Abstract
Background
As the second most common neurodegenerative disorder after Alzheimer’s disease (AD), Parkinson’s disease (PD) principally impacts the motor system in approximately 7 million patients worldwide. The present study aimed to explore the effects of cluster of differentiation (CD200) on adenosine triphosphate-sensitive potassium (KATP) channels and inflammatory response in PD mice.
Material/Methods
We created an in vivo PD model by intraperitoneal injection of 30 mg/kg/day 1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine hydrochloride (MPTP. HCL) for 5 consecutive days, and we created an in vitro PD model by injection of 100 μM 1-methyl-4-phenylpyridinium ion (MPP+) in primary microglia cells. Expression level of CD200/CD200R, inwardly rectifying potassium (Kir6.1/6.2), and sulfonylurea receptor (Sur1/2) were detected by Western blot (WB). Immunohistochemistry (IHC) was utilized to assess CD11b (microglia marker) and tyrosine hydroxylase (TH, a marker reveals dopamine level in neurons) expression levels. An in vitro PD model was applied to detect the influence of CD200 on ATP and inflammatory factors released from microglia. Interferon (IFN)-γ, tumor necrosis factor (TNF)-α, and interleukin (IL)-1β mRNA levels were explored by realtime quantitative polymerase chain reaction (RT-QPCR), and their protein levels were identified by enzyme-linked immunosorbent assay (ELISA).
Results
WB exhibited time-dependent down-regulation of CD200/CD200R in cerebra of PD mice compared to control mice, with Kir 6.1 and SUR 2 expressed mainly in microglia. IHC showed that CD11b reached a peak at the 1st day after MPTP treatment, followed by time-dependent reduction, and TH decreased noticeably after MPTP induction. RT-QPCR demonstrated that compared with controls, IFN-γ, TNF-α, and IL-1β mRNA levels were significantly elevated at MPTP-1d, was reduced at MPTP-3d, and then returned to baseline at MPTP-7d. IHC showed that MPP+ significantly elevated microglia release of ATP. Similar to the effect of pinacidil (K+ channel opener), CD200 remarkably depressed MPP+-induced ATP release. ELISA showed that MPP+ significantly increased IFN-γ, TNF-α, and IL-1β release, and CD200 and pinacidil remarkably suppressed this elevation.
Conclusions
Our results show a novel role of CD200 in promoting opening of the KATP channel, inhibiting microglia activation and release of ATP, as well as inflammatory factors, thus protecting dopaminergic (DA) neurons against damage and alleviating PD.
MeSH Keywords: KATP Channels, Microglia, Parkinson Disease
Background
As the second most common neurodegenerative disorder after AD, PD principally impacts the motor system in approximately seven million patients worldwide [1,2]. PD is more common in the elderly, and its morbidity in the population over 80 years old is 4%, which is much higher than the 1% morbidity rate found in people over age 60 [2]. The mean age of PD onset is around 60 years, but 5–10% of cases are as young as 20–50 years old [3]. There are 8–18 new cases of PD per 100 000 population per year [2]. In 2013, PD caused approximately 103 000 deaths globally, up from 44 000 deaths in 1990, and the mortality rate rose from 1.5 to 1.8 per 100 000 during that period [4].
The primary pathological characteristic of PD is chronic and progressive loss of DA neurons in substantia nigra pars compacta (SNPC), leading to irreversible striatal dopamine loss, which causes the major clinical symptoms of PD: akinesia, muscular rigidity, and resting tremor [5,6]. Although the mechanisms underlying PD pathogenesis are not fully elucidated, studies indicate that inflammation plays a crucial role in the degeneration of nigral DA neurons [7,8].
In 1919, microglia were initially identified as a type of mesodermal cell by Hortega [9]. The role of microglia in the pathology of PD has been investigated [10]. In addition, the activation of microglia results in progressive inflammation, leading to further microglia activation, which brings about a cycle of inflammation [11,12]. The induction of pro-inflammatory factors and cytokines (e.g., IFN-γ, TNF-α, and IL-1β) aggravates nitric oxide (NO)-induced DA neuron injury, as well as accelerating the activation of microglia and their infiltration across the blood–brain barrier, which generates the activation of further/resident microglia and perivascular macrophages [13], resulting in chronic inflammatory response. When this inflammatory response persists, it may cause the progression of PD.
CD 200 (14) is a type-1 membrane glycoprotein encoded by the CD200 gene [15], which contains 2 immunoglobulin domains. CD200 was initially demonstrated to be expressed in the brain, and a subsequent study demonstrated that it could be localized to subsets of neurons, vascular endothelial cells, smooth muscle cells, and B lymphocytes [16]. Studies on related genes in mice and rats suggest that the CD200 gene delivers an inhibitory signal for macrophage lineage in multiple tissues [15]. Fifteen years after the discovery of CD200, the CD200 receptor (CD200R) was identified [17]. However, how CD200 inhibits inflammatory factor release via microglia remains largely elusive.
