Abstract
Objective
The aim of this study is to compare the sedative properties and haemodynamic and respiratory effects of dexmedetomidine and a ketamine-propofol combination (ketofol), which are expected to have minimal effects on spontaneous breathing.
Methods
Sixty patients were enrolled in this prospective randomised study. Patients were divided into 2 groups according to the administration of dexmedetomidine (Group D) and ketofol (Group K). Target sedation level was determined as a Ramsay Sedation Score of 3. In Group D, 0.5 mcg kg−1 dexmedetomidine was administered via intravenous route in 10 minutes versus 0.125 mL kg−1 of a solution containing 200 mg propofol and 100 mg ketamine in Group K. Haemodynamic and respiratory effects, postoperative awakening time, analgesic properties and satisfaction levels of the patients and surgeon were assessed.
Results
There was a statistically significant decrease in mean arterial pressures following drug administration compared to initial measurements in both groups. However, there was a statistically significant decrease in heart rate only in Group D. There was no significant difference between the two groups regarding respiratory rate and protection of spontaneous respiration. Although the time for Aldrete score to be 9 was 16.1 minutes for Group K, it was 24.9 minutes for Group D, and this difference was statistically significant (p<0.01). There was no significant difference between the two groups regarding adverse effects, pain scores and satisfaction levels of the patients and surgeon.
Conclusion
Compared to dexmedetomidine, at similar sedation levels, sedation provided by ketofol enables satisfactory analgesia. Moreover, ketofol has a more rapid onset of action and a shorter recovery period from anaesthesia without causing significant haemodynamic or respiratory adverse effects.
Keywords: Dexmedetomidine, ketofol, sedation, cataract surgery
Introduction
Cataract surgery and vitreoretinal surgery are the most common ophthalmic surgical procedures, and they are generally performed under local anaesthesia (LA) with or without sedation (1, 2). Propofol, benzodiazepines, opioids and combinations of them are frequently used for sedation. Cataract surgery is more often applied to elderly patients. At advanced ages, the choice of anaesthetic agent becomes more important because systemic diseases appear and the responses given to the drugs change. It has been reported that sensitivity to propofol increases by 30%–50% and the elimination half-life of midazolam extends more than twofold in elderly patients (3, 4). Moreover, it has been stated that the hallucinogenic effect of ketamine is not apparent in the elderly patients; therefore, it can be used safely as an analgesic and sedative agent (5).
Because benzodiazepines and propofol do not have any analgesic effect, opioids are often administered as a rescue analgesic in painful procedures. Additional opioid administration can lead to excessive sedation and some side effects. It has been reported that ketamine can be an alternative to opioids because it provides good analgesia even at low doses, and it has opposite respiratory and cardiovascular effects compared to opioids (6).
Dexmedetomidine is a selective α-2 agonist with sedative and analgesic properties and its most important advantage is that it does not cause respiratory depression (7). Propofol is frequently used in sedation because of a rapid onset of action and a short recovery profile (8). It has haemodynamic and respiratory depressor effects depending on the dose. It has been reported that propofol can be used in combination with ketamine at a sub-hypnotic dose for providing an analgesic effect and the incidence of side effects can be decreased because of a reducible dose of propofol (9).
The aim of our study was to compare dexmedetomidine and ketamine-propofol combination (ketofol), which are among the analgesic drugs expected to have minimal effects on spontaneous breathing, for sedation in cataract surgery. The primary outcomes were considered to be haemodynamic, and respiratory effects and secondary outcomes were determined to be awakening features and side effects, respectively.
Methods
After receiving approval from the ethics committee of the Harran University Faculty of Medicine Ethics Advisory and Monitoring Board (Date: 06.05.2013, No: 2013/128), the study included 60 patients for whom phacoemulsification cataract surgery and intraocular lens implantation under retrobulbar block at elective conditions were planned and whose written informed consent was obtained. The inclusion criteria were determined to be American Society of Anaesthesiologists (ASA) physical status of I–III and an age over 45 years. Patients with histories of an additional surgery plan, communication problem (such as hearing and speech impairment), uncontrolled systemic disease, allergy to local anaesthetic or study drugs, chronic analgesic or sedative usage and alcohol or substance abuse were excluded from the study. The study was conducted in the Education and Research Hospital of the Medicine Faculty at Ordu University between June and December 2013.
