
The neuroendocrine tumors of the upper gastrointestinal tract are common in NF1, and characteristically found in the periampullary region [19]. These tumors are mostly carcinoid and can clinically manifest as intestinal bleeding or bowel obstruction [20]. Many of these carcinoids are classified as somatostatinomas, and in NF1 they have only rarely been associated with insulinomas or gastrinomas [21,22].
Gastrinomas can be primary or secondary tumors. Although primary pancreatic gastrinomas usually reside within the pancreas tissue, some are located extra-pancreatically and are thus referred to as “duodenal gastrinomas” due to the proximal relationship with the duodenal wall [23,24]. These extra-pancreatic tumors are difficult to differentiate from the primary duodenal gastrinomas which originate from the first or second portions of the duodenal wall [25,26].
In our patient, the isolated mass was large (3.5 cm), benign, and solitary. The mass was similar in its location and size to the gastrinoma reported by Lee et al. in 2005.
Based on the tumor size, the gastrinoma in our patient was probably of pancreatic origin. Primary duodenal tumor sizes range from 0.2 cm to 2 cm in diameter, with the majority less than 1 cm [26]. Extra-pancreatic gastrinomas are larger in size (>2 cm) and tend to reside close to the duodenum.
The World Health Organization (WHO) classifies neuroendocrine tumors (NETs) of the digestive system, based on the extent of the tumor, into two broad categories:
Well-differentiated NETs, which are further classified according to proliferative rate into low-grade (G1) and intermediate-grade (G2) subgroups.
Poorly differentiated neuroendocrine carcinomas, all of which are high-grade (G3).
The mass in our patient fell into the first category as a well-differentiated endocrine carcinoma with low-grade malignant behavior. Up to 50–80% of gastrinomas belong to this category and are generally larger than 2 cm.
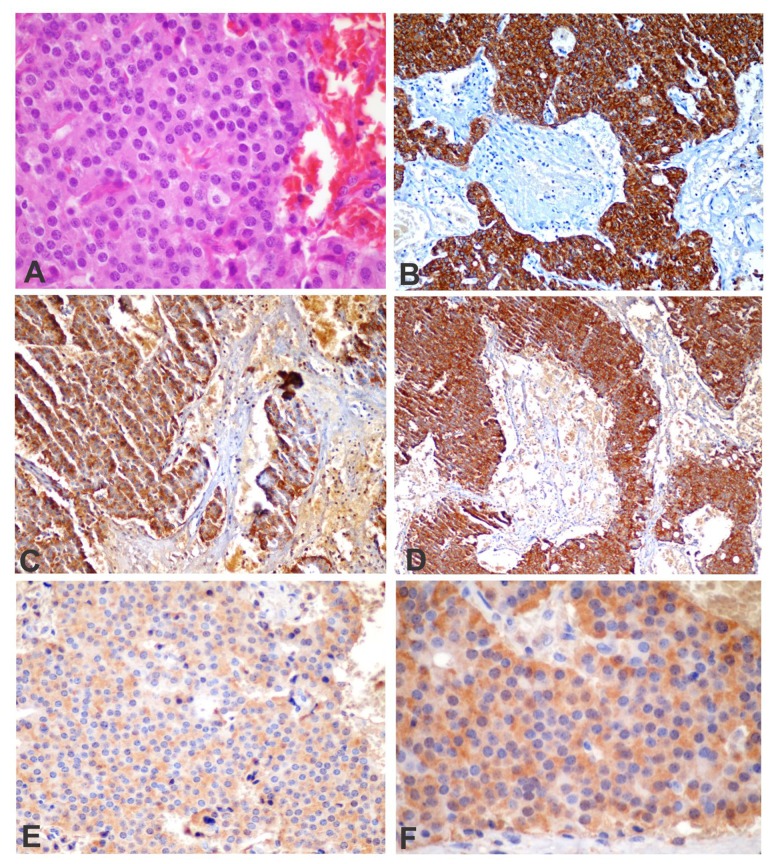
In our case, radiological studies were useful for localizing the gastrinoma and for follow-up of its behavior. Finally, the pathology report of the excised tumor confirmed the diagnosis of ZES (Figure 4).
Figure 4.
