Abstract
Background
The present study explored the effects of propofol on hippocampal autophagy and synaptophysin in depression-model rats undergoing electroconvulsive shock (ECS).
Material/Methods
The rat depression model was established by exposing Sprague-Dawley rats to stress for 28 consecutive days. Forty rats were assigned randomly into the depression group (group D; no treatment), the ECS group (group E), the propofol group (group P), and the propofol + ECS group (group PE). Open field tests and sucrose preference tests were applied to evaluate the depression behavior; and Morris water maze tests were used to assess the learning and memory function of the rats. Western blotting was used to detect the expression of Beclin-1 and LC3-II/I; and ELISA was applied to assess the expression of synaptophysin.
Results
Rats in group E and group PE scored higher in the open field and sucrose preference tests compared with those in group D. Furthermore, rats in group E also had a longer escape latency, a shorter space exploration time, and increased expression of Beclin-1, LC3-II/I, and synaptophysin. Compared with group E, rats in group PE possessed a shorter escape latency, a longer space exploration time, reduced expression of Beclin-1, LC3-II/I, and synaptophysin.
Conclusions
Propofol could inhibit excessive ECS-induced autophagy and synaptophysin overexpression in the hippocampus, thus protecting the learning and memory functions in depressed rats after ECS. The inhibitory effects of propofol on the overexpression of synaptophysin may result from its inhibitory effects on the excessive induction of autophagy.
MeSH Keywords: Autophagy, Depression, Electroconvulsive Therapy, Propofol, Synaptophysin
Background
Electroconvulsive therapy (ECT) is the most rapid and effective treatment for depression, particularly when depression is severe and resistant to other forms of treatment [1]. However, ECT also has adverse effects, including the impairment of learning and memory [2,3]. In addition, studies have shown that ECT can increase morphological synaptic growth in the hippocampus and induce changes that resemble functional long-term potentiation (LTP). These changes are considered to be the mechanisms that underlie the antidepressant effects of ECT, but they might also be the pathological basis for learning and memory impairment [4,5]. Recent studies have found that repeated electroconvulsive shock (ECS; simulated ECT in animal models) can cause a significant activation of autophagy in the hippocampus of healthy adult rats [6].
Autophagy is the process in which intracellular materials are degraded via a lysosomal pathway. A moderate level of autophagy plays an important role in mediating cellular stress responses, the resistance to cellular damage, and maintaining intracellular homeostasis [7,8]. Autophagy in neuronal cells can regulate synaptic morphology and the number of synapses, and can also affect nerve conduction and synaptic plasticity [9–11]. However, it is currently unknown whether ECS can activate autophagy in the central nervous system in rat models of depression to contribute to the changes in hippocampal synaptic morphology and function.
Propofol, an intravenous general anesthetic, can mitigate ECT-induced learning and memory impairments [3,12], but the mechanism behind these effects is unclear. Propofol exerts protective effects on a variety of cells, such as myocardial and neuron cells, and is thought to suppress the excessive activation of autophagy that occurs as a stress response in those cells [13–15]. However, it is unclear whether similar effects occur in the hippocampus of depressed rats receiving ECS, and whether this could explain the protective effects of propofol on learning and memory functions.
Synaptophysin (SYP) is a synaptic vesicle glycoprotein that is a marker of the presynaptic membrane and reflects the number of synapses. SYP plays an important role in mediating nerve conduction and synaptic plasticity, and abnormal SYP expression can lead to impaired synaptic plasticity [16,17].
In the current study, we speculated that ECT-induced changes in hippocampal synaptic morphology and function and excessive autophagy activation could lead to learning and memory impairment. Furthermore, we hypothesized that propofol may mitigate the ECT-induced impairments in learning and memory function by suppressing the excessive activation of autophagy. In this study, depressed rats were used as subjects to explore the effects of propofol on the levels of the autophagy markers Beclin-1 and microtubule-associated protein light chain 3-II/I (LC3-II/I), as well as on SYP expression in the ECS-treated rat hippocampus. The mechanisms by which propofol mitigates ECT-induced learning and memory impairment were explored in terms of the regulation of synaptic growth by autophagy.