KATP channels are expressed in diverse tissues throughout the body, including pancreatic β-cells [18] and the brain [19]. KATP channel is a hetero-octamer composed of 4 Kir6 subunits and 4 SUR subunits. The 4 centrally located Kir6 subunits form pores in the KATP channel and regulate K+ efflux, while the 4 peripherally located SUR subunits modulate channel activity, depending on intracellular ATP/ADP level [20,21]. Opening of the KATP channel has been reported to inhibit rotenone-induced neuroinflammation and glia activation [22]. Nevertheless, it is unclear whether CD200 attenuates PD via modulating KATP opening. In the present study, we tested the hypothesis that pretreatment of CD200 attenuated PD, possibly by suppressing glia activation, resulting in reduced release of pro-inflammatory factors and cytokines.
Material and Methods
Animals and agents
SPF C57BL/6 mice within the postnatal (P) period, 3–4 or 8 weeks, were purchased from the Chinese Academy of Science-Shanghai SLRC Experimental Animal Co. Ltd. CD200, MPTP.HCL, MPP+, ATP, and pinacidil (KATP inhibitor) were purchased from Sigma-Aldrich Co. Ltd. ELISA kits of IFN-γ, TNF-α, and IL-1β were purchased from R&D Systems. The ATP release kit was purchased from Beyotime Biotechnology Co., Ltd. Agents of RT-QPCR were purchased from Takaya Biotechnology Co. Ltd. Antibodies were purchased from Santa Cruz Co. Ltd. Cell culture medium and fetal bovine serum (FBS) were purchased from GIBCO.
In vivo and in vitro PD models
We established in vivo and in vitro subacute PD models separately. For the in vivo model, there were 3 mice in each group; 8-week-old mice received intraperitoneal injection of MPTP·HCL at a dosage of 30 mg/kg per day for 5 days, and were killed at day 1, 3, or 7 after the last injection. The controls received an equivalent volume of phosphate buffered saline (PBS). Each animal experiment was approved by the Nanjing Benq Hospital Animal Care and Use Committee.
For the in vitro model, primary cultured microglia cells were administrated different concentrations of MPP+, after which 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay was performed to measure cytotoxicity (data not shown) and ELISA assay was applied to assess cytokine release. Taking the least cell damage and highest cytokine concentration together, we finally adopted 100 μM MPP+ as the experimental concentration.
Primary microglia culture
Briefly, primary culture of mouse brain microglia was prepared as follows: P3–4 C57BL/6 mice were soaked in 75% ethyl alcohol for 5 min, then the whole brains were separated from the skulls in a biosafety cabinet and rinsed in ice-cold Dulbecco’s modified Eagle’s medium (DMEM) 3 times. Meninges and blood vessels were removed carefully under an anatomical microscope, and cerebral cortices were separated and digested into cell suspension with 0.125% trypsin for 5–10 min. Digestion was terminated with FBS and the cell suspension was agitated 12 times to disperse big tissue blocks. After that, 2.5×105/mL cells were filtered and seeded in a 75-cm2 flask pre-coated with Poly-D-Lysine (PDL, Sigma), maintained in DMEM/F-12 and 10% FBS, and cultured in a 37°C incubator containing 5% CO2. After 7 days, microglia cells were separated from astrocytes and oligodendrocytes by shaking for 3 h in an orbital shaker at the speed of 80 rpm/min. When microglia cells were completely suspended, we centrifuged the cell suspension at 1500 r/min for 10 min, reseeded them in flasks pre-coated with PDL, and changed the medium 4 h later to remove non-adherent cells. Trypan blue assay displayed >95% cell survivability.
Western blot
Expression levels of CD200/CD200R in subacute PD mice and Kir6.1/6.2, Sur1/2 in microglia were detected by WB. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as internal control. Briefly, samples were removed, rapidly washed with ice-cold PBS, and homogenized in RIPA lysis buffer (Roche) with a cocktail of protease inhibitors and phosphatase inhibitors included. Sample lysates were separated by SDS-PAGE, electrotransferred onto polyvinylidene fluoride (PVDF, Millipore Corp., Bedford, MA, USA) membranes, blocked in 5% bovine serum albumin (BSA) for 1 h at room temperature, and incubated overnight at 4°C with their respective antibodies. After washing with TBST, membranes were incubated with corresponding HRP-conjugated secondary antibodies for 1 h at room temperature.
