Abstract
A proposed Wilms tumor gene, WT1, which encodes a zinc finger protein, has previously been isolated from human chromosome 11p13. Chemical mismatch cleavage analysis was used to identify point mutations in the zinc finger region of this gene in a series of 32 Wilms tumors. Two exonic single base changes were detected. In zinc finger 3 of a bilateral Wilms tumor patient, a constitutional de novo C----T base change was found changing an arginine to a stop codon. One tumor from this patient showed allele loss leading to 11p hemizygosity of the abnormal allele. In zinc finger 2 of a sporadic Wilms tumor patient, a C----T base change resulted in an arginine to cysteine amino acid change. To our knowledge, a WT1 gene missense mutation has not been detected previously in a Wilms tumor. By comparison with a recent NMR and x-ray crystallographic analysis of an analogous zinc finger gene, early growth response gene 1 (EGR1), this amino acid change in WT1 occurs at a residue predicted to be critical for DNA binding capacity and site specificity. The detection of one nonsense point mutation and one missense WT1 gene point mutation adds to the accumulating evidence implicating this gene in a proportion of Wilms tumor patients.
Full text
PDF


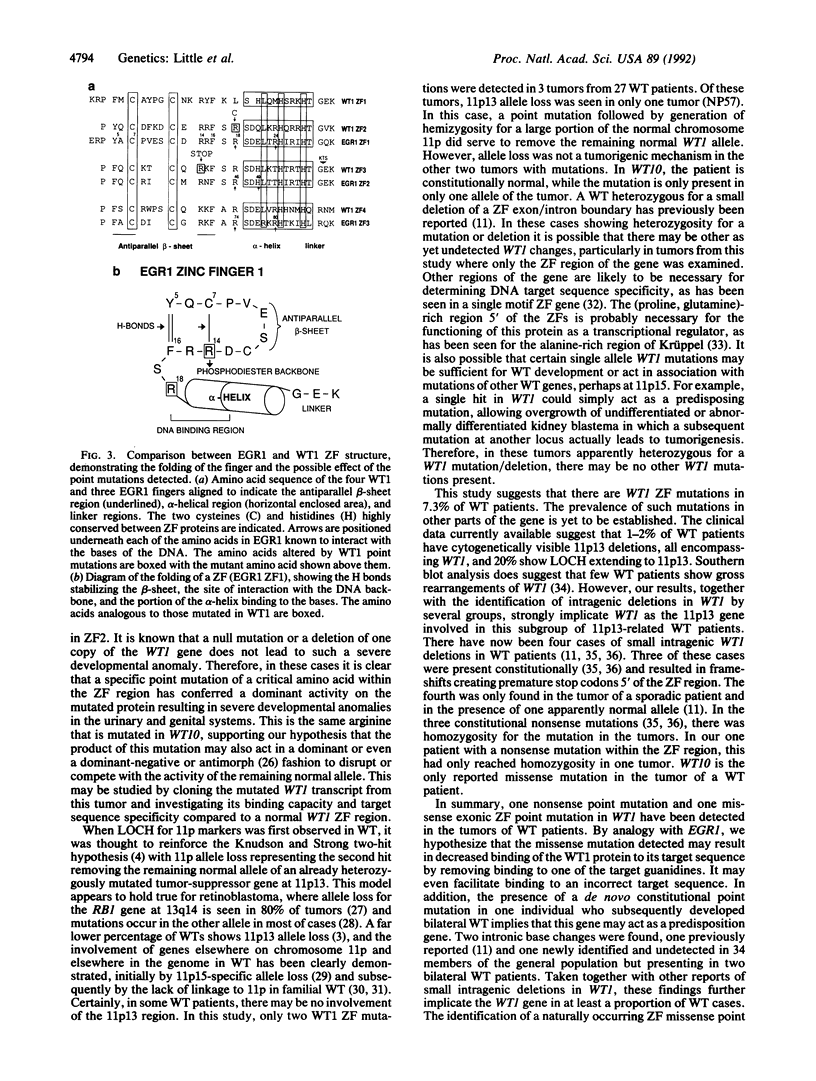

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedict W. F., Srivatsan E. S., Mark C., Banerjee A., Sparkes R. S., Murphree A. L. Complete or partial homozygosity of chromosome 13 in primary retinoblastoma. Cancer Res. 1987 Aug 1;47(15):4189–4191. [PubMed] [Google Scholar]
- Bickmore W. A., Porteous D. J., Christie S., Seawright A., Fletcher J. M., Maule J. C., Couillin P., Junien C., Hastie N. D., van Heyningen V. CpG islands surround a DNA segment located between translocation breakpoints associated with genitourinary dysplasia and aniridia. Genomics. 1989 Nov;5(4):685–693. doi: 10.1016/0888-7543(89)90109-2. [DOI] [PubMed] [Google Scholar]
- Call K. M., Glaser T., Ito C. Y., Buckler A. J., Pelletier J., Haber D. A., Rose E. A., Kral A., Yeger H., Lewis W. H. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell. 1990 Feb 9;60(3):509–520. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- Compton D. A., Weil M. M., Bonetta L., Huang A., Jones C., Yeger H., Williams B. R., Strong L. C., Saunders G. F. Definition of the limits of the Wilms tumor locus on human chromosome 11p13. Genomics. 1990 Feb;6(2):309–315. doi: 10.1016/0888-7543(90)90571-b. [DOI] [PubMed] [Google Scholar]
- Corton J. C., Johnston S. A. Altering DNA-binding specificity of GAL4 requires sequences adjacent to the zinc finger. Nature. 1989 Aug 31;340(6236):724–727. doi: 10.1038/340724a0. [DOI] [PubMed] [Google Scholar]
- Cotton R. G., Rodrigues N. R., Campbell R. D. Reactivity of cytosine and thymine in single-base-pair mismatches with hydroxylamine and osmium tetroxide and its application to the study of mutations. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4397–4401. doi: 10.1073/pnas.85.12.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell J. K., Wadey R. B., Haber D. A., Call K. M., Housman D. E., Pritchard J. Structural rearrangements of the WT1 gene in Wilms' tumour cells. Oncogene. 1991 Apr;6(4):595–599. [PubMed] [Google Scholar]