The patients were divided into two groups with block randomization formed via a computer before the intervention: Group D (patients who would be administered with dexmedetomidine) and Group K (patient who would be administered with ketofol). The patients taken into the operation room underwent standard monitorization, including electrocardiography, non-invasive blood pressure and peripheral oxygen saturation (SpO2) (Mindray, BeneViev T8, Shenzhen, P.R. China). For Group D, 2 mL of dexmedetomidine was diluted with 48 mL of saline and a solution including 4 mcg/mL of dexmedetomidine was prepared. Then, 0.5 mcg kg−1 loading dose was administered intravenously to the patients in 10 min. For Group K, 20 mL of propofol (Propofol-Lipuro 1%, 10 mg mL−1, 20 mL vial; B Braun, Melsungen, Germany), 2 mL of ketamine (Ketalar 50 mg mL−1, 10 mL; Eczacıbaşı, Turkey) and 28 mL of saline were combined and a solution containing 4 mg of propofol and 2 mg mL−1 of ketamine was prepared. Subsequently, 0.125 mL kg−1 loading dose of the solution, including propofol and ketamine in the proportion of 2:1, was intravenously administered to patients in 10 min. Study medications were given with a volumetric infusion pump (Perfusor Space, B Braun, Melsungen, Germany). All patients were monitored using the Ramsay sedation score (RSS) (10) every 5 min, and the target score was considered to be 3 (Table 1). After loading doses, infusion was started at the rate of 0.2–0.7 mcg kg−1 hr−1 in Group D and at the rate of 0.05–0.125 mL kg−1 hr−1 in Group K. Patients were given 4 L min−1 of O2 with a facial mask. After administering the loading doses of the medications, the retrobulbar block was applied with 3 mL of lidocaine (Jetokain 20 mg mL−1, 2 mL, Adeka, Turkey). Surgical intervention and block procedure were performed by the same surgeon (second author) in all patients.
Table 1.
Ramsay sedation score
| Score | State |
|---|---|
| 1 | Awake, agitated and restless patient |
| 2 | Cooperative, oriented and calm patient |
| 3 | Patient responding to only commands |
| 4 | Patient sleeping and responding rapidly to glabellar tap |
| 5 | Patient sleeping and responding slowly to stimuli |
| 6 | Patient not responding to painful stimulus |
Intraocular pressures (IOP) of the patients were measured as baseline values and values after administration of drugs via a hand-held tonometer (i-Care TA01i, Tiolat Oy, Helsinki, Finland) from the non-operated side. During the operation, the patients were monitored for their heart rate (HR), mean arterial pressure (MAP), SpO2 and respiratory rate (RR). Monitoring times were determined as follows: T1: baseline value; T2: after loading dose; T3: after block procedure; T4: beginning of intervention; T5, T6 and T7: 5th, 10th and 15th min during procedure; T8: end of intervention and T9 and T10: postoperative 5th and 15th min. The heart rate lower than 50 beats per minute was recorded as bradycardia and 0.5 mg of atropine was administered intravenously. The decrease of 30% from the baseline value in the MAP was considered to be hypotension and 5 mg of ephedrine was administered intravenously. Bradypnea was defined as the condition with an RR below 10 per min, and desaturation was defined as the value of SpO2 lower than 90%. Airway opening manoeuvres were implemented when necessary. Drug infusions were ended when the surgery was completed, and patients were transferred to the recovery room. The patients, who were followed up with the Modified Aldrete Scoring system (MAS) in the recovery room, were sent to the service with the score of 9 and over. Other follow-up parameters were as follows:
- The time of RSS: 3
- Pain during the procedure and block (retrobulbar block) [evaluated with 10-point visual analogue scale (VAS); 0 point: no pain, 10 points: severe pain]
- The time of MAS: 9
- Postoperative pain (evaluated with VAS at the 1st, 2nd and 4th h)
- Patient satisfaction [evaluated according to the response given to the question “How do you evaluate your experience of anaesthesia that you had taken during your operation?” in the 7-point Likert-like verbal rating scale (11). 1: strongly dissatisfied, 2: dissatisfied, 3: partly dissatisfied, 4: undecided, 5: partly satisfied, 6: satisfied, 7: strongly satisfied]
- Surgeon satisfaction (evaluated similar to patient satisfaction)
According to our evaluation based on a previous study (12), when the main result was considered to be the time of reaching the targeted sedation level, it was calculated that the difference of 10% with respect to haemodynamic parameters between the groups, each of which included at least 26 patients, could be detected as 90% power and 5% significance values (α: 0.05, β: 0.90). Considering possible overlooks, it was planned to include 30 patients in each group (Minitab Inc. State College PA, USA).
Statistical analysis
The data obtained in the study were analysed using the Statistical Package for the Social Sciences (SPSS) 20.0 software (IBM SPSS Statistics, Chicago, IL, USA). The Shapiro–Wilk test was used in comparisons to detect the distribution of variable groups. Descriptive statistics were demonstrated as mean±standard deviation for continuous variables and as the number of patients and percentage (%) for nominal variables. While comparing the differences between two different drug administrations, Student’s t-test was employed for data showing normal distribution and Mann–Whitney U test was used for data not showing normal distribution. Moreover, the data related to MAP and respiration were evaluated with repeated measures one way analysis of variance (ANOVA) and the Bonferroni correction as a post hoc test. Chi-square analysis was used for the evaluation of data obtained as a result of cross tabulation. In all analyses, the value of p<0.05 was accepted to be statistically significant.