Histological features of gastrinoma. Hematoxylineosin (H&E) immunohistochemical staining was performed for cytokeran (CK), chromogranin A (CgA), synaptophysin (Syn), and gastrin (G). (A) Histological section (H&E, 60×) showing tumor cells with a glandular pattern; (B) CK-positive cells represent the epithelial differentiation of the tumor cells (CK, 20×); (C) chromogranin A (CgA, 20×) showing the neuroendocrine features of the tumor; (D) synaptophysin immunostaining (Syn., ×10); (E) the tumor cells were strongly immunopositive for the gastrin stain (G, 40×); and (F) G, 60×.
Conclusions
Well-defined gastrinomas can readily be excised. Early diagnosis and a suitable management plan can be life-saving. These tumors must be removed after a detailed investigation to locate their position and to determine their size, type, and stage. Although gastrinoma has a rare association with NF1, it can be life-threatening if it is not managed appropriately.
Herein, we report the first case of ZES associated with NF1 in the Middle East region and the third case reported in the global medical literature.
Acknowledgments
The authors would like to thank the patient and her family for their consent to publish these data and for their cooperation with the medical procedures.
The authors highly appreciate and are grateful for the assistance of Dr. Nihad Mahli, who performed the surgery; Dr. Mamoun Dabea, who participated in the endoscopy sessions and follow-up visits; Dr. Antoine Nasimian, who performed the imaging studies and provided clinical clues; and Dr. Mohanad Malhis, who did the histological examinations and provided the final diagnosis of the disease.
Footnotes
Statements and declarations regarding conflicts of interest
The authors have no conflicts of interest to disclose.
References:
- 1.Jensen RT, Berna MJ, Bingham DB, Norton JA. Inherited pancreatic endocrine tumor syndromes: Advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer. 2008;113(7 Suppl.):1807–43. doi: 10.1002/cncr.23648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee HY, Garber PE. Von recklinghausen’s disease associated with pheochromocytoma and carcinoid tumor. Ohio State Med J. 1970;66(6):583–86. [PubMed] [Google Scholar]
- 3.Weichert RF, III, Roth LM, Krementz ET, et al. Carcinoid-islet cell tumors of the duodenum. report of twenty-one cases. Am J Surg. 1971;121(2):195–205. doi: 10.1016/0002-9610(71)90098-5. [DOI] [PubMed] [Google Scholar]
- 4.Barber PV. Carcinoid tumour of the ampulla of vater associated with cutaneous neurofibromatosis. Postgrad Med J. 1976;52(610):514–17. doi: 10.1136/pgmj.52.610.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson L, Weaver M. Von recklinghausen’s disease and gastrointestinal carcinoids. JAMA. 1981;245(24):2496. [PubMed] [Google Scholar]
- 6.Kapur BM, Sarin SK, Anand CS, Varma K. Carcinoid tumour of ampulla of vater associated with viscero-cutaneous neurofibromatosis. Postgrad Med J. 1983;59(697):734–35. doi: 10.1136/pgmj.59.697.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dayal Y, Doos WG, O’Brien MJ, et al. Psammomatous somatostatinomas of the duodenum. Am J Surg Pathol. 1983;7(7):653–65. doi: 10.1097/00000478-198310000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Zollinger R, Hedinger C. Pheochromocytoma and sympathetic paraganglioma. 2. combination with typical associated diseases. familial occurrence. Schweiz Med Wochenschr. 1983;113(31–32):1086–92. [PubMed] [Google Scholar]
- 9.Weder W, Saremaslani P, Maurer R. Calcitonin-producing duodenal carcinoid in recklinghausen’s neurofibromatosis. Clinical case report and review of the literature. Schweiz Med Wochenschr. 1983;113(24):885–92. [PubMed] [Google Scholar]
