Abstract
Background
We investigated whether an intrathecal transplantation of mesenchymal stem cells (MSCs) activates extracellular adjusting protein kinase1 and 2(ERK1/2) in the spinal cords of rats following an ischemia-reperfusion injury, resulting in improved spinal cord function and inhibition of apoptosis.
Material/Methods
We observed the relationship between the activation of ERK1/2 in the rat spinal cord and intrathecal transplantation of MSCs, as well as the effect of U0126, a MEK1/2 (upstream protein of ERK1/2) inhibitor, on a spinal cord ischemia-reperfusion injury model in rats using Basso Beattie Bresnahan (BBB) scoring, somatosensory evoked potentials (SSEPs), immunohistochemistry, and Western blot analysis.
Results
After transplantation of MSCs, the lower limb motor function score increased, and the incubation period of SSEPs and amplitude were improved. Moreover, following transplantation of MSCs, Bcl2 expression increased, whereas Bax expression decreased after reperfusion. Transplantation of MSCs significantly enhanced pERK1/2 expression in the spinal cord, as well as pERK1/2 in immunoreactive cells located in the grey matter of the L4/5 levels of the spinal cord, following ischemia reperfusion injury in rats. The effective dose of U0126 required to inhibit pERK1/2 expression was 200 μg/kg. Bcl-2 decreased and the level of Bax expression increased in the spinal cord after ischemia reperfusion injury, and the protective effects of MSCs were attenuated.
Conclusions
Our findings suggest that intrathecal transplantation of MSCs activates ERK1/2 in the spinal cord following ischemia reperfusion injury, partially improves spinal cord function, and inhibits apoptosis in rats.
MeSH Keywords: Mesenchymal Stem Cell Transplantation, Mitogen-Activated Protein Kinase 3, Spinal Cord Ischemia
Background
Extensive surgical procedures are complicated by the occurrence of spinal cord ischemia-reperfusion injury, causing spinal cord dysfunction, postoperative sensory loss, and motor function damage (e.g., in the bladder and bowel, and irreversible sexual dysfunction) [1–3]. These adverse events impose huge psychological and economic burdens on patients and their families. In recent years, many scholars have used mesenchymal stem cells (MSCs) transplantation to investigate spinal cord ischemia-reperfusion injury, with positive results [4–6]. Antiapoptosis is an important aspect of the protective mechanism associated with MSCs transplantation in modulating spinal cord ischemia reperfusion injury [7]. Extracellular adjusting protein kinase1 and 2 (ERK1/2) are important intracellular signaling molecules that are members of the MAPK family. ERK1/2 are mainly phosphorylated and activated by various growth factors, such as ion beam, hydrogen peroxide, and transcription factors, within the nucleus. ERK1/2 also promote transcription and expression of certain genes and are closely related to cell proliferation and differentiation [8,9]. Previous studies have determined that ERK1/2 phosphorylation in ischemia-reperfusion injury plays an antiapoptotic role [10,11]. Studies have demonstrated that ERK1/2 activation in the spinal cord can improve spinal cord function following ischemia-reperfusion injury [12,13]. We investigated whether intrathecal transplantation of MSCs activates ERK1/2 in the spinal cord following ischemia-reperfusion injury and whether this is associated with improvement in spinal cord function.
We investigated the effects of intrathecal transplantation of MSCs on pERK1/2 expression in the spinal cord and nerve function in rats subjected to spinal cord ischemia-reperfusion injury. Simultaneously, we used the MEK1/2-specific inhibitor, U0126, to block pERK1/2 expression in the spinal cord, then observed the effect of MCSs on pERK1/2 expression in the spinal cord and on nerve function in these rats. We sought to determine whether intrathecal transplantation of MSCs activates ERK1/2 in the spinal cord following ischemia-reperfusion injury, leading to an improvement in spinal cord function.