Material and Methods
Animals and protocol
The study was approved by the Animal Ethics Committee at Chongqing Medical University, and complied with international guidelines for the care and use of laboratory animals. Clean healthy adult male Sprague-Dawley rats (200–250 g; 2–3 months old) were provided by the Laboratory Animal Center at Chongqing Medical University. Rats were acclimatized to a standard laboratory environment (ad libitum water and food intake, 12 hour/12 hour light-dark cycle, temperature of 22±2ºC) for seven days. Sucrose preference and open field tests were conducted during days (D) 8–10. A method based on chronic unpredictable mild stress (CUMS) was used from D11 for 28 consecutive days to establish a rat model of depression [18]. The sucrose preference and open field tests were conducted again on D39–41. After successfully establishing the model of depression, 40 rats undertook the Morris water maze test to evaluate the learning and memory function on six consecutive days (on D42–47). Rats were randomly divided into four groups (n=10/group): the model group (group D), the ECS group (group E), the propofol group (group P), and the propofol + ECS group (group PE). The rats in group PE received 80 mg/kg of propofol (10 mg/mL; batch number KB895; AstraZeneca, Italy) by intraperitoneal injection. ECS was then performed when the righting reflex and corneal reflex had disappeared. Rats in group E were administered saline (8 mL/kg) by intraperitoneal injection followed by ECS treatment. Animals in groups D and P were injected intraperitoneally with saline or propofol, and the received sham ECS treatments. The above interventions were conducted daily for seven consecutive days. After the interventions were completed, the rats again underwent sucrose preference, open field, and Morris water maze tests. The rats were then sacrificed and specimens collected for the measurements described in the next section. A detailed flowchart is shown in Figure 1.
Figure 1.
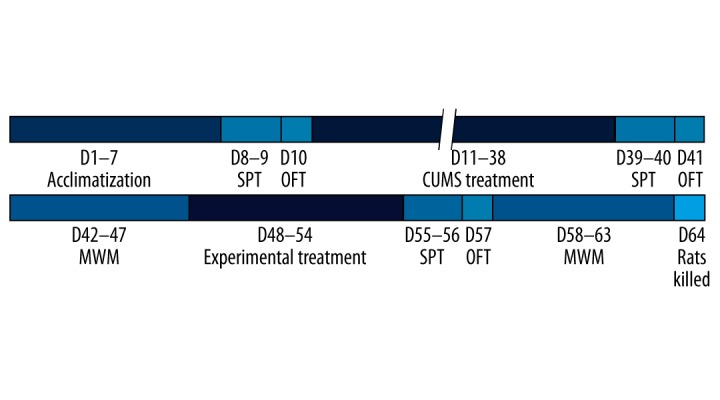
Schematic overview of the experimental protocol. SPT – sucrose preference test; OFT – open field test; CUMS – chronic unpredictable mild stress; MWM – Morris water maze.
CUMS method
Rats were subjected randomly to 10 mild stressors: (1) isolation (one rat per cage), (2) swimming in ice water at 4ºC for 5 minutes, (3) swimming in warm water at 45ºC for 5 minutes, (4) cages tilted to 45° for 24 hours, (5) cages shaken horizontally once per second for 15 minutes, (6) food deprivation for 24 hours, (7) water deprivation for 24 hours, (8) tail pinch for one minute, (9) soiled litter for 24 hours, and (10) exposure to a reversed light/dark cycle. All rats were exposed randomly to one stressor every day for 28 days, and the same type of stressor was not applied on consecutive days.
ECS
Rats were treated with 80 mg/kg of propofol (or 8 mL/kg saline) via intraperitoneal injection. When the righting reflex and corneal reflex had disappeared ECS was performed using a modified ECT system (Niviqure ECT System, Niviqure Meditech Pvt. Ltd., India) using a biphasic square wave (frequency 125 Hz, pulse amplitude 0.8 A, pulse width 1.5 ms). The appearance of a tonic-clonic seizure was used as the criterion for success. For the sham ECS treatment the ears of the rats were clamped with electrodes, but no power was applied. During anesthesia and ECS treatment the rats inhaled 50% oxygen, and their oxygen saturation (SpO2) was monitored.
