Abstract
Chronic rhinosinusitis (CRS) is a common inflammatory disease that results in a significant decrease in patient quality of life and a large economic burden. However, the lack of population-based epidemiologic studies and robust model systems has made it difficult to fully elucidate the key inflammatory pathways that drive the chronic inflammatory responses observed in CRS. This review will highlight the wide variety of factors that likely contribute to CRS disease pathogenesis. Defects in the innate immune function of the airway epithelium, including decreases in barrier function, mucociliary clearance, and production of antimicrobial peptides, all likely play a role in the initial inflammatory response. Subsequent recruitment and activation of eosinophils, mast cells, and innate lymphoid cells (ILCs) further contributes to the chronic inflammatory response and directly activates adaptive immune cells, including T and B cells. However, development of new tools and model systems is still needed to further understand the chronicity of this inflammatory response and which specific factors are necessary or sufficient to drive CRS pathogenesis. Such studies will be critical for the development of improved therapeutic strategies aimed at treating this highly prevalent and costly disease.
Keywords: Adaptive immunity, Chronic inflammation, Chronic rhinosinusitis, Epithelial cells, Innate immunity
Introduction
Chronic rhinosinusitis (CRS) is a common disease that is associated with significant patient morbidity and socio-economic burden. Despite the large impact of CRS, there have been few population-based epidemiologic studies focused on elucidating the prevalence and characterization of this disease, and the mechanisms that drive inflammation in CRS remain unclear. In general, CRS patients are divided into two distinct subgroups, those without nasal polyps (CRSsNP) and those with nasal polyps (CRSwNP). It is now well accepted that these two forms of disease are driven by unique inflammatory mechanisms and do not represent different ends of a single disease spectrum. Despite this, much of the published data focus either on CRSwNP pathogenesis alone or group all CRS patients together and do not comment on any specific properties associated with a particular subgroup. As a result, there is a large literature available on the distinct inflammatory characteristics associated with CRSwNP, but few studies have identified any unique inflammatory signature profiles associated with CRSsNP. Thus, this review will highlight the more recent advances in our understanding of the mechanisms that underlie CRS pathogenesis, and will focus mainly on CRSwNP.
Epidemiology
As a whole, CRS is characterized by inflammation of the sinonasal mucosa lasting for greater than 12 weeks and resulting in four cardinal symptoms: nasal obstruction, smell loss, drainage, and facial pain or pressure [1••]. It has been estimated that CRS is associated with over $8 billion in annual spending in the USA for medical and surgical management [2]. Patients are most often treated with extended courses of antibiotics and intranasal and systemic steroids. If these treatments are unsuccessful in alleviating symptoms, patients can undergo functional endoscopic sinus surgery to remove their inflamed sinus tissues. Due to the lack of population-based epidemiology studies, it is not clear what proportion of CRS patients eventually require sinus surgery, but it is thought that patients with more severe disease are less likely to respond to medical therapy alone [3••]. Surgical treatment has been shown to result in significant long-term improvement for many patients, but some patients do require multiple sinus surgeries to adequately control their symptoms. Again, it has been suggested that patients with more severe disease will require multiple surgeries, and these patients are also much more likely to have CRSwNP [3••].
It is estimated that CRS affects up to 10 % of the population in the USA, with similar prevalence estimates in Europe and Asia [4–6]. Interestingly, few studies have evaluated the frequency of patients with CRSsNP compared to those who have CRSwNP in the general population. Overall, it is estimated that CRSsNP patients make up the majority of patients. Importantly, however, studies have suggested that CRSwNP and CRSsNP patients are equally represented among those undergoing sinus surgery. Thus, CRSwNP appears to be more severe and requires surgical intervention more often than CRSsNP [3••]. Studies also indicate a predominance of men among the CRSwNP population, while women are more likely to have CRSsNP [1••]. Interestingly, our data from a single tertiary care population suggest that while women are less likely to have CRSwNP than men, women tend to have more severe sinus inflammation and require more surgeries than men [7]. This suggests that women with CRSwNP have more severe disease than men, although the mechanisms responsible for this apparent gender difference remain unclear. Gender-based differences in disease severity are well established in asthma and autoimmunity [8–10], but more work is needed to replicate these findings in CRSwNP and to determine whether a similar gender difference in disease severity occurs in CRSsNP patients.
