Abstract
A 46-year-old man with HIV infection and active intravenous drug use presented with approximately two weeks of fevers and body aches. On physical examination he was somnolent, had a new systolic murmur, bilateral conjunctival hemorrhages, diffuse petechiae, and left-sided arm weakness. Echocardiography revealed a large mitral valve vegetation and brain imaging demonstrated numerous embolic infarctions. Blood cultures grew Serratia marcescens. Despite aggressive treatment with meropenem the patient died due to intracranial hemorrhage complicated by herniation. Serratia marcescens is an uncommon cause of infective endocarditis. While this disease has historically been associated with intravenous drug use, more recent reports suggest that it is now largely a consequence of opportunistic infections of the chronically ill. Our case highlights several characteristic features of this infection, including isolation of a non-pigmented strain of the organism, an antibiotic susceptibility profile suggestive of AmpC β-lactamase production, and rapid clinical deterioration with multiple embolic complications resulting in death. In this review we discuss the history, epidemiology, and management of endovascular infections due to Serratia spp., emphasizing the continued importance of considering this organism in the differential diagnosis of endocarditis among intravenous drug users and as a potential indication for surgical therapy.
Keywords: Serratia marcescens, infective endocarditis, intravenous drug use, AmpC beta-lactamase
Case presentation
A 46-year-old man with a history of human immunodeficiency virus (HIV) infection presented to our hospital with fever and myalgias. Ten days prior to presentation he sought care at an outpatient clinic for fevers as high as 39.3°C, myalgias, and migratory arthralgias. He was diagnosed with a viral syndrome and prescribed fluids and anti-pyretics. His symptoms persisted and he also developed nausea, vomiting and watery non-bloody diarrhea, prompting an evaluation in the emergency room. He had no headache, visual disturbances, dyspnea, cough, or abdominal pain.
He had been diagnosed with HIV in 2000. In 2006 his CD4 count fell to a nadir of 204 per microliter (13.3% CD4 cells), and in 2007 he was initiated on antiretroviral therapy with atazanavir, ritonavir, and fixed dose emtricitabine/tenofovir. Within three months of starting this regimen his HIV viral load became undetectable (<50 copies/milliliter) and he had developed a chronically elevated bilirubin. He continued this regimen and approximately four months prior to this presentation his CD4 count had risen to 340 per microliter (25.6%). His medical history also included previously untreated chronic hepatitis C virus infection, recurrent herpes simplex virus keratitis for which he took suppressive valacyclovir, and previously treated chlamydia urethritis and syphilis. He did not use tobacco and drank alcohol socially. He had been an intravenous drug user (IVDU) for many years – most recently he reported injecting oxycodone tablets, and he was using boiled municipal tap water both to dissolve the tablets and to clean his needles prior to self-injection.
On examination he appeared cachectic, was febrile to 38.1°C, with a blood pressure of 118/75 mmHg, a heart rate of 77 beats per minute, and an oxygen saturation of 99% while breathing ambient air. He was somnolent, but arousable and able to cooperate with the examination. Notable findings included bilateral conjunctival petechiae, a blowing IV/VI holosystolic murmur that was loudest over the apex and radiated to the axilla, scattered petechiae on the trunk and all four extremities, and prominent left-sided upper extremity weakness.
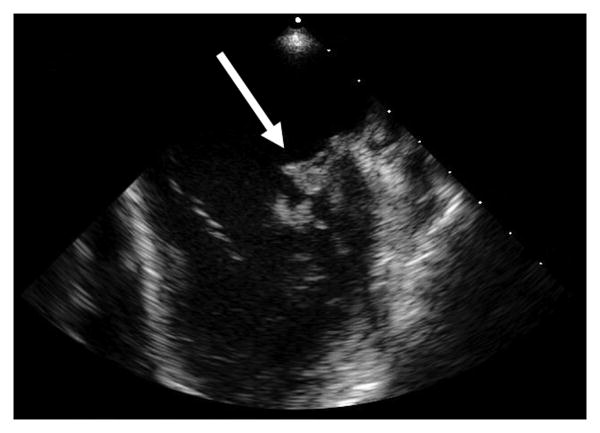
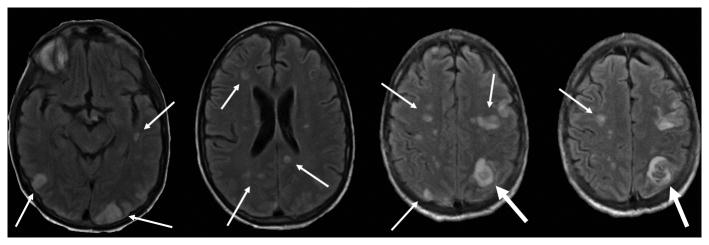
Laboratory studies were notable for: white blood cell (WBC) count, 13,100 cells per microliter with 91% neutrophils; hemoglobin, 11.3 grams per deciliter; platelet count, 105,000 per microliter; sodium, 126 mmol per liter; total bilirubin 4.2 mg per deciliter; aspartate aminotransferase, 165 units per liter; alanine aminotransferase, 106 units per liter; alkaline phosphatase, 566 units per liter; and a urinalysis demonstrating 32 WBCs per high power field and 192 red blood cells per high power field. The CD4 count was 150 per microliter (25.3%) and his HIV viral load was <48 copies/milliliter. Two sets of blood cultures were obtained on admission and within 12 hours both were growing gram negative rods that appeared as non-pigmented colonies on solid media, subsequently identified as Serratia marcescens. Based on antibiotic susceptibility testing by broth microdilution the isolate was reported as having resistance to ampicillin, ampicillin/sulbactam, cefazolin, and cefuroxime, intermediate susceptibility to cefoxitin, piperacillin/tazobactam, and tobramycin, and full susceptibility to all other routinely tested antibiotics (including third and fourth-generation cephalosporins, levofloxacin and meropenem). Transesophageal echocardiography (TEE) demonstrated a large 1.7×1.1 centimeter mobile vegetation on the anterior mitral leaflet/annulus with leaflet perforation, severe mitral regurgitation and a left ventricular ejection fraction of 60% (Figure 1a and b). Magnetic resonance imaging (MRI) of the brain demonstrated multiple hematogenously distributed embolic infarctions and a 0.5×0.5cm subcortical hemorrhage in the left parietal lobe (Figure 1c).
Figure 1a and b. Transesophageal echocardiogram demonstrating (a) large vegetation on the anterior leaflet of mitral valve (thick white arrow), and (b) Doppler flow imaging indicative of severe regurgitant flow (thin white arrows).
