Abstract
Objective
In Robot-assisted radical prostatectomy (RARP) patients, preoperative bowel preparation and intraoperative fluid restriction may cause dehydration and electrolyte imbalance. In these patients, laboratory results that are considered “normal” in the pre-anaesthesia clinic may be misleading, and cardiac arrhythmia due to hypokalaemia and hypocalcaemia, as well as problems, such as prolonged non-depolarising blockade and delayed recovery from anaesthesia, may be observed during anaesthesia practice. In this study, we aimed to determine these disturbances by comparing the preoperative (T1) laboratory values with those at the beginning of the operation (T2) and at the 6th hour of the operation (T3) and values at discharge.
Methods
This prospective study comprised 49 American Society of Anesthesiologists (ASA) I-II patients. Bowel preparation was made with a rectal enema (NaP) twice in 12 hours and with one single dose of oral laxative soda (NaP). During surgery, 1 mL kg−1 h−1 0.09% NaCl and 1 mL kg−1 h−1 6% HES 200/05 infusions were applied.
Results
The potassium level at T2 was significantly lower than at T1 and T3. The calcium levels at T2 and T3 were significantly lower than at T1, and the level at T3 was significantly lower than at T2. The creatinine level at T3 was significantly higher than at T1 and T2.
Conclusion
Although there were no severe increases or decreases in laboratory test values due to bowel preparation and fluid restriction in RARP operations, which reflected on the clinical outcome in this ASA I–II patient group, these changes may be important in critically ill or ASA III–IV patients.
Keywords: Remote operations (robotic), dehydration, water-electrolyte imbalance, bowel preparation
Introduction
Prostate cancer is the most frequently seen cancer of the male reproductive system and often occurs in the 5th and 6th decades of life. The presence of comorbidities in patients of this age renders the pre-anaesthesia workup more important.
As in many forms of surgery, the use of minimally invasive surgical techniques in urologic surgery has reduced postoperative complications, shortened hospital stay and increased patient satisfaction. However, as this surgical technique is not minimally invasive from the anaesthetist’s perspective, several risks may be caused by aggressive bowel preparation applied preoperatively, in addition to perioperative fluid restriction and excessive Trendelenburg position.
In current practice, anaesthesia applications are made on the basis of the patient’s examination and laboratory test values (1), although bowel preparation and fluid restriction applied to patients who will undergo major abdominal surgery may cause the laboratory values seen preoperatively to be misleading, particularly in respect of electrolytes. This may be the reason for unwanted side effects that are seen, secondary to electrolyte imbalance, during the application of anaesthesia.
Preoperative preparation begins in the department where the patient has been admitted. Preparation protocols are defined in each clinic according to the type of surgery to be applied. In major abdominal and pelvic surgery, the importance of nutritional support that is started preoperatively, antibiotic prophylaxis, deep vein thrombosis prophylaxis and bowel preparation is known, but there is no standardisation on the bowel preparation topic. The aim of bowel cleansing is to reduce the bacterial load of the intestinal contents as much as possible. Bowel preparation can be applied in two ways: mechanical preparation and antibiotic application. Mechanical bowel preparation aims to reduce the amount of faeces, and antibiotic application aims to reduce the amount of bacteria in the faeces (2, 3).
In the process from pre-anaesthetic examination to the operating theatre, bowel preparation applied to patients for whom major abdominal surgery is planned may cause fluid electrolyte shifts and dehydration. In robot-assisted radical prostatectomy procedures, a factor that can increase dehydration is fluid restriction, which is applied to this patient group to prevent a percentage of oedema of the respiratory mucosa depending on the position and to not have an impaired surgical view during ureterovesical anastomosis. In addition, the steep Trendelenburg position given during surgery may mask the expected clinical symptoms of dehydration (4, 5). In these patients, due to bowel preparation and fluid restriction, metabolic acidosis may be seen with electrolyte imbalances, such as hypocalcaemia, hypokalaemia and hypernatraemia, and these may cause clinical arrhythmia, prolonged muscle relaxation, prolongation of recovery from anaesthesia and even acute tubular necrosis and renal failure (6–8).