CRS is also associated with significant morbidity and decreases in quality of life. It is important to note that the majority of studies on patient quality of life have been done in tertiary care patient populations, and many do not distinguish between the two subsets of CRS patients. CRS patients seen by tertiary care specialists have a significantly decreased quality of life that is on par with patients who suffer from congestive heart failure or chronic obstructive pulmonary disease [11]. Interestingly, the most bothersome symptoms reported by patients with CRSsNP are distinct from those reported by CRSwNP patients. While CRSsNP are more bothered by facial pain and pressure, those with CRSwNP are more bothered by rhinorrhea and smell/taste loss [12]. It is also well established that patients with CRS, especially those with CRSwNP, are more likely than the general population to suffer from comorbid respiratory diseases, including asthma and allergic rhinitis [13•, 14]. A recent study investigated the association of a wide variety of premorbid medical conditions in the 5 years before a patient was diagnosed with CRS [13•]. This study found that prior to diagnosis, CRSwNP patients had a higher prevalence of acute rhinosinusitis, allergic rhinitis, and asthma, while CRSsNP patients had a higher prevalence of upper and lower respiratory tract infections [13•]. These data further support the notion that pathogenesis of these two forms of CRS is likely distinct, and suggest that CRSsNP is more associated with specific infections, while CRSwNP is more associated with inflammatory airway disease. Finally, there is accumulating evidence that indicates that the mechanisms that drive CRSwNP pathogenesis are not uniform in different populations throughout the world [1••]. CRSwNP is characterized by type 2 inflammation and eosinophilia in the USA and Europe (see below), but it is characterized by a more mixed inflammatory phenotype and neutrophilia in many Asian countries, especially in China [15]. The mechanisms that drive these unique regional phenotypic differences are not yet clear, although recent work suggests that there may be a strong genetic component [16, 17]. As such, this review will focus on the pathogenesis of CRSwNP characterized by type 2 inflammation. Larger studies of the general population are still needed in order to gain a better overall understanding of the factors that contribute to CRS pathogenesis and morbidity as it applies to the general population.
Innate Immune Functions of Epithelial Cells
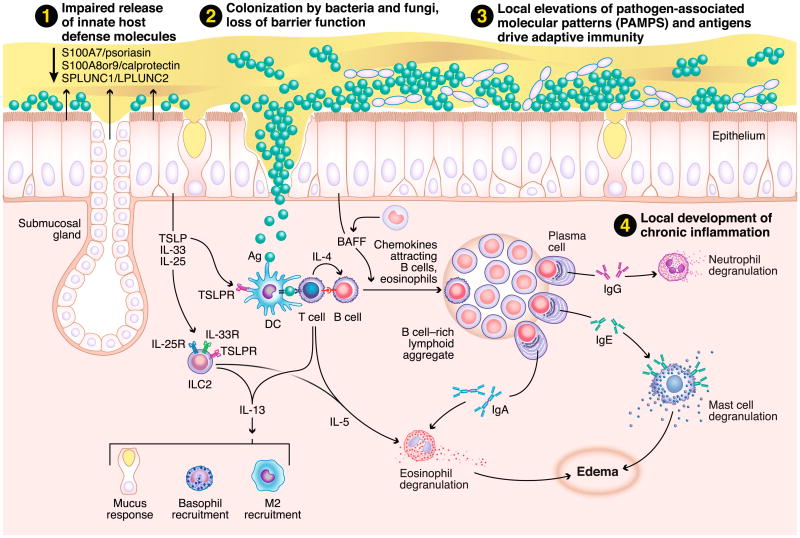
Although not traditionally thought of as immune cells, epithelial cells play a critical role in innate immune responses, and they can help to shape the nature of ensuing adaptive responses (Fig. 1) [3••, 18]. The specialized tight junctions that form between epithelial cells form the first line of defense against pathogens. These junctions provide a physical barrier that prevents entry of microbes and antigens to the underlying mucosal tissue. Sinonasal epithelial cells from CRSwNP patients have been shown to have defects in their ability to form tight junctions [19•]. Moreover, stimulation of airway epithelial cells with either IL-4 or IFN-γ, but not IL-17, resulted in decreased epithelial barrier function in vitro [19•]. Since type 2 cytokines are elevated in CRSwNP, this may provide one mechanism to account for the decreased barrier function seen in cells from these patients. In another study levels of the IL-6 family member oncostatin M (OSM) were found to be highly elevated in nasal polyps, and the authors found that stimulation of airway epithelial cells with OSM resulted in a loss of barrier function and mis-localization of tight junction proteins to the cytoplasm [20]. Together, these studies suggest that inflammatory cytokines may play an important role in diminishing the barrier function of airway epithelial cells in CRSwNP. This defective barrier may cause and/or perpetuate chronic inflammation by allowing microbes, antigens, and allergens into the mucosal tissue, where they can trigger immune responses.
Fig. 1.
