Abstract
Objective
The aim of this study is to compare the effects of 3 different kinds of anaesthesia on stress response induced by surgery.
Methods
Sixty patients aged between 25–70 American Society of Anesthesiologists (ASA) I–II group to undergo inguinal herniography were included in this study. Patients were randomly divided into 3 groups of 20. Group 1 received general anaesthesia with sevoflurane/air/remifentanil, patients in Group 2 received total intravenous anaesthesia (TIVA) with propofol/air/remifentanil and Group 3 received spinal anaesthesia induced by hyperbaric bupivacaine, adjoined by remifentanil sedation. Mean arterial pressure (MAP), heart rate and SpO2 values were recorded preoperatively, intraoperatively and postoperatively at certain periods. Cortisol, leptin and glucose levels were preoperatively detected. Intervals were as; 15 minutes prior to the induction of anaesthesia, at intraoperative first hour and at the postoperative third and twenty-forth hours.
Results
MAP and heart rate values were similar in the inhalational anaesthesia and TIVA groups but relatively higher in the spinal anaesthesia group. Blood glucose levels were elevated, insulin levels were decreased in all groups, at the intraoperative first hour. Biphasic variation in blood leptin levels was observed in all groups, as the levels were lower than the preoperative control values at the intraoperative first and postoperative third hours and significantly higher at the postoperative twenty-forth hour. There was a significant decrease in cortisol level percentage change in the TIVA group at the intraoperative 1st hour, increasing in the other groups.
Conclusion
We concluded that TIVA supresses the stress response induced by surgery better by lowering cortisol levels, leading to a lower increase in blood glucose levels and a lower decrease in blood insulin levels when compared to others. Furthermore, leptin levels were increased at the postoperative twenty-forth hour. The lower increase at the postoperative twenty-forth hour in the TIVA group can be correlated with the anaesthetic agent.
Keywords: Stress response of surgery, leptin, TIVA, inhalation anaesthesia, spinal anaesthesia
Introduction
Autonomous, endocrine, metabolic and immunological responses given to the harmful stimuli for providing and maintaining homeostasis in the body are generally called stress. These responses develop in the presence of major traumas, surgery, sepsis, fasting, infection, burns and pain. Response to stress is seen as mobilization of energy sources for survival, hypermetabolism, activation of cardiovascular system and increased blood supply in the vital organs. However, these responses can be harmful by increasing the consumption of energy and the task of the myocardium during surgical intervention. Therefore, their being under control can reduce the rates of postoperative morbidity and mortality (1).
Surgical stress response is influenced by many factors. These factors include the size of surgery, type of surgical procedure, duration of operation, age of patient, amount of intraoperative bleeding, level of postoperative pain, and anaesthesia technique that is selected (2, 3). Excessive amount of intraoperative stress also affects the duration of hospitalization, time of discharge from the hospital, and hospital expenses in a negative manner (1).
There have been various studies conducted on the suppression of surgical stress-induced endocrine and metabolic responses through different anaesthesia techniques. In clinical studies, it has been observed that various anaesthesia techniques initiate neurohumoral, autonomic, and immunological changes through some pathophysiological ways and affect the stress response (4). Decreased peroperative stress plays an important role in reducing hospital expenses because of a more rapid recovery and reduced hospitalization time (5). Remifentanil is a short-acting μ-agonist opioid with a rapid onset of action. It can be used for intraoperative analgesia and sedation (6). In our study, the patients who would undergo elective inguinal hernia surgery were provided a balanced anaesthesia with sevoflurane/air/remifentanil, total intravenous anaesthesia with propofol/air/remifentanil and remifentanil sedation, and they were administered spinal anaesthesia with hyperbaric bupivacaine. By using remifentanil as the common opioid in three different anaesthesia techniques, it was aimed to compare its effects on haemodynamic, neuroendocrine and metabolic responses and on leptin levels.
Methods
For the study, the approval was received from the ethics committee of the Ankara Atatürk Training and Research Hospital under the Ministry of Health. The study included 60 patients between the ages of 25 and 70 years, who were involved in the American Society of Anesthesiologists (ASA) I–II group and would undergo elective inguinal hernia surgery. Written informed consents were obtained from the patients. Patients with cardiac, kidney, liver, psychiatric, allergic, metabolic and endocrine diseases; those with the suspect of malignancy and those receiving hormone and steroid treatment were excluded from the study.
The patients who were forbidden to receive an oral intake after 24.00 o’clock before the surgery were given 0.9% NaCl as a maintenance fluid to reach the value of 3 mL kg h−1 until the operation. The study included patients who would undergo inguinal hernia surgery between 08:00 and 11:00 p.m. The operations that were shorter than 1 h and longer than 2.5 h were excluded. After the patients were taken to the operation room, they were monitored with an electrocardiogram, non-invasive blood pressure, pulse oximeter, and bispectral index (BIS), and their baseline values were recorded. The patients were randomly divided into three groups of 20, patients in the sevoflurane group (Group 1) underwent induction with 1 μg kg−1 remifentanil intravenous (IV) bolus and 8% sevoflurane in 100% O2 by asking them to breathe normally with the tidal breathing technique. The gas flow was adjusted to 6 L min−1. Endotracheal intubation was performed at the third minute, following the administration of 0.15 mg kg−1 cisatracurium IV bolus after the loss of eyelash reflex. The maintenance of anaesthesia was sustained with 2% sevoflurane in 50% O2 and 50% air mixture and 0.125 mg kg min−1 remifentanil infusion. For the cases in the total intravenous anaesthesia (TIVA) group (Group 2), 2 mg kg−1 propofol and 1 μg kg−1 remifentanil IV bolus were administered for induction. After the loss of eyelash reflex, 0.15 mg kg−1 cisatracurium IV bolus was administered, and endotracheal intubation was performed after waiting for 3 min. The maintenance of anaesthesia was provided with 5 mg kg−1 h−1 propofol and 0.125 μg kg−1 min−1 remifentanil infusion. The patients were ventilated with 50% O2 and 50% air mixture. BIS values were ensured to be between 40 and 45 in Group 1 and Group 2, respectively. For the patients in the spinal anaesthesia group (Group 3), a 15 mg dose of 0.5% bupivacaine was administered intrathecally from the L3–L4 interval with an atraumatic spinal needle in the lateral position, and 0.125 μg kg−1 min−1 remifentanil infusion was used for sedation. After waiting for the sensory block to reach up to T6 dermatoma, surgery was allowed and motor block was evaluated with the Bromage Scale. When the spinal block was unsuccessful, the case was excluded from the study.
