Abstract
Background
This study aimed to evaluate the combined effect of vacuum sealing drainage (VSD) and antibiotic-loaded bone cement on soft tissue defects and infection.
Material/Methods
This prospective non-blinded study recruited 46 patients with soft tissue defects and infection from January 2010 to May 2014 and randomly divided them into experimental and control groups (n=23). Patients in the experimental group were treated with VSD and antibiotic-loaded bone cement, while the patients in the control group were treated with VSD only.
Results
In the experimental group, the wound was healed in 23 cases at 4 weeks postoperatively, of which direct suture was performed in 12 cases, and additional free flap transplantation or skin grafting was performed in 6 cases and 5 cases, respectively. No infection reoccurred in 1-year follow-up. In the control group, the wound was healed in 15 cases at 6 weeks postoperatively, of which direct suture was performed in 8 cases, and additional free flap transplantation or skin grafting was performed in 3 cases and 4 cases, respectively. In the other 8 cases the wound was healed at 8 weeks postoperatively. Infection reoccurred in 3 cases during the follow-up. The experimental group had significantly fewer VSD dressing renewals, shorter time needed until the wound was ready for surgery, shorter duration of antibiotic administration, faster wound healing, and shorter hospital stay than the control group (p<0.01).
Conclusions
The combination of VSD and antibiotic bone cement might be a better method for treatment of soft tissue defects and infection.
MeSH Keywords: Antibiotics, Antineoplastic; Soft Tissue Infections; Vacuum
Background
Soft tissue infection often occurs after injuries, which, if not treated appropriately, could slow wound healing and further deteriorate the patient’s condition [1–3]. Thus, prevention and treatment of infection remains a major challenge for clinical surgeons. Vacuum sealing drainage (VSD) has been increasingly recognized as a valid treatment approach in the management of various types of tissue injuries [4,5]. It facilitates complete suction of seepage, necrotic tissues, and pus from the wound area by using a negative-pressure device [4]. Emerging studies have demonstrated that VSD treatment can speed wound healing and reduce incidence of infection [6–8]. However, its inability to kill microorganisms appears to limit its clinical utility.
Bone cement has been primarily used to anchor artificial joints. Recent evidence indicates that if antibiotic is added to bone cement, infected joint arthroplasties can be prevented and treated [9,10]. It has been suggested that prophylactic use of antibiotic-impregnated bone cement decreases the deep infection rate in total joint arthroplasty [11,12]. These findings suggest that antibiotic-impregnated bone cement may be a promising therapy for prevention of soft tissue infections. To the best of our knowledge, the effect of antibiotic-impregnated bone cement on soft tissue defects and infection has not been reported before.
We conducted a prospective randomized study to compare the combination of VSD therapy and antibiotic-impregnated bone cement versus the single VSD therapy for the therapeutic effect in patients with soft tissue defects and infection. To the best of our knowledge, this is the first study to test whether open flesh wounds benefit from the combined treatment of VSD therapy and antibiotic-impregnated bone cement. The treatment appeared to speed wound healing and prevent infection. The study might shed new light on clinical treatment of soft tissue defects and infection.
Material and Methods
General data
This prospective randomized non-blinded study enrolled 46 cases with soft tissue defects and infection in our hospital between January 2010 and May 2014. The study included 24 males and 22 females, who were aged 35–72 years with a mean age of 53 years. Their wound causes were: diabetic foot in 16 cases, postoperative infection following fracture internal fixation in 6 cases, sacral decubitus in 10 cases, and post-traumatic limb infection in 14 cases. The wound area ranged from 2×4 cm to 4×5 cm.
These patients were randomly divided into 2 groups by random numbers generated by computer: the experimental group was treated with VSD combined with antibiotic bone cement, while the control group received VSD treatment only. In order to avoid bias due to different injury causes, the cases with the same injury cause and similar size of tissue defect were randomly divided into 2 groups. Therefore, both groups (n=23) included 8 cases with diabetic foot, 3 cases with postoperative infection following fracture internal fixation, 5 cases with sacral decubitus, and 7 cases with post-traumatic limb infection. The study was approved by the Ethic Committee of the First Hospital of Harbin. Informed consent was obtained from each enrolled patient before the study.