Figure 1c. Magnetic resonance image of the brain demonstrating multiple widely distributed infarctions (thin white arrows), presumably of embolic origin, with a left parietal lobe hemorrhage (thick white arrow).
He had been started empirically on intravenous ceftriaxone, and was changed by the infectious diseases service to high dose intravenous meropenem for treatment of infective endocarditis due to S. marcescens with cerebral emboli. The cardiac surgery service deferred immediate intervention due to the acute cerebral hemorrhage. On hospital day 9 the patient became unresponsive and bradycardic and required endotracheal intubation and mechanical ventilator support. Computed tomography of the head demonstrated marked interval worsening of the left parietal hemorrhage with downward transtentorial and left uncal herniation. The patient's family declined neurosurgical intervention and on hospital day 10 the patient expired.
Microbiology
Serratia marcescens is an aerobic (facultative anaerobe), motile, oxidase-negative, non-lactose-fermenting gram negative bacillus. The organism was first identified in 1819 by Bartolemeo Bizio, but it did not acquire its modern taxonomic classification until 1980.1, 2 Within the genus Serratia, S. marcescens is the most frequently recovered species in clinical practice, and has been identified as a pathogen in virtually every anatomic site including, but not limited to, the central nervous system and eye, the respiratory, hepatobiliary, and genitourinary tracts, as well as the skin, soft tissue, bones, and joints. In the laboratory the organism is readily isolated on routine media (sheep's blood, chocolate, or MacConkey agar) using standard bacteriologic techniques, with optimal growth at 37°C.
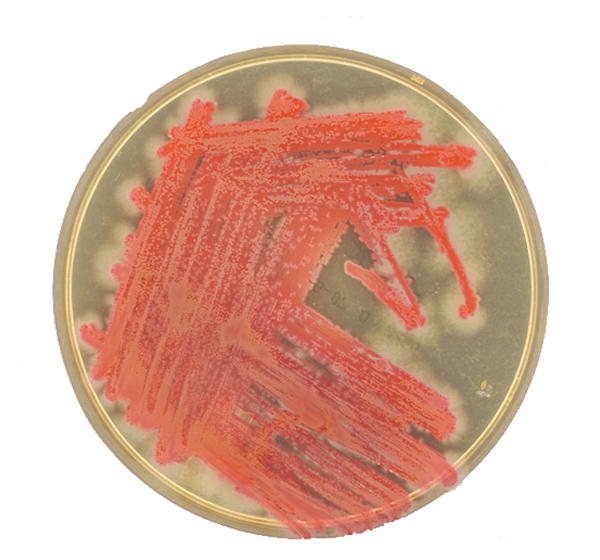
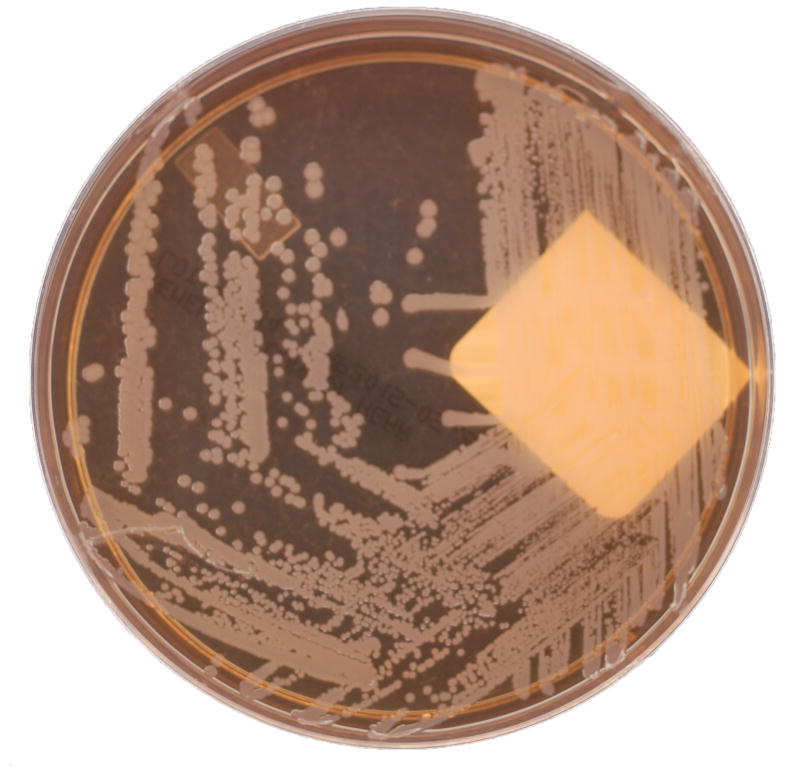
Perhaps the most well-known and defining characteristic of certain Serratia spp., including S. marcescens, is the production of a reddish pigment known as prodigiosin (Figure 2a).2 Certain strains can also produce another pigment, known as pyrimine.2 Colony pigmentation can be an important aid in laboratory identification, though this feature is certainly not unique to Serratia spp. Importantly, prodigiosin (from the Latin prodigiosus, meaning marvelous) production seems to be temperature-dependent with production declining above 28°C,3 which might partially explain the relative infrequency of pigmentation observed in clinical isolates (Figure 2b).
Figure 2. Representative clinical isolates of Serratia marcescens, including both (a) pigmented and (b) non-pigmented strains.