- Dianzani I., Forrest S. M., Camaschella C., Gottardi E., Cotton R. G. Heterozygotes and homozygotes: discrimination by chemical cleavage of mismatch. Am J Hum Genet. 1991 Feb;48(2):423–424. [PMC free article] [PubMed] [Google Scholar]
- Francke U., Holmes L. B., Atkins L., Riccardi V. M. Aniridia-Wilms' tumor association: evidence for specific deletion of 11p13. Cytogenet Cell Genet. 1979;24(3):185–192. doi: 10.1159/000131375. [DOI] [PubMed] [Google Scholar]
- Gessler M., Poustka A., Cavenee W., Neve R. L., Orkin S. H., Bruns G. A. Homozygous deletion in Wilms tumours of a zinc-finger gene identified by chromosome jumping. Nature. 1990 Feb 22;343(6260):774–778. doi: 10.1038/343774a0. [DOI] [PubMed] [Google Scholar]
- Grundy P., Koufos A., Morgan K., Li F. P., Meadows A. T., Cavenee W. K. Familial predisposition to Wilms' tumour does not map to the short arm of chromosome 11. Nature. 1988 Nov 24;336(6197):374–376. doi: 10.1038/336374a0. [DOI] [PubMed] [Google Scholar]
- Haber D. A., Buckler A. J., Glaser T., Call K. M., Pelletier J., Sohn R. L., Douglass E. C., Housman D. E. An internal deletion within an 11p13 zinc finger gene contributes to the development of Wilms' tumor. Cell. 1990 Jun 29;61(7):1257–1269. doi: 10.1016/0092-8674(90)90690-g. [DOI] [PubMed] [Google Scholar]
- Huff V., Compton D. A., Chao L. Y., Strong L. C., Geiser C. F., Saunders G. F. Lack of linkage of familial Wilms' tumour to chromosomal band 11p13. Nature. 1988 Nov 24;336(6197):377–378. doi: 10.1038/336377a0. [DOI] [PubMed] [Google Scholar]
- Huff V., Meadows A., Riccardi V. M., Strong L. C., Saunders G. F. Parental origin of de novo constitutional deletions of chromosomal band 11p13. Am J Hum Genet. 1990 Jul;47(1):155–160. [PMC free article] [PubMed] [Google Scholar]
- Huff V., Miwa H., Haber D. A., Call K. M., Housman D., Strong L. C., Saunders G. F. Evidence for WT1 as a Wilms tumor (WT) gene: intragenic germinal deletion in bilateral WT. Am J Hum Genet. 1991 May;48(5):997–1003. [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y., Egues M. C., Rowley J. D. Interstitial deletion of short arm of chromosome 11 limited to Wilms' tumor cells in a patient without aniridia. Cancer Res. 1981 Nov;41(11 Pt 1):4577–4578. [PubMed] [Google Scholar]
- Knudson A. G., Jr, Strong L. C. Mutation and cancer: a model for Wilms' tumor of the kidney. J Natl Cancer Inst. 1972 Feb;48(2):313–324. [PubMed] [Google Scholar]
- Lee W. H., Bookstein R., Hong F., Young L. J., Shew J. Y., Lee E. Y. Human retinoblastoma susceptibility gene: cloning, identification, and sequence. Science. 1987 Mar 13;235(4794):1394–1399. doi: 10.1126/science.3823889. [DOI] [PubMed] [Google Scholar]
- Licht J. D., Grossel M. J., Figge J., Hansen U. M. Drosophila Krüppel protein is a transcriptional repressor. Nature. 1990 Jul 5;346(6279):76–79. doi: 10.1038/346076a0. [DOI] [PubMed] [Google Scholar]
- Mannens M., Devilee P., Bliek J., Mandjes I., de Kraker J., Heyting C., Slater R. M., Westerveld A. Loss of heterozygosity in Wilms' tumors, studied for six putative tumor suppressor regions, is limited to chromosome 11. Cancer Res. 1990 Jun 1;50(11):3279–3283. [PubMed] [Google Scholar]