In this study, we aimed to determine the effects of aggressive bowel preparation on the laboratory test values of patients who were evaluated in the anaesthesia examination based on Turkish Anaesthesia and Reanimation Society (TARD) guidelines of pre-anaesthetic evaluation. The effects of fluid restriction were also examined to determine whether or not it causes dehydration, electrolyte imbalance, acidosis and renal impairment during the operation by comparing preoperative (T1) laboratory test values with values at the beginning of the operation (T2) and values at the 6th hour of the operation (T3).
Methods
Approval for the study was granted by the ethics committee of Umraniye Training and Research Hospital, Istanbul, Turkey (Chairperson Prof Naderi, April 30, 2013, B.10.1.TKH.4.34.H.G.P.0.01), and informed consent was obtained from all participants. This prospective study comprised 49 adult patients with American Society of Anesthesiologists (ASA) status I–II, with a mean age of 61.78±6.9 years (range, 50–80 years) and body mass index (BMI) >25, who were scheduled for RARP under general anaesthesia. Renal and hepatic diseases were considered exclusion criteria.
Patients were examined 1 week before the operation (T1) in the pre-anaesthesia clinic, including routine laboratory tests: blood urea nitrogen (BUN), creatinine (Crea), aspartate aminotransferase (AST), alanine transaminase (ALT), Na+, K+, Cl−, Ca+ and specific gravity of urine. Patients were admitted to the relevant clinic 24 hours before the operation. The night before the surgery, bowel preparation was made with a rectal enema of sodium biphosphate/sodium phosphate (NaH2PO4-Na2HPO4) twice in 12 hours and one single dose of oral laxative soda sodium biphosphate/sodium phosphate (NaH2PO4-Na2HPO4). Oral intake was stopped 8 hours before the surgery. Patients were taken to the operating room 8 hours after the last dose of bowel preparation. In the operating room (T2), blood and urine samples for ABG, Na, Cl, Ca, K, BUN, Crea, AST, ALT and specific gravity of urine were taken from the patients. During the surgery, 1 mL kg−1 h−1 0.09% NaCl and 1 mL kg−1 h−1 6% HES 200/05 infusions were applied, and the steep Trendelenburg position was given after the anaesthesia induction, and haemodynamic data, mean arterial pressure (MAP) and heart rate were recorded. At 6 hours from the beginning of the operation (T3), blood and urine samples were retaken. Patients were discharged with control laboratory tests, including the same parameters, 48 hours postoperatively.
Statistical analysis
For all the statistical analyses, the Number Cruncher Statistical System (NCSS) 2007&Power Analysis and Sample Size (PASS) 2008 statistical software (Utah, USA) program was used. In the evaluation of the study data, besides descriptive statistical methods (mean, standard deviation, median, minimum, maximum), the repeated-measures test was used in the comparison between the 3 follow-up intervals of the parameters showing normal distribution, and the Bonferroni test was used in the post hoc evaluation. A level of p<0.05 was accepted as statistically significant.
Results
The 49 patients had a median BMI of 28.43 kg m2–1 (range 25–35 kg m2–1), and the median age was 61.78 years (range, 50–78 years). The median duration of general anaesthesia was 212.65 minutes (range 180–260 min), and the median time in the Trendelenburg position was 182.76 minutes (range 140–220 min) (Table 1).
Table 1.
Distribution of descriptive characteristics
| n | Minimum | Maximum | Mean | SD | |
|---|---|---|---|---|---|
| Age (years) | 49 | 50 | 78 | 61.78 | 6.99 |
| BMI | 49 | 23 | 35 | 28.43 | 2.84 |
| Duration of Trendelenburg (mins) | 49 | 140 | 220 | 182.76 | 18.17 |
| Duration of operation (mins) | 49 | 180 | 260 | 212.65 | 22.80 |
SD: standard deviation; BMI: body mass index
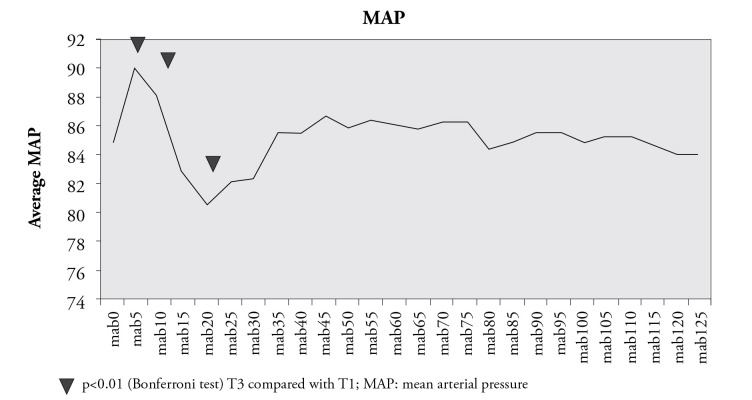
The mean arterial pressure (MAP) values at 5 and 10 minutes were found to be significantly higher, and the 20th minute values were found to be significantly lower than the initial values (p<0.01) (Figure 1).