(Photos courtesy of Lori Racsa, DO – Department of Pathology & Laboratory Medicine, Emory University School of Medicine)
Whether prodigiosin (or pyrimine) production has any impact on the natural history of clinical infections is unknown. As noted above, pigmentation is actually less common among clinical isolates than environmental strains of S. marcescens.4 This may be attributable to some of the characteristics of prodigiosin that promote growth in the environment, including its influence on bacterial adhesion to hydrophobic surfaces5 and energy utilization during stationary phase,6 and also because its expression is co-regulated with swarming motility.7 Prodigiosin also has broad, albeit weak, in vitro antimicrobial activity8 – in some insect vectors, colonization of the midgut with prodigiosin-producing organisms such as S. marcescens can attenuate the viability of parasite species ingested during a bloodmeal.9 On the other hand, while prodigiosin is not considered a traditional virulence factor in vivo, it does exhibit important immuno-modulatory properties,8 and the same transcription factor (PigP) that regulates its expression may also control hemolysis.7 In some early studies pigment production was found to negatively impact the transferability of R-factor plasmid-mediated drug resistance;10 however other studies have detected no difference in drug susceptibility profiles between pigmented and non-pigmented clinical isolates.11
Largely because of this pigmentation Serratia, among all Enterobacteriaceae, also has perhaps the most colorful history. For example Serratia has been implicated in the “miraculous” appearance of blood in various contexts for millennia, and one of these events prompted its ultimate identification in 1819.1, 2 More recently, but prior to its recognition as a human pathogen, pigmented Serratia strains were used as biologic markers in experiments ranging from the study of airborne transmission of microbes in the British House of Commons,12 to weaponized aerosolization of potential ocean-borne pathogens in San Francisco Bay.13 Despite speculation that such experiments were responsible for subsequent clusters of Serratia infections in various geographic regions this link has never been definitively proven.14
Serratia spp. can be members of the human intestinal flora, but most clinical infections are thought to be exogenous. These organisms are ubiquitous in the environment and S. marcescens has been isolated from soil, a variety of plants and animals, and most importantly a wide range of water sources, both natural and municipal.2 This predilection for water likely accounts for the prominent contribution of S. marcescens to sporadic ventilator-associated pneumonias,15 catheter-associated urinary tract infections,16 and other hospital-acquired infections,17 as well as multiple nosocomial outbreaks associated with contaminated intravenous medications and medical devices.1, 2
Risk factors and epidemiology
Infective endocarditis (IE) due to S. marcescens was first described in 1951,18 and the first case of IE due to a non-pigmented strain of Serratia was reported in 1970.19 Serratia marcescens also played an important role in historical experiments that elucidated the pathogenesis of endocarditis.20 Yet, IE due to this organism remained an obscure disease for decades until its association with IVDU, and even today endovascular infections are highly uncommon. For example, in the International Collaboration on Endocarditis (ICE) prospective cohort, out of 2,761 definite IE cases identified over a 5 year period, only 49 (1.8%) were due to non-HACEK (Haemophilus spp., Aggregatibacter actinomyetemcomitans, Cardiobacterium hominis, Eikenella corrodens, or Kingella spp.) gram negative bacilli, and of those only 4 (0.14% of the total) were due to S. marcescens.21 In fact, only 19 cases of Serratia endocarditis have been reported in the English-language literature since 1980 (Table 1).21-35 Similar to the case presented here, virtually all of these previously reported patients had chronic medical conditions or cardiovascular abnormalities, more than half had involvement of left-sided valves, and more than one fifth of the patients died. A few non-valvular endovascular Serratia infections have also been reported, and most of these cases were associated with preceding cardiac surgery and the presence of prosthetic material.36-38
Table 1. Published (English-language) cases of infective endocarditis due to Serratia spp. since 1980.
| Study/ Year/Ref | Location | Age | Risk factor(s) | Organism | Valve(s) | Antimicrobial therapy | Surgery | Outcome |
|---|---|---|---|---|---|---|---|---|
| Hyams/ 1981/22 | USA | 7 years | Extensive third-degree burns, central venous catheter | S. marcescens | TV | Cefotaxime × 6 weeks | None | Survived |
|
| ||||||||
| Hobbs/ 1982/23 | USA | 56 years | Diabetes mellitus, ESRD, intra-abdominal surgery | S. marcescens | TV, PV, AV, MV | Ampicillin + tobramycin | None | Died |
|
| ||||||||
| Berkowitz/1983/24 | South Africa | 14 years | Mitral valve repair for rheumatic mitral regurgitation | S. marcescens | MV | Ceftazidime × 6 weeks | Mitral valve replacement x2 | Survived |
|
| ||||||||
| Ena/ 1991/25 | Spain | 29 years | IVDU | S. marcescens | AV, MV, TV? | Ciprofloxacin × 4 weeks | None | Survived |
| 56 years | Post-sternotomy mediastinitis | S. liquefaciens | AV (prosthetic) | Ciprofloxacin × 4 weeks | None | Survived | ||
|
| ||||||||
| Korner/ 1994/26 | UK | 50 years | Non-Hodgkin's lymphoma, central venous catheter | S. marcescens | AV, MV | Azlocillin + gentamicin × 6 weeks | None | Survived |
|
| ||||||||
| Riberi/ 1997/27 | France | NS | NS | Serratia spp. | AV | NS | Aortic valve replacement | NS |
|
| ||||||||
| Pearlman/ 1998/28 | USA | 27 weeks | Prematurity | S. marcescens | Right ventricle | Cefotaxime + gentamicin | None | Died |
|
| ||||||||
| Mossad/ 2000/29 | USA | 55 years | Short gut syndrome, central venous catheter with suppurative thrombophlebitis | S. liquefaciens | Right atrium | Ceftriaxone | None | Survived |
|
| ||||||||
| Chuang/ 2007/30 | Taiwan | 41 years | IVDU, cirrhosis | S. liquefaciens | AV | Imipenem-cilastatin | None | Died |
|
| ||||||||
| Morpeth/ 2007/21 | Multi-national | NS (n=4) | NS | NS | NS | β-lactam + fluoroquinolone (2/4), β-lactam + aminoglycoside (2/4) | Yes, details NS | Survived |
|
| ||||||||
| Yang/ 2007/31 | China | 11 years | Intracardiac foreign body | S. marcescens | Right and left atria | Cefminox + etimicin | Resection of foreign body | Survived |
|
| ||||||||
| Baggish/ 2007/32 | USA | 43 years | Splenectomy | S. marcescens | MV | Cefepime × 6 weeks + gentamicin × 2 weeks | None | Survived |
|
| ||||||||
| De Silva/ 2009/33 | USA | 67 years | Pacemaker | S. marcescens | Pacing wire | Meropenem + gentamicin, then ciprofloxacin × 2 weeks | Explantation of pace-maker | Survived |
|
| ||||||||
| Hadano/ 2012/34 | Japan | 85 years | Diabetes mellitus | S. marcescens | MV | Ceftazidime + gentamicin | None | Died |
|
| ||||||||
| Lyall/2013/35 | UK | 65 years | Post-Bentall procedure | S. marcescens | AV (prosthetic) | Meropenem + ciprofloxacin + gentamicin | NS | Survived |
Abbreviations: ESRD = end-stage renal disease, IVDU = intravenous drug use, TV = tricuspid valve, PV = pulmonic valve, AV = aortic valve, MV = mitral valve, NS = not specified