Bacterial culture results and Antibiotics selection
Bacterial culture results were Pseudomonas aeruginosa-positive in 13 cases (sensitive to cefoperazone plus sulbactam), Escherichia coli-positive in 6 cases (sensitive to gentamicin), and Staphylococcus aureus-positive in 27 cases (sensitive to vancomycin) (Table 1). Antibiotics were selected based on the results of bacterial culture and drug sensitivity test. The following antibiotic-laden polymethyl-methacrylate bone cements (Heraeus, Beijing Landmover Medical Company, Beijing, China.) were used in the study based on our previous experiences: vancomycin bone cement (15% vancomycin), cefoperazone bone cement (10% cefoperazone), and gentamicin bone cement (1.6% gentamicin).
Table 1.
Bacterial culture results of all patients in two groups.
| Bacterial culture | Experimental group (n=23) | Control group (n=23) |
|---|---|---|
| Pseudomonas aeruginosa | 6 | 7 |
| Escherichia coli | 3 | 3 |
| Staphylococcus aureus | 14 | 13 |
Other materials used in our study were: VSD sponge, seaweed salt hydration alcohol polyethylene foam with pore diameter of 0.2–1.0 mm, and built-in multi-side holes and drainage pipes were purchased from Beijing Hongren Ningrui Technological Company (Beijing, China); semipermeable membranes comprised of polyurethane and acrylic acid were provided by Beijing Hongren Ningrui Technological Company (Beijing, China).
Surgical technique
The wound of patients in both groups was debrided thoroughly according to conventional procedures [8]. The VSD dressing was tailored according to the size of the wound. After thorough debridement, the wound was veiled by the tailored VSD sponge sutured with surrounding natural skin in the control group. In case of a deep wound, VSD dressing was used to fill the wound cavity, leaving no dead space. It was appropriate that the VSD dressing was attached within 2 cm of the drainage tube fringe. When treating large wounds, drainage tubes in cascading series were required. The drainage tubes should be positioned appropriately for the convenience of drainage tube sealing.
Then, the skin around the wound was cleaned with 75% alcohol and a semipermeable membrane was used to seal the wound and the VSD dressing. The membrane should extend 2 cm from the edge of the wound. Thus, the open wound was closed. All the drainage tubes were connected to a 3-way stopcock connected to a negative-pressure device (Beijing Hongren Ningrui Technological Company, Beijing, China). The negative pressure was sustained at between 125 mmHg and 450 mmHg. Presence of sunken VSD dressing and absence of fluid accumulation under the membrane was a reflection of effective negative pressure status.
Prior to the VSD treatment, prepared pillar- or pie-shaped antibiotic-laden bone cement was placed in the bone or soft tissue cavities in the experimental group. In case of large cavities, bone cement beads were used. Then, the VSD dressing was applied to veil the wound. Subsequently, the experimental group received the same treatment measures as the control group.
VSD dressing was replaced every 10–14 days. The wounds of patients in both groups were checked regularly. The VSD treatment was performed again until a clean, red, granulating wound bed was achieved (“ready for surgery”) [13]. The time until the wound was ready for surgery was recorded. Then, the wound was sutured directly. If much scar tissue remained and suturing was not suitable, free flap transplantation or skin grafting was chosen. Appropriate free flaps or skin grafts were selected to repair the wound. The flap edge was sutured with surrounding normal skin and veiled by a sponge. The surgery for the 2 groups was performed by the same surgeons.
Evaluation of treatment outcome
Patients in the 2 groups were followed up for 12 months. The following variables of the experimental and control groups were compared to evaluate the outcome of different treatment measures: length of hospital stay, the time needed until the wound was ready for surgery, speed of wound closure, duration of antibiotic administration, and the number of VSD dressing renewals.