Sucrose preference test
A decrease in sucrose preference reflects anhedonia, which is the core symptom of depression [19]. Rats were familiarized with the sucrose solution by placing two bottles of 1% (w/v) sucrose solution in each cage for 24 hours. For the test, two bottles, one containing 1% sucrose solution and the other containing water, were placed in each cage after a 23-hour period of food and water deprivation. To avoid measurement errors caused by position preferences the locations of the bottles containing water and sucrose solution were swapped after 30 minutes and the test continued for another 30 minutes. The volume of water and sucrose solution consumed by each rat was measured, and the percentage of sucrose preference was calculated (the percentage of sucrose preference = the volume of consumed sucrose solution/[the volume of consumed sucrose solution sugar + the volume of consumed water] ×100%).
Open field test
Open field tests were used to evaluate the locomotor and exploratory activities of rats in a novel environment. An open field box (100×100×50 cm) was made from black plexiglass. Rats were placed randomly in the center of the open field box, and their activity was observed for three minutes using a camera and a video computer-tracking system (ZH-ZFT; Zhenghua Biological Instrument Equipment Co., Huaibei, China), which were installed one meter above the center of the open field box. The total distance traveled (cm) and the number of times each rat reared (raised their front legs and stood on their hind legs) was observed. After each rat had performed the test, the open field box was swabbed thoroughly with alcohol to avoid any interference with tests involving subsequent rats.
Morris water maze
The spatial learning and memory function of rats were assessed using the Morris water maze. The test lasted for six days: the first five days consisted of familiarization (D1) and acquisition (D2–5), and the probe test was performed on D6. This test was carried out to determine the rats’ positioning and navigational abilities for the first five days of the experiment. The learning ability was defined as the mean escape latency from D3–D5. The swimming time of rats in the southeast quadrant (the space exploration time) was recorded to reflect memory function. Specifically, a platform was placed in the center of the southeast quadrant, and was submerged 1.5 cm under the surface of the water. Rats were placed randomly into the water facing away from the platform from starting points in the east, south, west, and north quadrants. The escape latency was defined as the time taken for the rats to navigate from the point of origin to the platform. If a rat could not find the platform within 60 seconds it was gently guided to the platform and the escape latency was recorded as 60 seconds. After the rat reached the platform it was allowed to stay there for 15 seconds before the next test was performed. The final score was the mean escape latency from D3–5.
On D6, a space exploration experiment was conducted. The platform was removed and rats were placed the water in the quadrant opposite the one where the platform was originally placed (northwest quadrant). The swimming time of rats in the southeast quadrant (the space exploration time) within a 60-second period was recorded.
Tissue preparation, western blotting, and ELISA
Two days after the last Morris water maze test the rats were anesthetized using 5% pentobarbital (50 mg/kg; intraperitoneal injection). They were then decapitated and their whole brains were removed. The bilateral hippocampal tissues were isolated carefully on ice and stored at −80ºC for future use.
The hippocampal tissue was weighed and placed into a homogenizer. The resulting lysates were centrifuged at 12,000 rpm for 15 minutes at 4ºC, and the supernatants were collected. A BCA protein assay kit (Pierce Biotechnology, USA) was used to determine the total protein concentration. Total proteins (50 μg) were denatured by boiling and separated using SDS-PAGE. After electrophoresis, the proteins were transferred to polyvinylidene fluoride membranes, which were then blocked in 5% nonfat milk overnight at 4ºC. The membranes were incubated at room temperature for 2 hours with rabbit anti-rat Beclin-1 (1:1000; 3738, Cell Signaling Technology, USA), rabbit anti-rat LC3-II/I (1:1000; 4108, Cell Signaling Technology), and rabbit anti-rat polyclonal glyceraldehyde 3-phosphate dehydrogenase (GAPDH; CWBIO, China) antibodies, and washed with Tris-buffered saline (TBS) containing 0.3% Tween-20. Subsequently the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Zhongshan-Golden Bridge, Beijing, China) at room temperature for one hour. The membranes were washed using TBS, and the immunoreactive protein bands were visualized using ECL reagent (Pierce). The optical density of the protein bands was analyzed semi-quantitatively using Image-ProPlus 6.0 software (Media Cybernetics, USA). GAPDH was used as a loading control for protein normalization.