Specific virulence factors that might predispose to invasive infection, and specifically endovascular invasion, have not been well studied. Like other Enterobacteriaceae, S. marcescens produces lipopolysaccharide (LPS),39 a pore-forming hemolysin (known as ShlA),40 as well as various adhesion factors (specifically, mannose-resistant and mannose-sensitive pili),4, 41 and extracellular products (such as chitinase, lipase, and chloroperoxidase);41 certain strains also exhibit the capacity for swarming motility7 and quorum-sensing regulated biofilm production,42 both partly under the control of the FlhDC system. Notably, the activity of many of these virulence factors is orchestrated through the RssAB regulatory system, and inactivation of RssAB is associated with increased virulence.2 Despite these numerous virulence factors, like other gram-negative bacteria, S. marcescens adheres much less readily to the valvular endothelium as compared to the gram positive bacteria typically associated with IE.43, 44
Serratia spp. are usually considered opportunistic pathogens. However, few host factors that would predispose to the development of IE due to S. marcescens have been identified. While bloodstream infections due to this organism are not uncommon, IE is quite rare (Table 1). Our patient was infected with HIV, which is an important risk factor for pyogenic bacterial infections, including bloodstream infections.45 However in the largest (n=17) reported case series of Serratia infections among HIV infected individuals, the authors found no patients with IE, and 64.7% of these infections were nosocomial in origin. No cases of IE due to S. marcescens in HIV-infected patients have been reported previously. HIV infection may be an independent risk factor for endocarditis,46 including recurrent endocarditis,47 particularly among intravenous drug users.48, 49 However it does not seem to confer an increased risk of complications or death in IVDU-related endocarditis.48
Two reports, published in 1976 and 1980 from the San Francisco Bay area, describing two clusters of cases of IE due to S. marcescens established the link between this disease and IVDU.50, 51 Together they described a total of 36 cases, more than triple the total number of such infections that had been reported in the preceding 25 years, and most (89%) were in IVDUs50, 51 – in fact, approximately 15% of all the IVDU-associated endocarditis at the University of California, San Francisco hospitals from 1968-1974 was due to S. marcescens.50 Interestingly a similar association with IVDU has not been seen in other geographic regions or even during subsequent years in the San Francisco Bay area.52 In the IVDU population gram positive organisms like the Staphylococci and Streptococci predominate, and even among gram negative bacilli, Pseudomonas has more frequently being described as a cause of IE, most extensively in Detroit,53-55 but also in Chicago56 and more recently in Spain as well.57 Some of this predilection for Pseudomonas infections was previously attributed to the predominant drugs of abuse at the time (pentazocine and tripelennamine),58 which led many to speculate that drug paraphernalia or municipal water sources were the reservoir for these unusual pathogens. Yet, microbiologic surveys of injection drugs and related paraphernalia have rarely yielded Serratia spp. or other causative organisms.51, 53, 59, 60 In our patient we speculate that he acquired the pathogen from the municipal tap water used for his IV drug use.
As noted previously, infective endocarditis due to Serratia and other non-HACEK gram negative bacilli has since become a disease of increased healthcare contact. In the ICE cohort, 57% of the cases of non-HACEK endocarditis were healthcare associated, and most of those were nosocomial in origin. Conversely only 4% were in IVDUs. In the 30 years following the landmark reports from San Francisco, the vast majority of the published cases of IE due to Serratia spp. have been in individuals with chronic illnesses, immuno-compromising conditions, or who have undergone recent surgery (Table 1) – in fact only two were in intravenous drug users. As such, the original 1951 description of IE due to S. marcescens in a chronically debilitated 67-year-old man with an indwelling urinary catheter was notably prescient for its time.
Diagnosis
Endocarditis due to S. marcescens is indistinguishable from that due to any other organism on clinical grounds alone. As noted above a history of IVDU can be suggestive, but empiric management of suspected IE in patients reporting active IVDU should account for the two more likely pathogens in that population, namely Staphylococcus aureus and Pseudomonas aeruginosa. While the minority of patients with bloodstream infections due to S. marcescens will develop IE, suggestive findings on physical examination or sustained bacteremia should prompt echocardiographic evaluation.
Intravenous drug users have higher rates of right-sided endocarditis compared to the general population.61 The mechanism for this predisposition is not fully understood, but likely involves a complex interplay between the infecting organism, the identity of the injection drug, other substances that are incidentally co-injected (e.g. particulate matter, diluent, etc.), and the underlying cardiac anatomy and immune status of the drug user.61 Based on the reported cases of Serratia endocarditis, the organism appears to have a predilection for the left-sided heart valves, even among IVDUs. In the two largest reports from San Francisco (total=36 patients, 89% IVDUs), 13/19 (68%) and 9/17 (53%) had involvement of the mitral or aortic valves, either alone or in combination with other valves.50, 51 Of the non-IVDU-associated cases that have been reported since 1980 (Table 1), the trend toward left-sided valvular involvement has persisted, and remains unexplained.
Treatment
Clinical isolates of Serratia marcescens can be susceptible to third and fourth generation cephalosporins, monobactams, carbapenems, fluoroquinolones, aminoglycosides, and cotrimoxazole.2, 62, 63 They are generally intrinsically resistant to penicillins and first and second generation cephalosporins due to plasmid encoded β-lactamases (e.g. TEM1), as well as tetracyclines, macrolides, chloramphenicol, and colistin.2, 63 Additional resistance can develop through a variety of mechanisms, including overexpression of AmpC β-lactamase, acquisition of plasmids encoding extended-spectrum β-lactamases (ESBLs), carbapenemases, or efflux pumps, and modifications to target proteins.2, 41, 63, 64
AmpC β-lactamase
Serratia marcescens, Enterobacter spp., Citrobacter spp., and several other organisms constitute a group of Enterobacteriaceae that possess a chromosomally-encoded AmpC β-lactamase (reviewed in detail by Jacoby, 2009).65 These enzymes readily hydrolyze penicillins and first, second, and third generation cephalosporins, and are uniformly resistant to all available β-lactamase inhibitors. They have significantly reduced activity against cefepime, carbapenems and monobactams. Their expression is typically maintained in a repressed state, but can increase to phenotypically apparent levels in two scenarios. First, exposure to β-lactam antibiotics can induce expression of AmpC enzymes via a signaling cascade initiated by the accumulation of peptidoglycan precursors. Certain β-lactams, particularly cefoxitin and perhaps later generation cephalosporins as well, tend to induce expression more than others. Second, mutations in AmpD, a regulatory protein which helps orchestrate the repression of AmpC, can lead either to hyperinducibility or constitutive expression of AmpC enzymes. In both scenarios AmpC overexpression confers phenotypic resistance.65 In our patient we suspected at least some level of AmpC expression given baseline resistance to ampicillin and first- and second-generation cephalosporins.