In all groups, when the mean arterial pressure (MAP) decreased by more than 25% from the baseline value, 4 mL kg−1 0.9% NaCl was primarily administered with a 5-min interval. When blood pressure did not increase despite this, the dose of remifentanil was decreased by 50% first and then, after 5 min, 10 mg ephedrine was IV administered. When the value of heart rate (HR) decreased below 50 beats per minute, 0.01 mg kg−1 atropine was IV administered. Compared to preoperative period, in case of elevated MAP by more than 25% and increased HR over 90 beats per minute for 1 min, the dose of remifentanil was increased by 50%. The administration of anaesthetic agent in Group 1 and Group 2 was discontinued after surgical procedure was completed. To reverse the neuromuscular block in all patients who have undergone general anaesthesia, 0.5 mg atropine and 1.5 mg neostigmine were administered. All patients waited in the recovery room for 1 h and then they were transferred to the clinic. For postoperative analgesia, 1 g paracetamol IV was administered to the patients whose visual analogue scale (VAS) value was over 4 and when it was insufficient, 50 mg tramadole (Contramal® Abdi İbrahim İlaç Sanayi ve Ticaret A.Ş. Zincirlikuyu, İstanbul, Turkey, Grunenthal GmbH, Germany) IM was administered. Before the initiation of the anaesthesia procedure, the baseline values of MAP, HR and SpO2 were measured in all patients and their measurement results at the intraoperative 5th, 10th, 15th, 20th, 30th, 60th, 90th and 120th min and at the postoperative 30th min, and 1st, 3rd and 24th h were recorded. For determining the levels of cortisol, insulin, leptin and blood glucose in the cases, blood samples were taken 15 min before induction (baseline value), at the intraoperative 1st h and at the postoperative 3rd and 24th h. Start and end times of both anaesthesia and surgery were recorded.
Biochemical analyses
Quantitative analyses of leptin in serum was performed with an immunoenzymatic method using the EASIA Biosource kit (Ref: KAP 2281, Lot: 080601/4 2009 02 28), and serum glucose concentration was spectrophotometrically measured with the glucose oxidase method at the Research Laboratories of Gazi University (7). Serum cortisol and insulin levels were measured with the chemiluminescent immunoassay.
Statistical analysis
Statistical analyses of the data were performed using the Statistical Package for the Social Sciences (SPSS, IBM, Chicago, IL, USA) for Windows 11.5 software program. The Shapiro– Wilk test was used for investigating whether continuous variables displayed normal distribution or not. Descriptive statistics were presented as mean ± standard deviation for demographic features and haemodynamic measurements, median (interquartile range) for laboratory findings and the number of cases and percentage (%) for nominal variables. The significance of the difference between the groups with respect to age, height, weight, body mass index and duration of operation was evaluated with one-way analysis of variance. Moreover, the significance of the difference with respect to the duration of anaesthesia, and haemodynamic and laboratory measurements was evaluated using the Kruskal–Wallis test. When the result of the Kruskal–Wallis test statistics was found to be significant, multiple comparison test was employed for identifying the groups causing this significant difference. Nominal variables were analysed using Pearson’s chi-square test. With respect to repeating measurements, the significance of the difference within the groups was examined with Friedman’s test. In case of the significant result of Friedman’s test statistics, Wilcoxon signed-rank test was used to detect the follow-up times leading to a significant difference compared to the baseline values. The extent of the linear relationship between the continuous variables was evaluated by determining Pearson’s “r” coefficient and significance level. Instead of original leptin measurements, logarithmic transformation data were used in correlation tests. The value of p<0.05 was accepted to be statistically significant in all evaluations. The Bonferroni correction was used for taking type one error under control in all intergroup and within-group comparisons that were performed for haemodynamic and laboratory findings in correlation tests and follow-up times.
Results
No statistically significant difference was found among the patients in three groups with respect to mean age, female-male distribution, mean body mass index and operation duration (Table 1). During the monitoring time, there was no statistically significant difference in saturation measurements among the groups (p>0.005 according to the Bonferroni correction). Similarly, among the groups, no significant difference was observed with respect to the need for analgesics (p=0.889).
Table 1.
Intergroup evaluation of demographic features of patients
| Variables | Group 1 (n=20) M±SD | Group 2 (n=20) M±SD | Group 3 (n=20) M±SD | p |
|---|---|---|---|---|
| Agea | 49.2±13.3 | 49.4±9.8 | 49.3±13.9 | 0.999b |
| Gender, F/M | 3/17 | 3/17 | 2/18 | 0.860c |
| Body mass indexa | 25.3±2.7 | 25.1±3.7 | 26.0±3.3 | 0.655b |
| Duration of surgerya | 65.3±7.9 | 64.3±8.4 | 65.1±10.0 | 0.244d |
M: mean; SD: standard deviation; F: female; M: male;
data are presented as mean±standard deviation.
One way analysis of variance.
Pearson’s Chi-Square test.
