Abstract
Background
Recent genome-wide association studies have identified rs6983267 polymorphism as a key locus in the 8q24 region associated with multisite cancers. However, the information on its association with thyroid cancer is inconclusive. The aim of this study was to determine whether this locus is a risk factor for susceptibility to thyroid cancer by conducting a meta-analysis.
Material/Methods
Relevant studies were identified by searching PubMed and Embase databases. The pooled odds ratio (OR) and corresponding 95% confidence interval (95% CI) were calculated.
Results
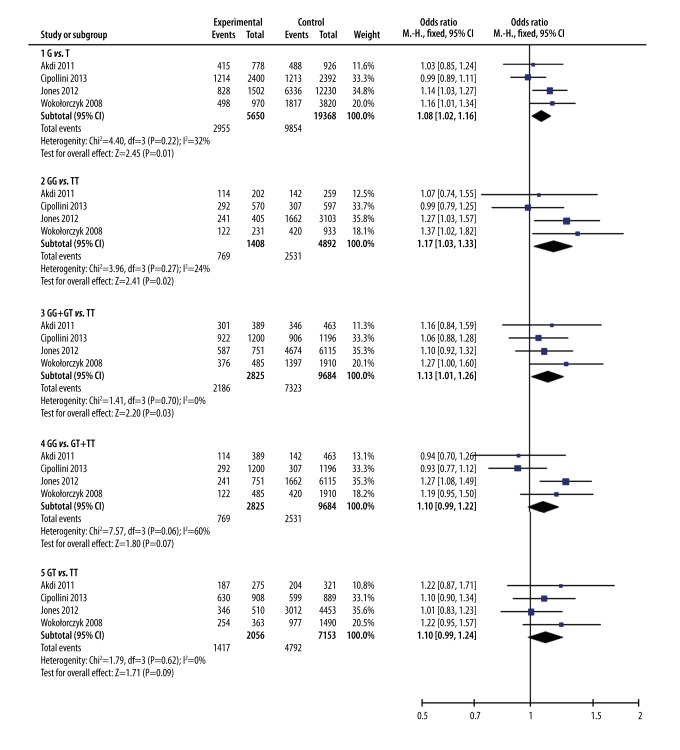
A total of 4 studies including 2825 cases and 9684 controls were enrolled to this meta-analysis. The pooled data showed the G allele of the rs6983267 polymorphism is a risk factor for susceptibility to thyroid cancer (OR=1.08, 95%CI: 1.02–1.16, P=0.01). Significant associations were also found in homozygote comparison (GG vs. TT: OR=1.17, 95%CI: 1.03–1.33, P=0.02) and dominant model (GG+GT vs. TT: OR=1.13, 95%CI: 1.01–1.26, P=0.03). Borderline significant associations in similar directions were found in the recessive model (GG vs. GT+TT: OR=1.10, 95%CI: 0.99–1.22, P=0.07) and heterozygote comparison (GT vs. TT: OR=1.10, 95%CI: 0.99–1.24, P=0.09).
Conclusions
Our meta-analysis shows that the rs6983267 G>T polymorphism might be associated with higher risk of thyroid cancer. Further research with larger sample sizes and full investigation of confounding risk factors is needed to confirm or revise our conclusions.
MeSH Keywords: Genetic Predisposition to Disease, Meta-Analysis, Thyroid Neoplasms
Background
Thyroid cancer (TC) is the most common endocrine malignancy [1] and may be caused by environmental and genetic factors. Family history is known to be a strong risk factor for thyroid cancer. An approximately 3- to 4-fold or higher increased risk for TC was reported among patients who have a first-degree relative with TC [2,3]. It has been acknowledged that single-nucleotide polymorphisms (SNP) could account for part of the hereditary susceptibility to cancer [4].
Recent genome-wide association studies (GWASs) have identified rs6983267 in chromosome 8q24 as a new susceptibility locus for several cancers, including breast, prostate, and colorectal cancer [5–8]. Associations between the rs6983267 polymorphism and TC in different populations have been investigated [9–14], but the published results are inconsistent due to some potential limitations, such as small sample size, different ethnicity, phenotypic heterogeneity, and study design. Therefore, we performed a systematic review and meta-analysis of the published studies to clarify this inconsistency and provide a more comprehensive picture of the relationship between rs6983267 polymorphism and TC susceptibility.