Mechanisms of inflammation associated with CRS. 1 A defective epithelial barrier likely plays a critical role in the initiation and maintenance of chronic inflammation in CRS. These defects include reduced secretion of innate host defense molecules and loss of the airway epithelial barrier, along with decreased mucociliary clearance. 2 This in turn may result in increased colonization by S. aureus and, in some cases, fungi. 3 As a result, there is a local accumulation of PAMPS, and other antigens and/or allergens, in the sinonasal cavity that can easily access the underlying mucosal tissues through the defective epithelial barrier. 4 Altogether, this results in the activation of innate effector immune cells (eosinophils, mast cells, ILC2s, etc.) and recruitment and activation of adaptive effector immune cells (T and B cells) to the tissue mucosa
Another function of airway epithelial cells that relates to innate immunity is their ability to produce mucus and clear away potentially harmful antigens through mucociliary clearance. It has long been established that patients with CRS have defects in their nasal mucociliary clearance function, but the mechanisms that drive this defect were not known [21]. Seshadri and colleagues recently reported increased expression of the epithelial anion transporter pendrin in nasal polyp tissue from CRSwNP patients [22]. Pendrin functions to increase mucus production, and its elevated expression in CRSwNP may lead to the increased mucus production and impaired mucociliary clearance seen in these patients. In addition, the authors found that pendrin expression by epithelial cells was enhanced by the type 2 inflammatory cytokines IL-4 and IL-13, and the addition of IL-17 or the TLR3 agonist polyI:C synergistically elevated pendrin expression [22]. These data suggest that the pro-inflammatory milieu within nasal polyps might function to perpetuate defects in mucociliary clearance and promote chronic inflammation [23]. Other recent studies have suggested an important role for bitter and sweet taste receptors in the mucociliary function of airway epithelial cells [24]. Cohen and colleagues first identified a role for the bitter taste receptor T2R38 in regulating ciliary beat frequency in airway epithelial cells, and they found that a specific polymorphism in the T2R38 gene resulted in non-functional receptors [25•]. This particular polymorphism was also strongly associated with CRS patients who did not respond to medical therapy and required surgical treatment, suggesting that defects in mucociliary clearance may play an important role in CRS disease severity [26, 27]. In a follow-up study, the same group demonstrated that the function of the T2R family of bitter taste receptors could be negatively regulated by the T1R family of sweet taste receptors, and they linked increased glucose concentration in the mucus from CRS patients to a potential increased function of the T1R sweet taste receptors, providing another mechanism for the decreased mucociliary function in CRS [28••]. Interestingly, the defects in the T2R38 bitter taste receptor were also associated with a decreased capacity of airway epithelial cells to kill bacteria [25•]. Production of innate antimicrobial peptides is yet another function of normal airway epithelial cells that helps to protect the underlying tissue mucosa from harmful microbes and antigens. Along with defects in the bitter taste receptor, defects in expression of other innate antimicrobials in CRSwNP have also been reported [1••]. These include S100A7, S100A8/A9, and PLUNC [29, 30]. Taken together, these studies suggest that the airway epithelium in CRS, especially in CRSwNP, cannot adequately prevent entry of microbes or antigens to the underlying tissue, and this may be an important trigger of chronic inflammation in CRS.
Innate Immune Effector Cells
Given the defects in the innate function of the airway epithelium in CRS, it is not surprising that a wide variety of innate immune effector cells have been reported to be elevated in CRS, particularly in CRSwNP (Fig. 1). These include eosinophils, neutrophils, basophils, mast cells, type 2 macrophages, and group 2 innate lymphoid cells (ILC2s) [3••]. Classically, CRSsNP has been described as neutrophilic and CRSwNP as eosinophilic. However, it is clear that neutrophils are present in roughly equal numbers in both forms of disease, and it is unclear whether neutrophils are truly elevated in CRSsNP compared to sinus tissues from control patients [31]. Eosinophils have long been known to play an important role in the type 2 inflammatory response and tissue damage seen in nasal polyps of CRSwNP patients, and elevations in key eosinophil chemoattractants and cytokines, such as the eotaxins and IL-5, are well documented in this tissue [1••, 3••, 31, 32•, 33]. Recent work has also uncovered a new role for eosinophils in the promotion of plasma cell survival and antibody production and the activation of T cells [34, 35]. Given the large increases in T cells, B cells, and antibodies reported in nasal polyps (see below), it is likely that eosinophils play an important part in driving these adaptive immune responses as well.
Mast cells have also long been associated with pathogenesis in diseases associated with type 2 inflammation, such as asthma. These cells are uniquely located at mucosal sites where they function as sentinels and early inducers of immune responses. Recently, a unique phenotype of mast cells was found to be highly elevated in the glandular epithelial cells of nasal polyp tissues [36]. These mast cells expressed tryptase, carboxypeptidase A3, and chymase, which was distinct from the mast cells found near the tissue epithelium that lacked chymase expression. Because chymase is a known stimulator of mucus secretion, it is possible then that the chymase+ mast cells found in the glandular epithelium play an important role in the increased production of mucus in CRSwNP patients [3••, 36]. Similarly, basophils have long been associated with type 2 inflammatory responses. However, until recently, there were no studies that investigated whether basophils might play a role in CRSwNP pathogenesis. Mahdavinia and colleagues have now reported that basophils are indeed elevated in nasal polyps, and their levels are positively correlated with levels of eosinophils [37•]. This suggests that the recruitment of these two potent innate effector cells may be coordinated in nasal polyps.