Kruskal–Wallis test
Heart rates
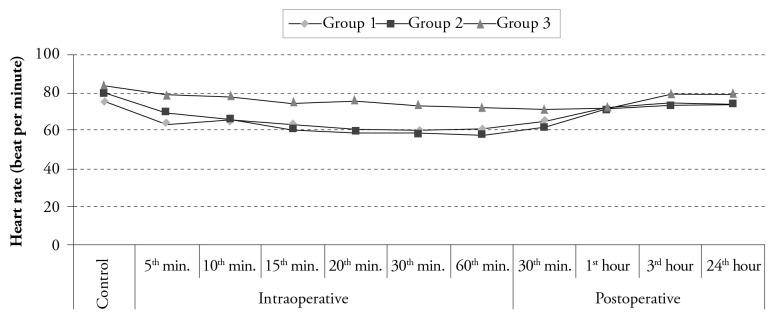
Among the groups, the values measured at the postoperative 30th min and at the postoperative 1st and 3rd h were found to be statistically similar (p>0.005 according to the Bonferroni Correction). On the other hand, the HR values obtained at the intraoperative 5th, 10th, 15th, 20th, 30th and 60th min and at the postoperative 24th h were different among the groups, which was statistically significant (p<0.005 according to the Bonferroni correction) (Figure 1). The HR levels at the intraoperative 5th, 10th, 15th, 20th, 30th and 60th min and postoperative 24th h were found to be significantly lower in Group 1 than those in Group 3 (p<0.001). Furthermore, the HR levels at these times were lower in Group 2 than those in Group 3, which was statistically significant (p<0.001). Although decreased HR values at the intraoperative 15th, 20th and 30th min were statistically significant in Group 1 compared to the control values, decreased values of HR at the intraoperative 10th, 15th, 20th, 30th and 60th min and at the postoperative 30th min were statistically significant in Group 2. The HR values of Group 3 displayed no change during the whole monitorization procedure (p>0.0003 according to the Bonferroni correction) (Figure 1).
Figure 1.
Distribution of heart rate values according to time among the groups
Mean arterial pressure
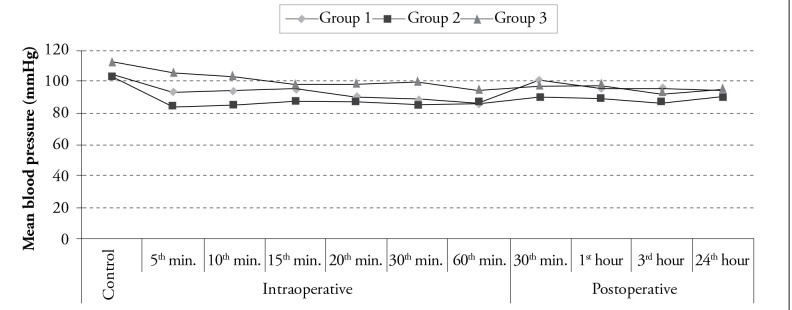
The control values and the values at the intraoperative 15th, 20th and 60th min and at the postoperative 1st and 24th h were found to be statistically similar among the groups (p>0.005 according to the Bonferroni Correction). On the other hand, the MAP values at the intraoperative 5th, 10th, 30th min and at the postoperative 30th min and 3rd h were statistically significantly different among the groups (p<0.005 according to the Bonferroni Correction). The MAP value at the intraoperative 30th min was lower in Group 1 than that in Group 3. In addition, the values at the intraoperative 5th, 10th and 30th min and at the postoperative 30th min were also observed to be lower in Group 2 than those in Group 3 (p<0.005 according to the Bonferroni Correction). The MAP values of Group 2 at the postoperative 30th min and 3rd h were found to be lower than those of Group 1, which was statistically significant (p<0.005 according to the Bonferroni Correction) (Figure 2). Compared to the control values, although a decrease in the MAP values at the intraoperative 30th and 60th min was statistically significant in Group 1 (p<0.003 according to the Bonferroni Correction), a decrease in the values at the intraoperative 10th, 20th, and 30th min and at the postoperative 30th min and 3rd and 24th h was statistically significant in Group 2 (p<0.003 according to the Bonferroni Correction). On the other hand, in Group 3, a decrease in MAP values observed in all postoperative follow-up periods was found to be statistically significant compared to the control values (p<0.0003 according to the Bonferroni Correction) (Figure 2).
Figure 2.
Distribution of mean blood pressure values according to time among the groups
Biochemical analyses
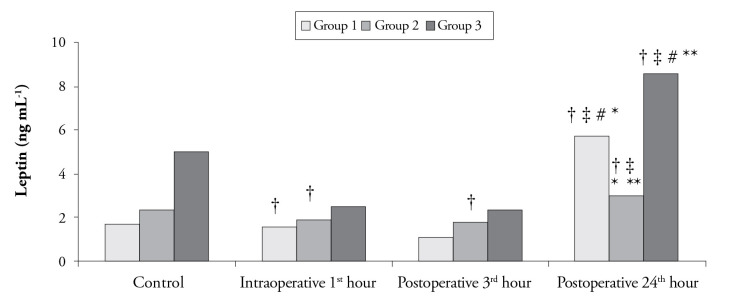
In intergroup comparisons, no statistically significant difference was detected with respect to the leptin levels at the intraoperative 1st h and postoperative 3rd h and control levels (p>0.0125 according to the Bonferroni Correction). However, the difference in leptin levels at the postoperative 24th h was statistically significant among the groups (p<0.0125). The leptin value of Group 2 at the postoperative 24th h was lower than both Group 1 and Group 3 (p<0.0125 according to the Bonferroni Correction) (Figure 3).
Figure 3.