Statistical analysis
Statistical analysis was performed using SPSS11.5 software. The results are presented as mean ± standard deviation (SD). Student’s t-test was used to compare the difference of each variable between the 2 groups. P-value <0.05 suggested a significant difference.
Results
Baseline characteristics of patients in two groups
General characteristics of patients in experimental and control groups are summarized in Table 2. There was no significant difference between the 2 groups in gender, age, average area of tissue defect before treatment, or the delay between infection and treatment (p>0.05).
Table 2.
General characteristics of patients in two groups.
| Parameters | Experimental group (n=23) | Control group (n=23) | P-value |
|---|---|---|---|
| Gender (male/female) | 12/11 | 11/12 | >0.05 |
| Age(year) | 53±14.5 | 51±15.2 | >0.05 |
| Area of tissue defect before treatment (cm2) | 14±1.5 | 12±1.35 | >0.05 |
| Delay between infection and treatment (day) | 38±5.6 | 40±3.1 | >0.05 |
P-value >0.05, there was no significant difference in each parameter between treatment and control groups.
Treatment outcome and follow-up
In the experimental group, the wound was healed in 23 cases within 4 weeks postoperatively, of which direct suture was performed in 12 cases, and additional free flap transplantation or skin grafting was performed in 6 cases and 5 cases, respectively. Notably, VSD treatment was performed only once in 6 cases in the experimental group and the drainage tube remained unobstructed for 14 days. The patients were followed up for 12 months postoperatively. No recurrence of infection was reported in any cases in the experimental group postoperatively. Function of the injured limbs of the patients and their vocational abilities recovered well (Typical cases: Figures 1, 2).
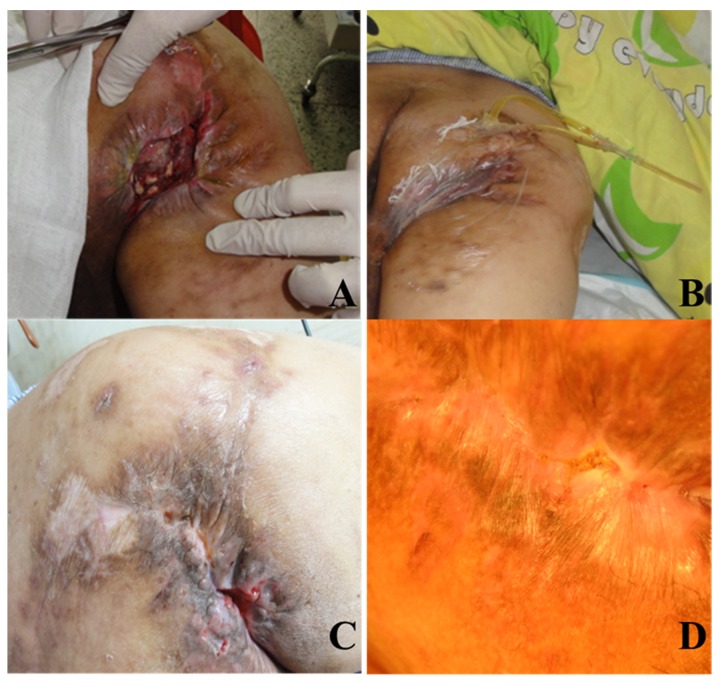
Figure 1.
Typical case 1 in experimental group: male, 53 years old, ischial tuberosity bedsores for 28 years. (A) His wound is surrounded by scar tissue, and antibiotic bone cement beads are placed in the wound cavity; (B) VSD dressing treatment; (C) His wound is almost healed; (D) His wound is completely healed.