Aliquots of the hippocampal supernatants obtained from centrifugation were analyzed using a synaptophysin enzyme-linked immunosorbent assay (ELISA) kit (BH4726, Hengfei Biotechnology, China) according to the manufacturer’s instructions. Tissue samples or synaptophysin standards (100 μL volume) were added to each well of a 96-well plate and incubated overnight at 4ºC. After the plate was washed four times, 100 μL of biotin-labeled mouse anti-synaptophysin monoclonal antibody (1: 1000) was added to each well, and the plate was incubated at 37ºC for 60 minutes. After an additional three washes, 100 μL of horseradish peroxidase conjugated working solution (1:1000) was added to each well, and the plate was again incubated at 37ºC for 30 minutes. The plate was washed five times, and 90 μL of tetramethylbenzidine substrate solution was added to each well. After incubation at 37ºC for 15 minutes 100 μL of stop solution was added, and the optical density (OD) of each well was measured immediately at a wavelength of 450 nm. The synaptophysin concentration of each sample was determined using a standard curve.
Statistical analysis
All measurement data are expressed as means ±standard deviations (M±SD), and statistical analyses were performed using SPSS 17.0 (Chicago, USA). All data were tested for homogeneity of variance using Levene’s test. The differences in behavioral data were analyzed using repeated-measures analysis of variance (ANOVA), and the other data were analyzed one-way ANOVA. Comparisons among groups were analyzed using Student-Newman-Keuls (SNK)-q tests. For data with heterogeneity of variance, comparisons were made using Kruskal–Wallis H tests. Values of p<0.05 were considered to be statistically significant.
Results
Depressive behavior
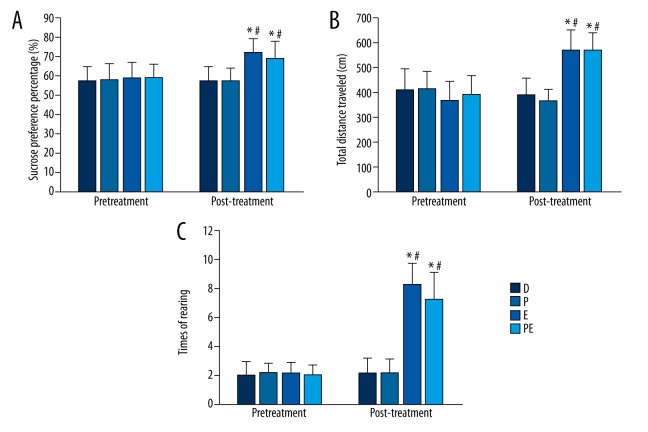
Before ECS treatment there was no significant differences in the percentage of sucrose preference, the total distance traveled, and the frequency of rearing among groups (the percentage of sucrose preference, p=0.976; total distance traveled, p=0.436; frequency of rearing, p=0.864). After ECS treatment the percentage of sucrose preference, the total distance traveled, and the frequency of rearing increased in groups E and PE compared with group D (the percentage of sucrose preference, p<0.001 and p=0.007; total distance traveled, p<0.001 and p<0.001; frequency of rearing p<0.001 and p<0.001, respectively). However, there were no statistically significant differences between groups D and P or groups E and PE (the percentage of sucrose preference, p=0.536 and p=0.711; total distance traveled, p=0.671 and p=0.873; frequency of rearing p=0.778 and p=0.519, respectively). The data are shown in Figure 2A–2C.
Figure 2.
The effects of ECS on the behavioral performance of animals according to the sucrose preference test and open field test. (A) ECS treatment increased the percentage of sucrose preference in groups E and PE. (B) ECS treatment increased the total distance traveled in groups E and PE. (C) ECS treatment increased the incidence of rearing in groups E and PE. Results are expressed as means ±SDs. * p<0.05 vs. group D. # p<0.05 vs. group P. & p<0.05 vs. group E. Significant differences are reported for post-treatment comparisons only.