Material and Methods
Bone marrow stromal cells gain, cultivation, and identification
Bone marrow was obtained from a tibial puncture in rats using a heparinized 24-gauge needle and was subjected to density gradient centrifugation using Percoll (1.073 g/ml) liquid layer. The isolated nucleated cells were cultured using a medium containing DMEM/F12, 20% fetal calf serum and ampicillin (1×105 U/ml) (37ºC, 5% CO2). MSCs were selected based on their adherence. The medium was replaced 3 days later and washed twice with phosphate-buffered saline to remove non-adherent cells. Purified cells were obtained after more than 50% MSCs fusion and after 3- to 5-cell propagation. Cell surface markers CD29, CD34, CD45, and CD90 of MSCs were detected using flow cytometry.
Experimental animals
We used male Sprague-Dawley rats weighing between 220 and 250 g that were bred in standard cages with free access to food and water and housed separately after surgery in a room at the First Affiliated Hospital, China Medical University. Our experiment was approved by the Ethics Committee of China Medical University and complied with the Guide for the Care and Use of Laboratory Animals (U.S. National Institutes of Health publication no. 85–23, National Academy Press, Washington DC, revised 1996).
The spinal cord ischemia reperfusion injury model and experimental protocol
We created the spinal cord ischemia reperfusion injury model by blocking the aortic arch for 14 min, as previously described [14]. In brief, rats were anesthetized with 4% sodium pentobarbital (40 mg/kg, i.p.). The abdominal cavity was exposed, and the position of the left renal artery was identified retroperitoneally. The abdominal aorta was blocked for 14 min below the left renal artery. The sham group of 8 animals underwent laparotomy followed by dissociation of the abdominal aorta without any block, avoiding spinal cord ischemia-reperfusion injury. All rats were randomly divided into the following 7 groups: naïve, sham, spinal cord ischemia-reperfusion injury (con), PBS, MSCs, U0126+MSCs, and DMSO+MSCs groups. To study the protective role of pERK1/2 mediated signaling pathways, rats were injected with U0126 (200 μg/kg, i.v.) or DMSO, then MSCs (1×108/ml) or an equivalent volume of PBS were injected intrathecally, with the latter serving as a control at L5–6 segments of the spine. We used the BBB scoring standard for evaluating the rats’ lower limb movement function, as previously described [15].
Measurement of somatosensory evoked potentials (SSEPs) of lower limbs
The somatosensory evoked potentials (SSEPs) of the lower limbs were measured by a somatosensory evoked potentiometer (KEYPOINT, Denmark). According to the system developed by the International Society for EEG, recording electrodes were placed in front at the intersection of the ears and coronal suture. The reference electrode was placed on the back of the brain. A stimulation electrode was inserted on the skin outside of the gastrocnemius of lower limb on one side, and the earth electrode was placed on the abdomen. Warm salt water was sprinkled around the electrode for humidity. The resistance between poles was less than 5 ohms, and the stimulation intensity included 1.5 m A of stimulation frequency 1.5 Hz, superimposed 100 times. The latency (L) refers to the period from the beginning of stimulation to the emergence of (downward and upward) wave peak, in millisecond (ms) as a unit. The first downward wave was the P1 wave, and the first upward wave was N1 wave. The amplitude was measured as the distance from P-wave trough or N-wave peak to the baseline, in microvolts (μV) as a unit. The incubation periods of the P1 wave and the N1 wave and the amplitude were recorded for analysis and comparison.
Immunohistochemistry
Rats were deeply anesthetized with 4% sodium pentobarbital (40 mg/kg, i.p.) and perfused through the ascending aorta with saline, followed by 4% paraformaldehyde. L4/5 spinal cord segments were dissected and post-fixed for 2~4 h. Transverse spinal cord sections (40 μm) were cut and processed for immunohistochemistry using the ABC method as described previously [16]. Briefly, sections were blocked with 2% goat serum in 0.3% Triton X-100 for 1 h at room temperature (RT) and incubated overnight at 4°C with primary antibody. The sections were then incubated for 2 h with biotinylated secondary antibody (1:200) and 1 h with ABC complex (1:50; Vector Laboratories, Burlingame, CA) at RT. Finally, the reaction product was visualized with 0.05% DAB – 0.002% hydrogen peroxide in 0.1 M acetate buffer, pH 6.0, containing 2% ammonium nickel sulfate, for 5 min.
