Abstract
The whitefly Bemisia tabaci is a genetically diverse complex with multiple cryptic species, and some are the most destructive invasive pests of many ornamentals and crops worldwide. Encarsia sophia is an autoparasitoid wasp that demonstrated high efficiency as bio-control agent of whiteflies. However, the immune mechanism of B. tabaci parasitization by E. sophia is unknown. In order to investigate immune response of B. tabaci to E. Sophia parasitization, the transcriptome of E. sophia parasitized B. tabaci nymph was sequenced by Illumina sequencing. De novo assembly generated 393,063 unigenes with average length of 616 bp, in which 46,406 unigenes (15.8% of all unigenes) were successfully mapped. Parasitization by E. sophia had significant effects on the transcriptome profile of B. tabaci nymph. A total of 1482 genes were significantly differentially expressed, of which 852 genes were up-regulated and 630 genes were down-regulated. These genes were mainly involved in immune response, development, metabolism and host signaling pathways. At least 52 genes were found to be involved in the host immune response, 33 genes were involved in the development process, and 29 genes were involved in host metabolism. Taken together, the assembled and annotated transcriptome sequences provided a valuable genomic resource for further understanding the molecular mechanism of immune response of B. tabaci parasitization by E. sophia.
Introduction
The whitefly Bemisia tabaci (Hemiptera: Aleyrodidae), is well known as a worldwide invasive pest and may cause severe damage to various vegetables by feeding on phloem sap and transmitting many viruses [1]. It is a complex species containing at least 30 cryptic species [2]. B and Q-types are two most economically damaging and invasive species [3]. There are many studies focus on biological characterization, resistance, invasive mechanism, and biological control of B. tabaci [4–12]. Over the past years, B. tabaci has demonstrated a remarkable resistance to many groups of chemical insecticides [13–16]. Due to the rapid resistance development, it is necessary to explore an alternative and effective management strategy to control B. tabaci. Parasitoid or parasitoid–produced regulatory molecules can be used to improve conventional pest control strategies.
Endoparasitoids have been identified as very important natural enemies of various arthropods, and could be used as biological control agents[17–19]. Hymenopteran endoparasitoids deposit their eggs into the host insect haemocoel, whose larvae feed on the host until its death [20–21]. Encarsia sophia is one of the specific parasitoids of Aleyrodidae species and has been used as efficacious classic biological control agents in many regions [22]. It can parasitize all instar nymphs of B. tabaci, especially the third and fourth instar nymphs [23]. The female wasp is generated by a bisexual process, but the male wasp is produced by autoparasitism [24]. Homogeneous E. sophia prefers to lay male eggs in the host parasitized by the heterogeneous wasp. When E. sophia and other kinds of wasps are raised or released together, the antecedent colonizers should inhibit the colonization of followers [25]. Previous studies have shown that E. sophia has strong plasticity adaption abilities[26].
However, the relationships between endoparasitoids and their hosts are complicated and involve long-term co-evolution. Many studies have investigated parasitoid biological characteristics, chemical communication, phylogenetic co-evolution, and physiological responses [27]. An increasing number of researchers have focused on revealing the physiological mechanism underlying the parasite induced immune defensive system and the biological development of hosts in order to estimate the co-evolution process between parasitoids and their hosts [28–31]. Although several reports have concentrated on the molecular regulation mechanisms, there have only been a few descriptions of related, functional genes [32,33]. Furthermore, the limitations of previous research methods has led to the development of high-throughput RNA sequencing technology (RNA-Seq)[34].
RNA-Seq is widely used to obtain transcriptomes of the organism, tissue, or organ, to identify genes that were regulated under certain conditions, and to reveal the regulatory mechanisms in different organisms [35–39]. In recent years, RNA-Seq has increasingly being applied in the biological agents to reveal the interaction mechanisms in the complex parasitoid-host system. Transcriptome profiling of organism under parasitization helps us to obtain a better understanding of host responses and effect on host’s growth, development. As a model species, Drosophila melanogaster and its parasitoid wasp Asobara tabida (Hymenoptera: Braconidae) is a well-studied system. Most genes associated with insect immunity appeared to be differentially expressed after wasp parasitized [40]. Most transcriptome studies on parasitoid-host systems have focused on Lepidoptera and Coleoptera, such as Plutella xylostella, Chilo suppressalis, Tenebrio molitor and Octodonta nipae [41–44]. A previous study showed that another parasitoid, Eretmocerus mundus may parasitize B. tabaci and induce the specific transcription of functional genes related to immune responses in the host [45]. However, the host manipulation by the parasitoid is species-specific, and the molecular mechanism of immune system in B. tabaci parasitization by E. sophia has not yet been explored. In this study, we used deep sequencing to explore B. tabaci response to E. sophia parasitization. Our results demonstrate that immune- and metabolic-related genes that are differentially expressed in parasitized versus non-parasitized B. tabaci nymph.
Materials and Methods
Insects Rearing and Parasitization
The biotype Q of Bemesia tabaci was obtained from the greenhouse at the Beijing Academy of Agriculture and Forestry. All experimental populations were derived from one pairs of newly emerged B. tabaci female and male. In our laboratory, the B. tabaci was reared on cotton plants (Zhong-mian-suo 49) in insect proof cages at 26 ± 1°C, and with a photoperiod of 15L: 9D. The purity of the cultures was monitored every three to five generations using the random amplified polymorphic DNA-polymerase chain reaction technique with COI gene [46]. E. sophia was obtained from the greenhouse at Beijing Academy of Agriculture and Forestry. All whitefly instar nymph stages were provided as hosts to E. sophia. Then approximate fifty E. sophia (female to male ratio of 8:1) individuals were released into cages to breed and the newly emerged female and male as parents for five generations breeding.
Thirty pairs of whiteflies were fed on cotton leaf in a micro insect cage and the fresh cotton leaf were provided every 24 hours. When they had reached later 3rd or early 4th instar, they were transferred in culture dish with a piece of cotton leaf, whose petiol were wrapped into soggy cotton, and then the mated E. sophia was released into B. tabaci rearing cage for parasitization. Sixty paired E. sophia were released into one culture dish. Wasp E. sophia were removed after 2 hours parasitization. The first group of samples was collected at 24-hr after parasitization (24AP). At this time period, the parasitoids were at the egg stage in which the embryo had formed and gradually began to move. The brown substance in the egg began to accumulate and chorion had appeared. In other words, the parasitoid possessed immune regulation ability, but the ability was not strong at the egg stage. Therefore, we could identify the immune defense response of the host against the parasitoid. The second sampling period was 72-hr after parasitization (72AP) when the wasps reach larval stage move around and absorb nutrition from the host. At this time, E. sophia may start to regulate host development and metabolism to finish their own development in whiteflies. Each treatment and control had three replicates.
cDNA Library Construction and Illumina Sequencing
Total RNA was extracted from all nymph samples using TRIzol™ reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction and treated with DNaseI. The concentration and integrity of RNA sample were determined using 2100 Bioanalyzer (Agilent Technologies). The first- and second- strand cDNA synthesis, end reparation, addition of “A” bases to 3' ends, ligation of adapters at the end of DNA fragments, and PCR amplification. The cDNA library was qualified and quantified with an Agilent 2100 Bioanalyzer and ABI StepOnePlus Real-time PCR system, respectively, and then sequenced using the Illumina HiSeq™2000 platform at the Beijing Genomics Institute (BGI, Shenzhen, China).
Transcriptome Analysis
In order to obtain clean reads, the low quality and adapter-polluted reads were removed from raw data. The good quality reads were assembled using Trinity[47] and assembled sequences were output as unigenes. All raw sequencing data have been deposited in NCBI Sequence Read Archive (SRA) database (http://www.ncbi.nlm.nih.gov/sra) with the following accession numbers: SRR1909644 (24AP), SRR1909651(72AP), SRR1909652 (CK-24AP), and SRR1909653 (CK-72AP). All the open reading frames (ORF) of unigene in B. tabaci were identified. If a unigene had many ORFs, we selected the longest one.
The unigenes were used for BlastX search and annotation against the NCBI non-redundant (nr) (http://blast.ncbi.nlm.nih.gov/Blast.cgi), Swiss-Prot (http://expasy.org/tools/blast), Kyoto Encyclopedia of Genes and Genome (KEGG, http://www.genome.jp/kegg/) databases with an E-value cut-off of 10-5. Gene Ontology (GO) annotation of unigenes was analyzed using the Blast2Go software [48], and GO functional classification for all unigens was performed using the WEGO software [49]. In the absence of B. tabaci and E. sophina genome sequences, we selected eight transcriptome datasets of B. tabaci from the NCBI database, and try to utilize the annotation that were the most closely related to B. tabaci gene in the parasitized library.
Differentially Expressed Gene (DEG) Analysis
In order to find all the differentially expressed genes, the same FPKM (Fragments Per Kilobase per Million fragments) value of unigene was first calculated for the treatment and control groups [50]. The results were displayed as fold changes, p-values and q-values. According to the q-value (p-value’s statistical result after PFR (Positive False Rate) correction), a q-value less than 0.05 or the absolute value of fold change greater than 2 represented a significant difference between the treatment and the control.
Quantitative Real-time PCR (qRT-PCR) Validation
The quantitative real-time PCR technique was used to verify the reliability of the deep sequencing. Nine differentially expressed genes were randomly selected. The β-actin gene was used for normalization. The four RNA samples represented nymphs at 24AP and 72AP, and their respective control (non-parasitized nymphs) at the same developmental stages.