Innate lymphoid cells (ILCs) are a newly identified subset of innate effector cells. ILCs share many properties with CD4+ T cells, but they lack expression of specific lineage markers, such as CD3 [38]. Group 2 ILCs (ILC2s) are known to produce type 2 cytokines, especially IL-5 and IL-13, and are activated by cytokines from epithelial cells such as IL-25, IL-33, and thymic stromal lymphopoietin (TSLP), which are also associated with type 2 inflammatory responses [39]. Spits and colleagues have demonstrated that ILC2s are highly elevated in nasal polyp tissues [40•]. Since ILC2 function has been associated with pathogenesis of allergic asthma, it is possible that they play a similar pathogenic role in CRSwNP [41]. In addition, while it remains unclear whether protein expression of IL-25 and IL-33 is elevated in polyps, it has been demonstrated that expression and activity of TSLP are elevated, which could contribute to ILC2 activation in this tissue [3••, 42].
Adaptive Immunity
Along with increased numbers of innate immune effector cells, CRS tissues are associated with increased numbers of adaptive immune cells (Fig. 1). These adaptive immune cells are likely recruited to the sinonasal mucosa as a result of the activation of the innate immune effector cells discussed above. Many studies have focused on the potential role of T cells in CRS. As previously mentioned, CRSwNP has classically been associated with type 2 inflammatory responses, and Th2 cells have been thought to play an important role in nasal polyps. CD3+ T cells have been found to be elevated in both CRSsNP and CRSwNP tissues, but early studies relied on measuring cytokine expression in tissue homogenates to classify responses as Th1 or Th2 [1••, 31]. The discovery of ILC2s in nasal polyps has now called into question whether the elevated levels of IL-5 and IL-13 found in nasal polyps are truly due to T cells, and it is likely that both cell types contribute to the production of these cytokines in nasal polyps. More recently, Gevaert and colleagues used intracellular cytokine staining and flow cytometry analysis to identify specific subsets of cytokine-positive T cells in sinus tissues from both CRSsNP and CRSwNP patients [43••]. Interestingly they did find an elevated level of CD3+ T cells in CRSwNP tissues, but not in CRSsNP [43••]. Further, they found that T cells from both subsets of CRS patients, as well as those from controls, expressed a wide array of cytokines. Overall, there did not appear to be a difference in the T cell subsets found in CRSsNP tissue compared to control sinus tissue, but the CRSwNP tissue did contain an elevated frequency of IL-5+CD3+CD4+ T cells [43••]. However, the T cell profile was not restricted to Th2 cells in CRSwNP tissues, and the authors found cells expressing a wide variety of cytokines, including IFN-γ, IL-17, and IL-10 [43••]. Altogether, this study suggests that the patterns of T cell-associated inflammation in the sinus tissue of CRS patients are more complex than originally thought.
In addition to inflammatory T cell subsets, some groups have also investigated whether there are defects in T regulatory cells (Tregs) in CRS patients. Some early studies relied on semi-quantitative methods to identify Tregs in CRS, such as measuring expression of the regulatory transcription factor Foxp3 by PCR or immunohistochemistry. These studies found decreased expression of Foxp3 in CRSwNP, along with decreased expression of the regulatory cytokines IL-10 and TGF-β, but no differences in these markers in CRSsNP [44, 45]. More recently, one study has reported elevated expression of Foxp3 and regulatory cytokines in CRS tissue, and another found no differences in the frequencies of Tr1 cells between either CRS subgroup and control sinus tissues [46, 47]. Interpretation of much of these data is somewhat limited by the fact that both Foxp3 and CD25, markers which specifically identify regulatory T cells in mice, are expressed by activated T cell subsets in humans as well as Tregs, and additional markers to identify true Tregs were not used.
B cells have also been thought to play a role in the pathogenesis of CRSwNP [48]. While early studies found no difference in the presence of CD20+ B cells in sinus tissues from CRS patients, it has become clear that CD19+ B cells and CD138+ plasma cells are highly elevated in polyps from CRSwNP patients [31, 49, 50]. In addition, antibodies of almost every isotype have been found to be elevated in nasal polyp tissue, but not in sinus tissue from CRSsNP [49, 50]. These findings are in line with other studies demonstrating elevated expression of the B cell attracting chemokines CXCL12 and CXCL13 in polyp tissues [51]. In addition, expression of the key B cell survival and activation cytokines IL-6 and B cell-activating factor of the TNF family (BAFF) is elevated in nasal polyps [52]. Other studies also demonstrate elevated expression of germline transcripts for IgE and the presence of tertiary lymphoid structures in polyps, which would support the notion that B cells can become activated and undergo class switch recombination locally in nasal polyps [53]. While we have been unable to detect an elevated presence of tertiary lymphoid structures, we have identified increased expression of the extrafollicular plasmablast marker Epstein-Barr virus-induced protein 2 (EBI2) in nasal polyps, which also supports the notion that local activation of B cells can occur in nasal polyps [50]. To date, the antigen specificity of the majority of the antibody repertoire is not known, although, IgE to S. aureus enterotoxin B and IgG and IgA to a variety of auto-antigens have been reported to be elevated [54, 55•, 56]. Whether these antibodies contribute to pathogenesis also remains unclear. A recent study by Geveart and colleagues investigated the effects of the humanized anti-IgE, omalizumab, in a specific subset of CRSwNP patients [57•]. They found that treatment resulted in a significant reduction in the total nasal polyp score and a significant increase in many quality of life measures compared to placebo [57•]. This suggests that, at least in some CRSwNP patients, IgE does play a critical role in pathogenesis. In addition, many cells that express Fc receptors, including eosinophils and mast cells, are elevated in nasal polyps [3••]. Thus, it is plausible that antibody binding and crosslinking of these receptors could play an important role in the activation of these cells. Finally, recent work from Tan and colleagues suggests that antibody-mediated complement activation is highly elevated in nasal polyps, which is also likely to contribute to inflammation at this site [58].