Material and Methods
Literature search strategy
All genetic association studies on rs6983267 polymorphism and TC published before March 1, 2015 were sought by searching databases (PubMed, Embase, ISI Web of Knowledge, Science Direct, and Cochrane databases) using “thyroid carcinoma” or “thyroid cancer” as free-text words combined with “8q24”, “rs6983267”, “polymorphism” or “susceptibility”. Related MeSH terms were used in the PubMed database. Two authors (Li JD and Wang XF) independently scanned the title and abstract of every retrieved record to exclude those that were obviously irrelevant. Full articles of the remaining trials were retrieved for further assessment to identify relevant articles. In addition, we also reviewed all references cited in the identified articles.
Inclusion and exclusion criteria
The inclusion criteria for eligible studies were: (1) cohort or case-control study; (2) evaluated the association of rs6983267 polymorphism and TC risk; (3) included at least 2 comparison groups (TC and control groups); and (4) provided sufficient data to calculate the odds ratio (OR) and corresponding 95% confidence interval (CI). Overlapping studies, case-only studies, and review articles were excluded.
Data extraction and quality assessment
Two investigators (Li JD and Wang XF) independently extracted data from each original article, including first author name, publication year, ethnicity, source of controls, genotyping method, sample size (numbers of cases and controls), and genotype frequency. The Newcastle-Ottawa Scale (NOS) for cohort or case-control studies was used to assess the quality of included studies. Detailed information for quality assessment by NOS was previously described [15]. The full score was 9 stars, with ≥7 stars defined as a high-quality study. Discrepancies were discussed and resolved by consensus.
Statistical analysis
We investigated Hardy-Weinberg equilibrium in the control groups of individual studies by using the goodness-of-fit χ2 statistic with 1 degree of freedom. A P value more than 0.05 was considered to be consistent with Hardy-Weinberg equilibrium.
To evaluate the strength of the association between rs6983267 polymorphism and TC risk, the summarized odds ratio (OR) and its 95% confidence interval (CI) were calculated under various genetic models: the dominant model (GG+GT vs. TT), the recessive model (GG vs. GT+TT), the homozygote comparison (GG vs. TT), the heterozygote comparison (GT vs. TT), and allele contrast (G vs. T) by fixed-effects model [16] or random-effects model [17] based on the heterogeneity. The heterogeneity among individual trials was assessed by the I2 test and Q-test [18]. The P value of Q-test <0.1 indicated evidence of significant statistical heterogeneity. When heterogeneity was present, the random-effects model was used to pool the data; otherwise the fixed-effects model was used. Publication bias was examined using a funnel plot. All P values were 2-sided and the type I error was set at 0.05. All data analyses were performed using RevMan software version 5.02 (Cochrane Collaboration, Oxford, England). This article was performed according to the PRISMA statement for reporting systematic reviews [19].
Results
Study characteristics
The literature review identified 826 reports that met the search criteria. As shown in Figure 1, after further evaluation for eligibility, only 4 studies [10,13,20,21] fulfilled the inclusion criteria, including 2825 cases and 9684 controls. All studies were published in English and they were all case-control study designs. All were conducted in Europe. Table 1 presents the main characteristics of these studies. The genotype distribution in the controls was consistent with Hardy-Weinberg equilibrium in all studies. Three studies used the same method of genotyping for cases and controls, but the cases and controls were genotyped with a different method in another study [21]. All cases were histologically confirmed.
Figure 1.
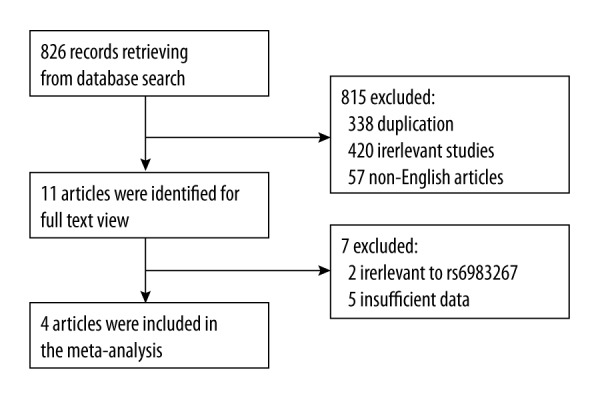
Flow chart of article selection.
Table 1.