Whether AmpC-mediated resistance emerging as a consequence of antibiotic therapy (either through induction or selection) is clinically relevant has been an issue of some controversy since the phenomenon was first described on a large-scale in a landmark study of Enterobacter bloodstream infections in 1991.66 In that study 11 of 118 cases (9%) were complicated by bacteriologic failure, defined as recovery of a second Enterobacter isolate of the same spp. with a more resistant antibiotic profile while on antibiotic therapy. Seven of those isolates were identical to the original clone by molecular typing, implying emergence of resistance while on therapy, and in 6 of those 7 cases the patient had been receiving treatment with a third-generation cephalosporin. These patients accounted for 19% of the patients being treated with such agents.66 Other subsequent studies have recapitulated this finding for Enterobacter infections at other sites that are treated with third-generation cephalosporins.67
Based on these findings, many experts now recommend against the use of all cephalosporins for any AmpC-producing organism.68 However, in this regard Enterobacter and Serratia may be quite different. For example, in one study from South Korea that described outcomes of treatment for a variety of infections due to AmpC-producing organisms, none of 113 patients with Serratia infections had emergence of resistance while on therapy, including 33 who had bacteremia and 37 that were treated with a broad-spectrum cephalosporin (cefotaxime, ceftriaxone, ceftazidime).67 Yet, in another study from the same center of 129 episodes of Serratia bacteremia, of the 91 cases that were treated appropriately 13 had been treated with third-generation cephalosporin monotherapy and three of those developed resistance while on therapy (23%).69 Moreover in their multivariate analysis, exposure to second or third-generation cephalosporins in the preceding three months was a risk factor for baseline third-generation cephalosporin resistance, however it is unclear if that resistance was AmpC-mediated.69 In a more recent propensity score matched retrospective comparison of cefepime and meropenem for the treatment of infections due to AmpC-producing organisms, only 15% of Serratia isolates tested positive for AmpC production at baseline compared to 38% of all Enterobacter isolates.70
Antimicrobial therapy
As is the case for other non-HACEK gram negative bacilli where data are sparse, appropriate antimicrobial therapy for IE due to S. marcescens is not well defined. The 2005 Infectious Diseases Society of America (IDSA) and 2009 European Society of Cardiology (ESC) infective endocarditis guidelines make no specific antimicrobial recommendations, though both suggest combination therapy with a β-lactam and an aminoglycoside, extrapolating from data on Pseudomonas spp.71, 72
Most of the data describing antimicrobial regimens and treatment outcomes for Serratia endocarditis again comes from the two case series from San Francisco. In those series all patients were treated with an aminoglycoside, either alone, or more often in combination with carbenicillin, cefazolin, chloramphenicol, or cotrimoxazole.50, 51 Of note, both of the β-lactams used in those series are known substrates of AmpC β-lactamase. The reported outcomes were generally poor with 13/19 patients dying (68% mortality) in the original series.50 There have been a few case reports in the era of extended-spectrum and anti-pseudomonal β-lactams and fluoroquinolones (Table 1), but treatment strategies have been heterogeneous, making it difficult to draw any definitive conclusions regarding relative efficacy.
Given the theoretical possibility of developing AmpC-mediated resistance, particularly in deep-seated infections (e.g. endovascular, bone, central nervous system) with a high inoculum,73 treatment with a carbapenem or fluoroquinolone may be the most reliable option. There are emerging data that cefepime,70 extended-spectrum penicillins (e.g. piperacillin), and cotrimoxazole may be suitable alternatives.68 However, global surveillance programs indicate that the rates of baseline resistance of S. marcescens to many of these agents vary substantially between regions,62, 74 therefore for serious infections such as IE, empiric carbapenem use may be warranted while awaiting the results of susceptibility testing.
The added benefit of combination therapy is unclear. In experimental rabbit models of endocarditis due to Enterobacter concurrent aminoglycoside administration led to more rapid sterilization of affected valves, but the clinical significance of this finding remains unclear.75 In our patient given the initial antibiotic resistance profile and concern for baseline AmpC production, we elected to change his therapy from ceftriaxone to meropenem. His repeat blood cultures remained sterile so combination aminoglycoside therapy was deferred.
Surgical therapy
Endocarditis due to Serratia spp. is a highly morbid disease. Valve destruction, paravalvular complications, and distant embolic events are frequently described. This propensity for rapidly progressive disease likely reflects a selection for particularly virulent strains of the organism. As such, based largely on the experience in San Francisco from the 1970-80s, current guidelines recommend prompt (within 7-10 days of diagnosis) surgical intervention.71, 72 Whether this recommendation is equally valid in the era of more reliably active antibiotics (e.g. carbapenems, fluoroquinolones, etc.) is unclear, but the pathogenicity of this organism should certainly be carefully considered in addition to the standard surgical indications when managing patients with IE.
Prevention
Serratia marcescens is frequently a hospital-acquired pathogen and it has been implicated in scores of nosocomial outbreaks, with sources including contaminated fluids, medications, medical devices, and hospital surfaces.1, 2 Intensification of routine infection control measures, including scrupulous hand hygiene and cohorting of infected or colonized patients, has played a central role in terminating many of these outbreaks63, even in cases where the source could not be adequately identified. Given the propensity for S. marcescens to cause invasive disease that is often associated with significant morbidity and mortality, clusters of hospital-acquired infections due to S. marcescens warrant exhaustive epidemiologic investigation as well as heightened vigilance for breakdowns in infection control practices.
Conclusion
Infective endocarditis with Serratia marcescens is rare, but may be increasing. While historically associated with intravenous drug use, recent trends suggest that it is frequently a disease of healthcare contact. With a growing population of chronically ill, debilitated, and immunocompromised patients with frequent healthcare exposure and thus a higher risk for Serratia infections, there will certainly be more reports of IE due to Serratia spp. Yet, as illustrated by the patient discussed here, who exhibited many of the characteristic epidemiologic and clinical features that have been previously described for this condition, S. marcescens should remain an important consideration in IVDUs presenting with suspected IE. In our case, despite appropriate antibiotic therapy the patient's disease rapidly progressed and he was not a candidate for surgical intervention. Treatment options for serious infections due to AmpC-producing organisms like S. marcescens remain limited therefore a multidisciplinary therapeutic approach may optimize patient outcomes.