Results
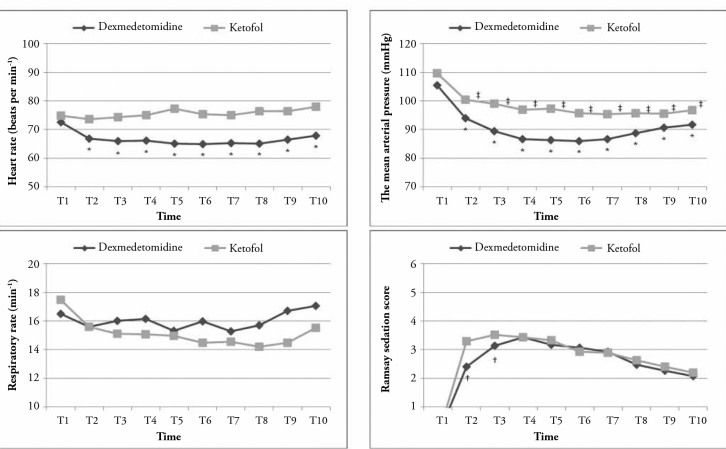
Of 78 patients evaluated for our study, eight patients with uncontrolled systemic disease, five patients having undergone additional surgical procedures, three patients using chronic analgesics and two patients with whom no communication could be established were excluded from the study. The data obtained from 60 cases who satisfied the inclusion criteria were evaluated. No significant difference was found between the groups with respect to the age, gender, ASA score and duration of surgery (Table 2). The time passing from the beginning of the administration of study drugs to reaching the targeted sedation score (RSS: 3) was 16.10±2.67 min in the dexmedetomidine group and 8.93±1.72 min in the ketofol group; the difference between them was statistically significant (p<0.001). Although there was a statistically significant difference between the groups with respect to RSS in T2 (after drug administrations) and T3 (after nerve blockade) monitoring times (p<0.001 and p<0.017, respectively), no significant difference was found in other measurement times (Figure 1).
Table 2.
Demographic data and duration of surgery
| Group D (n: 30) | Group K (n: 30) | Significance* (p) | |
|---|---|---|---|
| Age (years) | 68.6±8.4 | 66.4±6.3 | 0.434 |
| Gender (F/M) | 14/16 | 17/13 | 0.688 |
| ASA I/II/III | 4/20/6 | 3/18/8 | 0.356 |
| Duration of surgery (min) | 38.1±11.3 | 36.4±10.2 | 0.562 |
Data are presented as mean±standard deviation and number of patients. F: female; M: male; ASA: American Society of Anaesthesiologists
chi-square and t tests
Figure 1.
Perioperative haemodynamic and respiratory changes and the depth of sedation
* and †significant according to the baseline value, ‡Significant between the groups, t-test and ANOVA
T1: basal value; T2: after loading dose; T3: after block procedure; T4: beginning of the intervention; T5, T6 and T7: 5th, 10th and 15th min during the surgery; T8: end of the intervention and T9 and T10: postoperative 5th and 15th min
No statistically significant difference was detected between the control IOP values of the groups (19.10±7.01 mmHg in Group D AND 15.07±4.02 mmHg in Group K). After the administrations of the drugs, the values of IOP were significantly decreased to 14.73±5.27 mmHg in Group D and 11.43±2.34 mmHg in Group K. The rates of these decreases in IOP values were not statistically significant.
In the dexmedetomidine group, a statistically significant decrease was found between the baseline values and following measurements for the values of HR and MAP. In the ketofol group, while MAP values significantly decreased compared to the baseline values, no significant change was observed in HR values (Figure 1). With respect to RR, no significant change was found in both groups compared to the baseline values.
In one patient in the ketofol group, it was required to open the airway through the chin lift manoeuvre because of deep sedation (RSS:5). Manual ventilation was not needed, and the protection of spontaneous airway was provided in a short time. On the other hand, cough developed in one patient. Other side effects such as apnoea, desaturation, hypotension, nausea-vomiting, bradycardia, agitation and hallucination were not observed in any case. These side effects did not also develop in the dexmedetomidine group.
In the dexmedetomidine group, the time when MAS was 9 was longer than that in the ketofol group (24.9±4.5 min vs. 16.1±2.1 min) and the difference between them was statistically significant (p<0.001). With respect to VAS values during the block procedure and at the postoperative 1st, 2nd and 4th h, no statistically significant difference was observed between the groups. VAS value at any measurement time did not require the administration of additional analgesics (VAS <4). Moreover, with respect to patient and surgeon satisfaction levels, any statistically significant difference was not found between the groups (Table 3).