Figure 1.
Mean arterial pressure
The K+ level at T2 was significantly lower than at T1 and T3 (p=0.001, p=0.003, p<0.01) (p=0.101). The Cl− levels at T2 and T3 were significantly higher than at T1 (p=0.001, p=0.001, p<0.01). The Ca++ levels at T2 and T3 were significantly lower than at T1 (p=0.004, p=0.001, p<0.01), and the level at T3 was also significantly lower than at T2 (p<0.01). The Crea level at T3 was significantly higher than at T1 and T2 (p=0.001, p=0.001, p<0.01). The urine specific gravity levels at T2 and T3 were significantly higher than at T1 (p=0.001, p=0.001, p<0.01), and the level at T3 was also significantly higher than at T2 (p<0.05) (Table 2).
Table 2.
Laboratory test values
| BUN | Cre | AST | ALT | Na | K | Ca | Cl | Urine Specific Gravity | pH | pCO2 | LAC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1±SD | 34.31±8.62 | 0.91±0.19 | 19.08±6.26 | 20.78±8.66 | 138.55±2.39 | 4.29±0.36 | 9.02±0.46 | 102.57**±1.62 | 1013.87±3.6 | |||
| T2±SD | 35.121±2.04 | 0.92±0.30 | 20.31±5.83 | 21.51±9.56 | 139.18±3.34 | 3.72*±0.50 | 8.74**±0.77 | 107.84±4.50 | 1019.04**±6.75 | 7.42±0.04 | 33.27±4.27 | 10.10±4.07 |
| T3±SD | 36.16±11.47 | 1.13**±0.39 | 22.39±13.81 | 20.49±9.84 | 138.73±3.12 | 4.1±0.66 | 8.29±066 | 107.55±2.91 | 1022.20±8.99 | 7.30±0.06 | 44.30±8.20 | 14.12±7.20 |
| Discharge | 35.41±16.04 | 34.20±28.50 | 20.59±9.40 | 138.76±2.76 | 3.90±0.34 | 8.49±0.50 | 105.88±3.69 | 1018.4±4.75 | 7.40±0.06 | 39.49±4.40 | 13.02±5.35 |
SD: standard deviation; BUN: blood urine nitrogen; Cre: creatinine; AST: aspartate transaminase; ALT: alanine transaminase; Na: sodium; K: potassium; Ca: calcium; CI: clor; pH; pCO2; carbon dioxide partial pressure; LAC: lactate;
p<0.05; repeated-measures test
p<0.01; repeated-measures test
Discussion
Tests that are requested for patients in the anaesthesia clinic are defined according to specific disease, age and ASA evaluation. Taking potential complications and existing comorbidities into account, the basic laboratory tests have been defined as blood count, coagulation profile and the examination of liver enzymes with serum electrolytes (1). Routine anaesthesia applications are made on the basis of clinical laboratory values when there is no requirement for new laboratory tests. Preoperative preparation begins in the department where the patient has been admitted. Preparation protocols are defined in each clinic according to the type of surgery to be applied.
Many solutions are used for bowel preparation. When current side effect profiles are taken into consideration, polyethylene glycol-electrolyte (PEG) solutions with sodium phosphate (NaP) solutions are preferred over prokinetic and laxative agents (9). As PEG solutions have a high volume, the salty taste and sulphur smell have a negative effect on patient tolerance (10). As NaP preparations have a low volume and are better tolerated, they are preferred more by clinicians. However, NaP solutions may cause severe fluid-electrolyte shifts and acute phosphate nephropathy (9, 11).