Acknowledgments
Varun K. Phadke is supported by the Emory Vaccinology Training Program, Award Number T32AI074492 from the National Institute of Allergy and Infectious Diseases. The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Sources of support: Varun K. Phadke is supported by the Emory Vaccinology Training Program, Award Number T32AI074492 from the National Institute of Allergy and Infectious Diseases. The content of this review is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
Footnotes
Conflicts of interest: The authors have no potential conflicts of interest to disclose
References
- 1.Yu VL. Serratia marcescens: historical perspective and clinical review. The New England journal of medicine. 1979;300:887–893. doi: 10.1056/NEJM197904193001604. [DOI] [PubMed] [Google Scholar]
- 2.Mahlen SD. Serratia infections: from military experiments to current practice. Clinical microbiology reviews. 2011;24:755–791. doi: 10.1128/CMR.00017-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams RP, Gott CL, Qadri SM, et al. Influence of temperature of incubation and type of growth medium on pigmentation in Serratia marcescens. Journal of bacteriology. 1971;106:438–443. doi: 10.1128/jb.106.2.438-443.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aucken HM, Pitt TL. Antibiotic resistance and putative virulence factors of Serratia marcescens with respect to O and K serotypes. Journal of medical microbiology. 1998;47:1105–1113. doi: 10.1099/00222615-47-12-1105. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg M, Blumberger Y, Judes H, et al. Cell surface hydrophobicity of pigmented and nonpigmented clinical Serratia marcescens strains. Infection and immunity. 1986;51:932–935. doi: 10.1128/iai.51.3.932-935.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haddix PL, Jones S, Patel P, et al. Kinetic analysis of growth rate, ATP, and pigmentation suggests an energy-spilling function for the pigment prodigiosin of Serratia marcescens. Journal of bacteriology. 2008;190:7453–7463. doi: 10.1128/JB.00909-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shanks RM, Lahr RM, Stella NA, et al. A Serratia marcescens PigP homolog controls prodigiosin biosynthesis, swarming motility and hemolysis and is regulated by cAMP-CRP and HexS. PloS one. 2013;8:e57634. doi: 10.1371/journal.pone.0057634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stankovic N, Senerovic L, Ilic-Tomic T, et al. Properties and applications of undecylprodigiosin and other bacterial prodigiosins. Applied microbiology and biotechnology. 2014;98:3841–3858. doi: 10.1007/s00253-014-5590-1. [DOI] [PubMed] [Google Scholar]
- 9.Azambuja P, Garcia ES, Ratcliffe NA. Gut microbiota and parasite transmission by insect vectors. Trends in parasitology. 2005;21:568–572. doi: 10.1016/j.pt.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 10.Muto Y, Tsuji A, Kaneko Y, et al. Relationship between pigment producibility and drug resistance in Serratia marcescens. Microbiology and immunology. 1981;25:1101–1108. doi: 10.1111/j.1348-0421.1981.tb00118.x. [DOI] [PubMed] [Google Scholar]
- 11.Cooksey RC, Bannister ER, Farrar WE., Jr Antibiotic resistance patterns of clinical isolates of Serratia marcescens. Antimicrobial agents and chemotherapy. 1975;7:396–399. doi: 10.1128/aac.7.4.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon MH. Appendix to Parliamentary Paper Cd 3035. Cd 3035. London: 1906. Report on investigation of the ventilation of the debating chamber of the House of Commons. [Google Scholar]
- 13.Biological testing involving human subjects by the Department of Defense, 1977: hearings before the Subcommittee on Health and Scientific Research of the Committee on Human Resources, United States Senate, Ninety-fifth Congress, first session … March 8 and May 23, 1977. U.S. Govt. Print. Off.; 1977. [Google Scholar]
- 14.Farmer JJ, 3rd, Davis BR, Grimont PA, et al. Source of American Serratia. Lancet. 1977;2:459–460. doi: 10.1016/s0140-6736(77)90650-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2010;51(Suppl 1):S81–87. doi: 10.1086/653053. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa K, Matsumoto T, Yasuda M, et al. The nationwide study of bacterial pathogens associated with urinary tract infections conducted by the Japanese Society of Chemotherapy. Journal of infection and chemotherapy : official journal of the Japan Society of Chemotherapy. 2011;17:126–138. doi: 10.1007/s10156-010-0174-1. [DOI] [PubMed] [Google Scholar]
- 17.Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2013;34:1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 18.Wheat RP, Zuckerman A, Rantz LA. Infection due to chromobacteria; report of 11 cases. AMA archives of internal medicine. 1951;88:461–466. doi: 10.1001/archinte.1951.03810100045004. [DOI] [PubMed] [Google Scholar]
- 19.Williams JC, Jr, Johnson JE., 3rd Serratia marcescens endocarditis. Report of a patient. Archives of internal medicine. 1970;125:1038–1040. [PubMed] [Google Scholar]
- 20.Rodbard S. Blood velocity and endocarditis. Circulation. 1963;27:18–28. doi: 10.1161/01.cir.27.1.18. [DOI] [PubMed] [Google Scholar]
- 21.Morpeth S, Murdoch D, Cabell CH, et al. Non-HACEK gram-negative bacillus endocarditis. Annals of internal medicine. 2007;147:829–835. doi: 10.7326/0003-4819-147-12-200712180-00002. [DOI] [PubMed] [Google Scholar]
- 22.Hyams KC, Mader JT, Pollard RB, et al. Serratia endocarditis in a pediatric burn patient. Cure with cefotaxime. Jama. 1981;246:983–984. [PubMed] [Google Scholar]
- 23.Hobbs RD, Downing SE, Andriole VT. Four-valve polymicrobial endocarditis caused by Pseudomonas aeruginosa and Serratia marcescens. The American journal of medicine. 1982;72:164–168. doi: 10.1016/0002-9343(82)90604-0. [DOI] [PubMed] [Google Scholar]
- 24.Berkowitz FE, Colsen P, Raw K. Serratia marcescens endocarditis treated with ceftazidime. A case report. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 1983;64:105–106. [PubMed] [Google Scholar]
- 25.Ena J, Amador C, Parras F, et al. Ciprofloxacin as an effective antibacterial agent in serratia endocarditis. The Journal of infection. 1991;22:103–105. doi: 10.1016/0163-4453(91)91346-y. [DOI] [PubMed] [Google Scholar]