Immunohistochemistry
Midbrain slices were prepared from C57BL/6 mice. In brief, mice were deeply anesthetized with 1% chloral hydrate. Perfusion was performed with PBS followed by 4% paraformaldehyde (PFA, Sigma) in 0.1 M PBS at pH 7.4. Then brains were dissected and embedded in OCT. The embedded blocks were sectioned into 20-μm thickness and slices containing substantia nigra were selected. Microglia activation and DA neuron loss at day 1, 3, and 7 were investigated by anti-CD11b antibody and anti-TH antibody, respectively.
The CD200/MPP+ group was treated with recombinant CD200 for 30 min before exposure to MPP+. Negative controls were exposed to DMEM medium, while positive controls were treated with pinacidil. ATP was exogenously added into untreated controls to investigate whether CD200 inhibited ATP-induced microglia activation.
RT-QPCR
RT-QPCR was adopted to determine mRNA levels of IFN-γ, TNF-α, and IL-1β in the midbrain of subacute PD mice. Midbrains of mice were homogenized rapidly in Trizol reagent (Invitrogen Life Technologies). Total RNA was treated by DNAseI (Invitrogen Life Technologies) and reversely transcribed into cDNA according to the manufacturer’s instruction (Takara, Dalian, China). The QPCR reaction system contained 0.5 μl cDNA, 5 μl 2X iQSYBR-green mix, 0.5 μl forward primer, 0.5 μl reverse primer, and 3.5 μl nanopure water. Then we used ABI Prism 7300 (Applied Biosystems, Foster City, CA, USA) and SYBR Green I dye (Biotium, Inc., Hayward, CA). Conditions were as following: 1 initial step of 10 min at 95°C for polymerase activation, 5 s at 95°C for denaturation, 30 s at 60°C for annealing/extension, and 40 cycles for all the primers, followed by a DNA melting curve. The GAPDH gene was used as an endogenous control to normalize differences in each sample. Gene expression amount was calculated as the differences between the cycle threshold (ΔCT) value of target gene and GAPDH. The primer sequences were as follows: IL-1β (forward: 5′-GAGCCCATCCTCTGTGACTCAT-3′, reverse: 5′-AGCCTGTAGTGCAGCTGTCTAATG-3′), TNF-α (forward: 5′GACCCTCACACTCAGATCATCT-T-3′, reverse: 5′-CCACTTGGTGGTTTGCTACGA-3′), IFN-γ (forward: 5′-TCAAGTGGCATAGATGTGGAAAGAA-3′, reverse: 5′-TGGCTCTGCAGGATTTTCATG-3′), GAPDH (forward: 5′-ATGTGTCCGTCGTGGATCTGA-3′, reverse: 5′-TGCCTGCTTCACCACCTTCT-3′).
ELISA assay
ELISA assay was utilized to analyze protein levels of IFN-γ, TNF-α, and IL-1β in microglia cells after exposure to 100 μM MPP+ for 1/3/7/24 h. Briefly, 96-well plates were sensitized by synthetic peptide. Sensitized plates were left in the oven until dry and placed overnight at 4°C. After antigen sensitization, plates were blocked with 2% BSA for 2 h at 37°C and treated successively with samples for 1 h at 37°C. After washing, biotinylated labeled antibodies human-IgG (Sigma, St. Louis, MO) were diluted at 1: 5000 and added to the plate for 1 h at 37°C. Then, we added the streptavidin-peroxidase conjugate diluted at 1: 1000 for 30 min at 37°C. After washing 3 times, substrate 3,39,5,59-tetramethylbenzidine (TMB) in citrate buffer containing hydrogen peroxide was added to the corresponding plates. Reactions were stopped by addition of H2SO4 2N. Optical densities were read at 450 nm in an ELISA reader (BioRad, Hercules, CA).
Statistical analyses
Results are presented as means ± SD (standard deviation). Differences were tested with 2-way analysis of variance (ANOVA) followed by Bonferroni post hoc tests. Significance was determined on a criterion of P< 0.05.
Results
Primary cultured mouse microglia expressed Kir6.1 and Sur2
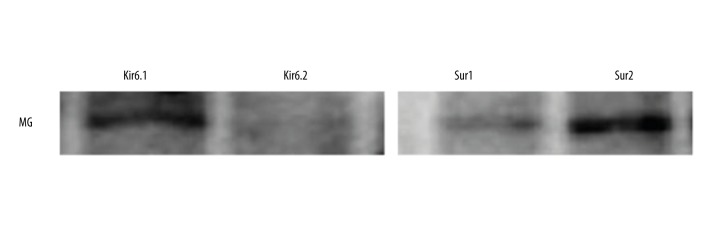
Studies suggest that CD200 gene delivers an inhibitory signal for macrophage lineage (15). Therefore, to identify whether CD200 has the possibility of modulating KATP channel, we first tested whether primary mouse microglia expressed KATP-associated proteins Kir6.1/Kir6.2 and Sur1/2. Primary cultured microglia from mouse brains mainly expressed Kir6.1 and Sur2 (Figure 1).