Conclusions
A substantial amount of progress has been made towards understanding the mechanisms of inflammation that drive CRS, and it is clear that many distinct processes likely play a role in this process. These include defects in the innate immune function of airway epithelial cells and the recruitment and activation of both innate and adaptive effector immune cells. It is also important to note that, due to the lack of a robust animal model of CRS, it is difficult to know whether any of these factors truly contribute to disease pathogenesis or if they are simply the consequence of the ongoing inflammation. Currently there have been some efforts at developing a murine model of CRSwNP, which may help to address these questions [59], but models of CRSsNP are still lacking. Thus, it is clear that new model systems are still needed in order to fully elucidate the mechanisms that underlie this prevalent and costly disease. Until a better understanding of the inflammatory mechanisms associated with CRS is obtained, it will be difficult to design improved therapeutic strategies for these patients, and this disease will continue to present a large socio-economic burden.
Acknowledgments
Funding This study was funded by NIH P01 106683-01, K12 HD055884, and the Ernest S. Bazley Trust.
Abbreviations
- BAFF
B cell-activating factor of the TNF family
- CRS
Chronic rhinosinusitis
- CRSsNP
CRS without nasal polyps
- CRSwNP
CRS with nasal polyps
- EBI2
Epstein-Barr virus-induced protein 2
- ILC
Innate lymphoid cell
- OSM
Oncostatin M
- PLUNC
Palate lung and nasal epithelium clone protein
- TLR
Toll-like receptor
- TSLP
Thymic stromal lymphopoietin
Footnotes
Compliance with Ethical Standards: Conflict of Interest Dr. Hulse declares a grant from NIH.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1••.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology Supplement. 2012;(23):3 p preceding table of contents, 1–298. Comprehensive review of the latest definitions, practice standards and research on chronic rhinosinusitis. [PubMed] [Google Scholar]
- 2.Smith KA, Orlandi RR, Rudmik L. Cost of adult chronic rhinosinusitis: a systematic review. Laryngoscope. 2015;125(7):1547–56. doi: 10.1002/lary.25180. [DOI] [PubMed] [Google Scholar]
- 3••.Hulse KE, Stevens WW, Tan BK, Schleimer RP. Pathogenesis of nasal polyposis. Clin Exp Allergy. 2015;45(2):328–46. doi: 10.1111/cea.12472. Comprehensive review on the current understanding of the mechanisms that drive chronic rhinosinusitis with nasal polyps. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan BK, Kern RC, Schleimer RP, Schwartz BS. Chronic rhinosinusitis: the unrecognized epidemic. Am J Respir Crit Care Med. 2013;188(11):1275–7. doi: 10.1164/rccm.201308-1500ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvis D, Newson R, Lotvall J, Hastan D, Tomassen P, Keil T, et al. Asthma in adults and its association with chronic rhinosinusitis: the GA2LEN survey in Europe. Allergy. 2012;67(1):91–8. doi: 10.1111/j.1398-9995.2011.02709.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim YS, Kim NH, Seong SY, Kim KR, Lee GB, Kim KS. Prevalence and risk factors of chronic rhinosinusitis in Korea. Am J Rhinol Allergy. 2011;25(3):117–21. doi: 10.2500/ajra.2011.25.3630. [DOI] [PubMed] [Google Scholar]
- 7.Stevens WW, Peters AT, Suh L, Norton JE, Kern RC, Conley DB, et al. A retrospective, cross-sectional study reveals that women with CRSwNP have more severe disease than men. Immun Inflammation Dis. 2015;3(1):14–22. doi: 10.1002/iid3.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee TP, Chiang BL. Sex differences in spontaneous versus induced animal models of autoimmunity. Autoimmun Rev. 2012;11(6–7):A422–9. doi: 10.1016/j.autrev.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 9.Townsend EA, Miller VM, Prakash YS. Sex differences and sex steroids in lung health and disease. Endocr Rev. 2012;33(1):1–47. doi: 10.1210/er.2010-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SA, et al. Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med. 2012;185(4):356–62. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soler ZM, Wittenberg E, Schlosser RJ, Mace JC, Smith TL. Health state utility values in patients undergoing endoscopic sinus surgery. Laryngoscope. 2011;121(12):2672–8. doi: 10.1002/lary.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Loos Dietz DA, Hopkins C, Fokkens WJ. Symptoms in chronic rhinosinusitis with and without nasal polyps. Laryngoscope. 2013;123(1):57–63. doi: 10.1002/lary.23671. [DOI] [PubMed] [Google Scholar]