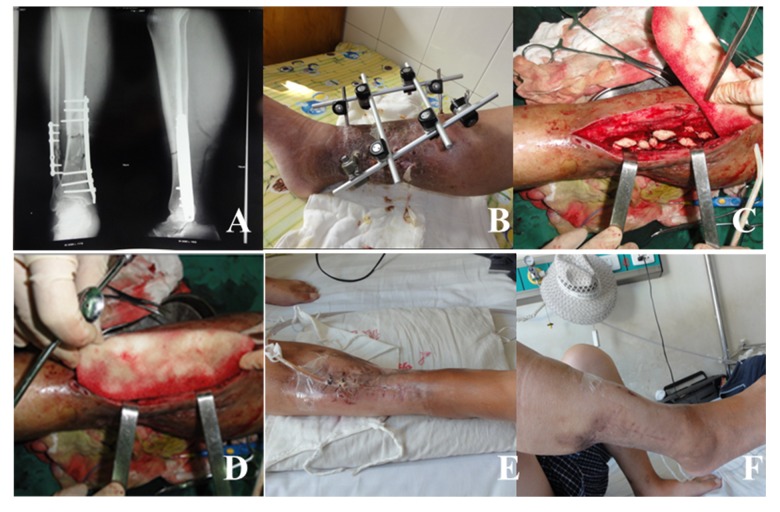
Figure 2.
Typical case 2 in experimental group: male, 54 years old, postoperative infection following tibia and fibula fracture internal fixation for a year. (A, B) Tibial plate is removed, and his fracture is fixed with outer frame. Many sinus tracts develop in his calf. (C) The outer frame is removed, followed by debridement and subsequent placement of antibiotic bone cement beads. (D) VSD dressing treatment. (E) His wound is almost healed, and sinus tracts disappear. (F) His wound is completely closed.
For the control group (n=23), the wound was healed in 15 cases within 6 weeks postoperatively. Among the 15 cases, the wound was directly sutured in 8 cases, and additional free flap transplantation or skin grafting was performed in 3 cases and 4 cases, respectively. The other 8 cases were healed within 8 weeks postoperatively. The patients in the control group were also followed up for 12 months. The wound was ruptured in 3 cases within 2 months following VSD treatment, and these were re-admitted to the hospital.
The experimental group was compared with the control group for the number of VSD dressing renewals (times), the time needed until the wound was ready for surgery (days), the speed of wound closure (cm2/week), the duration of antibiotic administration (days), and the length of hospital stay (days) (Table 3). Statistical analyses revealed that the experimental group had significantly fewer VSD renewals, shorter time needed until the wound was ready for surgery, shorter duration of antibiotic administration, faster speed of wound closure, and shorter hospital stay compared with the control group (p<0.01).
Table 3.
Comparison of clinical results between treatment and control groups.
| Parameters | Experimental group (n=23) | Control group (n=23) | P-value |
|---|---|---|---|
| Time needed untile the wound was ready for surgery (day) | 21±3.5 | 35±5.5 | <0.01 |
| Wound closure speed (cm2/week) | 2.8±0.4 | 1.5±0.2 | <0.01 |
| Number of VSD dressing renewals | 2.5±0.5 | 7.0±0.5 | <0.01 |
| Duration of antibiotic administration (day) | 6.5±2.5 | 14.8±3.1 | <0.01 |
| Length of hospital stay (day) | 28.5±6.5 | 49.6±7.0 | <0.01 |
P-value <0.01, there was significant difference in each parameter between treatment and control groups.
Discussion
This study compared the combination of VSD and antibiotic-impregnated bone cement with VSD only in management of soft tissue defects and infection. To the best of our knowledge, this is the first study to evaluate the impact of the combined treatment on soft tissue defects and infection. Our results showed that 23 cases in the experimental group were healed within 4 weeks postoperatively, and no infection recurrence was reported in 1-year follow-up. In contrast, only 15 of the 23 cases in the control group were healed within 6 weeks postoperatively and infection recurrence was reported in 3 cases in the follow-up. We found that the combined treatment might be useful for accelerating wound healing and preventing infection recurrence in patients with soft tissue defects and infection.