Figure 1.
Kir6.1 and Sur2 were expressed mainly in microglia.
In MPTP-induced PD mice, CD200/CD200R protein levels were down-regulated time-dependently compared to control mice
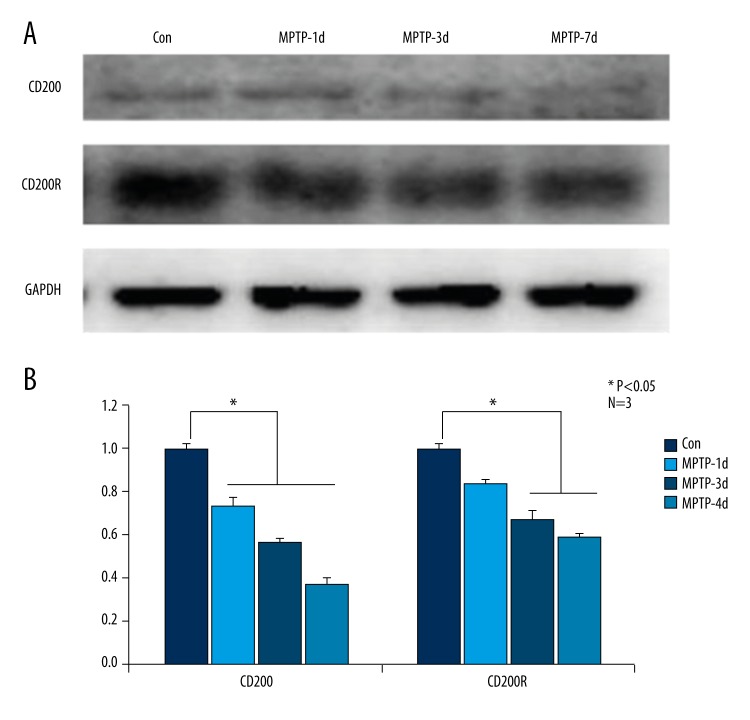
We examined the protein levels of CD200/CD200R in C57BL/6 mouse brains of MPTP-1d, MPTP-3d, MPTP-7d, and control groups by WB. Compared with control group, the expression level of CD200 remarkably decreased time-dependently (Figure 2A). Statistical data shown in Figure 2B were consistent with the result of WB, and with increasing time, the CD200 protein level reached minimum in MPTP-7d group.
Figure 2.
In MPTP-induced PD mice, CD200/CD200R protein levels were down-regulated time-dependently compared to control mice. Compared with the control group, the expression level of CD200 remarkably decreased time-dependently, and CD200R significantly decreased in MPTP-3d and MPTP-7d groups (A). The statistical data shown in (B) are consistent with the results of Western blot. * Manifests P<0.05.
We also tested CD200R expression level. CD200R significantly decreased in MPTP-3d and MPTP-7d groups as compared with the control group (Figure 2A, 2B).
In MPTP-induced PD mice, CD11b was up-regulated and TH was down-regulated compared with control mice
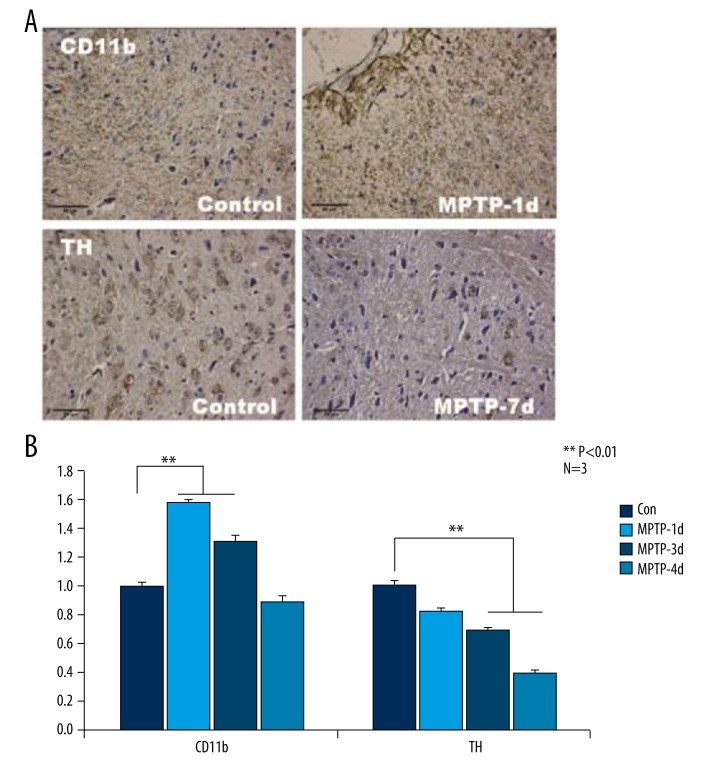
CD11b-positive cells were used to evaluate the activation level of microglia in the midbrain slices of PD mice. As shown by the IHC results Figure 3A, non-activated microglia cell bodies were slender or oval-shaped, branched, and possessed several long processes, which was nearly the same as in the control group. However, the morphology of CD11b-positive microglia in MPTP-1d slices was round, bearing less processes or spreading, consistent with activated microglia morphological characteristics. The statistical results indicated that MPTP administration promoted microglia activation as evidenced by elevated CD11b expression. The intensity of CD11b staining cells reached a peak at day 1 after MPTP treatment, then decreased over time (Figure 3B).