- 26.Korner RJ, Nicol A, Reeves DS, et al. Ciprofloxacin resistant Serratia marcescens endocarditis as a complication of non-Hodgkin's lymphoma. The Journal of infection. 1994;29:73–76. doi: 10.1016/s0163-4453(94)95141-1. [DOI] [PubMed] [Google Scholar]
- 27.Riberi A, Caus T, Mesana T, et al. Aortic valve or root replacement with cryopreserved homograft for active infectious endocarditis. Cardiovascular surgery. 1997;5:579–583. doi: 10.1016/s0967-2109(97)00074-4. [DOI] [PubMed] [Google Scholar]
- 28.Pearlman SA, Higgins S, Eppes S, et al. Infective endocarditis in the premature neonate. Clinical pediatrics. 1998;37:741–746. doi: 10.1177/000992289803701205. [DOI] [PubMed] [Google Scholar]
- 29.Mossad SB. The world's first case of Serratia liquefaciens intravascular catheter-related suppurative thrombophlebitis and native valve endocarditis. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2000;6:559–560. doi: 10.1046/j.1469-0691.2000.00164.x. [DOI] [PubMed] [Google Scholar]
- 30.Chuang TY, Chuang CP, Cheng HH, et al. Aortic valve infective endocarditis caused by Serratia liquefaciens. The Journal of infection. 2007;54:e161–163. doi: 10.1016/j.jinf.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Yang L, Wang JF, Wu W. Delayed Serratia marcescens endocarditis associated with intracardiac foreign body. Chinese medical journal. 2007;120:437–440. [PubMed] [Google Scholar]
- 32.Baggish AL, Nadiminti H. Intracranial abscess from embolic Serratia marcescens endocarditis. The Lancet Infectious diseases. 2007;7:630. doi: 10.1016/S1473-3099(07)70213-X. [DOI] [PubMed] [Google Scholar]
- 33.De Silva K, Fife A, Murgatroyd F, et al. Pacemaker endocarditis: an important clinical entity. BMJ case reports. 2009:2009. doi: 10.1136/bcr.02.2009.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hadano Y, Kamiya T, Uenishi N. A fatal case of infective endocarditis caused by an unusual suspect: Serratia marcescens. Internal medicine. 2012;51:1425–1428. doi: 10.2169/internalmedicine.51.6648. [DOI] [PubMed] [Google Scholar]
- 35.Lyall DA, Gregory ME, McDonnell J, et al. Bilateral endogenous Serratia marcescens endophthalmitis secondary to endocarditis following cardiac surgery. Scottish medical journal. 2013;58:e1–6. doi: 10.1177/0036933013482647. [DOI] [PubMed] [Google Scholar]
- 36.Nishimura Y, Okamura Y, Hiramatsu T, et al. Successful treatment of mycotic thoracic aortic aneurysm by in situ graft replacement with omental wrapping. The Japanese journal of thoracic and cardiovascular surgery : official publication of the Japanese Association for Thoracic Surgery = Nihon Kyobu Geka Gakkai zasshi. 2006;54:78–80. doi: 10.1007/BF02744606. [DOI] [PubMed] [Google Scholar]
- 37.Kawajiri H, Kanda K, Oka K, et al. Infectious pseudoaneurysm at the proximal edge of the endograft, after hybrid aortic arch repair. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2013;44:1151–1152. doi: 10.1093/ejcts/ezt240. [DOI] [PubMed] [Google Scholar]
- 38.Zoli S, Kassem S, Tessitore G, et al. Mycotic ascending aortic pseudoaneurysm following reduction aortoplasty. Journal of cardiac surgery. 2011;26:100–101. doi: 10.1111/j.1540-8191.2010.01153.x. [DOI] [PubMed] [Google Scholar]
- 39.Makimura Y, Asai Y, Sugiyama A, et al. Chemical structure and immunobiological activity of lipid A from Serratia marcescens LPS. Journal of medical microbiology. 2007;56:1440–1446. doi: 10.1099/jmm.0.47327-0. [DOI] [PubMed] [Google Scholar]
- 40.Kurz CL, Chauvet S, Andres E, et al. Virulence factors of the human opportunistic pathogen Serratia marcescens identified by in vivo screening. The EMBO journal. 2003;22:1451–1460. doi: 10.1093/emboj/cdg159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hejazi A, Falkiner FR. Serratia marcescens. Journal of medical microbiology. 1997;46:903–912. doi: 10.1099/00222615-46-11-903. [DOI] [PubMed] [Google Scholar]
- 42.Bakkiyaraj D, Sivasankar C, Pandian SK. Inhibition of quorum sensing regulated biofilm formation in Serratia marcescens causing nosocomial infections. Bioorganic & medicinal chemistry letters. 2012;22:3089–3094. doi: 10.1016/j.bmcl.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 43.Gould K, Ramirez-Ronda CH, Holmes RK, et al. Adherence of bacteria to heart valves in vitro. The Journal of clinical investigation. 1975;56:1364–1370. doi: 10.1172/JCI108216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogawa SK, Yurberg ER, Hatcher VB, et al. Bacterial adherence to human endothelial cells in vitro. Infection and immunity. 1985;50:218–224. doi: 10.1128/iai.50.1.218-224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huson MA, Stolp SM, van der Poll T, et al. Community-acquired bacterial bloodstream infections in HIV-infected patients: a systematic review. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;58:79–92. doi: 10.1093/cid/cit596. [DOI] [PubMed] [Google Scholar]
- 46.Sogaard OS, Lohse N, Ostergaard L, et al. Morbidity and risk of subsequent diagnosis of HIV: a population based case control study identifying indicator diseases for HIV infection. PloS one. 2012;7:e32538. doi: 10.1371/journal.pone.0032538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alagna L, Park LP, Nicholson BP, et al. Repeat endocarditis: analysis of risk factors based on the International Collaboration on Endocarditis - Prospective Cohort Study. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014;20:566–575. doi: 10.1111/1469-0691.12395. [DOI] [PubMed] [Google Scholar]
- 48.Nahass RG, Weinstein MP, Bartels J, et al. Infective endocarditis in intravenous drug users: a comparison of human immunodeficiency virus type 1-negative and -positive patients. The Journal of infectious diseases. 1990;162:967–970. doi: 10.1093/infdis/162.4.967. [DOI] [PubMed] [Google Scholar]
- 49.Manoff SB, Vlahov D, Herskowitz A, et al. Human immunodeficiency virus infection and infective endocarditis among injecting drug users. Epidemiology. 1996;7:566–570. doi: 10.1097/00001648-199611000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Mills J, Drew D. Serratia marcescens endocarditis: a regional illness associated with intravenous drug abuse. Annals of internal medicine. 1976;84:29–35. doi: 10.7326/0003-4819-84-1-29. [DOI] [PubMed] [Google Scholar]
- 51.Cooper R, Mills J. Serratia endocarditis. A follow-up report. Archives of internal medicine. 1980;140:199–202. [PubMed] [Google Scholar]