Fractionation of proteins and Western blot
The rats were anesthetized with 4% sodium pentobarbital (40 mg/kg, i.p.), decapitated, and the whole spinal cord was extracted by pressure expulsion with saline into an ice-cooled glass dish. The L4/5 regions were identified as required, excised, and snap-frozen in liquid nitrogen. Western blots were processed as described previously [16]. We separated the cytosolic and nuclear proteins that underwent electrophoresis and transfer to NC membranes (Amersham Corp, USA), which were blocked with 3% bovine serum for 3 h. Then, membranes were incubated overnight with the following primary antibodies: mouse anti Bcl2 (1:500) or Bax (1:1000); mouse anti pERK1/2(1:200) or mouse anti-β-tubulin. The membranes were extensively washed with TTBS and incubated for 1 h with the horseradish peroxidase (HRP)-conjugated goat secondary antibody against mouse IgG (1:1000; Abcam). Subsequently, the blots were probed. Detection and quantitation were performed with a Typhoon 9400 Variable Mode Imager (GE Healthcare) and Lumi-Light Western Blotting Substrate (Roche Diagnostics) for HRP-labeled blots.
Statistical analysis
Data are expressed as the mean ±SEM. The results were analyzed using the unpaired t test or one- or two-way ANOVA in behavioral experiments (ANOVA with repeated measures). Statistical analyses of the data were performed using GraphPad Prism5 (GraphPad Software Inc., San Diego, CA). All p values are based on two-tailed tests. A P<0.05 was considered statistically significant.
Results
MSCs cultivation and identification of MSCs by flow cytometry
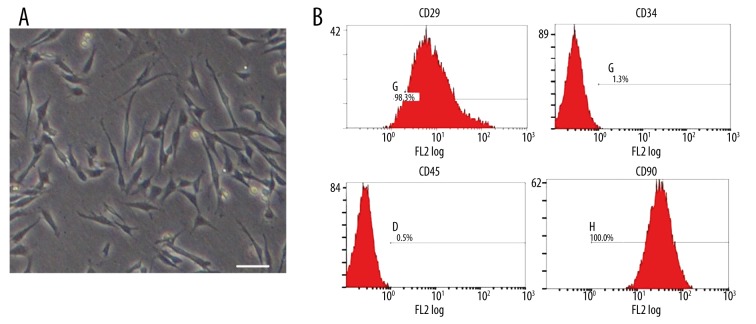
The majority of cells with adherent colony growth was observed at 3–5 generations of MSCs. The fiber spindles appeared as shown in the Figure 1A. Then, we determined the 4 cell surface markers and their expression percentage by flow cytometry as follows: CD29 was 98.3%, CD34 was 1.3%, CD45 was 0.5%, and CD90 was 100% (Figure 1B). The analysis showed that the MSCs were in conformity with the standards of the International Association of Cell Therapy for bone marrow mesenchymal stem cells.
Figure 1.
The identification of MSCs by flow cytometry. (A) The form of the third-generation MSCs, Scale bar=50 μm. (B) Flow cytometry of mesenchymal stem cells, labeled with antibodies specific for CD29, CD34, CD45, and CD90; the MSCs in line with the standards of the International Cell Therapy Association.
The influence of MSCs on lower limb movement function
We used the BBB scoring criteria to evaluate the lower limb motor function of rats at 2 days after spinal cord ischemia reperfusion injury. The lower limb motor function score was 3.8±0.87, the sham group score was 20.2±1.13, and the PBS group score did not increase (score 4.2±0.52). However, the intrathecal transplanting MSCs group score increased significantly: score 12.5±2.01, compared with the control group, P<0.01. The U0126+MSCs group score was 8.2±1.52 and had increased compared with the control group but not much as the increase noted with MSCs. The DMSO+MSCs group score was 12.7±2.15, significantly different compared with the control group, P<0.01; however, it was not significantly different from the MSCs group. These findings indicated that DMSO did not affect the lower limb motor function of the rats following intrathecal transplantation of MSCs (Figure 2).