Figure 3.
In MPTP-induced PD mice, CD11b was up-regulated and TH was down-regulated compared with control mice. The intensity of CD11b staining cells in IHC reached a peak at 1 day after MPTP treatment, then it reduced time-dependently. In MPTP-1d and MPTP-3d groups, CD11b was predominantly higher than in the control group (A, B). As shown in (A, B), TH clearly decreased due to MPTP induction from day 1 to day 7 in comparison with the control group. ** Manifests P<0.01.
TH was applied to evaluate the amount of DA-ergic neuron in substantia nigra striatum. TH was clearly decreased by MPTP induction from day 1 to day 7 day in comparison with the control group (Figure 3A, 3B).
In MPTP-induced PD mice, IFN-γ, TNF-α, and IL-1β transcriptional levels were up-regulated in comparison with control mice
We further performed RT-QPCR to investigate mRNA levels of IFN-γ, TNF-α, and IL-1β in PD and control mice. The supreme mRNA expression level significantly increased in MPTP-1d mice, decreased at MPTP-3d, and mainly returned to basal line in the MPTP-7d group, and even in the MPTP-7d group the mRNA levels were still higher than in controls (Figure 4), which was in line with changes in CD11b.
Figure 4.
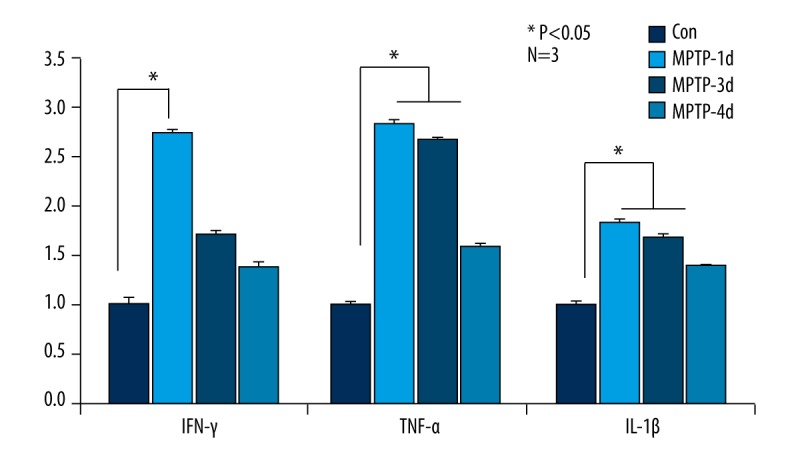
In MPTP-induced PD mice, IFN-γ, TNF-α, and IL-1β transcriptional levels were up-regulated in comparison with control mice. The supreme mRNA expression level significantly increased in MPTP-1d mice, decreased in MPTP-3d mice, and mainly returned to baseline in the MPTP-7d group. * Manifests P<0.05.
CD200 inhibited ATP release from Mpp+-induced microglia
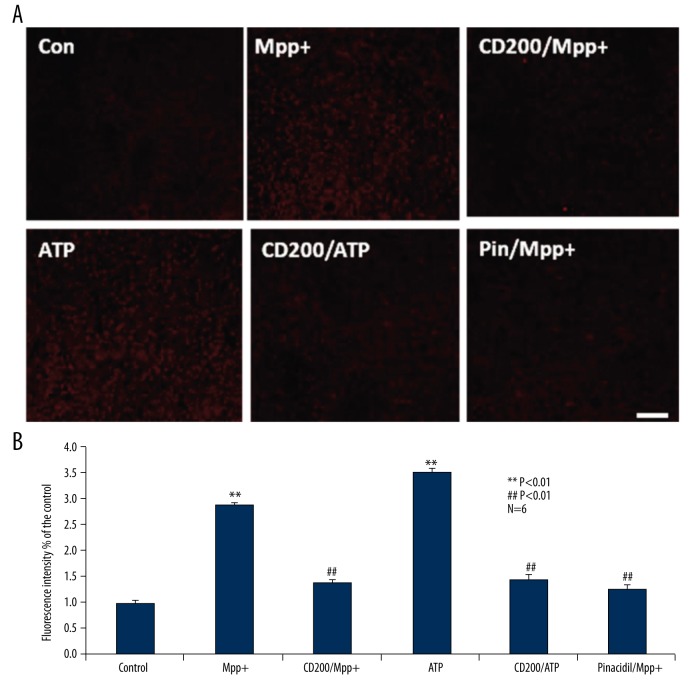
Consequently, we examined the effect of CD200 on ATP release from MPP+-induced microglia via IHC. We randomly divided primary cultured cells into 6 different groups: control, MPP+, CD200/MPP+, ATP, CD200/ATP, and Pin/MPP+ groups. Compared with control group, ATP concentration in MPP+ group was significantly higher, and similar with the effect of pinacidil, CD200 remarkably depressed MPP+-induced ATP release, and CD200 inhibited ATP-induced microglia activation (Figure 5A).