Morris water maze
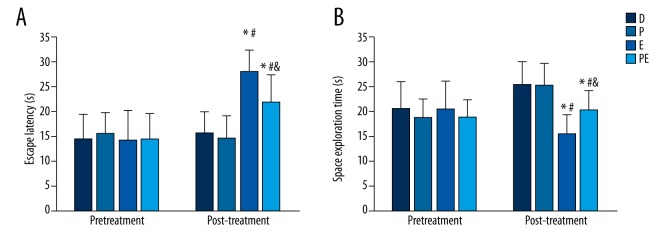
There were no significant differences in escape latency and space exploration times among groups before ECS treatment (p=0.915 and p=0.715, respectively). After ECS treatment the escape latency increased in groups E and PE compared with group D (p<0.001 and p=0.013, respectively), whereas the space exploration time of groups E and PE decreased (p<0.001 and p=0.032, respectively). The escape latency of rats in group PE decreased compared with those in group E (p=0.026), whereas the space exploration time increased (p=0.038). There were no statistically significant differences between groups P and D (p=0.759 and p=0.635, respectively). The data are shown in Figure 3A, 3B.
Figure 3.
Comparisons of rat learning and memory among groups based on the Morris water maze task. (A) Treatment with ECS alone increased the escape latency in group E, but decreased the latency in group PE. (B) ECS only treatment decreased and increased the space exploration time (indicating memory) in groups E and PE, respectively. Results are expressed as mean ±SD. * p<0.05 vs. group D. # p<0.05 vs. group P. $ p<0.05 vs. group E. Significant differences are reported for post-treatment comparisons only.
Expression of the autophagy markers Beclin-1 and LC3-II/I
The expression of autophagy markers Beclin-1 and LC3-II/I increased in group E compared with group D (p<0.001 and p<0.001, p=0.759 and p=0.635, respectively). There were no statistically significant differences in the expression of Beclin-1 among groups D, P, and PE (group D vs. group P, p=0.759; group D vs. group PE, p=0.771; group P vs. group PE, p=0.261). No statistically significant difference was found in the expression of LC3-II/I between groups D and P (p=0.705). The expression level of LC3-II/I in group PE was lower than that of group E (p=0.008), and higher than those of groups D and P (p<0.001 and p<0.001, respectively). The data are shown in Figure 4.
Figure 4.
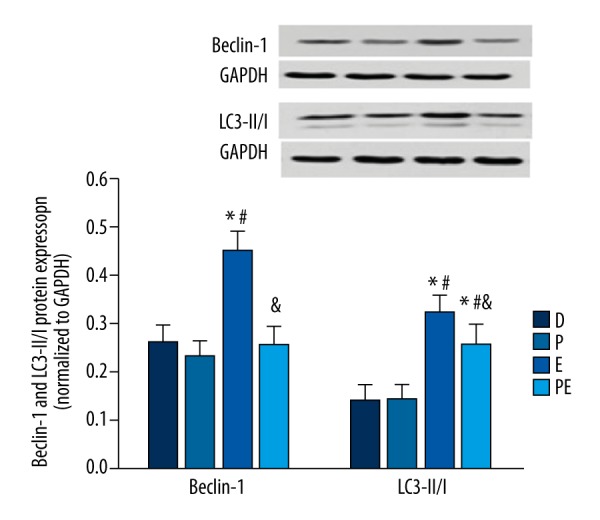
The effects of propofol on the hippocampal expression of Beclin 1 and LC3-II/I in ECS-treated depressed rats. The expression of Beclin 1 and LC3-II/I was increased in group E but decreased in group PE. The results are expressed as means ± SDs. * p<0.05 vs. group D. # p<0.05 vs. group P. $ p<0.05 vs. group E.
Expression of synaptophysin
The expression of SYP was increased in group E compared with group D (p<0.001). However, the expression of SYP was significantly lower in group PE than in group E (p<0.001), but higher than in groups D and P (p<0.001). There was no statistically significant difference in the expression of SYP between groups D and P (p=0.419). The data are shown in Figure 5.
Figure 5.