Figure 2.
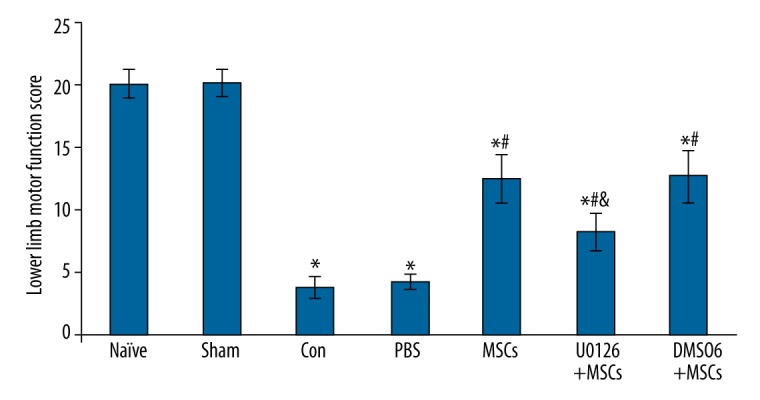
The effect of intrathecal transplantation of MSCs on lower limb motor function of rats by BBB scores after spinal cord ischemia-reperfusion injury. Data are shown as the mean ±SEM (n=8). * P<0.01, vs. naïve group, # P<0.01 vs. con group, & P<0.01, vs. MSCs group.
The influence of MSCs based on the SSEPs
In this experiment, the SSEPs of rats in the naive group and sham groups were normal. Two days after spinal cord ischemia reperfusion injury, the incubation periods of the P1 and N1 of SSEPs were extended, and the P1/N1 amplitude of SSEPs was shorter, suggesting that the spinal cord ischemia-reperfusion injury inhibited spinal nerve function in rats. Compared with the control group, the incubation periods of the P1 and N1 had shortened, and the P1/N1 amplitude was increased in the MSCs group, showing that intrathecal transplantation of MSCs improves spinal cord function following ischemia-reperfusion injury. Compared with the control group, the incubation periods of P1 and N1 were also shortened, and the P1/N1 amplitude was also increased in the U0126+MSCs group, P<0.01. However, the improvement was not as obvious as that noted in the MSCs group, suggesting that U0126 reduces the protective effect of MSCs in improving spinal cord function (Figure 3).
Figure 3.
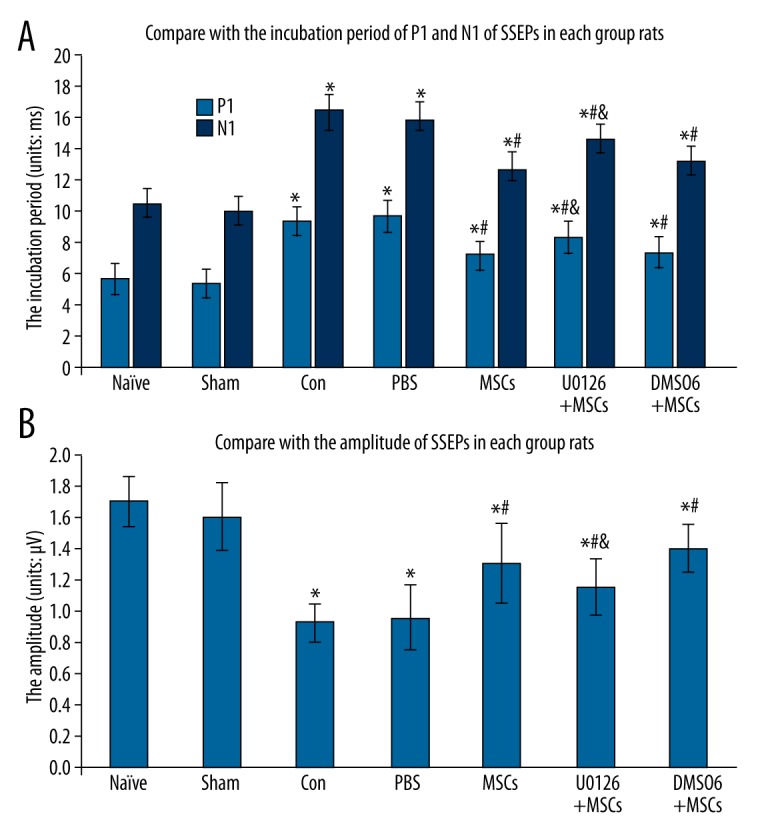
Effect of intrathecal transplantation of MSCs to SSEPs in rats following spinal cord ischemia-reperfusion injury. (A) The statistical analysis of the incubation periods of the P1 and N1 of SSEPs in every group. (B) The statistical analysis of the amplitude of SSEPs in every group. Data are shown as the mean ±SEM (n=8). * P<0.01, vs. naïve group, # P<0.01 vs. con group, & P<0.01, vs. MSCs group.