First-strand cDNA was synthesized from the total RNA (1.2 μg) by using PrimeScripTM 1st Strand cDNA Synthesis Kit (TaKaRa) with oligo (dT)18 as primer following the manufacture’s protocols. The reaction system consisted of 10 μl of SYBR Green, 0.4 μl of ROX, 2 μl of diluted cDNA, 0.4 μl of each primer and 6.8 μl of distilled water. The reactions were loaded on the CFX96™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA) under the following conditions: 50°C for 2 min; 95°C for 2 min; and 40 cycles of 95°C for 10s, 60°C for 15s, and 72°C for 20s, followed by melting curve generation (68°C to 95°C). Data analysis was performed by one-way ANOVA following by Tukey’s test using SPSS software.
Results and Discussion
Illumina Sequencing and de novo Assembly
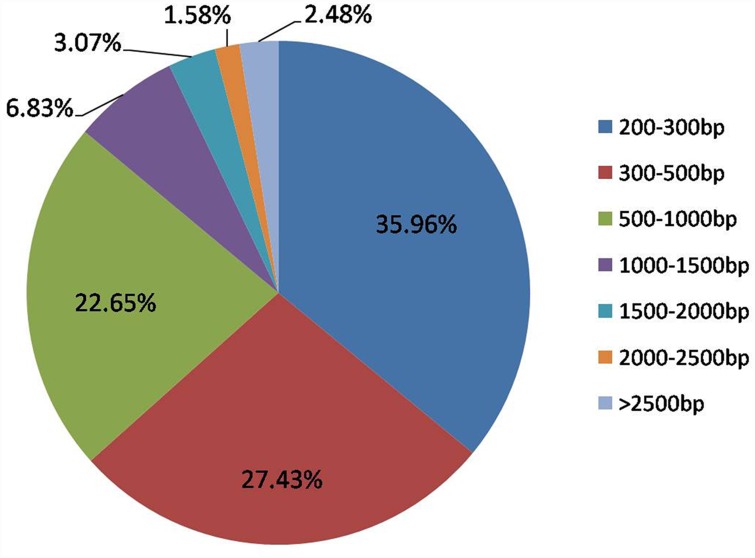
In order to know how E. sophia parasitization regulated B. tabaci development, immune-response and the differences in regulatory mechanisms between E. sophia egg- and larvae-stages. Approximately, 35 million and 39 million reads were generated from non-parasitized and parasitized B. tabaci nymphs at 24AP, respectively, and 31million and 37 million reads were from non-parasitized and parasitized B. tabaci nymphs at 72AP, respectively. De novo assembly produced 292,696 B. tabaci unigenes with an average size of 616 bp. Of these unigenes, 35.96% were between 200 and 300bp, 27.43% were between 300 and 500bp, 22.65% were between 500 and 1000bp and 13.96% had nucleotide lengths above 1000bp (Fig 1).
Fig 1. Distribution of unigene lengths in the B. tabaci transcriptome.
De novo assembly of RNA-seq data produced 292,696 unigenes between 201–28,036bp in length.
Functional Annotation and Classification
For functional annotation, the 292,696 unigenes were aligned to the GenBank protein databases with a cut-off E-value of 10−5 using BLASTx. Using this approach, 46,406 unigenes (15.8% of all unigenes) were successfully mapped. In order to predicate protein function, the unigenes were further given a gene ontology (GO) classification and subjected to KEGG pathway analysis. A total of 35,688 unigenes were annotated and assigned to GO terms, which consisted of three main categories: biological process, cellular component and molecular function. A total of 11,993 unigenes were categorized as cellular components, 12,102 unigenes were grouped under the molecular function, and 11,593 unigenes under biological processes. KEGG pathway analysis indicated that there were 4,721 unigenes assigned to different pathways in which translation, signal transduction, neurodegenerative diseases, infectious diseases, and endocrine system were the main B. tabaci pathways after E. sophia parasitizzation.
Enrichment Analysis of DEGs
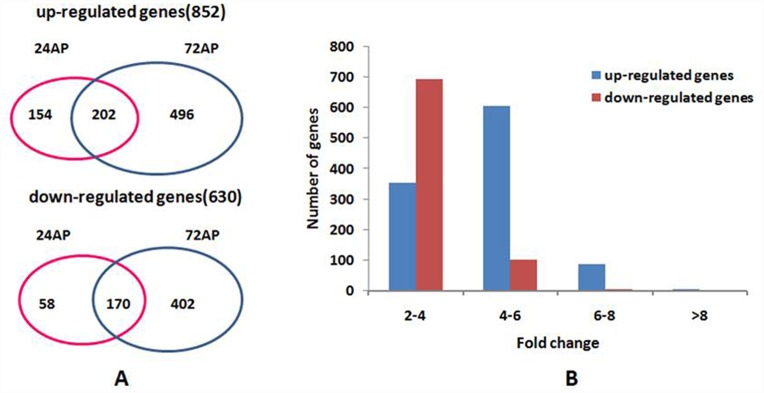
A total of 1,482 genes appeared to be significantly differentially expressed in the parasitized and non-parasitized B. tabaci, of which 852 genes were differentially up-regulated and 630 genes were differentially down-regulated (Fig 2A). At 24AP, there were 584 genes differentially expressed, of which 356 genes were up-regulated and 228 genes were down-regulated. At 72AP, there were 1,270 genes differentially expressed, of which 698 genes were up-regulated and 572 genes were down-regulated. Out of all of regulated genes, 202 up- and 170 down-regulated genes were found at both time points (Fig 2B) and more genes were up-regulated than that of the down-regulated genes at both 24AP and 72AP (Fig 2A). Furthermore, there was a significant difference in the numbers of differentially expressed genes at 24 hours than at 72 hours after parasitization. When E. sophia emerge in the larvae stage, more genes seemed to be involved in regulatory responses as compared to the egg stage. During the larvae stage, the parasitoid could move freely and began to feed on the host tissues. The distribution of the regulated genes indicated that their expression levels (>95%) were between two- to six-fold higher than at the egg stage (24 AP). Only a few genes changed more than six-fold (Fig 2).
Fig 2. General information about genes that were differentially expressed in response to parasitization.
The left figure shows the numbers of genes that were up-regulated and down-regulated at 24AP and 72AP. The right figure shows distribution of up-regulated (blue bars) and down-regulated (red bars) genes based on their fold change.
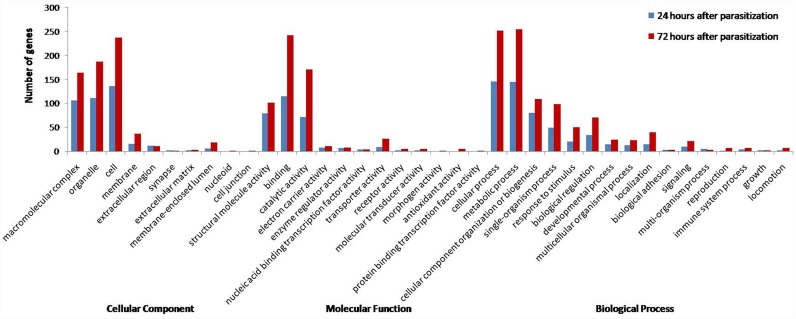
GO analysis revealed that the DEGs were mainly categorized in the cellular component cluster, that focus on macromolecular, organelle, and cellular levels. In the molecular function cluster, the DEGs were mainly found in structural molecule, binding, and catalytic activity. In the biological process cluster, the DEGs were mainly categorized in cellular and metabolic processes, and cellular component organization or biogenesis (Fig 3). In addition, more genes were involved in cellular processes, metabolic processes, single-organism processes, response to stimuli, biological regulation, localization, and cellular component organization or biogenesis at 72AP. Translation and signal transduction were the two most important pathways according to the KEGG pathways analysis. For KEGG enrichment analysis, genes involved in the immune system, nervous system, endocrine system, and metabolic activities were differentially expressed. The above results showed that parasitization had a great impact on the normal life activities of the host.
Fig 3. GO annotation of differentially expressed genes at 24APand 72AP (level 2).
At 24AP, the program categorized 390 unigenes in the cellular component category, 297 unigenes in the molecular function category, and 542 unigenes in the biological process category. At 72AP, the program categorized 732 unigenes in the cellular component category, 580 unigenes in the molecular function category, and 971 unigenes in the biological process category.
Effects of Parasitism on the Transcription of Host Immune-related Genes
Vertebrates have a set of immune defense mechanisms that include innate immunity and adaptive immunity, but invertebrates only have innate immunity protection [51]. Insects will initiate their innate immune response when encounting foreign agents, such as bacteria, fungi, virus, and parasitoid. The immune system of insects can be divided into two categories: 1) humoral defense, including the antimicrobial peptides, reactive intermediates of oxygen, melanin formation and clotting; and 2) cellular defense mainly based on haemocytes, such as phagocytosis, encapsulation, microaggregation and nodulation [52–54]. Two defense mechanisms are associated with a wide range of immune-related genes.
Our sequencing results indicated that E. sophia parasitism had a significant impact on the transcription of immune-related genes in B. Tabaci nymph (Table 1). We identified several up-regulated genes with homologs known to be involved in immune responses in insects, such as: defensin, knottin, serpin I2, laminin, spectrin, and apolipophorin. Defensin is an antimicrobial peptide, which acts as an innate immunity effector molecule and provides the first protection from pathogen infection. After parasitization by E. sophia at 24AP, we found that the transcription of defensin was up-regulated in B. tabaci nymph. Our results were consistent with previous studies that the mRNA levels of defensin in D. melanogaster and Phlebotomus duboscqi were significantly increased after parasitization [55, 56]. Although the main action targets of defensin are bacteria and fungi, it also plays a role in the host-parasitoid system. Knottins are mini proteins that are present in many different organisms and have various biological functions [57]. After parasitization by E. sophia at both 24AP and 72AP, four knottins were over-expressed. Like defensin, it is also an important antimicrobial peptide.