Leptin levels according to time among the groups
†The difference compared to the control values (p<0.003 according to the Bonferroni correction)
‡The difference compared to the value at the intraoperative 1st hour (p<0.003 according to the Bonferroni correction)
#The difference compared to the value at the postoperative 3rd hour (p<0.003 according to the Bonferroni correction)
*The difference between Group 1 and Group 2 (p<0.0125 according to the Bonferroni correction)
**The difference between Group 2 and Group 3 (p<0.0125 according to the Bonferroni correction)
In Group 1, a decrease in leptin level at the postoperative 3rd h was found to be statistically significant compared to the control value (p<0.003 according to the Bonferroni Correction). Moreover, compared to the control value and the values at the intraoperative 1st h and postoperative 3rd h, leptin level at the postoperative 24th h was significantly higher (p<0.003 according to the Bonferroni Correction) (Figure 3). In Group 2, a decrease in leptin level at the intraoperative 1st h and postoperative 3rd h was statistically significant compared to the control value (p<0.003 according to the Bonferroni Correction). Furthermore, compared to the intraoperative 1st h and postoperative 3rd h, leptin level at the postoperative 24th h was higher, which was statistically significant (p<0.003 according to the Bonferroni Correction) (Figure 3). In Group 3, leptin level measured at the postoperative 24th h was significantly higher than the control value and the values at the intraoperative 1st h and postoperative 3rd h (p<0.003 according to the Bonferroni Correction) (Figure 3).
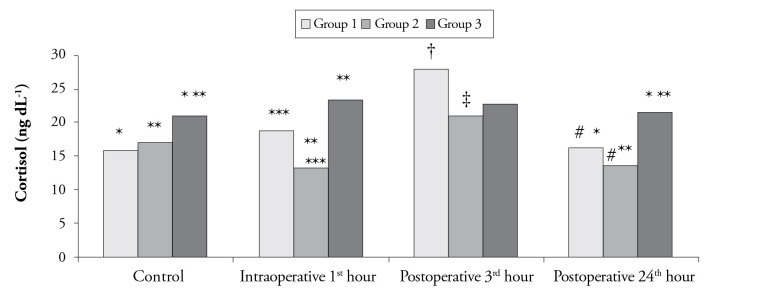
A statistically significant difference was detected among the groups with respect to cortisol levels at the intraoperative 1st h and postoperative 24th h and control values (p<0.0125 according to the Bonferroni Correction). Although the cortisol level at the postoperative 24th h and the control value were significantly higher in Group 3 than that in Group 1 and Group 2, cortisol level at the intraoperative 1st h was significantly lower in Group 2 than that in Group 1 and Group 3 (p<0.0125 according to the Bonferroni Correction). No statistically significant difference was observed in cortisol levels at the postoperative 3rd h among the groups (p>0.0125 according to the Bonferroni Correction) (Figure 4). In Group 1, cortisol level at the postoperative 3rd h was significantly higher than the control level and the value at the postoperative 24th h. In Group 2, cortisol level at the postoperative 3rd h was significantly higher than that at the intraoperative 1st h and postoperative 24th h (p<0.003 according to the Bonferroni Correction). However, in Group 3, no difference was found among cortisol levels (p>0.003 according to the Bonferroni Correction) (Figure 4).
Figure 4.
Cortisol levels according to time among the groups
†The difference compared to the control values (p<0.003 according to the Bonferroni correction)
‡The difference compared to the value at the intraoperative 1st hour (p<0.003 according to the Bonferroni correction)
#The difference compared to the value at the postoperative 3rd hour (p<0.003 according to the Bonferroni correction)
*The difference between Group 1 and Group 3 (p<0.0125 according to the Bonferroni correction)
**The difference between Group 2 and Group 3 (p<0.0125 according to the Bonferroni correction)
***The difference between Group 1 and Group 2 (p<0.0125 according to the Bonferroni correction)
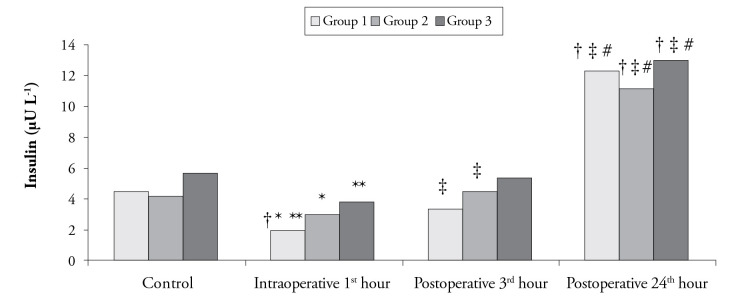
No statistically significant difference was found in insulin levels measured at the postoperative 3rd and 24th h and control values among the groups (p>0.0125 according to the Bonferroni Correction). On the other hand, among insulin levels at the intraoperative 1st h, there was a statistically significant difference in all groups (p<0.001), and the insulin level in Group 1 was observed to be significantly lower than that in Group 2 and Group 3 (p=0.010 and p<0.001) (Figure 5). In Group 1, compared to the control values, the decrease in insulin levels at the intraoperative 1st h and postoperative 3rd h was statistically significant. Moreover, increased insulin level at the postoperative 24th h was found to be statistically significant compared to the baseline values and values at the intraoperative 1st h and postoperative 3rd h (p<0.003 according to the Bonferroni Correction). In Group 2, elevated insulin level at the postoperative 3rd hour was statistically significant, considering the value at the intraoperative 1st h. Furthermore, in comparison with the control value and the values at the intraoperative 1st h and postoperative 3rd h, the increase in insulin level at the postoperative 24th h was evaluated to be statistically significant. On the other hand, in Group 3, the increase in insulin level obtained at the postoperative 24th h was statistically significant compared to the control value and the values at the intraoperative 1st h and postoperative 3rd h (p<0.003 according to the Bonferroni Correction) (Figure 5).
Figure 5.