In retrospective studies conducted on laparoscopic radical prostatectomy and colorectal surgery, no difference has been determined in terms of complications between cases who underwent mechanical bowel preparation and those did not (3, 12). However, in many countries, including Turkey, bowel cleansing is still applied routinely before major abdominal surgery.
In the current study, as the urology clinic protocol required, patients were admitted to the relevant department 1 day before surgery, and a nutritional regimen was started. Then, bowel preparation was made with a rectal enema of sodium biphosphate/sodium phosphate (NaH2PO4-Na2HPO4) twice in 12 hours and with a single dose of oral laxative soda sodium biphosphate/sodium phosphate (NaH2PO4-Na2HPO4). None of the patients showed toleration problems, and 2 of them had transient mild abdominal pain. Patients were taken to the operating room 8 hours after the last dose of bowel preparation.
After removal of cancerous prostate tissue in RARP operations, anastomosis is made between the ureter and the bladder. During the application of anastomosis, urine coming from the ureter may obscure the surgical visual field in the pelvic cavity (5).
The application of intraoperative fluid restriction is helpful in reducing the outflow of urine and provides an improved surgical field of vision during anastomosis between the bladder neck and ureter. In addition, fluid restriction reduces the airway oedema and facial oedema, which occur with the steep Trendelenburg position (13, 14).
In RARP operations, a practical approach in fluid replacement is recommended as a 1-L bolus of lactated Ringer’s solution before ureterovesical anastomosis and 1 L after completion of ureterovesical anastomosis and 2 L of crystalloid given throughout all cases and then continued at 150 mL h−1 for 12–24 hours (12, 14, 15). In some studies (5), fluid replacement is recommended as 30 mL kg−1 h−1 of hourly urine outflow after anastomosis, although in this method, the time to anastomosis can not be evaluated due to urine drainage into the abdominal cavity.
In the current study, due to possible variations in the duration of the operation, a standard infusion of 1 mL kg−1 h−1 0.09% NaCl with 1 mL kg−1 h−1 0.6% HES 200/05 was started following anaesthesia induction and was continued throughout the operation in all cases.
Since 2007, more than 450 robotic radical prostatectomy operations have been performed in our clinic. During that time, difficulties were experienced in the early cases related to impairment of the surgical view, as fluid restriction was not applied. More recently, in light of both our own experience and information in the literature, we created a perioperative fluid restriction protocol, as used in this current study in robotic surgery. From the patients who were followed up with this protocol, there have been no complications, such as leakage of anastomosis, or complaints from surgeons of the loss of surgical vision due to urine drainage.
The effect of gravity in the steep Trendelenburg position may cause oedema in the face, eyes and upper respiratory tract mucosa. Therefore, crystalloids are recommended together with colloids (5). As severe facial and conjunctival oedema was seen in the first cases to which we applied robotic surgery, we started to use a crystalloid-colloid combination in fluid restriction, and this risk was significantly reduced.
In the current study, although the anaesthesia technique, fluid restriction regimen, BMI and position were almost standard between the patients, facial oedema was observed in 63.3% (n=31) of the cases included. Conjunctival oedema was observed in 55.1% (n=27) of cases. We concluded that the facial oedema was related to the gravity force and structure of the connective tissue of the patients.
Previous studies have shown that fluid restriction and bowel preparation in patients cause a slight rise in creatinine values (4, 5). Most of the time, this is corrected before the patient is discharged from the hospital (4, 5). In the current study, although the creatinine values at 6 hours (T3) were determined as statistically significantly high compared to those in the polyclinic (T1) and at the start of the operation (T2), there was no statistically significant increase in BUN values. In addition, due to the reference value intervals of these values, they were not used as a clinical marker of increased creatinine and kidney damage. No statistically significant increase in creatinine or BUN was determined in any patient at the laboratory examination before discharge compared with the polyclinic values.
In RARP patients, hypovolemia due to routine preoperative bowel preparation and intraoperative fluid restriction may be a strong cause of perioperative dehydration (16). Hypotension, which is one of the most common clinical signs of dehydration, may be masked in this patient group due to the volume effect of the steep Trendelenburg position, which was given following anaesthesia induction.