Table 1. Immune-related genes differentially expressed in B. tabaci after being parasitized by E. sophia.
| Gene ID | Length | Gene name | NP-FPKM | P-FPKM | Fold change | P-value | q-value |
|---|---|---|---|---|---|---|---|
| Genes up-regulated at 24AP | |||||||
| Unigene_131748 | 1458 | Spectrin alpha chain (Drosophila melanogaster) | 0.131 | 1.893 | 3.85 | 2.77E-05 | 0.0163 |
| Unigene_111268 | 648 | Probable chitinase 3 (Drosophila melanogaster) | 0.211 | 4.937 | 4.55 | 1.18E-05 | 0.00865 |
| Unigene_145476 | 225 | Defensin (Galleria mellonella) | 0.795 | 10.671 | 3.75 | 0.000119 | 0.0489 |
| Unigene_183100 | 744 | Hemocyanin (Palinurus vulgaris) | 0.421 | 5.305 | 3.66 | 3.50E-05 | 0.0195 |
| Unigene_190217 | 2130 | Protein toll (Drosophila melanogaster) | 0.0154 | 1.793 | 6.86 | 5.15E-06 | 0.00455 |
| Unigene_194849 | 909 | Apolipophorins (Locusta migratoria) | 0.264 | 4.275 | 4.02 | 4.63E-06 | 0.00417 |
| Unigene_155504 | 573 | Protein disulfide-isomerase (Drosophila melanogaster) | 0.189 | 4.503 | 4.57 | 1.20E-08 | 4.20E-05 |
| Unigene_83201 | 1410 | Cytospin-A (Takifugu rubripes) | 0.0439 | 1.013 | 4.53 | 0.000115 | 0.0476 |
| Unigene_154109 | 477 | Apoptosis 2 inhibitor (Drosophila melanogaster) | 0.0845 | 11.267 | 7.06 | 2.71E-06 | 0.00275 |
| Unigene_134383 | 2097 | Zinc finger MIZ domain-containing protein 1 (Homo sapiens) | 0.112 | 1.585 | 4.93 | 2.40E-05 | 0.0148 |
| Unigene_329886 | 2853 | Ankyrin-3 (Homo sapiens) | 3.389 | 22.543 | 2.73 | 1.69E-05 | 0.0114 |
| Unigene_268316 | 1131 | Cytochrome P450 6a2 (Drosophila melanogaster) | 3.265 | 26.698 | 3.03 | 1.66E-06 | 0.00190 |
| Unigene_288430 | 1278 | Cytochrome P450 6k1 (Blattella germanica) | 1.451 | 60.115 | 5.37 | 1.78E-15 | 1.46E-10 |
| Unigene_277195 | 249 | Bemisia tabaci putative antimicrobial knottin protein Btk-4 (Bemisia tabaci) | 7.0717 | 113.992 | 4.01 | 1.14E-09 | 6.57E-06 |
| Genes up-regulated in the 72AP | |||||||
| Unigene_154832 | 372 | Arginine kinase (Apis mellifera) | 0.349 | 2.993 | 3.10 | 3.48E-05 | 0.0194 |
| Unigene_156269 | 633 | Serpin I2 (Mus musculus) | 0.0552 | 2.228 | 5.33 | 9.73E-05 | 0.0419 |
| Unigene_330609 | 1089 | Serine protease homolog 42 isoform 2 (Nasonia vitripennis) | 0.0441 | 3.424 | 6.28 | 3.75E-06 | 0.00353 |
| Unigene_136705 | 681 | GILT-like protein C02D5.2 Caenorhabditis elegans (Caenorhabditis elegans) | 0.1009 | 8.852 | 6.45 | 1.94E-06 | 0.00214 |
| Unigene_55874 | 699 | cAMP-dependent protein kinase catalytic subunit (Drosophila melanogaster) | 0.1532 | 3.324 | 4.44 | 4.84E-05 | 0.0249 |
| Unigene_126734 | 516 | guanine nucleotide-binding protein G (q) subunit alpha (Homarus americanus) | 0.112 | 2.325 | 4.37 | 7.49E-06 | 0.00607 |
| Unigene_70023 | 1074 | Alpha-actinin, sarcomeric (Drosophila melanogaster) | 0.191 | 2.429 | 3.67 | 6.57E-05 | 0.0314 |
| Unigene_116220 | 753 | Apoptosis inhibitor 5 (Homo sapiens) | 0.0541 | 2.220 | 5.36 | 9.03E-05 | 0.0396 |
| Unigene_116141 | 804 | Casein kinase II subunit alpha (Spodoptera frugiperda) | 0.281 | 3.880 | 3.78 | 3.64E-05 | 0.0201 |
| Unigene_231151 | 894 | Serine/threonine-protein kinase PAK 1-like isoform 1 (Bombus impatiens) | 0.0328 | 1.632 | 5.63 | 3.64E-05 | 0.0209 |
| Unigene_213155 | 1212 | Cytochrome P450 4e3 (Drosophila melanogaster) | 0.327 | 3.011 | 3.20 | 6.21E-05 | 0.0302 |
| Unigene_214185 | 1041 | Probable cytochrome P450 6a18 (Drosophila melanogaster) | 0.491 | 4.686 | 3.25 | 1.69E-05 | 0.0114 |
| Unigene_161936 | 1362 | Laminin subunit beta-1 (Drosophila melanogaster) | 0.131 | 2.682 | 4.35 | 6.93E-05 | 0.0326 |
| Unigene_232028 | 873 | Cytochrome P450 4g15 (Drosophila melanogaster) | 1.948 | 12.021 | 2.63 | 7.07E-05 | 0.0331 |
| Unigene_239924 | 948 | Cytochrome P450 4C1(Blaberus discoidalis) | 0.469 | 4.649 | 3.31 | 2.31E-05 | 0.0143 |
| Unigene_288430 | 1278 | Cytochrome P450 6k1 (Blattella germanica) | 0.919 | 6.934 | 2.91 | 1.50E-05 | 0.0103 |
| Genes up-regulated in the 24AP and 72AP | |||||||
| Unigene_143480 | 303 | Bemisia tabaci putative antimicrobial knottin protein Btk-1 (Bemisia tabaci) | 16.101 | 283.469 | 4.14 | 1.86E-10 | 1.64E-06 |
| 15.014 | 127.85 | 3.09 | 9.91E-07 | 0.00126 | |||
| Unigene_244685 | 195 | Bemisia tabaci putative antimicrobial knottin protein Btk-2 (Bemisia tabaci) | 33.883 | 419.491 | 3.63 | 5.82E-08 | 0.000142 |
| 21.196 | 226.199 | 3.42 | 1.19E-07 | 0.000247 | |||
| Unigene_138814 | 183 | Bemisia tabaci putative antimicrobial knottin protein Btk-3 (Bemisia tabaci) | 7.869 | 288.573 | 5.20 | 8.44E-15 | 5.79E-10 |
| 6.807 | 113.886 | 4.06 | 5.77E-10 | 3.94E-06 | |||
| Unigene_158224 | 552 | Ras-like protein 3 (Drosophila melanogaster) | 0.138 | 1.695 | 3.62 | 1.30E-05 | 0.00929 |
| 0.131 | 3.582 | 4.78 | 7.97E-09 | 3.13E-05 | |||
| Unigene_74024 | 576 | Ras-like GTP-binding protein Rho1 (Drosophila melanogaster) | 0.119 | 5.015 | 5.40 | 3.71E-06 | 0.00326 |
| 0.379 | 8.120 | 4.42 | 3.02E-07 | 0.000506 | |||
| Unigene_45734 | 2199 | Serine/threonine-protein kinase SRPK3 (Bombus impatiens) | 0.0793 | 0.907 | 3.61 | 9.18E-05 | 0.0402 |
| 0.0602 | 2.0787 | 5.11 | 1.25E-07 | 0.000255 | |||
| Unigene_154175 | 711 | Heat shock 70 kDa protein cognate 3 (Drosophila melanogaster) | 0.501 | 5.758 | 3.52 | 7.29E-07 | 0.000997 |
| 0.0908 | 5.741 | 5.98 | 4.75E-10 | 3.37E-06 | |||
| Unigene_244154 | 591 | Actin-5C (Anopheles gambiae) | 2.036 | 31.216 | 3.94 | 8.39E-08 | 0.000189 |
| 2.459 | 20.452 | 3.06 | 2.43E-05 | 0.0149 | |||
| Unigene_134315 | 663 | Casein kinase II subunit beta (Rattus norvegicus) | 0.112 | 1.585 | 3.82 | 8.55E-05 | 0.0381 |
| 0.0713 | 2.142 | 4.91 | 6.10E-06 | 0.00519 | |||
| Unigene_47109 | 912 | Guanine nucleotide-binding protein subunit beta-like protein (Drosophila melanogaster) | 5.457 | 31.448 | 2.53 | 5.61E-05 | 0.0278 |
| 3.347 | 66.897 | 4.32 | 3.95E-11 | 4.93E-07 | |||
| Unigene_185113 | 1365 | Glycogen synthase kinase 3 beta (Nasonia vitripennis) | 0.0137 | 0.797 | 5.85 | 0.000112 | 0.0466 |
| 0.0174 | 0.915 | 5.71 | 2.70E-05 | 0.0165 | |||
| Unigene_218239 | 321 | Cofilin (Drosophila melanogaster) | 0.774 | 8.850 | 3.51 | 4.06E-05 | 0.0218 |
| 1.279 | 14.450 | 3.50 | 7.13E-06 | 0.00584 | |||
| Genes down-regulated in the 72AP | |||||||
| Unigene_201980 | 897 | Paramyosin, short form (Drosophila melanogaster) | 143.013 | 21.903 | -2.71 | 3.04E-05 | 0.0176 |
| Unigene_201985 | 195 | Paramyosin, long form (Drosophila melanogaster) | 41.061 | 6.947 | -2.56 | 9.20E-05 | 0.0402 |
| Unigene_201725 | 1752 | Chorion peroxidase (Drosophila melanogaster) | 2.286 | 0.216 | -3.40 | 1.20E-05 | 0.00878 |
| Unigene_176466 | 1101 | Cathepsin B (Mus musculus) | 28.719 | 3.253 | -3.14 | 6.99E-07 | 0.000964 |
| Unigene_274030 | 624 | Superoxide dismutase [Cu-Zn](SODC) (Drosophila willistoni) | 2.831 | 0.180 | -3.97 | 3.74E-06 | 0.00352 |
| Unigene_286469 | 2118 | Peroxidase (PERO) (Drosophila melanogaster) | 32.555 | 3.919 | -3.05 | 1.45E-06 | 0.00171 |
| Unigene_224486 | 1527 | Catalase (CATA) (Riptortus pedestris) | 21.707 | 3.362 | -2.69 | 1.70E-05 | 0.0114 |
| Unigene_192238 | 462 | Troponin C, isoform 1 (Drosophila melanogaster) | 58.280 | 5.885 | -3.31 | 1.79E-07 | 0.000336 |