The effect of MSCs on cell apoptosis
As shown in Figure 4, the expression of Bcl-2 and Bax was assessed by Western blotting 2 days after spinal cord ischemia reperfusion injury. Compared with the sham group, the level of Bcl-2 protein in the control and PBS groups decreased (P<0.05), whereas MSCs treatment increased Bcl2 expression (P<0.05). Moreover, U0126 reduced the level of Bcl-2 by inhibiting the effect of MSCs treatment (P<0.05). In contrast, compared with the sham group, the expression of Bax protein in the control and PBS groups increased (P<0.05), whereas MSCs treatment reduced Bax level (P<0.05). U0126 enhanced the expression of Bax by inhibiting the effect of MSCs treatment (P<0.05). These results suggest that MSCs treatment inhibits apoptosis 2 days after spinal cord ischemia-reperfusion injury.
Figure 4.
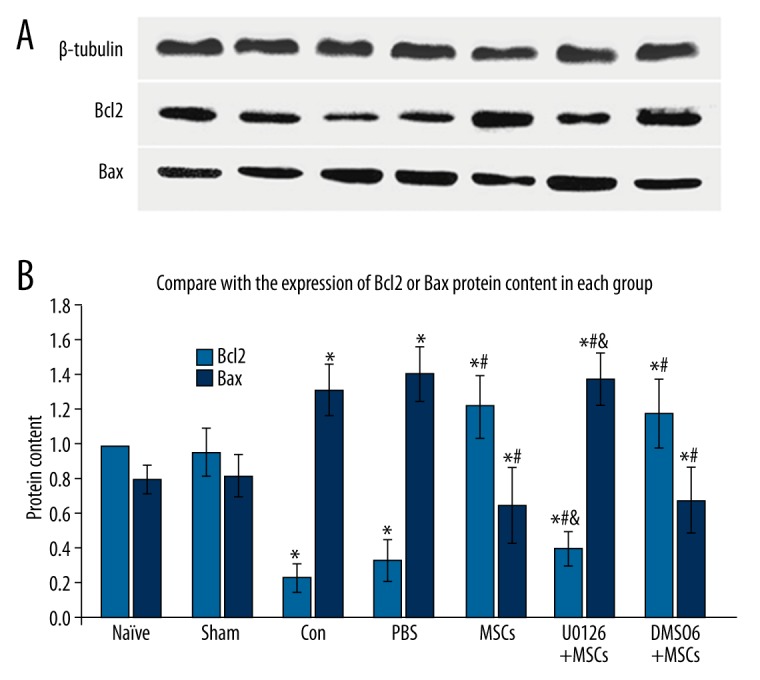
Effect of intrathecal transplantation of MSCs on cell apoptosis in the spinal cord following ischemia-reperfusion injury. (A) Representative Western blot for Bcl-2 and Bax proteins in every group. β-tubulin labeled band (up). (B, C) Statistical analysis of Bcl-2 and Bax protein expression. Data are shown as the mean ±SEM (n=8). * P<0.05, vs. naïve group, # P<0.05 vs. con group, & P<0.05, vs. MSCs group.