In the current study, due to the effect of intubation, MAP values at 5 and 10 minutes were found to be significantly higher than the initial values. Hypovolemic patients are sensitive to the vasodilator and the negative inotropic effects of volatile anaesthetics, barbiturates and agents, which induce histamine release. The decrease at the 20th minute was thought to be related to the depressant effect of the anaesthetic agents due to hypovolemia. After 20 minutes of induction, a steep Trendelenburg position was given to the patient. The volume effect of this position masked hypotension, and no significant decreases were seen in MAP values during the operation (Figure 1).
It is unfortunate that most of the literature about bowel preparation is related to endoscopic and colonoscopic procedures. No literature could be found related to bowel preparation and fluid restriction with patients under general anaesthesia. Therefore, the findings of the current study could not be compared with other studies, but to create awareness of this subject, potential complications and similar studies were examined.
The preoperative laboratory values of these patients to whom mechanical bowel preparation and fluid restriction were applied may be different and misleading, especially in respect of electrolytes. In addition, bowel preparation and fluid restriction applied during the operation may cause hyperphosphataemia, hyponatraemia, hypokalaemia and hypocalcaemia with acute tubular necrosis, metabolic acidosis and renal impairment in this group of patients (17–19).
Even though a particular interaction has not been defined during general anaesthesia and hyperphosphataemia, the renal functions must be carefully evaluated (20). As the vasodilator and cardiac depression effects of anaesthetics become more severe, hypernatraemia can lead to hypotension and tissue hypoperfusion. In animal experiments, hypernatraemia has also been shown to increase the MAC of volatile anaesthetics (20). Hypokalaemia causes hyperpolarisation in the cardiac cells, which may be encountered as ectopic tachycardia and delayed transmission.
In patients who will undergo cardiac surgery, K+ <3.5 mEq L−1 is related to increased perioperative dysrhythmias, especially with the incidence of atrial fibrillation/flutter. In some patients, hypokalaemia may cause increased neuromuscular blocker sensitivity, so their dosage should be reduced by 25%–50%, and monitoring should be applied with a nerve stimulator (21). Cardiovascular function may be impaired in hypocalcaemia, and cardiac failure, hypotension, impaired rhythm, digital insensitivity and impaired β adrenergic effects may occur. Hypocalcaemia may also potentially activate the negative inotropic effects of barbiturates and volatile anaesthetics (22). These disturbances may result in hypotension, arrhythmia and prolonged muscle relaxation in critically ill patients during anaesthetic administration.
In the current study, the patients were evaluated in the clinic preoperatively, at 6 hours after the start of the operation and before discharge with laboratory tests that are routinely used in practice. No statistically significant change was determined in the AST, ALT, Na and urea measurements.
In addition, the statistically significant reductions found in the K+ and Ca++ values preoperatively (T2) compared to the values seen in the clinic (T1) were not evaluated as hypokalaemia and hypocalcaemia clinically because of the reference value intervals. Severe dysrhythmias or cardiac depression that is thought to be associated with hypokalaemia and hypocalcaemia was not seen in any patient in anaesthesia induction and maintenance.
The specific gravity of urine with Cl− values preoperatively (T2) and at 6 hours after the start of the operation (T3) was determined as high compared to the preanaesthetic values (T1) and were evaluated as findings supporting dehydration. Although the increase in creatinine values at this level of dehydration was statistically significant at the 6th hour, as this increase was within the reference range, and the increase in BUN values were not statistically significant, the conclusion was reached that renal function was not greatly affected in the ASA I–II patient group.
The laboratory values of all patients were evaluated as being within normal limits prior to discharge, and no statistical difference was observed in comparison with the preanaesthetic values. No cardiac dysrhythmia was observed in any of the patients in the study, but in 4 patients, the 10-minute Aldrete score was determined to be low. This is thought to be due to the exclusion of patients with co-morbidities in the ASA I–II patient group of this study.
As the patient group that was selected for inclusion in the study comprised ASA I–II patients without any renal or liver disease, the predicted increases and decreases in laboratory values, which would not create a clinical effect, were observed. The application of the study to patients in the ASA III–VI patient group would enable a greater awareness of the problems that may be encountered in the anaesthesia of these patients.