Insulin levels according to time among the groups
†The difference compared to the control values (p<0.003 according to the Bonferroni correction)
‡The difference compared to the value at the intraoperative 1st hour (p<0.003 according to the Bonferroni correction)
#The difference compared to the value at the postoperative 3rd hour (p<0.003 according to the Bonferroni correction)
*The difference between Group 1 and Group 2 (p<0.0125 according to the Bonferroni correction)
**The difference between Group 1 and Group 3 (p<0.0125 according to the Bonferroni correction)
Discussion
In this study, TIVA with propofol-remifentanil, balanced anaesthesia with sevoflurane-remifentanil and spinal anaesthesia with remifentanil sedation were compared with respect to their effects on surgical stress response. Surgical trauma causes complex stress response defined by neurohumoral, immunological and metabolic changes (8). In the period after surgical trauma, metabolic response is divided into two phases: the early phase (Ebb phase or hypometabolic) and the hypermetabolic phase (9). The Ebb phase is an early response that is defined by a general and rapid catabolism and lasts for approximately 24 h. On the other hand, the hypermetabolic phase begins after the posttraumatic first 24 h (10, 11). It has been demonstrated that, in the ebb phase, an inflammatory response occurs with an increased release of cytokines such as tumour necrosis factor alpha, interleukin-1 and interleukin-6; activation of polymorphonuclear leucocytes and macrophages and excessive synthesis of reactive oxygen radicals (12). It has been revealed that the blockade of metabolic and hormonal changes induced by surgical stress will decrease postoperative morbidity and mortality (13).
In clinical studies, it has been specified that the anaesthesia technique will display different effects on HR variability and MAP and also on intraoperative stress response (14–16). In our study, it was detected that the values of HR and MAP were similar in the TIVA and inhalation groups during the operation but they were higher in the spinal anaesthesia group than the other two groups. However, this difference was not statistically significant. In our study, in which remifentanil was used in all three groups as the common agent, it was thought that the haemodynamic variations occurred because of the anaesthesia technique that was used, and their effects on stress response were similar.
In our study, because the baseline value of cortisol was found to be significantly higher in the spinal anaesthesia group than the baseline values of the other two groups, the amount of percentage change in cortisol levels between each measurement was evaluated. Although the percentage change of cortisol level at the intraoperative 1st h decreased in the TIVA group, it increased in the inhalation and spinal anaesthesia groups. This makes us assume that TIVA suppresses the stress response better in the intraoperative period. Similar results to ours were also obtained in the study conducted by Ledowski et al. (17). Segawa et al. (18) have reported that the phrenic nerve plays an important role in the conduction of neuronal information, and it does not suppress the surgical stress response. The increased cortisol level of the spinal anaesthesia group in the intraoperative period in our study suggests that the spinal anaesthesia level kept at the T6 level allows the transition of undesired stimuli to the hypothalamus.
We assume that the percentage of increase in cortisol level measured at the postoperative 3rd h was lower in the spinal anaesthesia group than that in the TIVA group can be associated with the continuance of spinal anaesthesia. In a study conducted with patients having undergone anorectal surgery, Büyükkoçak et al. (19) compared “saddle” block, which was performed without using a sedation agent, with general anaesthesia, in which they used thiopental, fentanyl and vecuronium for induction and O2/N2O/sevoflurane for maintenance, and they found the cortisol level at the postoperative 3rd h to be lower in saddle block than in general anaesthesia. On the other hand, in their other study comparing saddle block and general anaesthesia in haemorrhoidectomy surgeries, they reported that although there was a difference in cortisol levels at the postoperative 3rd h, no difference was observed between inhalation and spinal anaesthesia (20). Despite the fact that the technique used in our study was different from that in the study of Büyükkoçak et al. (19), no difference was also found in the inhalation and spinal anaesthesia groups in the intraoperative period in our study. Aono et al. (21) divided patients who would undergo laparoscopic cholecystectomy surgery into three groups, and they administered only general anaesthesia to Group 1, general anaesthesia with fentanyl to Group 2 and general anaesthesia with thoracal epidural anaesthesia to Group 3. They reported that general anaesthesia administered only with sevoflurane did not suppress the hypothalamic-pituitary adrenocortical axis sufficiently. The lower rate of biochemical changes in our inhalation group may have resulted from an inadequate suppression of the axis. Marana et al. (22) reported that TIVA induced a significant decrease in preoperative plasma catecholamines and corticotropin releasing hormone. This inhibitor effect was attributed mainly to the effect of remifentanilin on the central neuroadrenergic system and hypothalamus and partly to the effect of propofol on gamma-amino butyric acid (GABA) receptors (22–25). However, we suggested in our study that the role of propofol in intraoperative cortisol decrease was much more in the TIVA group because remifentanil infusion was administered to the three groups (26, 27). Engin et al. (28) performed a study with patients who were at two different stages of the disease and displayed a significant difference with respect to surgical scores. They found no significant difference in cortisol, hs-CRP and LDH levels between the groups in postoperative period. However, compared to the control values, there was a statistically significant increase in these levels. They also stated that the response to the trauma in the ebb phase was in an expected manner in their patients.
Another biomarker of surgical stress is the level of blood glucose (29). This is confirmed in different studies that have been conducted. In a study comparing propofol and sevoflurane, it was detected that intraoperative blood glucose concentrations increased significantly in the sevoflurane group (30). In another study conducted with sevoflurane by Oyama et al. (31), they reported that insulin level decreased but blood glucose level increased with surgical stress. In our study, intraoperative blood glucose levels increased in all groups and this increase was evaluated to be significantly lower in the TIVA group than those in both spinal anaesthesia and inhalation anaesthesia groups. At postoperative 3rd h, the concentration of blood glucose was higher in the inhalation group than that in the TIVA and spinal anaesthesia groups. Furthermore, in our study, it was detected that insulin level decreased in all groups, but insulin level in the intraoperative period was significantly lower in the inhalation group than that in the TIVA and spinal anaesthesia groups. It was thought that insulin mediated leptin secretion, but in vivo studies have revealed that insulin does not have an acute effect on leptin level. It has been found that leptin level increases in the cases when insulin level is chronically high (32–34). Similarly, in our study, no correlation was detected between the change percentages of insulin and leptin levels. Moreover, no correlation was detected between BMI and leptin, leptin and blood glucose and percentage changess of insulin levels in our study. Our results were similar to those of other researchers (35–37).