| Unigene_253831 | 1491 | Probable cytochrome P450 303a1 (Drosophila melanogaster) | 34.031 | 4.682 | -2.86 | 6.32E-06 | 0.00534 |
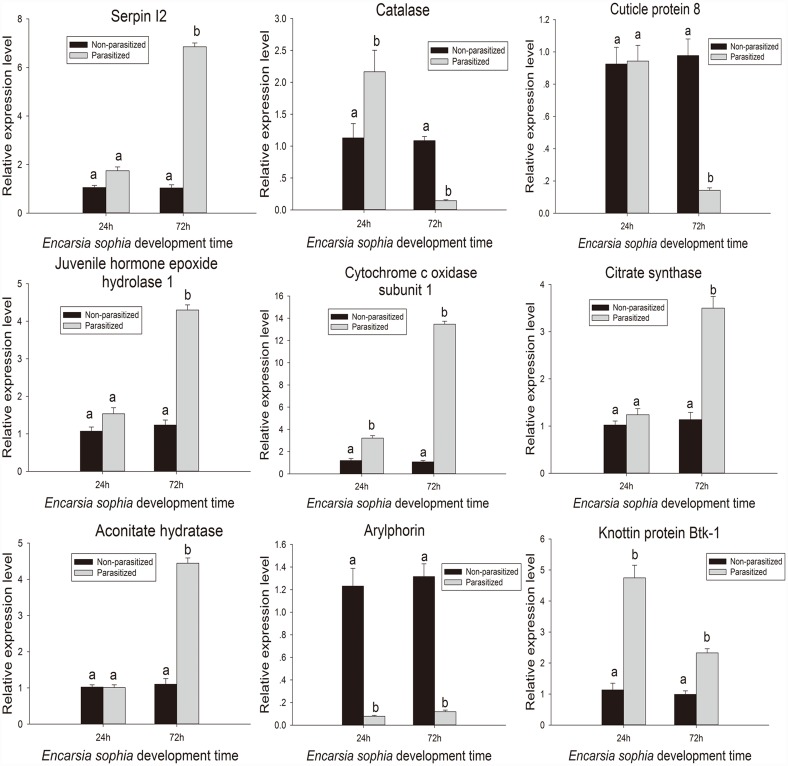
Serpin I2 was one of the genes having higher levels of up-regulation (5.33-fold) at 72AP in RNA-seq analysis. Quantitative RT-PCR analysis (Fig 4) also show that it was up-regulated by 6.85 folds. Serine proteases are important immune regulatory proteins which play a significant role in the activation of the prophenoloxidase (PPO) cascade. The cascade activation eventually causes melanization to kill parasitized wasp through choking [58], however, serine protease inhibitor (serpin) can prevent the serine proteases activated melanization and weaken host defense for wasp parasization. Although studies have shown that serpins can be regulated by the parasitoids infestation in many hosts, their transcriptional levels are different in different parasitoid-host systems, and even in the same parasitoid-host system, two opposite situations may occur. Mahadav et al. and Song et al. found that serpins were down-regulated in parasitized B. tabaci nymphs and P. xylostella larvae [26,59], while Etebari et al.[41] discovered that serpins were up-regulated 2- to 7-fold after P. xylostella parasitization by Diadegma semiclausum. In C. chilonis parasitized C. suppressalis, three up-regulated and three down-regulated serpins were identified in the fatbody [42]. Different serpins may play different roles in immune defense.
Fig 4. A qRT-PCR analysis of nine randomly selected genes from B.tabaci that showed relative expressions at 24AP and 72AP.
Black stands for the control and gray stands for a parasitized sample. The expression levels of the controls were regarded as 1. Error bars indicate standard deviations of the average from three replicates. The same letter above the error bar means that there was no significant difference at the 0.05 level by Duncan’s test.
Cellular immunity is another important component of the insect immune system. Laminin can stimulate cell adhesion and cell movement. Cofilin is an actin-binding protein which promotes cell migration and movement by changing the adhesion between cells and the extracellular matrix. Actin plays a significant role in facilitating cellular activities. The up-regulation of these genes showed that the host enhanced hemocyte encapsulation by reinforcing the extension and adhesion of hemocytes. laminin, cofilin, and actin were identified in our study and they were over-expressed at 24AP and 72AP. Ras3 and Rho1 are related to cellular immunity in D. melanogaster [40,60]. In our study, two genes were also identified significantly differentially expressed after parasization which may also be involved in the immune response of B. tabaci. At 24AP and 72AP, these genes were consistently over-expressed, which indicated that the cellular immunity not only defend parasitoid embryo and larval attacking.
Superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) are three common enzymes in organisms. Organisms produce reactive oxygen species (ROS) under environmental stresses, which is cytotoxic to cells. However, the organism utilizes these protective enzymes to eliminate redundant ROS and protect themselves from damage [61]. When Trichoplusia ni is infected by baculoviruses, the expression of manganese superoxide dismutase (MnSOD) significantly reduces oxidative damage [62]. Zhu et al. also discovered that the transcriptional levels of Tenebrio molitor superoxide dismutases were up-regulated following bacterial infection or parasitization by Scleroderma guani [63]. In our study, the expression of SODC, PERO, and CATA were suppressed two-to four-fold at 72AP, but did not show significant changes at 24AP. At 72AP, the E. sophia reaches the larval stage and the damage to host becomes worse than that of egg stage. The decrease in the transcription of protective enzymes showed the parasitoid immune suppressive strategy.
In our study, some genes involved in insecticide resistance or detoxification were found to be differentially expressed under parasitization. Although these genes have no direct connection with defense against parasitoids attacking, they can be regarded as a stress response caused by parasitoid secretions. Takeda et al.[64] confirmed that the activities of glutathione-S-transferase (GST) and cytochrome P450 (CYP) increased in parasitized P. xylostella larvae. We also found most of the cytochrome P450 genes were highly expressed after being parasitized by E. sophia. Some genes were over-expressed at 24AP and others were over-expressed at 72AP.
Heat shock proteins (HSPs) are recognized as a family of highly conserved chaperones which respond to all kinds of environmental stress factors, such as heat, toxins, UV radiation, and invading pathogens by protecting protein from misfolding and denaturation [65]. We identified three heat shock protein genes, which were homologous with D. melanogaster and Anopheles albimanus, and are involved in B. tabaci development. In addition, heat shock 70 kDa protein cognate 3 was found to participate in the immune response. Therefore, we deduced that heat shock protein families can defend the host from damage by participating in the immune response and B. tabaci development.
Effects of parasitism on the transcription of host development-related genes
The parasitoids complete their development by absorbing the host’s hemolymph and tissues. However, the development of the parasitoid and the host are synchronous. A previous study found that Aphidiu servi parasitized Acyrthosiphon pisum late-stage nymph stopped growth [30]. B. tabaci late nymphs parasitized by Encarsia bimaculata also stop growing [66]. After Encarsia formosa parasitized Trialeurodes vaporariorum Westwood nymph, the wasp didn’t molt to until host nymph reached to last instar [67]. In order to complete development, the parasitoids have to change the host’s development to match their own growth. In some cases, parasitoids suppress host’s development and accelerate the host’s early-maturity [68], while, other parasitoids prolong host’s development to meet their own developmental needs. Previous studies have proposed that the wasp might control host’s development through regulating the juvenile hormone and ecdysone levels [69,70].
Juvenile hormone epoxide hydrolases (JHEHs) have been identified as regulatory proteins in the catabolism of juvenile hormones [71,72]. A previous study showed that JHEH transcript levels were down-regulated more than two-fold in P. xylostella after parasitization by D. semiclausum [41]. However, Wu et al. [42] discovered that JHEH and juvenile hormone esterase (JHE) transcript levels increased in C. suppressalis after C. chiilonis parasitization. Based on our transcriptome data, parasitization by E. sophia led to JHEH1 up-regulation at 72AP (Table 2 & Fig 4). However, up-regulation of larvae cuticle protein and down-regulation of pupal cuticle protein might imply that the parasitoid suppressed the host’s development. Thus, the high concentrations of JH may lead to up-regulation of JHEHs and their activity in order to maintain the balance.