- Mannens M., Slater R. M., Heyting C., Bliek J., de Kraker J., Coad N., de Pagter-Holthuizen P., Pearson P. L. Molecular nature of genetic changes resulting in loss of heterozygosity of chromosome 11 in Wilms' tumours. Hum Genet. 1988 Dec;81(1):41–48. doi: 10.1007/BF00283727. [DOI] [PubMed] [Google Scholar]
- Nardelli J., Gibson T. J., Vesque C., Charnay P. Base sequence discrimination by zinc-finger DNA-binding domains. Nature. 1991 Jan 10;349(6305):175–178. doi: 10.1038/349175a0. [DOI] [PubMed] [Google Scholar]
- Pavletich N. P., Pabo C. O. Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 A. Science. 1991 May 10;252(5007):809–817. doi: 10.1126/science.2028256. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Bruening W., Kashtan C. E., Mauer S. M., Manivel J. C., Striegel J. E., Houghton D. C., Junien C., Habib R., Fouser L. Germline mutations in the Wilms' tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell. 1991 Oct 18;67(2):437–447. doi: 10.1016/0092-8674(91)90194-4. [DOI] [PubMed] [Google Scholar]
- Pelletier J., Bruening W., Li F. P., Haber D. A., Glaser T., Housman D. E. WT1 mutations contribute to abnormal genital system development and hereditary Wilms' tumour. Nature. 1991 Oct 3;353(6343):431–434. doi: 10.1038/353431a0. [DOI] [PubMed] [Google Scholar]
- Pritchard-Jones K., Fleming S., Davidson D., Bickmore W., Porteous D., Gosden C., Bard J., Buckler A., Pelletier J., Housman D. The candidate Wilms' tumour gene is involved in genitourinary development. Nature. 1990 Jul 12;346(6280):194–197. doi: 10.1038/346194a0. [DOI] [PubMed] [Google Scholar]
- Prosser J., Elder P. A., Condie A., MacFadyen I., Steel C. M., Evans H. J. Mutations in p53 do not account for heritable breast cancer: a study in five affected families. Br J Cancer. 1991 Feb;63(2):181–184. doi: 10.1038/bjc.1991.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser J., Thompson A. M., Cranston G., Evans H. J. Evidence that p53 behaves as a tumour suppressor gene in sporadic breast tumours. Oncogene. 1990 Oct;5(10):1573–1579. [PubMed] [Google Scholar]
- Rose E. A., Glaser T., Jones C., Smith C. L., Lewis W. H., Call K. M., Minden M., Champagne E., Bonetta L., Yeger H. Complete physical map of the WAGR region of 11p13 localizes a candidate Wilms' tumor gene. Cell. 1990 Feb 9;60(3):495–508. doi: 10.1016/0092-8674(90)90600-j. [DOI] [PubMed] [Google Scholar]
- Schroeder W. T., Chao L. Y., Dao D. D., Strong L. C., Pathak S., Riccardi V., Lewis W. H., Saunders G. F. Nonrandom loss of maternal chromosome 11 alleles in Wilms tumors. Am J Hum Genet. 1987 May;40(5):413–420. [PMC free article] [PubMed] [Google Scholar]
- Stanojević D., Hoey T., Levine M. Sequence-specific DNA-binding activities of the gap proteins encoded by hunchback and Krüppel in Drosophila. Nature. 1989 Sep 28;341(6240):331–335. doi: 10.1038/341331a0. [DOI] [PubMed] [Google Scholar]
- Winship P. R. An improved method for directly sequencing PCR amplified material using dimethyl sulphoxide. Nucleic Acids Res. 1989 Feb 11;17(3):1266–1266. doi: 10.1093/nar/17.3.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heyningen V., Bickmore W. A., Seawright A., Fletcher J. M., Maule J., Fekete G., Gessler M., Bruns G. A., Huerre-Jeanpierre C., Junien C. Role for the Wilms tumor gene in genital development? Proc Natl Acad Sci U S A. 1990 Jul;87(14):5383–5386. doi: 10.1073/pnas.87.14.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]