Leptin has a cytokine-like protein structure that is secreted from adipocytes. It acts in the central nervous system and peripheral organs, energy homeostasis and metabolism (38). There are some studies suggesting that stress affects leptin level and leptin is an acute phase reactant with a biphasic response that presents with a decrease at the beginning and then with an increase at the postoperative 24th h in acute surgical stress (39, 40). Stratton et al. (41) thought in their study that the leptin level elevating in circulation within the first 24 h after hip surgeries covered acute phase reaction. In various studies, intraoperative sudden decreases and increases at the postoperative 24th h in leptin levels were detected. The decrease was attributed to the secretion of catecholamine triggered by the surgery. It was reported that the release of leptin was not affected by the anaesthesia technique (42). Moreover, in another study, it was found that the highest rate of decrease in leptin level occurred in the 2nd h after the surgery and leptin level increased after 24 h compared to the control values (35). In our study, in all groups, there was a decrease in leptin level in the intraoperative period and at the postoperative 3rd h compared to the baseline value. On the other hand, there was a significant increase at the postoperative 24th h. However, it was revealed that the increase in leptin level at the postoperative 24th h was significantly lower in the TIVA group than in the inhalation and spinal anaesthesia groups. It was thought that this result may have been associated with that propofol suppressed surgical stress better because of its antioxidant feature (26, 27). Indeed, the fact that the percentage change of cortisol level in the intraoperative period was lower in the TIVA group than that in the other two groups supports this opinion.
In our study, all patients whose VAS value was over 4 were IV administered 1 g paracetamol for postoperative analgesia and when it was insufficient, 50 mg tramadole (Contramal®) was i.m. administered. When pain levels were VAS 4 in the spinal anaesthesia group, the protocol was initiated to be administered. The effects of postoperative pain on stress are known (43). However, no difference was detected among the groups with respect to postoperative pain in our study. Because the need for additional analgesia was found to be similar among the groups, we assume that it did not influence our results.
Conclusion
We assume that, in patients having undergone inguinal hernia surgery, the administration of TIVA suppresses surgical stress better because it reduces cortisol level in the intraoperative period and causes a lower increase in blood glucose level than the inhalation and spinal anaesthesia groups and that it leads to a lower decrease in the insulin level than inhalation anaesthesia. Furthermore, a variation in leptin levels was found to be biphasic in our study. Decreased levels of leptin in the intraoperative period and at the postoperative 3rd h and increased level of leptin at the postoperative 24th h were observed. The increase at the postoperative 24th h is lower in the TIVA group.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Ministry Health Ankara Atatürk Training and Research Hospital (31.03.2008-2008/03708).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - D.A., E.K.E., T.G.; Design -D.A., E.K.E., T.G., A.S.D.; Supervision - T.G., O.K.; Funding - D.A., E.K.E.; Materials - D.A., E.K.E.; Data Collection and/or Processing - D.A., E.K.E., T.G.; Analysis and/or Interpretation - D.A., E.K.E., D.Ş., A.S.D., T.G., O.K.; Literature Review - D.A., E.K.E., D.Ş.; Writer - D.A., E.K.E., A.S.D.; Critical Review - T.G., O.K., A.S.D.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Kayhan Z. Klinik Anestezi. III Baskı. Logos Yayıncılık; İstanbul: 2004. pp. 406–21. [Google Scholar]
- 2.Desborough JP. The stres responce to trauma and surgery. Br J Anaesth. 2000;85:109–17. doi: 10.1093/bja/85.1.109. http://dx.doi.org/10.1093/bja/85.1.109. [DOI] [PubMed] [Google Scholar]
- 3.Marana E, Scambia G, Maussier ML. Neuroendocrine stres responce in patients undergoing benign ovarian cyst surgery by laporoscopy, minilaparotomy and laparotomy. J Am Assoc Gynecol Laparosc. 2003;10:159–65. doi: 10.1016/s1074-3804(05)60291-5. http://dx.doi.org/10.1016/S1074-3804(05)60291-5. [DOI] [PubMed] [Google Scholar]
- 4.Marana E, Annetta MG, Meo F. Sevofluran improves the neuroendocrine stres responce during laparoscopic pelvic surgery. Can J Anaest. 2003;50:348–54. doi: 10.1007/BF03021031. http://dx.doi.org/10.1007/BF03021031. [DOI] [PubMed] [Google Scholar]