- 10.Hough DR, Chan A, Davidson H. Von recklinghausen’s disease associated with gastrointestinal carcinoid tumors. Cancer. 1983;51(12):2206–8. doi: 10.1002/1097-0142(19830615)51:12<2206::aid-cncr2820511209>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Chagnon JP, Barge J, Henin D, Blanc D. Recklinghausen’s disease with digestive localizations associated with gastric acid hypersecretion suggesting zollinger-ellison syndrome. Gastroenterol Clin Biol. 1985;9(1):65–69. [PubMed] [Google Scholar]
- 12.Lee WS, Koh YS, Kim JC, et al. Zollinger-ellison syndrome associated with neurofibromatosis type 1: A case report. BMC Cancer. 2005;5:85. doi: 10.1186/1471-2407-5-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirallie E, Pattou F, Malvaux P, et al. Value of endoscopic ultrasonography and somatostatin receptor scintigraphy in the preoperative localization of insulinomas and gastrinomas. experience of 54 cases. Gastroenterol Clin Biol. 2002;26(4):360–66. [PubMed] [Google Scholar]
- 14.Kisker O, Bastian D, Bartsch D, et al. Localization, malignant potential, and surgical management of gastrinomas. World J Surg. 1998;22(7):651–57. doi: 10.1007/s002689900448. ; discussion 657–58. [DOI] [PubMed] [Google Scholar]
- 15.Norton JA, Doppman JL, Jensen RT. Curative resection in Zollinger-Ellison syndrome. Results of a 10-year prospective study. Ann Surg. 1992;215(1):8–18. doi: 10.1097/00000658-199201000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz-Tovar J, Priego P, Martinez-Molina E, et al. Pancreatic neuroendocrine tumours. Clin Transl Oncol. 2008;10(8):493–97. doi: 10.1007/s12094-008-0238-1. [DOI] [PubMed] [Google Scholar]
- 17.Gutmann DH. Recent insights into neurofibromatosis type 1: Clear genetic progress. Arch Neurol. 1998;55(6):778–80. doi: 10.1001/archneur.55.6.778. [DOI] [PubMed] [Google Scholar]
- 18.Widemann BC, Salzer WL, Arceci RJ, et al. Phase I trial and pharmacokinetic study of the farnesyltransferase inhibitor tipifarnib in children with refractory solid tumors or neurofibromatosis type I and plexiform neurofibromas. J Clin Oncol. 2006;24(3):507–16. doi: 10.1200/JCO.2005.03.8638. [DOI] [PubMed] [Google Scholar]
- 19.Somani A, Jain AK, Dixit VK. Periampullary carcinoid: An uncommon tumor at an unusual site. Indian J Cancer. 2011;48(4):496–99. doi: 10.4103/0019-509X.92255. [DOI] [PubMed] [Google Scholar]
- 20.Han SH, Park SH, Cho GH, et al. Malignant gastrointestinal stromal tumor in a patient with neurofibromatosis type 1. Korean J Intern Med. 2007;22(1):21–23. doi: 10.3904/kjim.2007.22.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fung JW, Lam KS. Neurofibromatosis and insulinoma. Postgrad Med J. 1995;71(838):485–86. doi: 10.1136/pgmj.71.838.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chagnon JP, Barge J, Henin D, Blanc D. Recklinghausen’s disease with digestive localizations associated with gastric acid hypersecretion suggesting Zollinger-Ellison syndrome. Gastroenterol Clin Biol. 1985;9(1):65–69. [PubMed] [Google Scholar]
- 23.Hoffmann KM, Furukawa M, Jensen RT. Duodenal neuroendocrine tumors: Classification, functional syndromes, diagnosis and medical treatment. Best Pract Res Clin Gastroenterol. 2005;19(5):675–97. doi: 10.1016/j.bpg.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Thom AK, Norton JA, Axiotis CA, Jensen RT. Location, incidence, and malignant potential of duodenal gastrinomas. Surgery. 1991;110(6):1086–91. ; discussion 1091–93. [PubMed] [Google Scholar]
- 25.Metz DC, Jensen RT. Gastrointestinal neuroendocrine tumors: Pancreatic endocrine tumors. Gastroenterology. 2008;135(5):1469–92. doi: 10.1053/j.gastro.2008.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zogakis TG, Gibril F, Libutti SK, et al. Management and outcome of patients with sporadic gastrinoma arising in the duodenum. Ann Surg. 2003;238(1):42–48. doi: 10.1097/01.SLA.0000074963.87688.31. [DOI] [PMC free article] [PubMed] [Google Scholar]