Conclusion
In spite of the fact that there were no severe increases or decreases in laboratory test values (Table 2) that reflected on the clinical outcome in the ASA I–II patient group due to bowel preparation and fluid restriction in RARP operations, these changes may be important in critically ill or ASA III–IV patients. In these patients, laboratory results that are considered “normal” in the pre-anaesthesia clinic may be misleading, and cardiac arrhythmia due to hypokalaemia and hypocalcaemia, as well as problems, such as prolonged non-depolarising blockade and delayed recovery from anaesthesia, may be observed during practice.
In conclusion, it should be kept in mind that dehydration can be masked in patients scheduled for RARP operations. In the preoperative period, it would be appropriate to give balanced electrolyte solutions to these patients in order to prevent electrolyte disorders that may occur during preoperative bowel preparation. After this study, routine administration of balanced electrolyte solutions was implemented in our clinic after intestinal preparation applied the night before surgery in RARP patients.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Ümraniye Training and Research Hospital.
Informed Consent: Written informed consent was obtained from all participants who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - G.K.; Design - GK., N.B.; Supervision - G.K., N.B., C.T.Y.; Funding - C.T.Y., A.O.Ç.; Materials - A.O.Ç.; Data Collection and/or Processing - G.K., N.B.; Analysis and/or Interpretation - G.K., N.B., C.T.Y., A.O.Ç.; Literature Review - G.K., C.T.Y.; Writer - G.K.; Critical Review - N.B., C.T.Y., A.O.Ç.; Other - G.K., N.B., C.T.Y., A.O.Ç.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Türk Anestezi ve Reanimasyon Derneği Anestezi Uygulama Klavuzları, Preoperatif Hazırlık, Kasım2005 http://www.tard.org.tr/kilavuz/3.pdf
- 2.Ogün S, Aslan G. Bowel preparation before radical cystectomy. Uroloji Bülteni. 2012;1:62–5. [Google Scholar]
- 3.Ram E, Sherman Y, Weil R, Vishne T, Kravarusic D, Dreznik Z. Is mechanical bowel preparation mandatory for elective colon surery? Arch Surg. 2005;140:285–8. doi: 10.1001/archsurg.140.3.285. http://dx.doi.org/10.1001/archsurg.140.3.285. [DOI] [PubMed] [Google Scholar]
- 4.Mattei A, Di Pierro GB, Rafeld V, Konrad C, Beutler J, Danuser H. Positioning injury, rhabdomyolysis, and serum creatine kinase-concentration course in patients undergoing robot-assisted radical prostatectomy and extended pelvic lymph node dissection. J Endourol. 2013;27:45–51. doi: 10.1089/end.2012.0169. http://dx.doi.org/10.1089/end.2012.0169. [DOI] [PubMed] [Google Scholar]
- 5.Izdes S. Anesthesia in the robotic surgery. Anestezi Dergisi. 2012;20:63–72. [Google Scholar]
- 6.Matsou A, Vrakas G, Doulgerakis M, Hatzimisios K, Zandes N, Saliangas K. Mechanical bowel preparation before elective colorectal surgery:is it necessary? Tech Coloproctol. 2011;15(Suppl 1):59–62. doi: 10.1007/s10151-011-0733-1. http://dx.doi.org/10.1007/s10151-011-0733-1. [DOI] [PubMed] [Google Scholar]
- 7.Gainsburg DM. Anesthetic concerns for robotic-assisted laparoscopic radical prostatectomy. Minerva Anestesiol. 2012;78:596–604. [PubMed] [Google Scholar]