- 5.Kelbel l, Weiss M. Anaesthetics and immune function. Curr Opin Anaesthesiol. 2001;14:685–91. doi: 10.1097/00001503-200112000-00015. http://dx.doi.org/10.1097/00001503-200112000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Glass PS, Gan TJ, Howell S. A review of the pharmacokinetics and pharmacodynamics of remifentanil. Anesth Analg. 1999;89(4 Suppl):7–14. doi: 10.1097/00000539-199910001-00003. http://dx.doi.org/10.1097/00000539-199910001-00003. [DOI] [PubMed] [Google Scholar]
- 7.Caraay WT. Carbohydrate. In: Tietz NW, editor. Fundamentals of Clinical Chemistry. WB Saunders Company; Philadelphia: 1970. p. 156. [Google Scholar]
- 8.Croizer TA, Mueller JE, Quittkat D. Total Intravenous Anesthesia with methoxitone-alfentanil or propofol-alfentanil: clinic aspect and hemodynamic, endocrine and metabolic effects. Anaesthesist. 1994;43:59604. doi: 10.1007/s001010050098. [DOI] [PubMed] [Google Scholar]
- 9.Aller MA, Arias JL, Nava MP, Arias J. Posttraumatic inflammation is a complex response based on the pathological expression of the nervous, immune, and endocrine functional systems. Exp Biol Med (Maywood) 2004;229:170–81. doi: 10.1177/153537020422900206. [DOI] [PubMed] [Google Scholar]
- 10.Breznock EM. The systemic response of the traumatized patient: an overview. Vet Clin North Am Small Anim Pract. 1980;10:523–32. doi: 10.1016/s0195-5616(80)50052-5. http://dx.doi.org/10.1016/S0195-5616(80)50052-5. [DOI] [PubMed] [Google Scholar]
- 11.Lawrence WT. Physiology of the acute wound. Clin Plast Surg. 1998;25:321–40. [PubMed] [Google Scholar]
- 12.Menger MD, Vollmar B. Surgical trauma: hyperinflammation versus immunosuppression? Langenbecks Arch Surg. 2004;389:475–84. doi: 10.1007/s00423-004-0472-0. http://dx.doi.org/10.1007/s00423-004-0472-0. [DOI] [PubMed] [Google Scholar]
- 13.Kehlet H. Manipulation of the metabolic response in clinical practice. World J Surg. 2000;24:690–5. doi: 10.1007/s002689910111. http://dx.doi.org/10.1007/s002689910111. [DOI] [PubMed] [Google Scholar]
- 14.Grundman U. Total intravenous anaesthesia with propofol and remifentanil in paediatric patients: a comparison with a desflurane-nitrous oxide inhalation anaesthesia. Acta Anaesthesiol Scand. 1998;42:845–50. doi: 10.1111/j.1399-6576.1998.tb05332.x. http://dx.doi.org/10.1111/j.1399-6576.1998.tb05332.x. [DOI] [PubMed] [Google Scholar]
- 15.Ledowski T, Bein B, Hanss R, Paris A, Fudickar W, Scholz J, et al. Neuroendocrine stress responce and heart rate variability: a comparison of total intravenous versus balanced anesthesia. Anesth Analg. 2005;101:1700–5. doi: 10.1213/01.ane.0000184041.32175.14. http://dx.doi.org/10.1213/01.ane.0000184041.32175.14. [DOI] [PubMed] [Google Scholar]
- 16.Kanaya N, Hirata N, Kurosawa S, Nakayama M, Namiki A. Differential effects of propofol and sevoflurane on heart rate variability. Anesthesiology. 2003;98:102–9. doi: 10.1097/00000542-200301000-00009. http://dx.doi.org/10.1097/00000542-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Ledowski T, Bein B, Hanss R, Paris A, Fudickar W, Scholz J, et al. Neuroendocrine stress response and heart rate variability: a comparison of total intravenous versus balanced anesthesia. Anesth Analg. 2005;101:1700–5. doi: 10.1213/01.ane.0000184041.32175.14. http://dx.doi.org/10.1213/01.ane.0000184041.32175.14. [DOI] [PubMed] [Google Scholar]
- 18.Segawa H, Mori K, Kasai K, Fukata J, Nakao K. The role of the phrenic nerves in stress response in upper abdominal surgery. Anesth Analg. 1996;82:1215–24. doi: 10.1097/00000539-199606000-00020. http://dx.doi.org/10.1097/00000539-199606000-00020. [DOI] [PubMed] [Google Scholar]
- 19.Buyukkocak U, Daphan C, Caglayan O, Aydinuraz K, Kaya T, Saygun O, et al. Effects of different anesthetic techniques on serum leptin, C-reactive protein, and cortisol concentrations in anorectal surgery. Croat Med J. 2006;47:862–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Buyukkocak U, Caglayan O, Daphan C, Aydinuraz K, Saygun O, Agalar F. Similar effects of general and spinal anaesthesia on perioperative stress response in patients undergoing haemorrhoidectomy. Mediators Inflamm. 2006;2006:97257. doi: 10.1155/MI/2006/97257. http://dx.doi.org/10.1155/MI/2006/97257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aono H, Takeda A, Tarver SD, Goto H. Stress responses in Three different anesthetic Techniques for Carbon Dioxide Laparoscopic cholecystectomy. J Clin Anesth. 1998;10:546–50. doi: 10.1016/s0952-8180(98)00079-8. http://dx.doi.org/10.1016/S0952-8180(98)00079-8. [DOI] [PubMed] [Google Scholar]
- 22.Marana E, Scambia G, Colicci S, Maviglia R, Maussier ML, Marana R, et al. Leptin and perioperative neuroendocrine stress response with two different anaesthetic techniques. Acta Anaesthesiol Scand. 2008;52:541–6. doi: 10.1111/j.1399-6576.2008.01589.x. http://dx.doi.org/10.1111/j.1399-6576.2008.01589.x. [DOI] [PubMed] [Google Scholar]
- 23.Hall GH, Lacoumenta S, Hart GR. Site of action of fentanyl in inhibiting the pituitary adrenal response to surgery in man. Br J Anaesth. 1990;65:251–3. doi: 10.1093/bja/65.2.251. http://dx.doi.org/10.1093/bja/65.2.251. [DOI] [PubMed] [Google Scholar]