VSD is a negative-pressure technique that drains out seepage, pus and necrotic tissues [14]. It has been widely used for treatment of skin defects, soft tissue defects, and complicated wounds [15–17]. However, the VSD sponge could not kill microorganisms by itself. In case of severe infection, large amounts of pus might be produced, which might clog the pores of the VSD and inhibit its normal functioning. Polymethyl methacrylate bone cement has been widely recognized as one of most stable antibiotic carriers [18]. It aids in maintaining long-term and high concentrations of antibiotics for infected wounds [19,20]. Polymethyl-methacrylate bone cement was used in the current study.
Topical antibiotics have been increasingly used for prevention and treatment of various types of infections [21,22]. It has been suggested that perioperative topical prophylaxis is beneficial for several different surgical procedures [23]. Topical use of antibiotics has several potential advantages over systemic administration of antibiotics, such as limiting the possibility of systemic toxicity, sustaining high concentrations of antimicrobials locally, and reducing the potential development of antibiotic resistance [24]. Antibiotic-impregnated cement has been revealed to be similar to systemically administered antibiotics, and is independent and additive in combination with other prophylactic measures [25]. In the present study, the combined use of antibiotic-impregnated cement and VSD achieved better outcome than the VSD treatment only, as evidenced by significantly fewer VSD dress renewals, shorter time needed until the wound was ready for surgery, short duration of antibiotic administration, faster wound healing, shorter hospital stay, and lower incidence of infection recurrence in the experimental group. The better outcome may be explained by strong postoperative local resistance of the antibiotic-impregnated cement to infection because of elution of antibiotics [26]. Notably, the VSD dressing was used only once in 6 cases of the experimental group, and the drainage pipeline remained unobstructed for 14 days. One possible explanation for this is that topical antimicrobials from the antibiotic-impregnated bone cement might kill microorganisms or inhibit their growth, thereby leading to reduced production of secretions. It reveals that use of antibiotic-laden bone cement might a complementary measure to VSD in the prevention and treatment of soft issue defects and infection. Moreover, a sealed, effective and negative pressure status, and unobstructed pipelines are required during the surgery.
Topical application of antibiotics might cause adverse effects such as local hypersensitivity, contact dermatitis reactions, and possible disturbance of the wound-healing process [23,27]. There are also concerns with regard to higher cost of antibiotic-laden bone cement than plain bone cement and possible development of antibiotics resistance [28–32]. Unlike systemic antibiotics, rigorous trials to accurately determine the dose of topical antibiotics are not available, thus the dose of topical antibiotics is difficult to determine [24]. In this study, based on the results of bacterial culture and drug sensitivity test, vancomycin bone cement (15%), cefoperazone bone cement (10%), and gentamicin bone cement (1.6%) were used. The ratios of antibiotics in bone cement were determined based on previous clinical experiences of our surgeons in the present study. Further studies are needed to explore the optimum dose of antibiotics.
This is a preliminary study with some limitations. The small sample size and short-term follow-up limit the power of the study. More studies are required to address these concerns and to validate the findings regarding the 2 treatments.
Conclusions
Compared to application of VSD only, combination of VSD and antibiotic-laden bone cement accelerated wound healing, shortened hospital stay, and decreased infection recurrence in the management of soft tissue defects and infection. This combined therapy might be a better approach to speed wound healing and prevent infection. Further studies are warranted to verify the findings of this study.