Figure 5.
CD200 inhibited ATP release from Mpp+-induced microglia. MPP+ significantly elevated ATP release from microglia compared with the control group, and, the same as the effect of pinacidil, CD200 remarkably depressed ATP release from microglia induced by MPP+. Moreover, CD200 suppressed ATP-induced microglia activation (A). As shown in (B), statistical data verified the same results as IHC in (A). ** Manifests P<0.01 when compared with control, ## manifests P<0.01 when compared with MPP+ group.
The statistical data verified the same results as IHC (Figure 5B).
CD200 inhibited IFN-γ, TNF-α, and IL-1β release from Mpp+-induced microglia
To further elucidate the influence of CD200 on IFN-γ, TNF-α, and IL-1β release, we conducted ELISA assay in an in vitro PD model. We divided cultured cells into 6 different groups optionally, including control, MPP+, CD200/MPP+, CD200, and Pin/MPP+ groups. Consistent with the results of IHC, MPP+ significantly elevated inflammatory factor release from microglia compared with control group, and CD200 as well as pinacidil remarkably depressed inflammatory factor release induced by MPP+. However, without exposure to MPP+, no significant effect on ATP level was detected in the presence of CD200 (Figure 6).
Figure 6.
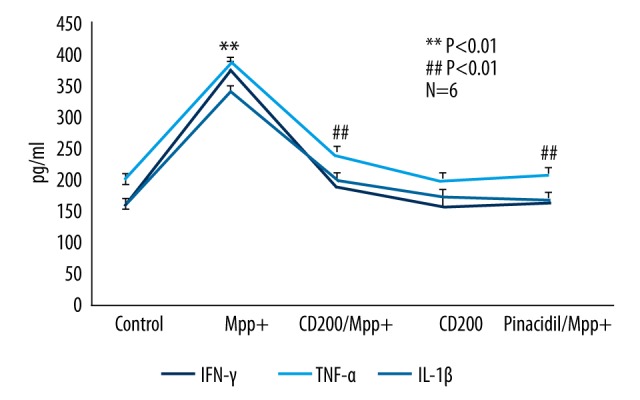
CD200 inhibited IFN-γ, TNF-α and IL-1β release from Mpp+-induced microglia. MPP+ significantly elevated inflammatory factor release from microglia compared with the control group. CD200 as well as pinacidil remarkably depressed inflammatory factor release from microglia induced by MPP+. However, without exposure to MPP+, no significant effect on ATP level was detected in the presence of CD200. ** Manifests P<0.01 when compared with control, ## manifests P<0.01 when compared with MPP+ group.
Discussion
Studies of neuro-inflammatory processes in human brain diseases began over 25 years ago, aiming to discover a regular anti-inflammatory drug to cure AD and PD. However, understanding/treating inflammation in diseased human brains is complex and not easily treatable.
In the current study we utilized CD200 in a PD model and found that MPP+ significantly elevates ATP release, and CD200 as well as pinacidil remarkably depresses MPP+-induced ATP release from microglia cells. Expression levels of inflammatory factors were in accordance with the changes in ATP concentration. Taken together, we elucidate for the first time that CD200 has the ability to attenuate MPP+-induced PD via modulating KATP channel opening and inflammatory factor release. Our study sheds light on identifying new therapeutic targets for treating PD.
Opening of the KATP channel has been reported to inhibit rotenone-induced neuroinflammation and glia activation [20]. Therefore, we first investigated whether the KATP channel was expressed in primary cultured microglia of mouse cerebral cortices. We discovered that Kir6.1 and Sur2 were dominantly expressed in microglia, which provides the possibility that the KATP channel responds to CD200.
Studies suggest that the CD200 gene delivers an inhibitory signal for macrophage lineage in multiple tissues [15]. The neurobiology of CD200/CD200R is relatively unexplored. A seminal series of studies, particularly by Lynch’s group, have elucidated many aspects of the neurobiology of CD200 in rodent disease models [23]. We constructed an MPTP-induced PD model, and discovered that CD200/CD200R was time-dependently down-regulated in mouse cerebral cortices, suggesting that CD200/CD200R indeed participates in the progression of PD.