The influence of MSCs on the spinal cord grey pERK1/2 neurons
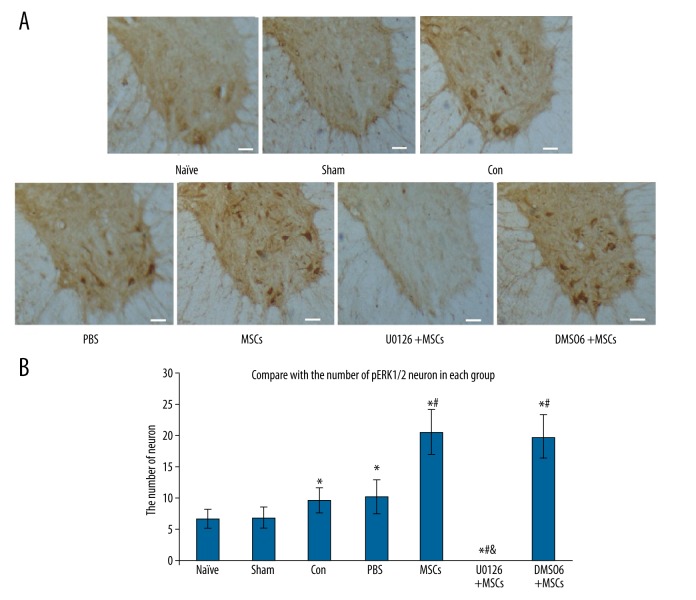
Two days after spinal cord ischemia-reperfusion injury, we performed immunohistochemical staining of the spinal cord slices and evaluated the number of pERK1/2 neurons. We found that, compared with the control group, the number of pERK1/2 neurons in the MSCs group was significantly increased (P<0.01). There were no pERK1/2 neurons in spinal cord grey matter in the U0126+MSCs group. Thus, U0126 (200 μg/kg) inhibits ERK1/2 activation in the spinal cord grey matter following ischemia-reperfusion injury. The number of pERK1/2 neurons in the DMSO+MSCs group was equal to the number of pERK1/2 neurons in the MSCs group, suggesting that DMSO solvent had no effect on the activation of ERK1/2 in the spinal cord following ischemia-reperfusion injury (Figure 5).
Figure 5.
Effect of intrathecal transplantation of MSCs on the numbers of pERK1/2 neurons in the spinal cord following ischemia-reperfusion injury. (A) The immunohistochemical staining of pERK1/2 neurons in the spinal cord slice in every group. Scale bar=50 μm. (B) The statistical analysis of the number of pERK1/2 positive neurons in every group. Data are shown as the mean ±SEM (n=8). * P<0.01, vs. naïve group, # P<0.01 vs. con group, & P<0.01, vs. MSCs group.
The influence of MSCs on the expression of pERK1/2 protein
Western blot analysis was performed to test the expression of pERK1/2 protein in the spinal cord 2 days after spinal cord ischemia reperfusion injury. We found that, compared with the naive and sham groups, the expression of pERK1/2 protein significantly increased in the MSCs group P<0.01, which demonstrates that intrathecal transplantation of MSCs promotes ERK1/2 phosphorylation in the spinal cord after ischemia-reperfusion injury. However, we also found that pERK1/2 protein was not expressed in the U0126+MSCs group. Thus, intravenously injected U0126 (200 μg/kg) inhibits activation of ERK1/2 in the spinal cord after ischemia-reperfusion injury (Figure 6).
Figure 6.