Table 2. Developmental-related genes differentially expressed in B. tabaci after being parasitized by E. sophia.
| Gene ID | Length | Gene name | NP-FPKM | P-FPKM | Fold change | P-value | q-value |
|---|---|---|---|---|---|---|---|
| Genes up-regulated in the 24AP | |||||||
| Unigene_119370 | 660 | RNA-binding protein squid (Drosophila melanogaster) | 0.0369 | 2.346 | 5.99 | 7.62E-05 | 0.0350 |
| Unigene_137439 | 1092 | Protein slit (Drosophila melanogaster) | 0.0214 | 1.340 | 5.97 | 8.09E-05 | 0.0365 |
| Unigene_122721 | 588 | Hormone receptor 4 (Drosophila melanogaster) | 0.218 | 3.772 | 4.12 | 8.24E-05 | 0.0370 |
| Unigene_58772 | 369 | Fatty acid-binding protein 3, muscle and heart (Camponotus floridanus) | 1.091 | 18.331 | 4.07 | 2.60E-05 | 0.0157 |
| Genes up-regulated in the 72AP | |||||||
| Unigene_47461 | 1074 | Plexin-B (Drosophila melanogaster) | 0.071 | 1.835 | 4.69 | 1.65E-05 | 0.0111 |
| Unigene_163674 | 741 | Larval cuticle protein A3A (Tenebrio molitor) | 0.095 | 10.464 | 6.77 | 2.50E-10 | 2.07E-06 |
| Unigene_300307 | 327 | Larval cuticle protein 8 (Drosophila melanogaster) | 0.209 | 96.444 | 8.85 | 5.56E-11 | 6.40E-07 |
| Unigene_96322 | 1440 | Juvenile hormone epoxide hydrolase 1(Ctenocephalides felis) | 0.079 | 2.362 | 4.89 | 4.46E-07 | 0.000687 |
| Unigene_55874 | 699 | cAMP-dependent protein kinase Catalytic subunit (Drosophila melanogaster) | 0.153 | 3.324 | 4.44 | 4.84E-05 | 0.0249 |
| Unigene_330433 | 2163 | Heat shock protein 83 (Drosophila melanogaster) | 0.045 | 17.839 | 8.62 | 1.11E-15 | 1.08E-10 |
| Unigene_240353 | 2013 | Heat shock protein 70 B2 (Anopheles albimanus) | 0.022 | 0.903 | 5.34 | 9.45E-05 | 0.0410 |
| Unigene_126734 | 516 | Guanine nucleotide-binding protein G (q) subunit alpha (Homarus americanus) | 0.112 | 2.32 | 4.37 | 7.49E-06 | 0.00607 |
| Unigene_156339 | 354 | Eukaryotic translation initiation factor 4E binding protein 1(Nasonia vitripennis) | 0.232 | 5.202 | 4.49 | 7.39E-07 | 0.001016 |
| Unigene_157748 | 2775 | Mediator of RNA polymerase II transcription subunit 13 (Nasonia vitripennis) | 0.033 | 0.919 | 4.80 | 1.00E-05 | 0.00763 |
| Genes up-regulated at 24AP and 72AP | |||||||
| Unigene_74024 | 576 | Ras-like GTP-binding protein Rho1(Drosophila melanogaster) | 0.119 | 5.016 | 5.40 | 3.37E-06 | 0.00326 |
| 0.379 | 8.120 | 4.42 | 3.02E-07 | 0.000506 | |||
| Unigene_185113 | 1365 | Glycogen synthase kinase 3 beta (Nasonia vitripennis) | 0.0138 | 0.797 | 5.85 | 0.000112 | 0.0466 |
| 0.017 | 0.915 | 5.71 | 2.79E-05 | 0.0165 | |||
| Unigene_218239 | 321 | Cofilin (Drosophila melanogaster) | 0.774 | 8.851 | 3.51 | 4.06E-05 | 0.0218 |
| 1.279 | 14.450 | 3.50 | 7.13E-06 | 0.00584 | |||
| Unigene_45734 | 2199 | Serine/threonine-protein kinase SRPK3 (Bombus impatiens) | 0.079 | 0.967 | 3.6 | 9.18E-05 | 0.0402 |
| 0.060 | 2.078 | 5.11 | 1.25E-07 | 0.000255 | |||
| Unigene_244154 | 738 | Small subunit ribosomal protein S6e (Manduca sexta) | 2.036 | 31.21 | 3.07 | 8.39E-08 | 0.000189 |
| 2.459 | 20.452 | 4.48 | 2.43E-05 | 0.0149 | |||
| Unigene_131412 | 1218 | Endoplasmin (Nasonia vitripennis) | 0.037 | 0.941 | 4.67 | 6.78E-05 | 0.0322 |
| 0.023 | 0.968 | 5.37 | 8.78E-05 | 0.0388 | |||
| Unigene_148396 | 1641 | Elongation factor 2 (Drosophila melanogaster) | 2.793 | 23.783 | 3.98 | 9.28E-05 | 0.0405 |
| 2.001 | 26.905 | 3.75 | 7.49E-06 | 0.00607 | |||
| Unigene_287521 | 561 | Myosin light chain 6 (Apis mellifera) | 0.996 | 7.218 | 2.86 | 6.01E-05 | 0.0294 |
| 1.267 | 15.772 | 3.64 | 1.23E-07 | 0.000253 | |||
| Unigene_244154 | 591 | Actin-5C (Anopheles gambiae) | 2.036 | 31.216 | 3.94 | 8.39E-08 | 0.000189 |
| 2.459 | 24.453 | 3.06 | 2.43E-05 | 0.0149 | |||
| Unigene_154175 | 711 | Heat shock 70 kDa protein cognate 3 (Drosophila melanogaster) | 0.502 | 6.758 | 3.52 | 7.29E-07 | 0.000997 |
| 0.908 | 5.741 | 5.98 | 4.75E-10 | 3.37E-06 | |||
| Genes down-regulated at 72AP | |||||||
| Unigene_231672 | 372 | Adult-specific cuticular protein ACP-20 (Tenebrio molitor) | 6.293 | 0.076 | -3.22 | 0.000108 | 0.0453 |
| Unigene_225094 | 939 | Opsin-2 (Schistocerca gregaria) | 14.373 | 2.031 | -2.82 | 7.56E-06 | 0.00612 |
| Unigene_192238 | 462 | Troponin C, isoform 1/ calmodulin (Drosophila melanogaster) | 58.280 | 5.885 | -3.31 | 1.79E-07 | 0.000336 |
| Unigene_196111 | 3882 | Fatty acid synthase (Gallus gallus) | 0.961 | 0.067 | -3.84 | 8.25E-06 | 0.00652 |
| Genes down-regulated at 24AP and 72AP | |||||||
| Unigene_178448 | 2010 | Arylphorin subunit alpha (Manduca sexta) | 2.532 | 0.255 | -3.31 | 1.13E-05 | 0.00838 |
| 4.377 | 0.555 | -2.98 | 1.29E-05 | 0.00923 | |||
| Unigene_198800 | 390 | Pupal cuticle protein Edg-84A (Drosophila melanogaster) | 139.029 | 21.277 | -2.71 | 8.31E-05 | 0.0372 |
| 169.474 | 23.808 | -2.83 | 5.56E-05 | 0.0277 | |||
| Unigene_196053 | 447 | Cuticle protein 8 (Blaberus craniifer) | 789.210 | 95.603 | -3.54 | 7.40E-05 | 0.0343 |
| 1674.11 | 101.192 | -2.84 | 2.30E-06 | 0.00245 | |||
| Unigene_257893 | 714 | Cuticle protein 7 (Locusta migratoria) | 436.781 | 50.575 | -3.11 | 0.000119 | 0.0487 |
| 683.999 | 45.784 | -3.90 | 5.98E-06 | 0.00511 | |||
| Unigene_203192 | 468 | Cuticle protein 19 (Locusta migratoria) | 13.851 | 2.438 | -2.51 | 6.23E-05 | 0.0302 |
| 19.539 | 3.361 | -2.54 | 4.69E-05 | 0.0243 | |||
Effects of parasitism on the transcription of host metabolism-related genes
Stearoyl-CoA desaturase (SCD) is an endoplasmic reticulum enzyme that catalyzes the biosynthesis of monounsaturated FA from saturated FA [73]. SCD inactivation causes obesity and abnormal lipid metabolism and one SCD activity, SCD1, was induced by insulin, but inhibited by leptin [74]. We found that at 24AP and 72AP, the transcript levels of SCD in B. tabaci nymph were up-regulated 6.69 and 4.52 times, respectively (Table 3). Furthermore, genes involved in the insulin signaling pathway were also significantly up-regulated. Our result implied that the wasp regulated the lipid metabolism of the host in order to get more nutrients available in host and meet their own needs. A report showed that the wasp preferred to parasitize late instar larvae because of adequate nutrition [75]. Stearoyl-CoA desaturase is an essential enzyme for the parasitic Trypanosoma brucei, and RNA interference of SCD caused a reduction of the parasitemia and an increase in host survival [76]. Environmental stress can influence the organism’s metabolism, same as parasitoid infestation, which is energetically consumption process [77]. We found a high number of differentially expressed transcripts were related to organism metabolism. Metabolic changes occurred at both time points, but a greater amount and different kinds of genes were affected at 72APthan at24AP.