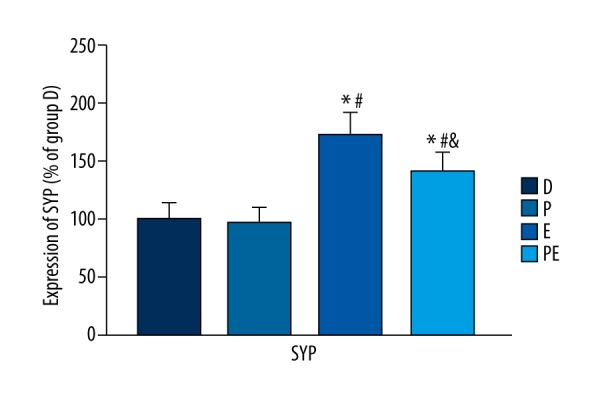
The effects of propofol on the hippocampal expression of synaptophysin in ECS-treated depressed rats. The expression of synaptophysin increased in group E but decreased in group PE. The results are expressed as means ±SDs. * p<0.05 vs. group D. # p<0.05 vs. group P. $ p<0.05 vs. group E.
Discussion
The current study demonstrated that ECS could cause learning and memory impairment in depressed rats, and also improve depressive behavior. ECS activated autophagy in the hippocampus of depressed rats and also upregulated the expression of SYP. Propofol alleviated ECS-induced learning and memory impairment in rats, inhibited the activation of ECS-induced autophagy in the hippocampus, and modulated the expression of SYP in rats that received ECS. Propofol had no significant effects in sham-treated rats.
Beclin-1 is a key molecule during the initiation of autophagy, and it is considered to be the “gatekeeper” of autophagy [20]. LC3-II/I is a specific marker for autophagy [21,22]. LC3-I is normally present in the cytoplasm. During the activation of autophagy LC3-I is converted into LC3-II, which is transferred to the autophagosomal membrane. In our study, after ECS treatment the expression of Beclin-1 and LC3-II/I increased significantly in the hippocampus of depressed rats, suggesting that the level of autophagy increased in the hippocampus after ECS. These results are consistent with the results reported by Otabe et al., who treated adult rats with ECS [6]. The current study employed a rat model of depression that was established using the CUMS method, which further clarified the effects of ECS on the induction of autophagy in an animal model of depression.
The excessive expression of SYP in response to ECS could be the basis of the resulting learning and memory impairment. The protective effects of propofol on learning and memory function could be related to the suppression of ECS-induced SYP. The present study found that the expression of SYP increased significantly in the rat hippocampus after ECS treatment, which is consistent with results of previous studies reporting that ECS enhanced hippocampal synapses [4,5,23].
The number and morphology of synapses are highly regulated. At the physiological level, the number of synapses is the basis of maintaining normal nerve conduction and synaptic plasticity, and excessive synaptic growth can impair synaptic plasticity [24,25]. The damage to the learning and memory function of depressed rats caused by ECS could be related to the excessive upregulation of SYP expression and the failure of homeostatic regulation of hippocampal synapses. In contrast, propofol suppresses ECS-induced SYP overexpression and restores synaptic homeostasis to a certain extent. This might be one of the mechanisms by which propofol mitigates ECS-induced learning and memory impairment.
The inhibitory effects of propofol on the ECS-induced overexpression of SYP may be related to its suppression of autophagy. Studies have shown that autophagy is involved in the regulation of synaptic events. Shen et al. demonstrated that the activation of autophagy indirectly upregulated and activated the JNK-AP-1 signaling pathway by inhibiting highwire, a signaling component of synaptogenesis, and thereby causing synaptic overgrowth at the neuromuscular junction [9,26]. Since autophagy is a highly conserved cellular process, there are a small number of differences in its activity among species, individuals, organs, and tissues. We speculated that ECS-activated autophagy might cause synaptic overgrowth and impair the learning and memory function in depressed rats via similar mechanisms. Propofol could have suppressed synaptic overgrowth by inhibiting the excessive activation of autophagy, thus mitigating ECS-induced learning and memory impairment.
Activation of the glutamatergic signaling pathway by ECS might be one of the major mechanisms of autophagy activation. We demonstrated previously that glutamate levels and the expression of the NMDA receptor NR2B subunit in the rat brain were increased significantly after repeated ECS treatment [27]. Kumari et al. found that glutamate treatment could cause mitochondrial damage in neuronal cells at the cellular level, which was accompanied by the activation of autophagy. Selenium could inhibit glutamate-induced autophagy activation and also prevent the resulting damage [28]. The activation of the δ2 glutamate receptor could significantly upregulate the level of autophagy and cause cellular damage due to glutamate excitotoxicity [29]. Our previous study found that propofol could partially inhibit the upregulation of glutamate expression after ECS [27]. Therefore, the inhibitory effects of propofol on the ECS-induced overexpression of glutamate might also be one of the mechanisms by which it suppresses the excessive activation of autophagy.