- 13•.Tan BK, Chandra RK, Pollak J, Kato A, Conley DB, Peters AT, et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;131(5):1350–60. doi: 10.1016/j.jaci.2013.02.002. An un-biased assesment of the clinical conditions associated with different forms of chronic rhinosinusitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan BK, Zirkle W, Chandra RK, Lin D, Conley DB, Peters AT, et al. Atopic profile of patients failing medical therapy for chronic rhinosinusitis. Int Forum Allergy Rhinol. 2011;1(2):88–94. doi: 10.1002/alr.20025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akdis CA, Bachert C, Cingi C, Dykewicz MS, Hellings PW, Naclerio RM, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2013;131(6):1479–90. doi: 10.1016/j.jaci.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato A, Hulse KE. Searching for distinct mechanisms in eosinophilic and noneosinophilic airway inflammation. Am J Respir Crit Care Med. 2014;190(6):596–8. doi: 10.1164/rccm.201408-1491ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahdavinia M, Suh LA, Carter RG, Stevens WW, Norton JE, Kato A, et al. Increased noneosinophilic nasal polyps in chronic rhinosinusitis in US second-generation Asians suggest genetic regulation of eosinophilia. J Allergy Clin Immunol. 2014 doi: 10.1016/j.jaci.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi-Peters A, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. Am J Rhinol. 2008;22(6):549–59. doi: 10.2500/ajr.2008.22.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Soyka MB, Wawrzyniak P, Eiwegger T, Holzmann D, Treis A, Wanke K, et al. Defective epithelial barrier in chronic rhinosinusitis: the regulation of tight junctions by IFN-gamma and IL-4. J Allergy Clin Immunol. 2012;130(5):1087–96 e10. doi: 10.1016/jjaci.2012.05.052. First study to show defects in epithelial tight junction function in chronic rhinosinusitis may be related to increased expression of inflammatory cytokines. [DOI] [PubMed] [Google Scholar]
- 20.Pothoven KL, N J, O'Campo C, Suh L, Carter R, Hulse KE, Seshadri S, Tan BK, Chandra R, Peters AT, Harris KE, Conley DB, Grammer LC, Kern R, Schleimer RP. Oncostatin M is elevated in chronic rhinosinusitis and decreases barrier function in human airway epithelium. J Allergy Clin Immunol. 2014;133(2):AB237. [Google Scholar]
- 21.Passali D, Ferri R, Becchini G, Passali GC, Bellussi L. Alterations of nasal mucociliary transport in patients with hypertrophy of the inferior turbinates, deviations of the nasal septum and chronic sinusitis. Eur Arch Otorhinolaryngol. 1999;256(7):335–7. doi: 10.1007/s004050050158. [DOI] [PubMed] [Google Scholar]
- 22.Seshadri S, Lu X, Purkey MR, Homma T, Choi AW, Carter R, et al. Increased expression of the epithelial anion transporter pendrin/SLC26A4 in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2015 doi: 10.1016/jjaci.2015.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiao J, Duan S, Meng N, Li Y, Fan E, Zhang L. Role of IFN-gamma, IL-13 and IL-17 on mucociliary differentiation of nasal epithelial cells in chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2015 doi: 10.1111/cea.12644. [DOI] [PubMed] [Google Scholar]
- 24.Lee RJ, Cohen NA. Bitter and sweet taste receptors in the respiratory epithelium in health and disease. J Mol Med (Berl) 2014;92(12):1235–44. doi: 10.1007/s00109-014-1222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25•.Lee RJ, Xiong G, Kofonow JM, Chen B, Lysenko A, Jiang P, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012;122(11):4145–59. doi: 10.1172/JCI64240. Demonstrated that a polymorphism in a bitter taste receptor gene resulted in significantly higher prevalence of upper respiratory infections. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adappa ND, Howland TJ, Palmer JN, Kennedy DW, Doghramji L, Lysenko A, et al. Genetics of the taste receptor T2R38 correlates with chronic rhinosinusitis necessitating surgical intervention. Int Forum Allergy Rhinol. 2013;3(3):184–7. doi: 10.1002/alr.21140. [DOI] [PubMed] [Google Scholar]