Table 3. Metabolism-related genes differentially expressed in B. tabaci after being parasitized by E. sophia.
| Gene ID | Length | Gene name | NP-FPKM | P-FPKM | Fold change | P-value | q-value |
|---|---|---|---|---|---|---|---|
| Genes up-regulated at 24 AP | |||||||
| Unigene_108039 | 489 | V-type H+-transporting ATPase 16kDa proteolipid subunit (Drosophila melanogaster) | 0.0841 | 4.129 | 5.62 | 1.25E-06 | 0.00151 |
| Unigene_71375 | 777 | Glutamine synthetase 2, isoform B (Drosophila melanogaster) | 0.0445 | 2.586 | 5.86 | 0.00011 | 0.0460 |
| Unigene_111268 | 900 | Probable chitinase 3 (Drosophila melanogaster) | 0.211 | 4.937 | 4.11 | 1.18E-05 | 0.00865 |
| Unigene_316078 | 861 | Stearoyl-CoA desaturase (Trichoplusia ni) | 0.0307 | 3.181 | 6.69 | 8.97E-06 | 0.00697 |
| Unigene_151198 | 1947 | Chitin synthase A (Spodoptera exiqua) | 0.028 | 1.216 | 5.40 | 3.30E-06 | 0.00321 |
| Unigene_130384 | 1677 | Fatty acyl-CoA reductase (Drosophila melanogaster) | 0.039 | 1.574 | 5.33 | 4.43E-06 | 0.00402 |
| Genes up-regulated at 72 AP | |||||||
| Unigene_106282 | 291 | Cytochrome c oxidase subunit 2 (Nasonia qiraulti) | 0.703 | 14.135 | 4.25 | 8.74E-07 | 0.00113 |
| Unigene_47076 | 450 | Cytochrome c oxidase subunit 5a (Nasonia vitripennis) | 0.208 | 9.349 | 5.50 | 5.53E-05 | 0.0276 |
| Unigene_77855 | 936 | F-type H+-transporting ATPase subunit beta (Drosophila melanogaster) | 1.119 | 10.000 | 3.16 | 6.67E-06 | 0.00554 |
| Unigene_142444 | 822 | F-type H+-transporting ATPase subunit gamma (Drosophila melanogaster) | 0.079 | 3.912 | 5.62 | 3.80E-05 | 0.0208 |
| Unigene_115206 | 333 | F-type H+-transporting ATPase subunit a (Aedes aegypti) | 0.829 | 31.105 | 5.23 | 1.01E-09 | 6.04E-06 |
| Unigene_72168 | 321 | F-type H+-transporting ATPase subunit f (Drosophila melanogaster) | 0.539 | 10.619 | 4.30 | 1.07E-05 | 0.00803 |
| Unigene_110914 | 996 | Glyceraldehyde 3-phosphate dehydrogenase (Drosophila pseudoobscura) | 0.679 | 15.247 | 4.49 | 1.09E-10 | 1.07E-10 |
| Unigene_261092 | 783 | Citrate synthase (Aedes aegypti) | 0.214 | 5.028 | 4.55 | 7.02E-09 | 2.83E-05 |
| Unigene_134666 | 1512 | Aconitate hydratase (Nasonia vitripennis) | 0.066 | 1.287 | 4.27 | 9.84E-05 | 0.0423 |
| Unigene_330649 | 972 | Succinyl-CoA synthetase alpha subunit (Drosophila melanogaster) | 0.083 | 2.182 | 4.71 | 1.54E-05 | 0.0106 |
| Unigene_110915 | 786 | Arylformamidase (Cerapachys biroi) | 0.262 | 7.736 | 4.88 | 4.63E-07 | 0.000765 |
| Unigene_110492 | 165 | F-type H+-transporting ATPase subunit alpha (Drosophila melanogaster) | 0.210 | 32.159 | 6.72 | 6.94E-07 | 0.000961 |
| Unigene_239710 | 1395 | Facilitated trehalose transporter Tret1(Culex quinquefasciatus) | 0.352 | 2.810 | 3.00 | 1.41E-05 | 0.0410 |
| Genes up-regulated at 24 AP and 72 AP | |||||||
| Unigene_120237 | 261 | Ubiquinol-cytochrome c reductase cytochrome b subunit (Philotrypesis pilosa) | 1.147 | 10.895 | 3.25 | 2.39E-06 | 0.00251 |
| 1.198 | 17.491 | 3.87 | 1.59E-08 | 5.24E-05 | |||
| Unigene_153762 | 150 | Cytochrome c oxidase subunit 1 (Locusta migratoria) | 0.865 | 9.243 | 3.42 | 2.53E-06 | 0.00262 |
| 0.856 | 16.724 | 4.29 | 3.12E-09 | 1.47E-05 | |||
| Unigene_228753 | 570 | Maltase 1 (Drosophila virilis) | 0.340 | 5.047 | 3.89 | 9.82E-06 | 0.00748 |
| 0.145 | 4.129 | 4.83 | 8.59E-06 | 0.00673 | |||
| Unigene_228756 | 1071 | Maltase 2 (Drosophila virilis) | 5.034 | 39.472 | 2.97 | 2.39E-06 | 0.00251 |
| 3.573 | 45.787 | 3.68 | 9.93E-09 | 3.70E-05 | |||
| Genes down-regulated at 24 AP | |||||||
| Unigene_241514 | 1599 | Pyruvate kinase (Drosophila melanogaster) | 25.087 | 4.04 | -2.63 | 3.09E-05 | 0.0178 |
| Genes down-regulated at 72 AP | |||||||
| Unigene_269576 | 432 | ATP synthase lipid-binding protein, mitochondrial (Manduca sexta) | 66.809 | 10.812 | -2.63 | 3.45E-05 | 0.0193 |
| Unigene_249063 | 1092 | Fructose-bisphosphate aldolase, class I (Drosophila melanogaster) | 7.807 | 0.927 | -3.07 | 5.59E-06 | 0.00484 |
| Unigene_263538 | 942 | Glucuronosyltransferase (Zootermopsis nevdensis) | 6.257 | 1.077 | -2.54 | 0.000105 | 0.0444 |
| Unigene_252632 | 306 | Acylphosphatase (Acyrthosiphon pisum) | 57.208 | 6.835 | -3.07 | 1.89E-05 | 0.0123 |
| Unigene_224486 | 1527 | Catalase (Riptortus pedestris) | 21.707 | 8.362 | -2.69 | 1.70E-05 | 0.0114 |
E.sophia infection influenced carbohydrate, lipid, and energy metabolism in the host. Some studies have found that trehalose content changed after parasitization [78,79]. In two treatment groups, maltose was degraded to glucose under the action of maltase. Beside the upregulation of maltase, other genes, in the citrate cycle and glycolysis, were over-expressed at 72AP, such as citrate synthase, aconitate hydratase, and glyceraldehydes 3-phosphate dehydrogenase. Glycolysis and the citrate cycle are carbohydrate metabolisms to produce ATP. Citrate synthase, aconitate hydratase and succinyl-CoA synthetase are three essential enzymes in the TCA cycle. Up-regulation of genes that control the synthesis of these enzymes showed that total ATP decreased in the organism. Therefore, the insect needed to obtain more energy by increasing the reaction rate of the TCA cycle. In addition, we found that the transcriptional level of cytochrome c oxidase and f-type H+-transporting ATPase were significantly enhanced. Cytochrome c oxidase is involved in ATP synthesis as a terminase of the mitochondrial inner membrane respiratory chain [80]. However, whether it can be regarded as evidence of enhanced respiration is not clear. Measurement of respiration rate should be investigated in future studies.
There are three types of ion transporting ATPases: P-type, V-type, and F-type. In organisms, their main function is to synthesize ATP and transport H+ [81]. There were more over-expressed f-type H+-ATPases at 72AP than at 24AP. This suggests that E. sophia parasitization of B. tabaci involved increased energy consumption. The host was regulated to produce more energy to supply to the parasitoid. Visser et al. found most wasps lacked a lipid synthesis mechanism and could not accumulate energy [82]. Therefore, it is reasonable to assume that the parasitoid may continually obtain energy from the host in order to complete its development.
Conclusions
In summary, our study first presented comprehensive transcriptome profiles of B. tabaci in response to E. sophia parasitization using RNAseq. The most of differentially expressed genes of B. tabaci after parasization have potential roles in immunity, development and metabolism to meet parasitoids needs. The transcriptome profiles provided a basis for future research in elucidate the host-parasitoid interaction. In addition, the identified immune-, development and detoxification–related genes may be target for B. tabaci control.
Acknowledgments
This work was supported by National Basic Research Program of China (Grant Number: 2013CB127600) to ZF. We would like to thank DCS, YCL, JWC, and LY for their bioinformatics analysis of the data.
Data Availability
All raw sequencing data have been deposited in NCBI Sequence Read Archive (SRA) database (http://www.ncbi.nlm.nih.gov/sra) with the following accession numbers: SRR1909644 (24AP), SRR1909651(72AP), SRR1909652 (CK-24AP), and SRR1909653 (CK-72AP).
Funding Statement
This research was supported by the National Key Basic Research and Development Program (973 program 2013CB127600) to ZF.