We further identified the activation level of microglia; the intensity of CD11b showed that in MPTP-induced PD mice, intensity decreased time-dependently. The primary pathological characteristic of PD is chronic and progressive loss of DA neurons, leading to irreversible striatal dopamine loss. Therefore, we evaluated TH, which indicates the dopamine level in neurons; our results showed that dopamine decreased time-dependently as well. Both of them elucidated the involvement of microglia and dopamine loss in PD, and our results were consistent with previous studies [5,6,10].
As acknowledged, microglia activation leads to progressive inflammation, which generates further microglia activation and a cycle of inflammation [11,12]. Inflammation was shown to play a pivotal role in the degeneration of nigral DA neurons [7,8]. The induction of IFN-γ, TNF-α, and IL-1β [13] results in chronic inflammatory response; therefore, we investigated their mRNA levels. The highest mRNA level significantly increased by MPTP-1d, decreased in MPTP-3d mice, and mainly returned to basal line in the MPTP-7d group. Even in the MPTP-7d group, mRNA levels were still higher than in controls, which is in line with the changes in CD11b and previous studies [7,8,11,12].
It remains unclear whether CD200 affects ATP release from microglia. To explore this, we performed IHC in different groups and discovered that in cultured primary microglia, CD200 reduced MPP+-induced ATP release. In paralleled with this, we carried out ELISA to detect inflammatory factor release by microglia; results were consistent with that of ATP release. Taking these 2 lines of evidence together, we conclude that CD200 plays a crucial role in attenuating PD in vitro via promoting KATP opening and inhibiting inflammation.
However, one question remains unanswered: how does CD200 inhibit inflammatory factor release by promoting KATP opening in microglia? The KATP channel is closed at high intracellular ATP level [24] and open at high intracellular ADP level [25], and it is an established drug target [26]. IFN-γ, TNF-α, and IL-1β aggravate NO-induced DA neuron injury and accelerates the activation of microglia [13], resulting in chronic inflammatory response. PI3Kγ/AKT/nNOS/NO/KATP is a recently discovered pathway modulating KATP channel opening in morphine analgesia [27]. Furthermore, morphine was discovered to affect Parkinson’s-related genes PINK1/2 [28]. We postulated that CD200 suppressed NO-induced DA neuron injury via decreasing IFN-γ, TNF-α, and IL-1β release from microglia. This process may depend on NO-induced KATP channel opening, ultimately attenuating PD. We plan further research on this topic in the near future.
Conclusions
The present study shows a novel role of CD200 in promoting the opening of the KATP channel, and inhibiting microglia activation and release of ATP as well as inflammatory factors, thus protecting dopaminergic (DA) neurons against damage and alleviating PD.
Footnotes
Source of support: The present study was supported by grants from the Natural Science Fund Project in Jiangsu Province (No. BK2010115)
Conflict of interest
The authors declare that they have no competing interests.
References
- 1.Yao SC, Hart AD, Terzella MJ. An evidence-based osteopathic approach to Parkinson disease. Osteopathic Family Physician. 2013;5:96–101. [Google Scholar]
- 2.de Lau LM, Breteler MM. Epidemiology of Parkinson’s disease. Lancet Neurol. 2006;5:525–35. doi: 10.1016/S1474-4422(06)70471-9. [DOI] [PubMed] [Google Scholar]
- 3.Samii A, Nutt JG, Ransom BR. Parkinson’s disease. Lancet. 2004;363:1783–93. doi: 10.1016/S0140-6736(04)16305-8. [DOI] [PubMed] [Google Scholar]
- 4.GBD 2013 Mortality and Causes of Death, Collaborators. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;385:117–71. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes AJ, Daniel SE, Kilford L, Leea AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–84. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergman H, Deuschl G. Pathophysiology of Parkinson’s disease: From clinical neurology to basic neuroscience and back. Mov Disord. 2002;17(Suppl 3):28–40. doi: 10.1002/mds.10140. [DOI] [PubMed] [Google Scholar]
- 7.Frank-Cannon TC, Alto LT, McAlpine FE, Tansey MG. Does neuroinflammation fan the flame in neurodegenerative diseases? Mol Neurodegener. 2009;4:47. doi: 10.1186/1750-1326-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGeer PL, McGeer EG. Glial reactions in Parkinson’s disease. Mov Disord. 2008;23:474–83. doi: 10.1002/mds.21751. [DOI] [PubMed] [Google Scholar]