- 8.Barkun A, Chiba N, Enns R, Marcon M, Natsheh S, Pham C, et al. Commonly used preparations for colonoscopy: Efficacy, tolerability and safety- A Canadian association of Gastroenterology position paper. Can J Gastroenterol. 2006;20:699–710. doi: 10.1155/2006/915368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kao D, Lalor E, Sandha G, Fedorak RN, Van der Knoop B, Doornweerd S, et al. A randomized controlled trial of four precolonoscopy bowel cleansing regimens. Can J Gastroenterol. 2011;25:657–62. doi: 10.1155/2011/486084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar A, Hussain A. Preoperative bowel preparation in children: Polyethylene glycol versus normal saline. Afr J Paediatr Surg. 2013;10:235–8. doi: 10.4103/0189-6725.120889. http://dx.doi.org/10.4103/0189-6725.120889. [DOI] [PubMed] [Google Scholar]
- 11.Heher EC, Their SO, Rennke H, Humphreys BD. Adverse renal and metaolic effects associated with oral sodium phosphate bowel preparation. Clin J Am Soc Nephrol. 2008;3:1494–503. doi: 10.2215/CJN.02040408. http://dx.doi.org/10.2215/CJN.02040408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugihara T, Yasunaga H, Horiguchi H, Fujimura T, Nishimatsu H, Kume H, et al. Is mechanical bowel preparation in laparoscopic radical prostatectomy beneficial? An analysis of Japanese national database. BJU Int. 2013;112:76–81. doi: 10.1111/j.1464-410X.2012.11725.x. http://dx.doi.org/10.1111/j.1464-410X.2012.11725.x. [DOI] [PubMed] [Google Scholar]
- 13.Irvine M, Patil V. Anaesthesia for robot-assisted laparoscopic surgery. Contin Educ Anaesth Crit Care Pain. 2009;9:125–9. http://dx.doi.org/10.1093/bjaceaccp/mkp020. [Google Scholar]
- 14.Danic MJ, Chow M, Alexander G, Bhandari A, Menon M, Brown M. Anesthesia considerations for robotic-assisted laparoscopic prostatectomy: a review of 1,500 cases. J Robotic Surg. 2007;1:119–23. doi: 10.1007/s11701-007-0024-z. http://dx.doi.org/10.1007/s11701-007-0024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Alonzo RC, Gan TJ, Moul JW, Albala DM, Polascik TJ, Robertson CN, et al. A retrospective comparison of anesthetic management of robot-assisted laparoscopic radical prostatectomy versus radical retropubic prostatectomy. J Clin Anesth. 2009;21:322–8. doi: 10.1016/j.jclinane.2008.09.005. http://dx.doi.org/10.1016/j.jclinane.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Lichtenstein GR, Cohen LB, Uribarri J. Review article: Bowel preparation for colonoscopy - the importance of adequate hydration. Aliment Pharmacol Ther. 2007;26:633–41. doi: 10.1111/j.1365-2036.2007.03406.x. http://dx.doi.org/10.1111/j.1365-2036.2007.03406.x. [DOI] [PubMed] [Google Scholar]
- 17.Ben Chaabane N, Ben Mansour W, Hellara O, Ben Mansour I, Melki W, Loghmeri H, et al. Bowel preparation before colonoscopy. Presse Med. 2012;41:37–42. doi: 10.1016/j.lpm.2011.04.017. http://dx.doi.org/10.1016/j.lpm.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 18.Kamolpornwijit W, Phupong V. Oral potassium chloride and oral rehydration solution supplement to prevent hypokalemia in sodium phosphate regimen for bowel preparation prior to gynecological laparoscopic surgery. J Med Assoc Thai. 2008;91:615–8. [PubMed] [Google Scholar]
- 19.Yakut M, Cinar K, Seven G, Cetinkaya H, Bahar K. The efficacy and safety of colonoscopy preparation with oral sodium phosphate in elderly patients. Turk J Gastroenterol. 2010;21:140–5. doi: 10.4318/tjg.2010.0072. [DOI] [PubMed] [Google Scholar]
- 20.Butterworth J, Mackey DC, Wasnick J. Clinical Anesthesia. 5th edition. United States: The Mc Graw-Hill Companies; 2013. [Google Scholar]
- 21.Wong KC. Hypokalemia and anesthetic implications. Anesth Analg. 1993;77:1238–60. doi: 10.1213/00000539-199312000-00027. http://dx.doi.org/10.1213/00000539-199312000-00027. [DOI] [PubMed] [Google Scholar]
- 22.Aguilera IM, Vaughan RS. Calcium and the anaesthetist. Anaesthesia. 2000;55:779–90. doi: 10.1046/j.1365-2044.2000.01540.x. http://dx.doi.org/10.1046/j.1365-2044.2000.01540.x. [DOI] [PubMed] [Google Scholar]