- 52.Jain V, Yang MH, Kovacicova-Lezcano G, et al. Infective endocarditis in an urban medical center: association of individual drugs with valvular involvement. The Journal of infection. 2008;57:132–138. doi: 10.1016/j.jinf.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 53.Reyes MP, Palutke WA, Wylin RF. Pseudomonas endocarditis in the Detroit Medical Center. 1969-1972. Medicine. 1973;52:173–194. doi: 10.1097/00005792-197305000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Crane LR, Levine DP, Zervos MJ, et al. Bacteremia in narcotic addicts at the Detroit Medical Center. I. Microbiology, epidemiology, risk factors, and empiric therapy. Reviews of infectious diseases. 1986;8:364–373. doi: 10.1093/clinids/8.3.364. [DOI] [PubMed] [Google Scholar]
- 55.Reyes MP, Ali A, Mendes RE, et al. Resurgence of Pseudomonas endocarditis in Detroit, 2006-2008. Medicine. 2009;88:294–301. doi: 10.1097/MD.0b013e3181b8bedc. [DOI] [PubMed] [Google Scholar]
- 56.Shekar R, Rice TW, Zierdt CH, et al. Outbreak of endocarditis caused by Pseudomonas aeruginosa serotype O11 among pentazocine and tripelennamine abusers in Chicago. The Journal of infectious diseases. 1985;151:203–208. doi: 10.1093/infdis/151.2.203. [DOI] [PubMed] [Google Scholar]
- 57.Miro JM, Moreno A, Mestres CA. Infective Endocarditis in Intravenous Drug Abusers. Current infectious disease reports. 2003;5:307–316. doi: 10.1007/s11908-003-0007-9. [DOI] [PubMed] [Google Scholar]
- 58.Botsford KB, Weinstein RA, Nathan CR, et al. Selective survival in pentazocine and tripelennamine of Pseudomonas aeruginosa serotype O11 from drug addicts. The Journal of infectious diseases. 1985;151:209–216. doi: 10.1093/infdis/151.2.209. [DOI] [PubMed] [Google Scholar]
- 59.Tuazon CU, Hill R, Sheagren JN. Microbiologic study of street heroin and injection paraphernalia. The Journal of infectious diseases. 1974;129:327–329. doi: 10.1093/infdis/129.3.327. [DOI] [PubMed] [Google Scholar]
- 60.Reiner NE, Gopalakrishna KV, Lerner PI. Enterococcal endocarditis in heroin addicts. Jama. 1976;235:1861–1863. [PubMed] [Google Scholar]
- 61.Frontera JA, Gradon JD. Right-side endocarditis in injection drug users: review of proposed mechanisms of pathogenesis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2000;30:374–379. doi: 10.1086/313664. [DOI] [PubMed] [Google Scholar]
- 62.Sader HS, Farrell DJ, Flamm RK, et al. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalized in intensive care units in United States and European hospitals (2009-2011) Diagnostic microbiology and infectious disease. 2014;78:443–448. doi: 10.1016/j.diagmicrobio.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 63.Herra C, Falkiner FR. Serratia marcescens. In: Yu VL, Weber R, Raoult D, editors. Antimicrobial Therapy and Vaccines. Vol. 1. New York: Apple Trees Production; 2002. [Google Scholar]
- 64.Begic S, Worobec EA. The role of the Serratia marcescens SdeAB multidrug efflux pump and TolC homologue in fluoroquinolone resistance studied via gene-knockout mutagenesis. Microbiology. 2008;154:454–461. doi: 10.1099/mic.0.2007/012427-0. [DOI] [PubMed] [Google Scholar]
- 65.Jacoby GA. AmpC beta-lactamases. Clinical microbiology reviews. 2009;22:161–182. doi: 10.1128/CMR.00036-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chow JW, Fine MJ, Shlaes DM, et al. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Annals of internal medicine. 1991;115:585–590. doi: 10.7326/0003-4819-115-8-585. [DOI] [PubMed] [Google Scholar]
- 67.Choi SH, Lee JE, Park SJ, et al. Emergence of antibiotic resistance during therapy for infections caused by Enterobacteriaceae producing AmpC beta-lactamase: implications for antibiotic use. Antimicrobial agents and chemotherapy. 2008;52:995–1000. doi: 10.1128/AAC.01083-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harris PN, Ferguson JK. Antibiotic therapy for inducible AmpC beta-lactamase-producing Gram-negative bacilli: what are the alternatives to carbapenems, quinolones and aminoglycosides? International journal of antimicrobial agents. 2012;40:297–305. doi: 10.1016/j.ijantimicag.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 69.Choi SH, Kim YS, Chung JW, et al. Serratia bacteremia in a large university hospital: trends in antibiotic resistance during 10 years and implications for antibiotic use. Infection control and hospital epidemiology : the official journal of the Society of Hospital Epidemiologists of America. 2002;23:740–747. doi: 10.1086/502004. [DOI] [PubMed] [Google Scholar]
- 70.Tamma PD, Girdwood SC, Gopaul R, et al. The use of cefepime for treating AmpC beta-lactamase-producing Enterobacteriaceae. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57:781–788. doi: 10.1093/cid/cit395. [DOI] [PubMed] [Google Scholar]
- 71.Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005;111:e394–434. doi: 10.1161/CIRCULATIONAHA.105.165564. [DOI] [PubMed] [Google Scholar]
- 72.Habib G, Hoen B, Tornos P, et al. Guidelines on the prevention, diagnosis, and treatment of infective endocarditis (new version 2009): the Task Force on the Prevention, Diagnosis, and Treatment of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and the International Society of Chemotherapy (ISC) for Infection and Cancer. European heart journal. 2009;30:2369–2413. doi: 10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 73.Kang CI, Pai H, Kim SH, et al. Cefepime and the inoculum effect in tests with Klebsiella pneumoniae producing plasmid-mediated AmpC-type beta-lactamase. The Journal of antimicrobial chemotherapy. 2004;54:1130–1133. doi: 10.1093/jac/dkh462. [DOI] [PubMed] [Google Scholar]
- 74.Pfaller MA, Jones RN, Group MS. Antimicrobial susceptibility of inducible AmpC beta-lactamase-producing Enterobacteriaceae from the Meropenem Yearly Susceptibility Test Information Collection (MYSTIC) Programme, Europe 1997-2000. International journal of antimicrobial agents. 2002;19:383–388. doi: 10.1016/s0924-8579(02)00009-2. [DOI] [PubMed] [Google Scholar]
- 75.Kobasa WD, Kaye D. Aztreonam, cefoperazone, and gentamicin in the treatment of experimental Enterobacter aerogenes endocarditis in rabbits. Antimicrobial agents and chemotherapy. 1983;24:321–324. doi: 10.1128/aac.24.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]