This is the first study to explore the effects of ECS on hippocampal autophagy in a rat model of depression. We attempted to explore the mechanisms underlying the ability of propofol to mitigate ECS-induced learning and memory impairment by investigating the regulation of synaptic growth by autophagy. However, we have not yet provided a definitive explanation for the mechanisms by which autophagy regulates SYP expression in this rat model of depression. Therefore, further research is needed.
Conclusions
Propofol can inhibit ECS-induced autophagy and SYP overexpression of SYP in the hippocampus and thereby protect the learning and memory function of depressed rats after ECS. The inhibitory effects of propofol on the overexpression of SYP may result from its inhibitory ability to inhibit excessive autophagy induction.
Footnotes
Source of support: This work was supported by funding from National Natural Science Foundation of China (grant no. 81271501) and the Natural Science Foundation of Chongqing Science & Technology committee (grant no. cstc2013jcyjA0433)
References
- 1.Pagnin D, de Queiroz V, Pini S, Cassano GB. Efficacy of ECT in depression: A meta-analytic review. J ECT. 2004;20(1):13–20. doi: 10.1097/00124509-200403000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Pigot M, Andrade C, Loo C. Pharmacological attenuation of electroconvulsive therapy – induced cognitive deficits: Theoretical background and clinical findings. J ECT. 2008;24(1):57–67. doi: 10.1097/YCT.0b013e3181616c14. [DOI] [PubMed] [Google Scholar]
- 3.Li X, Li W, Luo J, et al. Effects of propofol on the activation of hippocampal CaMKIIalpha in depressed rats receiving electroconvulsive therapy. J ECT. 2012;28(4):242–47. doi: 10.1097/YCT.0b013e31826140c7. [DOI] [PubMed] [Google Scholar]
- 4.Gombos Z, Spiller A, Cottrell GA, et al. Mossy fiber sprouting induced by repeated electroconvulsive shock seizures. Brain Res. 1999;844(1–2):28–33. doi: 10.1016/s0006-8993(99)01924-1. [DOI] [PubMed] [Google Scholar]
- 5.Stewart C, Jeffery K, Reid I. LTP-like synaptic efficacy changes following electroconvulsive stimulation. Neuroreport. 1994;5(9):1041–44. doi: 10.1097/00001756-199405000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Otabe H, Nibuya M, Shimazaki K, et al. Electroconvulsive seizures enhance autophagy signaling in rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2014;50:37–43. doi: 10.1016/j.pnpbp.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Kuma A, Hatano M, Matsui M, et al. The role of autophagy during the early neonatal starvation period. Nature. 2004;432(7020):1032–36. doi: 10.1038/nature03029. [DOI] [PubMed] [Google Scholar]
- 8.Matsui Y, Takagi H, Qu X, et al. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100(6):914–22. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- 9.Shen W, Ganetzky B. Autophagy promotes synapse development in Drosophila. J Cell Biol. 2009;187(1):71–79. doi: 10.1083/jcb.200907109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L, Miao Y, Chen L, et al. The role of elevated autophagy on the synaptic plasticity impairment caused by CdSe/ZnS quantum dots. Biomaterials. 2013;34(38):10172–81. doi: 10.1016/j.biomaterials.2013.09.048. [DOI] [PubMed] [Google Scholar]
- 11.Shehata M, Matsumura H, Okubo-Suzuki R, et al. Neuronal stimulation induces autophagy in hippocampal neurons that is involved in AMPA receptor degradation after chemical long-term depression. J Neurosci. 2012;32(30):10413–22. doi: 10.1523/JNEUROSCI.4533-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butterfield NN, Graf P, Macleod BA, et al. Propofol reduces cognitive impairment after electroconvulsive therapy. J ECT. 2004;20(1):3–9. doi: 10.1097/00124509-200403000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Noh HS, Shin IW, Ha JH, et al. Propofol protects the autophagic cell death induced by the ischemia/reperfusion injury in rats. Mol Cells. 2010;30(5):455–60. doi: 10.1007/s10059-010-0130-z. [DOI] [PubMed] [Google Scholar]