References
- 1.Oliveira M, Henneberry T, Anderson P (2001) History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot 20: 709–723. [Google Scholar]
- 2.Li ZX (2006) Molecular phylogenetic analysis reveals at least five genetic races of Bemisia tabaci in China. Phytoparasitica 34:431–440. [Google Scholar]
- 3.Costa H, Brown J (1991) Variation in biological characteristics and esterase patterns among populations of Bemisia tabaci, and the association of one population with silverleaf symptom induction. Entomologia experimentalis et applicata 61:211–219. [Google Scholar]
- 4.Horowitz AR, Kontsedalov S, Khasdan V, Ishaaya I (2005) Biotypes B and Q of Bemisia tabaci and their relevance to neonicotinoid and pyriproxyfen resistance. Arch Insect Biochem Physiol 58:216–225. [DOI] [PubMed] [Google Scholar]
- 5.Wan F, Zhang G, Liu S, Luo C, Chu D, Zhang Y, et al. (2009) Invasive mechanism and management strategy of Bemisia tabaci (Gennadius) biotype B: Progress report of 973 Program on invasive alien species in China. Science in China Series C: Life Sciences 52:88–95. 10.1007/s11427-008-0135-4 [DOI] [PubMed] [Google Scholar]
- 6.Hoddle M, Van Driesche R, Sanderson J (1998) Biology and use of the whitefly parasitoid Encarsia formosa. Annu Rev Entomol 43:645–669. [DOI] [PubMed] [Google Scholar]
- 7.Xiao Y, Chen J, Cantliffe D, Mckenzie C, Houben K, Osborne LS (2011) Establishment of papaya banker plant system for parasitoid, Encarsia sophia (Hymenoptera: Aphilidae) against Bemisia tabaci (Hemiptera: Aleyrodidae) in greenhouse tomato production. Biol Control 58:239–247. [Google Scholar]
- 8.Jaworski CC, Chailleux A, Bearez P, Desneux N (2015) Apparent competition between major pest reduces pest population densities on tomato crop, but not yield loss. J Pest Sci 88: 793–803. [Google Scholar]
- 9.Zhang YB, Yang NW, Sun LY, Wan FH (2015) Host instar suitability in two invasive whiteflies for the naturally occurring parasitoid Eretmocerus hayati in China. J Pest Sci 88: 225–234. [Google Scholar]
- 10.Lin KJ, Lu YH, Wan P, Yang YZ, Wyckhuys KAG, Wu KM (2015) Simultaneous reduction in incidence of Bemisia tabaci (Hemiptera: Aleyrodidae) and Sylepta derogate (Lepidoptera:Pyralidae) using velvetleaf, Abutilon theophrasti as a trap crop. J Pest Sci. 88:49–56. [Google Scholar]
- 11.Cruz PL, Baldin ELL, de Castro MJP (2014) Characterization of antibiosis to the silverleaf whitefly Bemisia tabaci biotype B (Hemiptera: Aleyrodidae) in cowpea entries. J Pest Sci. 87:639–645 [Google Scholar]
- 12.Moreno-Ripoll R, Gabarra R, Symondson WOC, King RA, Agusti N (2014) Do the interations among natural enemies compromise the biological control of the whitefly Bemisia tabaci? J Pest Sci 87:133–141. [Google Scholar]
- 13.Cahill M, Gorman K, Day S, Denholm I (1996) Baseline determination and detection of resistance to imidacloprid in Bemisia tabaci (Homoptera: Aleyrodidae). Bull Entomol Res 86: 343–349. [Google Scholar]
- 14.Horowitz AR, Kontsedalov S, Ishaaya I (2004) Dynamics of resistance to the neonicotinoids acetamiprid and thiamethoxamthiamethoxam in Bemisia tabaci (Homoptera: Aleyrodidae). J Econ Entomol 97: 2051–2056 [DOI] [PubMed] [Google Scholar]
- 15.Nauen R, Denholm I (2005) Resistance of insect pests to neonicotinoid insecticides: current status and future prospects. Arch Insect Biochem Physiol 58: 200–215. [DOI] [PubMed] [Google Scholar]
- 16.Liang P, Tian YA, Biondi A, Desneux N, Gao XW (2012) Short-term and transgenerational effects of the neonicotinoid nitenpyram on susceptibility to insecticides in two whitefly species. Ecotoxicology 21:1889–1898. 10.1007/s10646-012-0922-3 [DOI] [PubMed] [Google Scholar]
- 17.Turlings TC, Tumlinson JH, Lewis WJ (1990) Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250: 1251–1253. [DOI] [PubMed] [Google Scholar]
- 18.Han P, Niu CY, Desneux N (2014) Identification of top-down forces regulating cotton aphid population growth in transgenic Bt cotton in centra China. PLoS ONE. 9:e102980 10.1371/journal.pone.0102980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali A, Desneux N, Lu YH, Liu B, Wu KM (2016) Characterization of the natural enemy community attacking cotton aphid in the Bt cotton ecosystem in northern China. Sci Rep 6: 24273 10.1038/srep24273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Askew RR, Shaw MR (1986) Parasitoid communities: their size, structure and development In: Waage J, Greathead D, editors. Insect Parasitoids. London: Academic; pp. 225–263. [Google Scholar]
- 21.Desneux N, Barta RJ, Hoelmer KA, Hopper KR, Heimpel GE (2009) Multifaceted determination of host specificity in an aphid parasitoid. Oecologia 160:387–398. 10.1007/s00442-009-1289-x [DOI] [PubMed] [Google Scholar]
- 22.Simmons AM, Abd-Rabou S (2005) Parasitism of Bemisia tabaci (Homoptera: Aleyrodidae) after multiple releases of Encarsia sophia (Hymenoptera: Aphelinidae) in three vegetable crops. J Agr Urban Entomol 22:73–77. [Google Scholar]
- 23.Luo C, Liu TX (2011) Fitness of Encarsia sophia (Hymenoptera: Aphelinidae) parasitizing Trialeurodes vaporariorum and Bemisia tabaci (Hemiptera: Aleyrodidae). Insect Sci 18:84–91. [Google Scholar]
- 24.Zang LS, Liu TX, Wan FH (2011) Reevaluation of the value of autoparasitoids in biological control. PLoS ONE 6: e20324 10.1371/journal.pone.0020324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collier TR, Hunter MS (2001) Lethal interference competition in the whitefly parasitoids Eretmocerus eremicus and Encarsia sophia. Oecologia 129:147–154. [DOI] [PubMed] [Google Scholar]
- 26.Mahadav A, Gerling D, Gottlieb Y, Czosnek H, Ghanim M (2008) Parasitization by the wasp Eretmocerus mundus induces transcription of genes related to immune response and symbiotic bacteria proliferation in the whitefly Bemisia tabaci. BMC Genomics 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen XX, He JH, Shi ZH, Ma Y, Lou YG, Zhu ZR (2000) Research review of interaction between parasitoids and their host. China Entomology of 21th Century——The Entomological Society of China academic annual conference paper highlights, 242–246.
- 28.Zhang QQ, Wang F, Fang Q, Ye GY (2011) In vitro cellular response of Pieris rapae(Lepidoptera: Pieridae) hemocytes and the effects of Pteromalus puparum venom. Acta Entomologica Sinica 54: 1264–1273. [Google Scholar]
- 29.Stettler P, Trenczek T, Wyler T, Pfister-Wilhelm R, Lanzrein B (1998) Overview of parasitism associated effects on host haemocytes in larval parasitoids and comparison with effects of the egg-larval parasitoid Chelonus inanitus on its host Spodoptera littoralis. J Insect Physiol 44:817–831. [DOI] [PubMed] [Google Scholar]
- 30.Pennacchio F, Digilio M, Tremblay E (1995) Biochemical and metabolic alterations in Acyrthosiphon pisum parasitized by Aphidius ervi. Arch Insect Biochem Physiol 30: 351–367. [Google Scholar]
- 31.Gan M, Miao XX, Ding DC (2003) Biochemical and metabolic alterations in Aphis craccivora Koch parasitized by Lysiphlebus japonicas Ashmead. Acta Phytophylacica Sinica 30: 255–260. [Google Scholar]
- 32.Crozatier M, Ubeda J-M, Vincent A, Meister M (2004) Cellular immune response to parasitization in Drosophila requires the EBF orthologue collier. PLoS Biol 2: e196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams MJ, Ando I, Hultmark D (2005) Drosophila melanogaster Rac2 is necessary for a proper cellular immune response. Genes to Cells 10: 813–823. [DOI] [PubMed] [Google Scholar]
- 34.Ozsolak F, Milos PM (2011) RNA sequencing: advances, challenges and opportunities. Nat Rev Genet 12: 87–98. 10.1038/nrg2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiu P, Benbow L, Liu SX, Greene JR, Wang LQ (2002) Analysis of a human brain transcriptome map. BMC Genomics 3:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang F, Guo H, Zheng H, Zhou T, Zhou Y, Wang S, et al. (2010) Massively parallel pyrosequencing-based transcriptome analyses of small brown planthopper (Laodelphax striatellus), a vector insect transmitting rice stripe virus (RSV). BMC Genomics 11:303 10.1186/1471-2164-11-303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng V, Ewen-Campen B, Horch HW, Roth S, Mito T, Extavour CG (2013) Developmental gene discovery in a hemimetabolous insect: De novo assembly and annotation of a transcriptome for the Cricket Gryllus bimaculatus. PLoS ONE 8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gandhe AS, Arunkumar KP, John SH, Nagaraju J (2006) Analysis of bacteria-challenged wild silkmoth, Antheraea mylitta (lepidoptera) transcriptome reveals potential immune genes. BMC Genomics 7: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang N, Xie W, Jones CM, Bass C, Jiao X, Yang X, et al. (2013)Transcriptome profiling of the whitefly Bemisia tabaci reveals stage-specific gene expression signatures for thiamethoxam resistance. Insect Mol Biol 22: 485–496. 10.1111/imb.12038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wertheim B, Kraaijeveld AR, Schuster E, Blanc E, Hopkins M, Pletcher SD, et al. (2005) Genome-wide gene expression in response to parasitoid attack in Drosophila. Genome Biol 6: R94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Etebari K, Palfreyman RW, Schlipalius D, Nielsen LK, Glatz RV, Asgari S (2011) Deep sequencing-based transcriptome analysis of Plutella xylostella larvae parasitized by Diadegma semiclausum. BMC Genomics 12: 266–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu SF, Sun FD, Qi YX, Yao Y, Fang Q, Huang J, et al. (2013) Parasitization by Cotesia chilonis influences gene expression in fatbody and hemocytes of Chilo suppressalis. PLoS ONE 8: e74309 10.1371/journal.pone.0074309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu JY, Yang P, Zhang Z, Wu GX, Yang B (2013) Transcriptomic immune response of Tenebrio molitor pupae to parasitization by Scleroderma guani. PLoS ONE 8: e54411 10.1371/journal.pone.0054411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tang B, Chen J, Hou Y, Meng E (2014) Transcriptome immune analysis of the invasive beetle Octodonta nipae (Maulik) (Coleoptera: Chrysomelidae) parasitized by Tetrastichus brontispae Ferrière (Hymenoptera: Eulophidae). PLoS ONE 9:e91482 10.1371/journal.pone.0091482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hoffmann JA (2003) The immune response of Drosophila. Nature 426:33–38. [DOI] [PubMed] [Google Scholar]