Table 3.
Clinical data
| Group D (n: 30) | Group K (n: 30) | Significance (p) | |
|---|---|---|---|
| The time of RSS 3 (min) | 16.1±2.6 | 8.9±1.7* | <0.001 |
| VAS during block | 1.9±0.7 | 1.5±0.7 | 0.66 |
| The time of Aldrete: 9 (min) | 24.9±4.5 | 16.1±2.1* | <0.001 |
| VAS | |||
| Postoperative 1st h | 1.9±0.5 | 1.7±0.5 | 0.560 |
| Postoperative 2nd h | 1.8±0.6 | 1.7±0.4 | 0.490 |
| Postoperative 4th h | 2.1±0.6 | 2.0±0.6 | 0.565 |
| Patient satisfaction | 6.3±0.5 | 6.1±0.7 | 0.084 |
| Surgeon satisfaction | 6.4±0.6 | 6.2±0.8 | 0.067 |
Data are presented as mean±standard deviation. RSS: Ramsay sedation score; VAS: visual analogue scale
Statistically significant compared to Group D, Mann–Whitney U test
Discussion
During ophthalmic surgical procedures that would be performed under local/regional anaesthesia, it is desired that the patient should be sedated but also conscious for providing cooperation and the IOP level should be low (13). Therefore, many sedative agents are administered alone or in combination. The most commonly used sedative agents are propofol, midazolam and opioids (14, 15). It has also been reported that dexmedetomidine, which is a selective α2 adrenoreceptor agonist, is successfully used in the treatment of cataract (1, 12, 16–18). The most important advantage of dexmedetomidine seems to be its ability to exert analgesic and anxiolytic effects without causing respiratory depression (1). The analgesic efficiency of ketamine at sub-anaesthetic doses is known and it can be used with other sedative agents (15, 19). Therefore, the use of ketamine with propofol is popular. With this combination, a less amount of propofol is used and the side effects of propofol are avoided. In addition, the psychomimetic side effects of ketamine are reduced and a clinically important respiratory depression is not encountered (9, 20). This combination can be used by mixing in a single injector (9, 21), and it can have the content of propofol and ketamine at different proportions such as 1:1 (20), 2:1 (22) and 3:1 (15). The simplicity, efficiency and reliability of this type of administration in sedation and analgesia have been demonstrated in various procedural sedation applications (9, 22, 23).
For haemodynamic and respiratory effects, which were the primary outcomes of our study, similar results were reached in both groups. In the measurements of MAP, a significant decrease was detected after the drug administrations compared to the baseline values. With respect to the HR, a decrease was observed only in Group D compared to the baseline values. On the other hand, no statistically significant change was found in Group K.
Ghali et al. (12) compared sedation via dexmedetomidine and propofol in patients having undergone vitreoretinal surgery under local anaesthesia and they found a statistically significant decrease in the HR and MAP values of both groups compared to the baseline values.
Similar results with both dexmedetomidine and propofol have been reported in the literature (16, 17, 24). It has been shown that propofol is a strong inhibitor of sympathetic activity (25). Dexmedetomidine is also known to reduce the sympathetic activity and catecholamine levels in the circulation; therefore, it is expected to cause a decrease in MAP like propofol (26). It is thought that its effect on HR is a result of its sympatholytic and vagomimetic actions (27).
With respect to RR, no statistically significant change was observed in both groups compared to the baseline values. In one patient in the ketofol group, airway opening manoeuvre was needed. Except this case, adequate respiratory function was maintained spontaneously in all patients. In the study of Ghali et al. (12), in which they compared dexmedetomidine and propofol as sedative agents, they reported that the most impressive finding of their study was the significantly high level of RR and SpO2 values in the dexmedetomidine group. We think that the absence of respiratory depression in the ketofol group resulted from a lesser amount of propofol use in our study compared with that in the abovementioned study (0.5 mg kg−1 vs. 0.7 mg kg−1). The intravenous bolus dose of dexmedetomidine that was used in our study was lower (0.5 mcg kg−1 vs. 1 mcg kg−1). Because the patients for whom cataract surgery was planned were mostly at an advanced age and had comorbid diseases and our targeted sedation score was RSS:3, this dosage was preferred. In addition, in the literature, a similar dose regime has been used for cataract surgery (16).