Main characteristics of the studies included in this meta-analysis.
| Study | Year | Country | Sample size (case/control) | Genotype frequency (case/control) | Genotyping method | Quality score (★) | ||
|---|---|---|---|---|---|---|---|---|
| GG | GT | TT | ||||||
| Cipollini | 2013 | Italy | 1200/1196 | 292/307 | 630/599 | 278/290 | TaqMan | 7 |
| Wokolorczyk | 2008 | Poland | 485/1910 | 122/420 | 254/977 | 109/513 | PCR-RFLP | 9 |
| Akdi | 2011 | Spanish | 389/463 | 114/142 | 187/204 | 88/117 | iPLFX | 9 |
| Jones | 2012 | UK | 751/6115 | 241/1662 | 346/3012 | 164/1441 | KAspar; SNP array | 8 |
PCR-RFLP – polymerase chain reaction-restriction fragment length polymorphism assay.
Meta-analysis results
As shown in Figure 2, the G variant of the rs6983267 polymorphism was associated with a higher risk of TC. The pooled analysis of 4 studies showed a significant increase in TC risk in the homozygote comparison (GG vs. TT: OR=1.17, 95%CI: 1.03–1.33, P=0.02), dominant model (GG+GT vs. TT: OR=1.13, 95%CI: 1.01–1.26, P=0.03), and allele contrast (G vs. T: OR=1.08, 95%CI: 1.02–1.16, P=0.01). Borderline-significant associations with similar directions were found in the recessive model (GG vs. GT+TT: OR=1.10, 95%CI: 0.99–1.22, P=0.07) and heterozygote comparison (GT vs. TT: OR=1.10, 95%CI: 0.99–1.24, P=0.09). The heterogeneity was negligible among all the studies in each analysis (P>0.1), except for the recessive model analysis (I2=60%, P=0.06).
Figure 2.
Meta-analysis for the association between rs6983267 polymorphisms and thyroid cancer risk.
Sensitivity analyses and publication bias
Sensitivity analyses were performed by omitting each study one at a time to assess the effect of each individual study on the pooled OR. The results showed that the studies by Jones et al. [21] and Wokolorczyk et al. [13] significantly affected the pooled ORs of homozygote comparison, dominant model, and allele contrast. The symmetrical shape of the funnel plot indicated no publication bias for all the results.
Discussion
The etiology of TC is poorly understood at present. Accumulating evidence suggests that the contribution of genetics to the risk of TC is greater than in most other cancers. The common variation of rs6983267 is associated with the risk of several cancers, including those of prostate, colon, and breast, and was assessed as a TC susceptibility gene for this reason. A significant association between rs6983267 SNP and TC was found in the Polish [13] and United Kingdom [21] populations, but no association was found in Spanish [20], Italian [10], or Japanese [22] populations, perhaps due to small sample sizes and/or alleles distribution differences among different populations. Therefore, it is important to quantitatively evaluate the effect of rs6983267 SNP in different populations and to investigate potential heterogeneity of published data. To the best of our knowledge, this is the first systematic review and meta-analysis that assessed the rs6983267 SNP and susceptibility to TC. The present study involved 4 studies including 2825 cases and 9684 controls. Our findings demonstrated that the rs6983267 polymorphism may be a risk factor for TC.
It should be noted that the results were unstable because confidence intervals of ORs for homozygote comparison, dominant model, and allele contrast throughout were very close to the non-significant range, and 2 studies significantly affected these pooled ORs in sensitivity analyses. In addition, significant heterogeneity among studies was also detected in the recessive model analysis (I2=60%, P=0.06). Both of these can be explained by the different genetic and environmental backgrounds in different populations. The allele G at rs6983267 has a large effect on population prevalence of TC because of its high frequency, varying from 31% in native Hawaiians to 85% in African Americans [23]. Although the risk allele frequency of rs6983267 is approximately 50% (48~53%) in the 4 European populations, the allele distribution was significantly different using the χ2 statistic (P<0.01), which should affect the results. Furthermore, small sample size or prevalence of TC or some environmental factors may have affected the results. When other environmental risk factors prevail, the small-size effect of rs6983267 may be obscured. Therefore, it is not surprising that the associations between rs6983267 genotype and cancer risk were detectable in populations with low incidences of DTC (in Poland and the United Kingdom, the age-standardized rates are 4.1 and 3.8 cases out of 100 000 population, respectively), whereas lack of association was found in Texans and Italians, where the incidence is much higher (10.8 and 13.5, respectively) [10]. To eliminate the effects of potential confounding factors, it might be reasonable to perform subgroup analyses according allele frequency distribution, ethnic background, incidence of TC, and other environmental risk factors. However, because of the limited number of included studies, we could not do this.