- 46.Chu D, Zhang YJ, Cong B, Xu BY, Wu QJ, Zhu GR (2005) Sequence analysis of mtDNA COI gene and molecular phylogeny of different geographical populations of Bemisia tabaci (Gennadius). Scientia Agricultura Sinica 38: 76–85. [Google Scholar]
- 47.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, et al. (2011) Fulllength transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29: 644–652. 10.1038/nbt.1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Conesa A, Gotz S, Garcia-Gomez JM, Terol J, Talon M, Robles M (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21: 3674–3676. [DOI] [PubMed] [Google Scholar]
- 49.Ye J, Fang L, Zheng H, Zhang Y, Chen J, Zhang Z, et al. (2006) WEGO: a web tool for plotting GO annotations. Nucleic Acids Res 34: W293–W297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B (2008) Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods 5: 621–628. 10.1038/nmeth.1226 [DOI] [PubMed] [Google Scholar]
- 51.Hoffmann JA (2003) The immune response of Drosophila. Nature 426:33–38. [DOI] [PubMed] [Google Scholar]
- 52.Hoffmann JA (1995) Innate immunity of insects. Curr Opin Immunol 7: 4–10. [DOI] [PubMed] [Google Scholar]
- 53.Schmid-Hempel P (2005) Evolutionary ecology of insect immune defenses. Annu Rev Entomol 50: 529–551. [DOI] [PubMed] [Google Scholar]
- 54.Feldhaar H, Gross R (2008) Immune reactions of insects on bacterial pathogens and mutualists. Microbes Infect 10: 1082–1088. 10.1016/j.micinf.2008.07.010 [DOI] [PubMed] [Google Scholar]
- 55.Nicolas E, Nappi AJ, Lemaitre B (1996) Expression of antimicrobial peptide genes after infection by parasitoid wasps in Drosophila. Dev Comp Immunol 20:175–181. [DOI] [PubMed] [Google Scholar]
- 56.Boulanger N, Lowenberger C, Volf P, Ursic R, Sigutova L, Sabatiern L, et al. (2004) Characterization of a defensin from the sand fly Phlebotomus duboscqi induced by challenge with bacteria or the protozoan parasite Leishmania major. Infect Immun 72:7140–7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chiche L, Heitz A, Gelly J-C, Gracy J, Chau PT, Ha PT, et al. (2004) Squash inhibitors: from structural motifs to macrocyclic knottins. Curr Protein Pept Sci 5:341–349. [DOI] [PubMed] [Google Scholar]
- 58.Christensen BM, Li J, Chen CC, Nappi AJ (2005): Melanization immune responses in mosquito vectors. Trends Parasitol 21:192–199. [DOI] [PubMed] [Google Scholar]
- 59.Song KH, Jung MK, Eum JH, Hwang IC, Han SS (2008) Proteomic analysis of parasitized Plutella xylostella larvae plasma. J Insect Physiol 54:1271–1280. [DOI] [PubMed] [Google Scholar]
- 60.Bangi E, Pitsouli C, Rahme LG, Cagan R, Apidianakis Y (2012) Immune response to bacteria induces dissemination of Ras-activated Drosophila hindgut cells. Embo Rep 13:569–576. 10.1038/embor.2012.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corona M, Robinson G (2006) Genes of the antioxidant system of the honey bee: annotation and phylogeny. Insect mol biol 15:687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang P, Oberley LW, Howe D, Jarvis DL, Chauhan G, Murhammer DW (2004) Effect of expression of manganese superoxide dismutase in baculovirus-infected insect cells. Appl Biochem Biotech 119:181–193. [DOI] [PubMed] [Google Scholar]
- 63.Zhu JY, Ze SZ, Stanley DW, Yang B (2014) Parasitization by Scleroderma guani influences expression of superoxide dismutase genes in Tenebrio molitor. Arch Insect Biochem Physiol 87:40–52. 10.1002/arch.21179 [DOI] [PubMed] [Google Scholar]
- 64.Takeda T, Nakamatsu Y, Tanaka T (2006) Parasitization by Cotesia plutellae enhances detoxifying enzyme activity in Plutella xylostella. Pestic Biochem Physiol 86:15–22. [Google Scholar]
- 65.Parsell D, Lindquist S (1993) The function of heat-shock proteins in stress tolerance: degradation and reactivation of damaged proteins. Annu Rev Genet 27:437–496. [DOI] [PubMed] [Google Scholar]
- 66.Qian M, Ren S, Hu Q (2005) Effects of endoparasitism by Encarsia bimaculata on the titers of juvenile hormones and 20—hydroxyecdysone in Bemisia tabaci nymphs. Acta entomologica Sinica 49:568–573. [Google Scholar]
- 67.Hu JS, Gelman DB, Blackburn MB (2002) Growth and development of Encarsia formosa (Hymenoptera: Aphelinidae) in the greenhouse whitefly, Trialeurodes vaporariorum (Homoptera: Aleyrodidae): effect of host age. Arch Insect Biochem Physiol 49:125–136. [DOI] [PubMed] [Google Scholar]
- 68.Zhu JY, Ye GY, Hu C (2008) Advance in the studies on molecular mechanisms of parasitoid manipulation of host. Acta Phytophylacica Sinica 35:563–568. [Google Scholar]
- 69.Rath S, Sinha B (2005) Parasitization of fifth instar tasar silkworm, Antheraea mylitta, by the uzi fly, Blepharipa zebina; a host–parasitoid interaction and its effect on host’s nutritional parameters and parasitoid development. J Invertebr Pathol 88:70–78. [DOI] [PubMed] [Google Scholar]
- 70.Kaeslin M, Pfister-Wilhelm R, Lanzrein B (2005) Influence of the parasitoid Chelonus inanitus and its polydnavirus on host nutritional physiology and implications for parasitoid development. J Insect Physiol 51:1330–1339. [DOI] [PubMed] [Google Scholar]
- 71.Gilbert LI, Granger NA, Roe RM (2000) The juvenile hormones: historical facts and speculations on future research directions. Insect Biochem Mol Biol 30:617–644. [DOI] [PubMed] [Google Scholar]
- 72.Anspaugh DD, Roe RM (2005) Regulation of JH epoxide hydrolase versus JH esterase activity in the cabbage looper, Trichoplusia ni, by juvenile hormone and xenobiotics. J Insect Physiol 51:523–535. [DOI] [PubMed] [Google Scholar]
- 73.Ntambi JM, Miyazaki M, Dobrzyn A (2004) Regulation of stearoyl-CoA desaturase expression. Lipids 39:1061–1065. [DOI] [PubMed] [Google Scholar]
- 74.Zhang R, Zhang YH, Shao D, Wang LD, Gong DQ (2013) The function and regulation of stearoyl-CoA desaturase gene. Chinese Bulletin of Life Sciences 4: 008. [Google Scholar]
- 75.Zhang SZ, Wan FH, Zhang F, Hua BZ (2003) Parasitic suitability of two strains of Encarsia formosa on Bemisia tabaci. Chinese J Biol Control 19: 149–153. [Google Scholar]
- 76.Alloatti A, Gupta S, Gualdrón-López M, Nguewa PA, Altabe SG, Deumer G, et al. (2011) Stearoyl-CoA desaturase is an essential enzyme for the parasitic protist Trypanosoma brucei. Biochem Bioph Res Co 412:286–290. [DOI] [PubMed] [Google Scholar]
- 77.Holt HL, Aronstein KA, Grozinger CM (2013) Chronic parasitization by Nosema microsporidia causes global expression changes in core nutritional, metabolic and behavioral pathways in honey bee workers (Apis mellifera). BMC Genomics 14:799 10.1186/1471-2164-14-799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dahlman DL, Vinson SB (1977) Effect of calyx fluid from an insect parasitoid on host hemolymph dry weight and trehalose content. J Invertebr Pathol 29:227–229. [Google Scholar]
- 79.Nakamatsu Y, Tanaka T (2004) Correlation between concentration of hemolymph nutrients and amount of fat body consumed in lightly and heavily parasitized hosts (Pseudaletia separata). J Insect Physiol 50:135–141. [DOI] [PubMed] [Google Scholar]
- 80.Li LZ, Huang ZX (2001) Progresses in Cytochrome c Oxidase Studies. Chinese J Inorg Chem 17: 761–774. [Google Scholar]
- 81.Song LX, Wang RX (1989) Structure and function on ion transporting ATPase. Prog Physiol Sci 20: 334–338. [PubMed] [Google Scholar]
- 82.Visser B, Le Lann C, den Blanken FJ, Harvey JA, van Alphen JJ, Ellers J (2010) Loss of lipid synthesis as an evolutionary consequence of a parasitic lifestyle. PNAS 107: 8677–8682. 10.1073/pnas.1001744107 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All raw sequencing data have been deposited in NCBI Sequence Read Archive (SRA) database (http://www.ncbi.nlm.nih.gov/sra) with the following accession numbers: SRR1909644 (24AP), SRR1909651(72AP), SRR1909652 (CK-24AP), and SRR1909653 (CK-72AP).