- 9.Del Rio Hortega P. El ‘tercer elemento’ de los centros nerviosos. Poder fagocitario y movilidad de la microglia. Bol Soc Esp Biol Ano. 1919;ix:154–66. [in Spanish] [Google Scholar]
- 10.Teismann P, Schulz JB. Cellular pathology of Parkinson’s disease: Astrocytes, microglia and inflammation. Cell Tissue Res. 2004;318:149–61. doi: 10.1007/s00441-004-0944-0. [DOI] [PubMed] [Google Scholar]
- 11.Depboylu C, Stricker S, Ghobril JP, et al. Brain resident microglia predominate over infiltrating myeloid cells in activation, phagocytosis and interaction with T lymphocytes in the MPTP mouse model of Parkinson disease. Exp Neurol. 2012;238:183–91. doi: 10.1016/j.expneurol.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Dutta G, Barber DS, Zhang P, et al. Involvement of dopaminergic neuronal cystatin C in neuronal injury induced microglial activation and neurotoxicity. J Neurochem. 2012;122:752–63. doi: 10.1111/j.1471-4159.2012.07826.x. [DOI] [PubMed] [Google Scholar]
- 13.Stoll G, Jander S, Schroeter M. Cytokines in CNS disorders: neurotoxicity versus neuroprotection. J Neural Transm Suppl. 2000;59:81–89. doi: 10.1007/978-3-7091-6781-6_11. [DOI] [PubMed] [Google Scholar]
- 14.“P41217 (OX2G_HUMAN)””. Uniprot. Retrieved 16 May 2013
- 15.Wang QQ, Liu YJ, Zhou JW. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener. 2015;4:19. doi: 10.1186/s40035-015-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webb M, Barclay AN. Localisation of the MRC OX-2 glycoprotein on the surfaces of neurones. J Neurochem. 1984;43:1061–67. doi: 10.1111/j.1471-4159.1984.tb12844.x. [DOI] [PubMed] [Google Scholar]
- 17.Barclay AN, Ward HA. Purification and chemical characterisation of membrane glycoproteins from rat thymocytes and brain, recognised by monoclonal antibody MRC OX 2. Eur J Biochem. 1982;129:447–58. doi: 10.1111/j.1432-1033.1982.tb07070.x. [DOI] [PubMed] [Google Scholar]
- 18.Cook DL, Hales CN. Intracellular Atp directly blocks K+ channels in pancreatic B-Cells. Nature. 1984;311:271–73. doi: 10.1038/311271a0. [DOI] [PubMed] [Google Scholar]
- 19.Gehlert DR, Mais DE, Gackenheimer SL, et al. Localization of Atp sensitive potassium channels in the rat-brain using a novel radioligand, [125] Iodoglibenclamide. Eur J Pharmacol. 1990;186:373–75. doi: 10.1016/0014-2999(90)90465-i. [DOI] [PubMed] [Google Scholar]
- 20.Inagaki N, Gonoi T, Clement JP, et al. Reconstitution of I-Katp – an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–70. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 21.Burke MA, Mutharasan RK, Ardehali H. The sulfonylurea receptor, an atypical ATP-binding cassette protein, and its regulation of the K(ATP) channel. Circ Res. 2008;102:164–76. doi: 10.1161/CIRCRESAHA.107.165324. [DOI] [PubMed] [Google Scholar]
- 22.Zhou F, Yao HH, Wu JY, et al. Opening of microglial K-ATP channels inhibits rotenone-induced neuroinflammation. J Cell Mol Med. 2008;12:1559–70. doi: 10.1111/j.1582-4934.2007.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyons A, Downer EJ, Crotty S, et al. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: A role for IL-4. J. Neurosci. 2007;27:8309–13. doi: 10.1523/JNEUROSCI.1781-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashcroft FM. ATP-sensitive potassium channelopathies: Focus on insulin secretion. J Clin Invest. 2005;115:2047–58. doi: 10.1172/JCI25495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wind T, Prehn JHM, Peruche B, Krieglstein J. Activation of ATP-sensitive potassium channels decreases neuronal injury caused by chemical hypoxia. Brain Res. 1997;751:295–99. doi: 10.1016/s0006-8993(96)01419-9. [DOI] [PubMed] [Google Scholar]
- 26.Sattiraju S, Reyes S, Kane GC, Terzic A. K-ATP channel pharmacogenomics: From bench to bedside. Clin Pharmacol Ther. 2008;83:354–57. doi: 10.1038/sj.clpt.6100378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cunha TM, Roman-Campos D, Lotufo CM, et al. Morphine peripheral analgesia depends on activation of the PI3K gamma/AKT/nNOS/NO/K-ATP signaling pathway. Proc Natl Acad Sci USA. 2010;107:4442–47. doi: 10.1073/pnas.0914733107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snyder C, Mantione K. The effects of morphine on Parkinson’s-related genes PINK1 and PINK2. Med Sci Monit Basic Res. 2014;20:63–69. doi: 10.12659/MSMBR.890557. [DOI] [PMC free article] [PubMed] [Google Scholar]