The pseudogene of POU5F1 is the nearest gene to rs6983267 located in the 8q24 chromosomal gene-poor region (gene desert). POU5F1 encodes a transcription factor of Octamer-binding transcription factor (OCT4). The Oct4 gene, which is a key regulator of stem cell pluripotency, is specifically expressed in embryonic stem cells but can also be detected in adult stem cells [24]. In addition, recent studies have demonstrated that the Oct4 gene is expressed in several human cancer tissues and derived cell lines, including thyroid cancer [25–27]. Therefore, it is possible to hypothesize that the Oct4 gene might play a critical role in tumorigenesis, although the mechanistic role in cancer is unknown. Tomlinson et al. [28] detected differences in Oct4 gene expression according to rs6983267 status in colorectal cancer, but failed to find a clear association. However, there has no such study on thyroid cancer until now, and this requires further study. The nearest protein-coding gene, MYC, locates ~335 kb downstream from rs6983267. Some studies [29, 30] have suggested that this locus is a long-range cis-regulatory enhancer element for the MYC gene and is involved in regulating MYC expression. However, other studies [28] did not detect an unambiguous correlation between rs6983267 genotype and MYC expression. Therefore, the plausible regulatory role of this locus in MYC gene function needs further study.
Several potential limitations of our meta-analysis should be acknowledged. First, although the funnel plot did not detected significant publication bias, potential publication bias is probably present because we did not include unpublished studies and non-English language published studies in the meta-analysis. Second, the cancer cases in the included studies were not uniformly defined, and the data were not adjusted by other factors, such as age, sex, and lifestyle, because of the lack of detailed individual data. Third, there might be a selection bias because races/cultures of only 4 European regions were considered and genetic differences are likely to exist.
Conclusions
The rs6983267 G>T polymorphism in the 8q24 region might be a risk factor for thyroid cancer. However, the limitations and the small total sample size in our study make it impossible to draw a final conclusion. Therefore, further research with larger sample sizes and full consideration of confounding risk factors is needed to confirm or modify our conclusions. Potential gene-gene association should be included in future studies.
Footnotes
Source of support: Departmental sources
Declaration of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Brindel P, Doyon F, Bourgain C, et al. Family history of thyroid cancer and the risk of differentiated thyroid cancer in French polynesia. Thyroid. 2010;20(4):393–400. doi: 10.1089/thy.2009.0350. [DOI] [PubMed] [Google Scholar]
- 3.Xu L, Li G, Wei Q, et al. Family history of cancer and risk of sporadic differentiated thyroid carcinoma. Cancer. 2012;118(5):1228–35. doi: 10.1002/cncr.26398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adjadj E, Schlumberger M, de Vathaire F. Germ-line DNA polymorphisms and susceptibility to differentiated thyroid cancer. Lancet Oncol. 2009;10(2):181–90. doi: 10.1016/S1470-2045(09)70020-8. [DOI] [PubMed] [Google Scholar]
- 5.Bertucci F, Lagarde A, Ferrari A, et al. 8q24 Cancer risk allele associated with major metastatic risk in inflammatory breast cancer. PLoS One. 2012;7(5):e37943. doi: 10.1371/journal.pone.0037943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M, Zhou Y, Chen P, et al. Genetic variants on chromosome 8q24 and colorectal neoplasia risk: a case-control study in China and a meta-analysis of the published literature. PLoS One. 2011;6(3):e18251. doi: 10.1371/journal.pone.0018251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Troutman SM, Sissung TM, Cropp CD, et al. Racial disparities in the association between variants on 8q24 and prostate cancer: a systematic review and meta-analysis. Oncologist. 2012;17(3):312–20. doi: 10.1634/theoncologist.2011-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang YP, Zhang J, Zhu HY, et al. Common variation rs6983267 at 8q24.1 and risk of colorectal adenoma and cancer: evidence based on 31 studies. Tumour Biol. 2014;35(5):4067–75. doi: 10.1007/s13277-013-1532-2. [DOI] [PubMed] [Google Scholar]