The aim of anaesthesia administration for ophthalmic surgery is to quell patients’ anxiety and to prevent the occurrence of pain during peri/retro-bulbar block procedures. Moreover, it is recommended to avoid deep sedation because this can increase the risks for apnoea and undesirable patient movements (28). Therefore, in our study, the targeted sedation score was determined as RSS:3 (patient responding only to commands), as in similar studies in the literature (12, 16, 23). The time for reaching this score was significantly shorter in the ketofol group. In the literature, it has been reported that sedation with propofol has a rapid onset of action compared with that with dexmedetomidine (12). Frey et al. (15) compared propofol and the propofol-ketamine combination for sedation in ophthalmic surgery and they found the onset of action to be shorter in the propofol-ketamine group.
In day-case surgeries, the duration of patient’s recovery from the effects of anaesthetic agents is important. Modified Aldrete Scoring system (MAS) is frequently used for deciding on the transfer of patient from the recovery room to the service (29). Each of blood pressure, peripheral oxygen saturation, consciousness, motor activity and respiratory parameters are evaluated with MAS over two points. With the result of this evaluation, patients having 9 or 10 points are recommended to be transferred from the recovery room to the service.
In our study, the time for reaching MAS:9 was 16 min in the ketofol group and 25 min in the dexmedetomidine group and the difference was statistically significant. Arain et al. (30) found the time for reaching MAS:9 as 34 min in the dexmedetomidine group and 28 min in the propofol group in the intraoperative sedation performed with similar doses to ours.
In the study of Ghali et al. (12), in which they compared dexmedetomidine and propofol sedation in cataract surgery, MAS reached 10 points within 40 min in the dexmedetomidine group and within 37 min in the propofol group. The difference between their study and our study may have been due to the targeted MAS score.
It has been reported that dexmedetomidine cannot be appropriate for the procedural sedation administrations in which it is used as the single agent because recovery time can extend (31).
The recovery time of the propofol-ketamine combination seems to be dependent on the concentration of ketamine in the compound. In 100 procedural sedation administrations, the mean recovery time was 26 min in the group including propofol-ketamine at the proportion of 1:1 and 15 min in the group including the combination at the proportion of 4:1 (32). Frey et al. (15) found no difference between sedation implementations performed with propofol and the propofol-ketamine combination at the proportion of 3:1 in cataract surgery with respect to the hospitalization time in the recovery room.
In our study, no statistically significant difference was observed between the groups with respect to VAS values. The analgesic effect of dexmedetomidine is well-known and it seems superior to propofol with respect to providing analgesia in the studies in which both have been compared (12, 30, 33, 34). Ketamine also has an analgesic effect, which is thought to be from the spinal cord and opioid receptors in the brain (35, 36). Analgesia provided with propofol and ketamine combination for procedural sedation has been reported to be a reasonable choice compared to fentanyl and alfentanyl because it occurs with decreased airway complications (37–40).
The administration of sedative agents with local anaesthesia in ophthalmic surgery can offer desired surgical conditions such as patient’s remaining motionless and surgery site without congestion. It is stated that the chosen sedative drugs with the effect of decreasing intraocular pressure can help the success rate to increase and complication rates to decrease by preventing the ocular content to protrude from surgical or traumatic wound (41, 42). It is known that both dexmedetomidine and the ketamine-propofol combination decreases IOP (1, 15, 18, 43).
Furthermore, no significant difference was found between the groups with respect to patient and surgeon satisfaction scores. Psychomimetic side effects such as agitation and having a nightmare in the postoperative period were not observed in any patient.
Frey et al. (15) have reported the presence of increased IOP, delayed cognitive recovery or sedation with higher quality without psychomimetic side effects with the propofol and ketamine combination administered for sedation in cataract surgery.
The main limitation of our study is that we did not perform bispectral index (BIS) measurement, which is an objective technique used for evaluating the depth of sedation. However, the presence of the relationship between RSS and BIS has been specified in the literature (44, 45). Another limitation is the lack of monitoring discharge time, which is an important parameter for the assessment of anaesthesia techniques used in day-case surgeries.