- 27.Adappa ND, Zhang Z, Palmer JN, Kennedy DW, Doghramji L, Lysenko A, et al. The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int Forum Allergy Rhinol. 2014;4(1):3–7. doi: 10.1002/alr.21253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Lee RJ, Kofonow JM, Rosen PL, Siebert AP, Chen B, Doghramji L et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest. 2014;124(3):1393–405. doi: 10.1172/JCI72094. Demonstrated reciprocol functional regulation between bitter and sweet taste receptors in airway epithelial cells, and provides a potential mechanism for decreased innate immune function of airway epithelial cells in chronic rhinosinusitis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seshadri S, Lin DC, Rosati M, Carter RG, Norton JE, Suh L, et al. Reduced expression of antimicrobial PLUNC proteins in nasal polyp tissues of patients with chronic rhinosinusitis. Allergy. 2012;67(7):920–8. doi: 10.1111/j.1398-9995.2012.02848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tieu DD, Peters AT, Carter RG, Suh L, Conley DB, Chandra R, et al. Evidence for diminished levels of epithelial psoriasin and calprotectin in chronic rhinosinusitis. J Allergy Clin Immunol. 2010;125(3):667–75. doi: 10.1016/j.jaci.2009.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Zele T, Claeys S, Gevaert P, Van Maele G, Holtappels G, Van Cauwenberge P, et al. Differentiation of chronic sinus diseases by measurement of inflammatory mediators. Allergy. 2006;61(11):1280–9. doi: 10.1111/j.1398-9995.2006.01225.x. [DOI] [PubMed] [Google Scholar]
- 32•.Stevens WW, Ocampo CJ, Berdnikovs S, Sakashita M, Mahdavinia M, Suh L, et al. Cytokines in chronic rhinosinusitis. Role in eosinophilia and aspirin-exacerbated respiratory disease. Am J Respir Crit Care Med. 2015;192(6):682–94. doi: 10.1164/rccm.201412-2278OC. Comprehensive analysis of inflammatory cytokines and chemokines in distinct chronic rhinosinusitis patient groups. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schleimer RP, Kato A, Kern R. Eosinophils and chronic rhinosinusitis. In: Lee JJ, Rosenberg HF, editors. Eosinophils in health and disease. 1. San Diego, CA: Elsevier; 2012. pp. 508–18. 92101–4495. [Google Scholar]
- 34.Chu VT, Berek C. The establishment of the plasma cell survival niche in the bone marrow. Immunol Rev. 2013;251(1):177–88. doi: 10.1111/imr.12011. [DOI] [PubMed] [Google Scholar]
- 35.Jacobsen EA, Helmers RA, Lee JJ, Lee NA. The expanding role(s) of eosinophils in health and disease. Blood. 2012;120(19):3882–90. doi: 10.1182/blood-2012-06-330845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takabayashi T, Kato A, Peters AT, Suh LA, Carter R, Norton J, et al. Glandular mast cells with distinct phenotype are highly elevated in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2012;130(2):410–20 e5. doi: 10.1016/j.jaci.2012.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Mahdavinia M, Carter RG, Ocampo CJ, Stevens W, Kato A, Tan BK, et al. Basophils are elevated in nasal polyps of patients with chronic rhinosinusitis without aspirin sensitivity. J Allergy Clin Immunol. 2014;133(6):1759–63. doi: 10.1016/j.jaci.2013.12.1092. First report of elevated basophil levels in chronic rhinosinusitis with nasal polyps. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magri G, Miyajima M, Bascones S, Mortha A, Puga I, Cassis L, et al. Innate lymphoid cells integrate stromal and immunological signals to enhance antibody production by splenic marginal zone B cells. Nat Immunol. 2014;15(4):354–64. doi: 10.1038/ni.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim BS, Artis D. Group 2 innate lymphoid cells in health and disease. Cold Spring Harb Perspect Biol. 2015 doi: 10.1101/cshperspect.a016337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40•.Mjosberg JM, Trifari S, Crellin NK, Peters CP, van Drunen CM, Piet B et al. Human IL-25- and IL-33-responsive type 2 innate lymphoid cells are defined by expression of CRTH2 and CD161. Nat Immunol. 2011;12(11):1055–62. doi: 10.1038/ni.2104. First report of elevated levels of ILC2s in chronic rhinosinusitis with nasal polyps. [DOI] [PubMed] [Google Scholar]
- 41.Mjosberg J, Eidsmo L. Update on innate lymphoid cells in atopic and non-atopic inflammation in the airways and skin. Clin Exp Allergy. 2014;44(8):1033–43. doi: 10.1111/cea.12353. [DOI] [PubMed] [Google Scholar]