- 9.Brenner AV, Neta G, Sturgis EM, et al. Common single nucleotide polymorphisms in genes related to immune function and risk of papillary thyroid cancer. PLoS One. 2013;8(3):e57243. doi: 10.1371/journal.pone.0057243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cipollini M, Figlioli G, Garritano S, et al. Risk of differentiated thyroid carcinoma and polymorphisms within the susceptibility cancer region 8q24. Cancer Epidemiol Biomarkers Prev. 2013;22(11):2121–25. doi: 10.1158/1055-9965.EPI-13-0790. [DOI] [PubMed] [Google Scholar]
- 11.He H, Nagy R, Liyanarachchi S, et al. A susceptibility locus for papillary thyroid carcinoma on chromosome 8q24. Cancer Res. 2009;69(2):625–31. doi: 10.1158/0008-5472.CAN-08-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neta G, Yu CL, Brenner A, et al. Common genetic variants in the 8q24 region and risk of papillary thyroid cancer. Laryngoscope. 2012;122(5):1040–42. doi: 10.1002/lary.23209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wokolorczyk D, Gliniewicz B, Sikorski A, et al. A range of cancers is associated with the rs6983267 marker on chromosome 8. Cancer Res. 2008;68(23):9982–86. doi: 10.1158/0008-5472.CAN-08-1838. [DOI] [PubMed] [Google Scholar]
- 14.Wokolorczyk D, Gliniewicz B, Stojewski M, et al. The rs1447295 and DG8S737 markers on chromosome 8q24 and cancer risk in the Polish population. Eur J Cancer Prev. 2010;19(2):167–71. doi: 10.1097/CEJ.0b013e32832945c3. [DOI] [PubMed] [Google Scholar]
- 15.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 16.Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med. 1987;6(3):341–50. doi: 10.1002/sim.4780060325. [DOI] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akdi A, Perez G, Pastor S, et al. Common variants of the thyroglobulin gene are associated with differentiated thyroid cancer risk. Thyroid. 2011;21(5):519–25. doi: 10.1089/thy.2010.0384. [DOI] [PubMed] [Google Scholar]
- 21.Jones AM, Howarth KM, Martin L, et al. Thyroid cancer susceptibility polymorphisms: confirmation of loci on chromosomes 9q22 and 14q13, validation of a recessive 8q24 locus and failure to replicate a locus on 5q24. J Med Genet. 2012;49(3):158–63. doi: 10.1136/jmedgenet-2011-100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogounovitch TI, Bychkov A, Takahashi M, et al. The common genetic variant rs944289 on chromosome 14q13.3 associates with risk of both malignant and benign thyroid tumors in the Japanese population. Thyroid. 2015;25(3):333–40. doi: 10.1089/thy.2014.0431. [DOI] [PubMed] [Google Scholar]
- 23.Haiman CA, Le Marchand L, Yamamato J, et al. A common genetic risk factor for colorectal and prostate cancer. Nat Genet. 2007;39(8):954–56. doi: 10.1038/ng2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tai MH, Chang CC, Kiupel M, et al. Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis. 2005;26(2):495–502. doi: 10.1093/carcin/bgh321. [DOI] [PubMed] [Google Scholar]
- 25.Ma R, Minsky N, Morshed SA, Davies TF. Stemness in human thyroid cancers and derived cell lines: the role of asymmetrically dividing cancer stem cells resistant to chemotherapy. J Clin Endocrinol Metab. 2014;99(3):E400–9. doi: 10.1210/jc.2013-3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suo G, Han J, Wang X, et al. Oct4 pseudogenes are transcribed in cancers. Biochem Biophys Res Commun. 2005;337(4):1047–51. doi: 10.1016/j.bbrc.2005.09.157. [DOI] [PubMed] [Google Scholar]
- 27.Visciano C, Liotti F, Prevete N, et al. Mast cells induce epithelial-to-mesenchymal transition and stem cell features in human thyroid cancer cells through an IL-8-Akt-Slug pathway. Oncogene. 2015;34(40):5175–86. doi: 10.1038/onc.2014.441. [DOI] [PubMed] [Google Scholar]
- 28.Tomlinson I, Webb E, Carvajal-Carmona L, et al. A genome-wide association scan of tag SNPs identifies a susceptibility variant for colorectal cancer at 8q24.21. Nat Genet. 2007;39(8):984–88. doi: 10.1038/ng2085. [DOI] [PubMed] [Google Scholar]
- 29.Pomerantz MM, Ahmadiyeh N, Jia L, et al. The 8q24 cancer risk variant rs6983267 shows long-range interaction with MYC in colorectal cancer. Nat Genet. 2009;41(8):882–84. doi: 10.1038/ng.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wasserman NF, Aneas I, Nobrega MA. An 8q24 gene desert variant associated with prostate cancer risk confers differential in vivo activity to a MYC enhancer. Genome Res. 2010;20(9):1191–97. doi: 10.1101/gr.105361.110. [DOI] [PMC free article] [PubMed] [Google Scholar]