Footnotes
Source of support: This study was supported by Harbin Innovative Talent in Science and Technology special funds (Grant No. 2012RFQYS068)
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Lavery LA, Armstrong DG, Wunderlich RP, et al. Risk factors for foot infections in individuals with diabetes. Diabetes Care. 2006;29:1288–93. doi: 10.2337/dc05-2425. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham SC, Napolitano LM. Necrotizing soft tissue infection from decubitus ulcer after spinal cord injury. Spine. 2004;29:E172–74. doi: 10.1097/00007632-200404150-00028. [DOI] [PubMed] [Google Scholar]
- 3.Appelgren P, Farnebo F, Dotevall L, et al. Late-onset posttraumatic skin and soft-tissue infections caused by rapid-growing mycobacteria in tsunami survivors. Clin Infect Dis. 2008;47:e11–16. doi: 10.1086/589300. [DOI] [PubMed] [Google Scholar]
- 4.Qu J, Yan R, Wang L, et al. Free dermatoplasty combined with vacuum sealing drainage for the treatment of large-area soft tissue defects accompanied by bone exposure in the lower leg. Exp Ther Med. 2013;5:1375–80. doi: 10.3892/etm.2013.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin T, GUO W-c, Ling Y, ZHAO S-h. Clinical efficacy of artificial skin combined with vacuum sealing drainage in treating large-area skin defects. Chin J Traumatol. 2010;13:289–92. [PubMed] [Google Scholar]
- 6.Herscovici D, Sanders RW, Scaduto JM, et al. Vacuum-assisted wound closure (VAC therapy) for the management of patients with high-energy soft tissue injuries. J Orthop Trauma. 2003;17:683–88. doi: 10.1097/00005131-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Liu L, Tan G, Luan F, et al. The use of external fixation combined with vacuum sealing drainage to treat open comminuted fractures of tibia in the Wenchuan earthquake. Int Orthop. 2012;36:1441–47. doi: 10.1007/s00264-011-1404-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li R-G, Yu B, Wang G, et al. Sequential therapy of vacuum sealing drainage and free-flap transplantation for children with extensive soft-tissue defects below the knee in the extremities. Injury. 2012;43:822–28. doi: 10.1016/j.injury.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 9.Hanssen AD, Spangehl MJ. Practical applications of antibiotic-loaded bone cement for treatment of infected joint replacements. Clin Orthop Relat Res. 2004;427:79–85. doi: 10.1097/01.blo.0000143806.72379.7d. [DOI] [PubMed] [Google Scholar]
- 10.Hanssen AD, Rand JA, Osmon DR. Treatment of the infected total knee arthroplasty with insertion of another prosthesis: the effect of antibiotic-impregnated bone cement. Clin Orthop Relat Res. 1994;309:44–55. [PubMed] [Google Scholar]
- 11.Wang J, Zhu C, Cheng T, et al. A systematic review and meta-analysis of antibiotic-impregnated bone cement use in primary total hip or knee arthroplasty. PLoS One. 2013;8:e82745. doi: 10.1371/journal.pone.0082745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nowinski RJ, Gillespie RJ, Shishani Y, et al. Antibiotic-loaded bone cement reduces deep infection rates for primary reverse total shoulder arthroplasty: a retrospective, cohort study of 501 shoulders. J Shoulder Elbow Surg. 2012;21:324–28. doi: 10.1016/j.jse.2011.08.072. [DOI] [PubMed] [Google Scholar]
- 13.Mouës CM, Vos MC, van den Bemd GJ, et al. Bacterial load in relation to vacuum-assisted closure wound therapy: a prospective randomized trial. Wound Repair Regen. 2004;12:11–17. doi: 10.1111/j.1067-1927.2004.12105.x. [DOI] [PubMed] [Google Scholar]
- 14.Fleischmann W, Strecker W, Bombelli M, Kinzl L. [Vacuum sealing as treatment of soft tissue damage in open fractures]. Der Unfallchirurg. 1993;96:488–92. [in German] [PubMed] [Google Scholar]