Conclusion
In this study, it was found that sedation with ketofol provided adequate analgesia compared to dexmedetomidine at similar sedation levels, and it had a more rapid onset of action and shorter recovery time without leading to any haemodynamic and respiratory side effects. Ketofol can be a valuable alternative for the sedation of patients having undergone cataract surgery with phacoemulsification technique under retrobulbar block anaesthesia.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Harran University Faculty of Medicine Ethics Advisory and Monitoring Board (Date: 06.05.2013, No: 2013/128).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - Ö.Y., R.H.K., N.T., A.K.; Design - Ö.Y., R.H.K., N.T., A.K.; Supervision - Ö.Y., R.H.K., N.T., A.K.; Funding - Ö.Y., N.T.; Materials - Ö.Y., R.H.K.; Data Collection and/or Processing - Ö.Y., A.K.; Analysis and/or Interpretation - Ö.Y., N.T., A.K.; Literature Review - Ö.Y., N.T.; Writer - Ö.Y., R.H.K.; Critical Review - Ö.Y., R.H.K., N.T., A.K.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Vann MA, Ogunnaike BO, Joshi GP. Sedation and anesthesia care for ophthalmologic surgery during local/regional anesthesia. Anesthesiology. 2007;107:502–8. doi: 10.1097/01.anes.0000278996.01831.8d. http://dx.doi.org/10.1097/01.anes.0000278996.01831.8d. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld SI, Litinsky SM, Snyder DA, Plosker H, Astrove AW, Schiffman J. Effectiveness of monitored anesthesia care in cataract surgery. Ophthalmology. 1999;106:1256–60. doi: 10.1016/S0161-6420(99)00705-8. http://dx.doi.org/10.1016/S0161-6420(99)00705-8. [DOI] [PubMed] [Google Scholar]
- 3.Peacock JE, Spiers SP, McLauchlan GA, Edmondson WC, Berthoud M, Reilly CS. Infusion of propofol to identify smallest effective doses for induction of anaesthesia in young and elderly patients. Br J Anaesth. 1992;69:363–7. doi: 10.1093/bja/69.4.363. http://dx.doi.org/10.1093/bja/69.4.363. [DOI] [PubMed] [Google Scholar]
- 4.Reves JG, Fragen RJ, Vinik HR, Greenblatt DJ. Midazolam: pharmacology and uses. Anesthesiology. 1985;62:310–24. http://dx.doi.org/10.1097/00000542-198503000-00017. [PubMed] [Google Scholar]
- 5.Tüzüner F. Anestezi Yoğun Bakım Ağrı Ankara. 2010:1096–7. [Google Scholar]
- 6.Çelik N, Bombacı E, Çolakoğlu S, Ekinci O, Kaya GB, Aydın N. Propofol-ketamin ve propofol-fentanil ile yapılan total intravenöz anestezide hemodinami ve derlenmenin karşılaştırılması. J Kartal Tr. 2000;11:801–4. [Google Scholar]
- 7.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnesic, and analgesic properties of small-dose Dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. http://dx.doi.org/10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 8.Rao GP, Wong D, Groenewald C, McGalliard JN, Jones A, Ridges PJ. Local anesthesia for vitreoretinal surgery: a case control study of 200 cases. Eye. 1998;12:407–11. doi: 10.1038/eye.1998.96. http://dx.doi.org/10.1038/eye.1998.96. [DOI] [PubMed] [Google Scholar]
- 9.Badrinath S, Avramov MN, Shadrick M, Witt TR, Ivankovich AD. The use of a ketamine-propofol combination during monitored anesthesia care. Anesth Analg. 2000;90:858–62. doi: 10.1097/00000539-200004000-00016. http://dx.doi.org/10.1213/00000539-200004000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. BMJ. 1974;2:656–9. doi: 10.1136/bmj.2.5920.656. http://dx.doi.org/10.1136/bmj.2.5920.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Streiner DL, Norman GR. Scaling responses. In: Streiner DL, Norman GR, editors. Health Measurement Scales: A Practical Guide to Their Development and Use. Oxford: Oxford University Press; 1995. pp. 28–53. [Google Scholar]
- 12.Ghali A, Mahfouz AK, Ihanamaki T, El Btarny AM. Dexmedetomidine versus propofol for sedation in patients undergoing vitreoretinal surgery under sub -Tenon’s anesthesia. Saudi J Anaesth. 2011;5:36–41. doi: 10.4103/1658-354X.76506. http://dx.doi.org/10.4103/1658-354X.76506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karabıyık L, Çetin H, Çukur S. Yaşlılarda bilinçli sedasyon için uygulanan midazolam intraoküler basıncı azaltır mı? Çukurova Üniversitesi Tıp Fakültesi Dergisi. 1998;23:160–5. [Google Scholar]