- 14.Cui DR, Wang L, Jiang W, et al. Propofol prevents cerebral ischemia-triggered autophagy activation and cell death in the rat hippocampus through the NF-kappaB/p53 signaling pathway. Neuroscience. 2013;246:117–32. doi: 10.1016/j.neuroscience.2013.04.054. [DOI] [PubMed] [Google Scholar]
- 15.Cui D, Wang L, Qi A, et al. Propofol prevents autophagic cell death following oxygen and glucose deprivation in PC12 cells and cerebral ischemia-reperfusion injury in rats. PLoS One. 2012;7(4):e35324. doi: 10.1371/journal.pone.0035324. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Janz R, Sudhof TC, Hammer RE, et al. Essential roles in synaptic plasticity for synaptogyrin I and synaptophysin I. Neuron. 1999;24(3):687–700. doi: 10.1016/s0896-6273(00)81122-8. [DOI] [PubMed] [Google Scholar]
- 17.Qureshi HY, Han D, MacDonald R, Paudel HK. Overexpression of 14-3-3z promotes tau phosphorylation at Ser262 and accelerates proteosomal degradation of synaptophysin in rat primary hippocampal neurons. PLoS One. 2013;8(12):e84615. doi: 10.1371/journal.pone.0084615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banasr M, Valentine GW, Li XY, et al. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry. 2007;62(5):496–504. doi: 10.1016/j.biopsych.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. Psychopharmacology (Berl) 1997;134(4):319–29. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 20.Sinha S, Colbert CL, Becker N, et al. Molecular basis of the regulation of Beclin 1-dependent autophagy by the gamma-herpesvirus 68 Bcl-2 homolog M11. Autophagy. 2008;4(8):989–97. doi: 10.4161/auto.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabeya Y, Mizushima N, Ueno T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19(21):5720–28. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Urbanek T, Kuczmik W, Basta-Kaim A, Gabryel B. Rapamycin induces of protective autophagy in vascular endothelial cells exposed to oxygen-glucose deprivation. Brain Res. 2014;1553:1–11. doi: 10.1016/j.brainres.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Elfving B, Wegener G. Electroconvulsive seizures stimulate the vegf pathway via mTORC1. Synapse. 2012;66(4):340–45. doi: 10.1002/syn.21518. [DOI] [PubMed] [Google Scholar]
- 24.Mimura K, Kimoto T, Okada M. Synapse efficiency diverges due to synaptic pruning following overgrowth. Phys Rev E Stat Nonlin Soft Matter Phys. 2003;68(3 Pt 1):031910. doi: 10.1103/PhysRevE.68.031910. [DOI] [PubMed] [Google Scholar]
- 25.Shi W, Chen Y, Gan G, et al. Brain tumor regulates neuromuscular synapse growth and endocytosis in Drosophila by suppressing mad expression. J Neurosci. 2013;33(30):12352–63. doi: 10.1523/JNEUROSCI.0386-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.West RJ, Sweeney ST. Oxidative stress and autophagy: Mediators of synapse growth. Autophagy. 2012;8(2):284–85. doi: 10.4161/auto.8.2.18981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong J, Min S, Wei K, et al. Effects of electroconvulsive therapy and propofol on spatial memory and glutamatergic system in hippocampus of depressed rats. J ECT. 2010;26(2):126–30. doi: 10.1097/yct.0b013e3181a9947a. [DOI] [PubMed] [Google Scholar]
- 28.Kumari S, Mehta SL, Li PA. Glutamate induces mitochondrial dynamic imbalance and autophagy activation: Preventive effects of selenium. PLoS One. 2012;7(6):e39382. doi: 10.1371/journal.pone.0039382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yue Z, Horton A, Bravin M, et al. A novel protein complex linking the delta 2 glutamate receptor and autophagy: Implications for neurodegeneration in lurcher mice. Neuron. 2002;35(5):921–33. doi: 10.1016/s0896-6273(02)00861-9. [DOI] [PubMed] [Google Scholar]