- 24.Chrousos GP. The hypotalamic-pituitary-adrenal axis and immune-mediated inflammation. Review article. New Engl J Med. 1995;18:1350–63. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- 25.Cho YM, Kim SY, Cho BY. Dissociation between plasma adrenocorticotropin and serum cortizol level during the early postoperative period after gastrectomy. Hormone Res. 2000;53:246–50. doi: 10.1159/000023574. http://dx.doi.org/10.1159/000023574. [DOI] [PubMed] [Google Scholar]
- 26.Cucchiara RF, Miller ED, Roizen MF. Anesthesia. 5 ed. Churchill Livinstone; Philadelphia: 2000. [Google Scholar]
- 27.Tsuchiya M, Sato EF, Inore M. Open abdominal surgery incracases intraoperative oxidative stress: can it be prevented. Anesth Analg. 2008;107:1946–52. doi: 10.1213/ane.0b013e318187c96b. http://dx.doi.org/10.1213/ane.0b013e318187c96b. [DOI] [PubMed] [Google Scholar]
- 28.Engin A, Şahin TT, Kurukahvecioğlu O, Sepici-Dinçel A. Farklı şiddetteki cerrahi travmalara erken yanıtta serum albumin ve homosisteininin önemi. Turk J Biochem. 2010;35:77–82. [Google Scholar]
- 29.Bozkurt P, Kaya G, Altintas F, Yeker Y, Hacibekiroglu M, Emir H, et al. Systemic stress response during operations for acute abdominal pain performed via laparoscopy or laparotomy in children. Anaesthesia. 2000;55:5–9. doi: 10.1046/j.1365-2044.2000.01119.x. http://dx.doi.org/10.1046/j.1365-2044.2000.01119.x. [DOI] [PubMed] [Google Scholar]
- 30.Demirbilek S, Erk G, Reisli R, Postacı A. Sevoflurane ve propofolün stres endokrin yanıta etkileri. Türk Anest Rean Cem Mec. 1999;27:564–8. [Google Scholar]
- 31.Oyama T, Murakawa T, Matsuki A. Endocrine evaulation of sevoflurane, a new inhalation anestetic agent. ACTA Anestesiol Belg. 1989;40:269–74. [PubMed] [Google Scholar]
- 32.D’Adamo M, Buongiorno A, Maroccia E. Increased OB gene expression leads to elevated leptin concentrations in patients with choronic primary hyperinsulinemia. Diabetes. 1998;47:1625–9. doi: 10.2337/diabetes.47.10.1625. http://dx.doi.org/10.2337/diabetes.47.10.1625. [DOI] [PubMed] [Google Scholar]
- 33.Pratley RE, Nicolson M, Bogardus C. Plasma leptin responses to fasting in Pima Indians. Am J Physiol. 1997;273:644–9. doi: 10.1152/ajpendo.1997.273.3.E644. [DOI] [PubMed] [Google Scholar]
- 34.Schoeller DA, Cella LK, Sinha MK. Entrainment of the diurnal rhythm of plasma leptin to meal timing. J Clin Invest. 1997;100:1882–7. doi: 10.1172/JCI119717. http://dx.doi.org/10.1172/JCI119717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kain ZN, Zimolo Z, Heninger G. Leptin and the perioperative neuroendocrinological stress response. J Clin Endocrinol Metab. 1999;84:2438–42. doi: 10.1210/jcem.84.7.5850. http://dx.doi.org/10.1210/jcem.84.7.5850. [DOI] [PubMed] [Google Scholar]
- 36.Bougoulia M, Tzotzas T, Efthymiou H, Koliakos G, Konstantinidis T, Triantos A, et al. Leptin concentrations during oral glucose tolerance test (OGTT) in obese and normal weight women. Int J Obes Relat Metab Disord. 1999;23:625–8. doi: 10.1038/sj.ijo.0800891. http://dx.doi.org/10.1038/sj.ijo.0800891. [DOI] [PubMed] [Google Scholar]
- 37.Yoshimitsu N, Douchi T, Nagata Y. Perioperative changes in circulationg leptin levels in women undergoing total abdominal hysterectomy. Endocr J. 2001;48:509–13. doi: 10.1507/endocrj.48.509. http://dx.doi.org/10.1507/endocrj.48.509. [DOI] [PubMed] [Google Scholar]
- 38.Dalamaga M, Chou SH, Shields K, Papageorgiou P, Polyzos SA, Mantzoros CS. Leptin at the intersection of neuroendocrinology and metabolism: current evidence and therapeutic perspectives. Cell Metab. 2013;18:29–42. doi: 10.1016/j.cmet.2013.05.010. http://dx.doi.org/10.1016/j.cmet.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Newcomer JW, Selke G, Melson AK, Gross J, Vogler GP, Dagogo-Jack S. Dose-dependent cortisol-induced increases in plasma leptin concentration in healthy humans. Arch Gen Psychiatry. 1998;55:995–1000. doi: 10.1001/archpsyc.55.11.995. http://dx.doi.org/10.1001/archpsyc.55.11.995. [DOI] [PubMed] [Google Scholar]
- 40.Elimam A, Knutsson U. Variations in glucocorticoid levels within the physiological range affect plasma leptin levels. Eur J Endocrinol. 1998;139:615–20. doi: 10.1530/eje.0.1390615. http://dx.doi.org/10.1530/eje.0.1390615. [DOI] [PubMed] [Google Scholar]
- 41.Stratton RJ, Dewit O, Crowe E. Plasma leptin, energy intake and hunger following total hip replacement surgery. Clin Sci (Lond) 1997;93:113–7. doi: 10.1042/cs0930113. [DOI] [PubMed] [Google Scholar]
- 42.Cho YM, Kim MS, Shin CS, Park DJ, Park KS, Yang HK, et al. Dynamic change in plasma leptin level during the perioperative period. Horm Res. 2003;59:100–4. doi: 10.1159/000068579. http://dx.doi.org/10.1159/000068579. [DOI] [PubMed] [Google Scholar]
- 43.Çakar Turhan S. Postoperatif ağrı tedavisi. Turkiye Klinikleri J Anest Reanim-Special Topics. 2008;1:117–22. [Google Scholar]