- 14.Cok OY, Ertan A, Bahadir M. Comparison of midazolam sedation with or without fentanyl in cataract surgery. Acta Anaesthesiol Belg. 2008;59:27–32. [PubMed] [Google Scholar]
- 15.Frey K, Sukhani R, Pawlowski J, Pappas AL, Mikat-Stevens M, Slogoff S. Propofol versus propofol-ketamine sedation for retrobulbar nerve block: comparison of sedation quality, intraocular pressure changes, and recovery profiles. Anesth Analg. 1999;89:317–21. doi: 10.1097/00000539-199908000-00013. http://dx.doi.org/10.1097/00000539-199908000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Na HS, Song IA, Park HS, Hwang JW, Do SH, Kim CS. Dexmedetomidine is effective for monitored anesthesia care in outpatients undergoing cataract surgery. Korean J Anesthesiol. 2011;61:453–9. doi: 10.4097/kjae.2011.61.6.453. http://dx.doi.org/10.4097/kjae.2011.61.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apan A, Doganci N, Ergan A, Büyükkoçak U. Bispectral index-guided intraoperative sedation with dexmedetomidine and midazolam infusion in outpatient cataract surgery. Minerva Anestesiol. 2009;75:239–44. [PubMed] [Google Scholar]
- 18.Ayoglu H, Altunkaya H, Ozer Y, Yapakci O, Ozkocak I, Oz O, et al. Dexmedetomidine sedation during cataract surgery under regional anaesthesia. Br J Anaesth. 2007;99:448. doi: 10.1093/bja/aem226. http://dx.doi.org/10.1093/bja/aem226. [DOI] [PubMed] [Google Scholar]
- 19.Morse Z, Sano K, Kanri T. Effects of a propofol-ketamine ad-mixture in human volunteers. Pac Health Dialog. 2003;10:51–4. [PubMed] [Google Scholar]
- 20.Willman EV, Andolfatto G. A prospective evaluation of “ketofol” (ketamine/propofol combination) for procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2007;49:23–30. doi: 10.1016/j.annemergmed.2006.08.002. http://dx.doi.org/10.1016/j.annemergmed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Golden S. Combination propofol-ketamine anaesthesia in sick neonates. Paediatr Anaesth. 2001;11:119–22. doi: 10.1046/j.1460-9592.2001.00605.x. http://dx.doi.org/10.1046/j.1460-9592.2001.00605.x. [DOI] [PubMed] [Google Scholar]
- 22.Kogan A, Efrat R, Katz J, Vidne BA. Propofol-Ketamine mixture for anesthesia in pediatric patients undergoing cardiac catheterization. J Cardiothorac Vasc Anesth. 2003;17:691–3. doi: 10.1053/j.jvca.2003.09.008. http://dx.doi.org/10.1053/j.jvca.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Mahfouz AK, Ghali AM. Combined use of remifentanil and propofol to limit patient movement during retinal detachment surgery under local anesthesia. Saudi J Anaesth. 2010;4:147–51. doi: 10.4103/1658-354X.71570. http://dx.doi.org/10.1053/j.jvca.2003.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaygusuz K, Gokce G, Gursoy S, Ayan S, Mimaroglu C, Gultekin Y. A comparison of sedation with dexmedetomidine or propofol during shockwave lithotripsy: A randomized controlled trial. Anesth Analg. 2008;106:114–9. doi: 10.1213/01.ane.0000296453.75494.64. http://dx.doi.org/10.1213/01.ane.0000296453.75494.64. [DOI] [PubMed] [Google Scholar]
- 25.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–94. doi: 10.1097/00000542-200008000-00016. http://dx.doi.org/10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 26.Talke P, Chen R, Thomas B, Aggarwall A, Gottlieb A, Thorborg P, et al. The hemodynamic and adrenergic effects of perioperative dexmedetomidine infusion after vascular surgery. Anesth Analg. 2000;90:834–9. doi: 10.1097/00000539-200004000-00011. http://dx.doi.org/10.1097/00000539-200004000-00011. [DOI] [PubMed] [Google Scholar]
- 27.De Jonge A, Timmermans PB, Van Zwieten PA. Participation of cardiac presynaptic alpha 2-adrenoceptots in the bradycardic effects of clonidine and analogues. Naunyn Schmiedebergs Arch Pharmacol. 1981;317:8–12. doi: 10.1007/BF00506249. http://dx.doi.org/10.1007/BF00506249. [DOI] [PubMed] [Google Scholar]
- 28.Morgan GE, Mikhail MS, Murray MJ. Anesthesia for Ophtalmic Surgery. In: Morgan GE, Mikhail MS, Murray MJ, editors. Clinical Anesthesiology. 3rd edition. New York: Mc Graw-Hill; 2002. pp. 761–70. [Google Scholar]
- 29.Thomas WF, Macario A. The postanaesthesia care unit. In: Miller RD, editor. Anaesthesia. 6th ed. Philadelphia: Churchil Livingston; 2006. pp. 2703–27. [Google Scholar]