- 15.Yang Y-H, Jeng S-F, Hsieh C-H, et al. Vacuum-assisted closure for complicated wounds in head and neck region after reconstruction. J Plast Reconstr Aesthet Surg. 2013;66:e209–e16. doi: 10.1016/j.bjps.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Li RG, Yu B, Gang W, et al. Sequential therapy of vacuum sealing drainage and free-flap transplantation for children with extensive soft-tissue defects below the knee in the extremities. Injury. 2011;43:822–28. doi: 10.1016/j.injury.2011.09.031. [DOI] [PubMed] [Google Scholar]
- 17.Kanakaris NK, Thanasas C, Keramaris N, et al. The efficacy of negative pressure wound therapy in the management of lower extremity trauma: review of clinical evidence. Injury. 2008;38(Suppl 5):S8–S10. S11–S17. doi: 10.1016/j.injury.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 18.Saha S, Pal S. Mechanical properties of bone cement: a review. J Biomed Mater Res. 1984;18:435–62. doi: 10.1002/jbm.820180411. [DOI] [PubMed] [Google Scholar]
- 19.Grimsrud C, Raven R, Fothergill AW, Kim HT. The in vitro elution characteristics of antifungal-loaded PMMA bone cement and calcium sulfate bone substitute. Orthopedics. 2011;34:e378–81. doi: 10.3928/01477447-20110627-05. [DOI] [PubMed] [Google Scholar]
- 20.Curtis JM, Sternhagen V, Batts D. Acute renal failure after placement of tobramycin-impregnated bone cement in an infected total knee arthroplasty. Pharmacotherapy. 2005;25:876–80. doi: 10.1592/phco.2005.25.6.876. [DOI] [PubMed] [Google Scholar]
- 21.Sheikh A, Hurwitz B. Topical antibiotics for acute bacterial conjunctivitis: a systematic review. Br J Gen Pract. 2001;51:473–77. [PMC free article] [PubMed] [Google Scholar]
- 22.Drucker CR. Update on topical antibiotics in dermatology. Dermatol Ther. 2012;25:6–11. doi: 10.1111/j.1529-8019.2012.01493.x. [DOI] [PubMed] [Google Scholar]
- 23.McHugh S, Collins C, Corrigan M, et al. The role of topical antibiotics used as prophylaxis in surgical site infection prevention. J Antimicrob Chemother. 2011;66:693–701. doi: 10.1093/jac/dkr009. [DOI] [PubMed] [Google Scholar]
- 24.Lipsky BA, Hoey C. Topical antimicrobial therapy for treating chronic wounds. Clin Infect Dis. 2009;49:1541–49. doi: 10.1086/644732. [DOI] [PubMed] [Google Scholar]
- 25.Parvizi J, Saleh KJ, Ragland PS, et al. Efficacy of antibiotic-impregnated cement in total hip replacement. Acta Orthop. 2008;79:335–41. doi: 10.1080/17453670710015229. [DOI] [PubMed] [Google Scholar]
- 26.Bourne RB. Prophylactic use of antibiotic bone cement: an emerging standard – in the affirmative1 1No benefits or funds were received in support of this study. J Arthroplasty. 2004;19:69–72. doi: 10.1016/j.arth.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Tejwani NC, Immerman I. Myths and legends in orthopaedic practice: are we all guilty? Clin Orthop Relat Res. 2008;466:2861–72. doi: 10.1007/s11999-008-0458-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joseph TN, Chen AL, Di Cesare PE. Use of antibiotic-impregnated cement in total joint arthroplasty. J Am Acad Orthop Surg. 2003;11:38–47. doi: 10.5435/00124635-200301000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Jiranek WA, Hanssen AD, Greenwald AS. Antibiotic-loaded bone cement for infection prophylaxis in total joint replacement. J Bone Joint Surg. 2006;88:2487–500. doi: 10.2106/JBJS.E.01126. [DOI] [PubMed] [Google Scholar]
- 30.Hanssen AD. Prophylactic use of antibiotic bone cement: an emerging standard – in opposition1 1No benefits or funds were received in support of this study. J Arthroplasty. 2004;19:73–77. doi: 10.1016/j.arth.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 31.Al-Bar MH, Kuperan A, Casiano RR. Should topical antibiotics be routinely used following sinus surgery? Laryngoscope. 2014;124(12):2653–54. doi: 10.1002/lary.24673. [DOI] [PubMed] [Google Scholar]
- 32.Hoover WD, Davis SA, Fleischer AB, Feldman SR. Topical antibiotic monotherapy prescribing practices in acne vulgaris. J Dermatolog Treat. 2014;25:97–99. doi: 10.3109/09546634.2013.852297. [DOI] [PubMed] [Google Scholar]