- 42.Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;132(3):593–600. doi: 10.1016/j.jaci.2013.04.005.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43••.Derycke L, Eyerich S, Van Crombruggen K, Perez-Novo C, Holtappels G, Deruyck N, et al. Mixed T helper cell signatures in chronic rhinosinusitis with and without polyps. PLoS One. 2014;9(6):e97581. doi: 10.1371/journal.pone.0097581. Comprehensive analysis of T cell subsets in distinct chronic rhinosinusitis patients using intracellular cytokine staining. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang N, Van Zele T, Perez-Novo C, Van Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T-effector cells orchestrate mucosal inflammation in chronic sinus disease. J Allergy Clin Immunol. 2008;122(5):961–8. doi: 10.1016/j.jaci.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 45.Van Bruaene N, Perez-Novo CA, Basinski TM, Van Zele T, Holtappels G, De Ruyck N, et al. T-cell regulation in chronic paranasal sinus disease. J Allergy Clin Immunol. 2008;121(6):1435–41. 41 e1–3. doi: 10.1016/j.jaci.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 46.Pant H, Hughes A, Schembri M, Miljkovic D, Krumbiegel D. CD4(+) and CD8(+) regulatory T cells in chronic rhinosinusitis mucosa. Am J Rhinol Allergy. 2014;28(2):e83–9. doi: 10.2500/ajra.2013.28.4014. [DOI] [PubMed] [Google Scholar]
- 47.Li HB, Cai KM, Liu Z, Xia JH, Zhang Y, Xu R, et al. Foxp3+ T regulatory cells (Tregs) are increased in nasal polyps (NP) after treatment with intranasal steroid. Clin Immunol. 2008;129(3):394–400. doi: 10.1016/j.clim.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 48.Kato A, Hulse KE, Tan BK, Schleimer RP. B-lymphocyte lineage cells and the respiratory system. J Allergy Clin Immunol. 2013;131(4):933–57. doi: 10.1016/jjaci.2013.02.023. quiz58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van Zele T, Gevaert P, Holtappels G, van Cauwenberge P, Bachert C. Local immunoglobulin production in nasal polyposis is modulated by superantigens. Clin Exp Allergy. 2007;37(12):1840–7. doi: 10.1111/j.1365-2222.2007.02838.x. [DOI] [PubMed] [Google Scholar]
- 50.Hulse KE, Norton JE, Suh L, Zhong Q, Mahdavinia M, Simon P, et al. Chronic rhinosinusitis with nasal polyps is characterized by B-cell inflammation and EBV-induced protein 2 expression. J Allergy Clin Immunol. 2013 doi: 10.1016/j.jaci.2013.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patadia M, Dixon J, Conley D, Chandra R, Peters A, Suh LA, et al. Evaluation of the presence of B-cell attractant chemokines in chronic rhinosinusitis. Am J Rhinol Allergy. 2010;24(1):11–6. doi: 10.2500/ajra.2010.24.3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kato A, Peters A, Suh L, Carter R, Harris KE, Chandra R, et al. Evidence of a role for B cell-activating factor of the TNF family in the pathogenesis of chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2008;121(6):1385–92. 92 e1–2. doi: 10.1016/jjaci.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gevaert P, Nouri-Aria KT, Wu H, Harper CE, Takhar P, Fear DJ, et al. Local receptor revision and class switching to IgE in chronic rhinosinusitis with nasal polyps. Allergy. 2013;68(1):55–63. doi: 10.1111/all.12054. [DOI] [PubMed] [Google Scholar]
- 54.Van Zele T, Gevaert P, Watelet JB, Claeys G, Holtappels G, Claeys C, et al. Staphylococcus aureus colonization and IgE antibody formation to enterotoxins is increased in nasal polyposis. J Allergy Clin Immunol. 2004;114(4):981–3. doi: 10.1016/j.jaci.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 55•.Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2011;128(6):1198–206 e1. doi: 10.1016/j.jaci.2011.08.037. First demonstration of increased autoantibodies associated with chronic rhinosinusitis with nasal polyps. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jeffe JS, Seshadri S, Hamill KJ, Huang JH, Carter R, Suh L, et al. A role for anti-BP180 autoantibodies in chronic rhinosinusitis. Laryngoscope. 2013;123:2104–11. doi: 10.1002/lary.24016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57•.Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W, et al. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131(1):110–6 e1. doi: 10.1016/j.jaci.2012.07.047. First report of successful treatment of a subset of chronic rhinosinusitis patients with anti-IgE therapy. [DOI] [PubMed] [Google Scholar]
- 58.Van Roey GA, Vanison CC, Huang H, Kern RC, Chandra RK, Shintani Smith S, et al. Complement activation in nasal tissue of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2015;135(2):AB237. doi: 10.1016/j.jaci.2016.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim DW, Khaimuratova R, Hur DG, Jeon SY, Kim SW, Shin HW, et al. Staphylococcus aureus enterotoxin B contributes to induction of nasal polypoid lesions in an allergic rhinosinusitis murine model. Am J Rhinol Allergy. 2011;25(6):e255–61. doi: 10.2500/ajra.2011.25.3727. [DOI] [PubMed] [Google Scholar]