- 30.Arain SR, Ebert TJ. The efficacy, side effects, and recovery characteristics of dexmedetomidine versus propofol when used for intraoperative sedation. Anesth Analg. 2002;95:461–6. doi: 10.1097/00000539-200208000-00042. http://dx.doi.org/10.1213/00000539-200208000-00042. [DOI] [PubMed] [Google Scholar]
- 31.Makary L, Vornik V, Finn R, Lenkovsky F, McClelland AL, Thurmon J, et al. Prolonged recovery associated with dexmedetomidine when used as a sole sedative agent in office-based oral and maxillofacial surgery procedures. J Oral Maxillofac Surg. 2010;68:386–91. doi: 10.1016/j.joms.2009.09.107. http://dx.doi.org/10.1016/j.joms.2009.09.107. [DOI] [PubMed] [Google Scholar]
- 32.Daabiss M, Elsherbiny M, Alotibi R. Assessment of different concentration of Ketofol in procedural operation. BJMP. 2009;2:27–31. [Google Scholar]
- 33.Gertler R, Brown C, Mitchell DH, Silvius E. Dexmedetomidine: A novel sedative-analgesic agent. BUMC Proc. 2001;14:13–21. doi: 10.1080/08998280.2001.11927725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aantaa R, Kanto J, Scheinin M, Kallio A, Scheinin H. Dexmedetomidine, an α-2 adrenoceptor agonist, reduces anesthetic requirements for patients undergo-ing minor gynecologic surgery. Anesthesiology. 1990;70:230–5. doi: 10.1097/00000542-199008000-00007. http://dx.doi.org/10.1097/00000542-199008000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Hurley RW, Wu CL. Acute postoperative pain. In: Miller RD, editor. Miller’s anesthesia. 17th ed. Philadelphia: Churchil Livingstone Elsveier; 2010. p. 2767. http://dx.doi.org/10.1016/B978-0-443-06959-8.00087-X. [Google Scholar]
- 36.Reves JG, Glass P, lubarsky D, Matthe WD, Evoy MC, Martinez R, et al. Intravenous Anesthetics. In: Miller RD, editor. Miller’s anesthesia. 17th ed. Philadelphia: Churchil Livingstone Elsveier; 2010. pp. 742–7. [Google Scholar]
- 37.Messenger DW, Murray HE, Dungey PE, van Vlymen J, Sivilotti ML. Subdissociative-dose ketamine versus fentanyl for analgesia during propofol procedural sedation: a randomized clinical trial. Acad Emerg Med. 2008;15:877–86. doi: 10.1111/j.1553-2712.2008.00219.x. http://dx.doi.org/10.1111/j.1553-2712.2008.00219.x. [DOI] [PubMed] [Google Scholar]
- 38.Erden IA, Pamuk AG, Akinci SB, Koseoglu A, Aypar U. Comparison of propofol-fentanyl with propofol-fentanyl-ketamine combination in pediatric patients undergoing interventional radiology procedures. Paediatr Anaesth. 2009;19:500–6. doi: 10.1111/j.1460-9592.2009.02971.x. http://dx.doi.org/10.1111/j.1460-9592.2009.02971.x. [DOI] [PubMed] [Google Scholar]
- 39.Chiaretti A, Ruggiero A, Barone G, Antonelli A, Lazzareschi I, Genovese O, et al. Propofol/alfentanil and propofol/ketamine procedural sedation in children with acute lymphoblastic leukaemia: safety, efficacy and their correlation with pain neuromediator expression. Eur J Cancer Care (Engl) 2010;19:212–20. doi: 10.1111/j.1365-2354.2008.01006.x. http://dx.doi.org/10.1111/j.1365-2354.2008.01006.x. [DOI] [PubMed] [Google Scholar]
- 40.Miner JM. The surgical stress response, preemptive analgesia, and procedural sedation in the ED. Acad Emerg Med. 2008;15:955–8. doi: 10.1111/j.1553-2712.2008.00249.x. http://dx.doi.org/10.1111/j.1553-2712.2008.00249.x. [DOI] [PubMed] [Google Scholar]
- 41.Ejadjam JJ, Bruelle P, Lglourcey L, Viel E. Sedation and regional anaesthesia. ESRA. 1995:136–43. [Google Scholar]
- 42.Atkinson RS, Rushman GB, Davies NJ. Lee’s Synopsis of Anaesthesia. 11th edition. Oxford Butterwouth-Heinemann Ltd; 1993. pp. 680–1. [Google Scholar]
- 43.Aydoğan MS, Demirel S, Erdoğan MA, Fırat P, Çolak C, Durmuş M. Effects of ketamine-propofol mixture on intraocular pressure and hemodynamic in elderly patients: a randomized double-blind trial. Turk J Anaesth Reanim. 2014;42:12–8. doi: 10.5152/TJAR.2013.56. http://dx.doi.org/10.5152/TJAR.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamashita K, Terao Y, Inadomi C, Takada M, Fukusaki M, Sumikawa K. Age-dependent relationship between bispectral index and sedation level. J Clin Anesth. 2008;20:492–5. doi: 10.1016/j.jclinane.2008.05.004. http://dx.doi.org/10.1016/j.jclinane.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Consales G, Chelazzi C, Rinaldi S, De Gaudio AR. Bispectral Index compared to Ramsay score for sedation monitoring in intensive care units. Minerva Anestesiol. 2006;72:329–36. [PubMed] [Google Scholar]