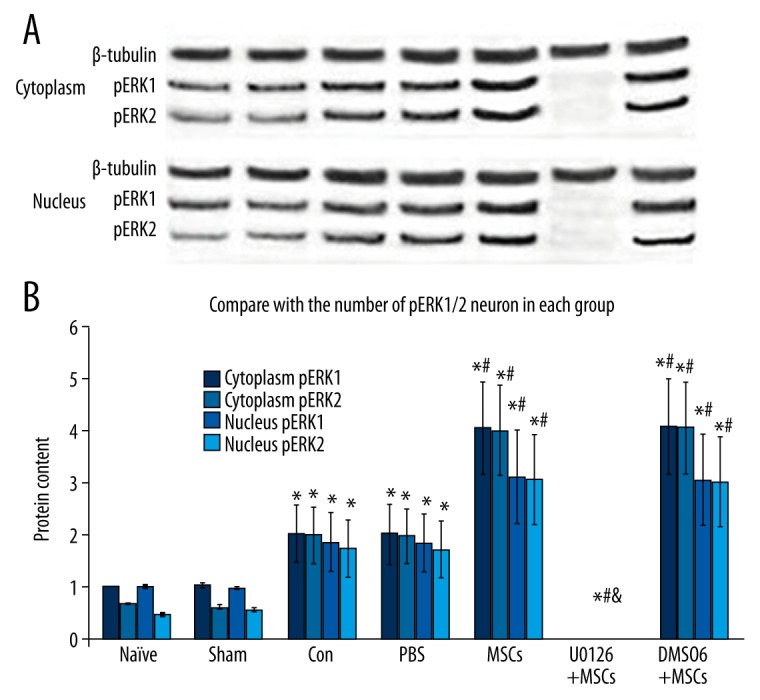
Effect of intrathecal transplantation of MSCs on pERK1/2 protein in the spinal cord following ischemia-reperfusion injury. (A) Representative Western blots are shown with the pERK1/2 and β-tubulin labeled band (up). (B) Densitometry of all Western blot results from spinal cord of the 7 groups. Data are shown as the mean ±SEM (n=8). * P<0.01, vs. naïve group, # P<0.01 vs. con group, & P<0.01, vs. MSCs group.
Discussion
Spinal cord ischemia-reperfusion injury causes spinal cord dysfunction and is associated with a huge economic burden and psychological stress for the patients and their families. As a result, researchers have sought ways to prevent and treat spinal cord ischemia-reperfusion injury. In recent years, there has been significant progress in the use of MSCs transplantation to prevent and cure spinal cord ischemia-reperfusion injury [5,6,17]. Shi performed intrathecal injections of marrow stromal cells in rabbits and reported a therapeutic benefit in the ischemia-injured spinal cords [5]. Bo Fang also conducted intrathecal injections of MSCs in rabbits and found that MSCs attenuated the disruption in the blood-spinal cord barrier induced by spinal cord ischemia-reperfusion injury, leading to recovery of spinal cord function [6]. In our experiment, lower limb movements increased, and the latency and amplitude of SSEPs improved, after intrathecal transplantation of MSCs 2 days after spinal cord ischemia-reperfusion injury in rats. Thus, MSCs transplantation promotes spinal cord function recovery in rats following spinal cord ischemia-reperfusion injury. Previous studies have shown that MSCs transplantation enhances the antiapoptotic effect in spinal cord neurons that promotes restoration of nerve function [18]. ERK1/2 activation has always been associated with cell differentiation and proliferation [8] rather than inhibition of apoptosis in spinal cord ischemia-reperfusion injury [10,11]. Fortunately, in our experiment, we found that the number of pERK1/2 neurons and their protein content increases in the spinal cord after intrathecal transplantation of MSCs 2 days after spinal cord ischemia-reperfusion injury. However, it is unclear whether the increase of pERK1/2 expression in the spinal cord is primarily responsible for the improvement in nerve function. We therefore administered U0126, a MEK1/2-specific inhibitor, to block ERK1/2 activation in the spinal cord of ischemia-reperfusion injury rats, then transplanted MSCs intrathecally. Surprisingly, lower limb motor function improved, though not as much as in the MSCs group. The incubation period and amplitude of SSEPs also did not improve as much as in the MSCs group. ERKl/2 antiapoptotic mechanisms are exerted through ERK1/2 phosphorylation of antiapoptotic molecules (e.g., BAD), simultaneous activation of transcription factors, and stimulation of the expression of related genes and preservation of antiapoptotic effects [19]. Our results suggest the MSCs treatment can inhibit apoptosis 2 days after spinal cord ischemia-reperfusion injury in rats. Furthermore, consistent with previous studies, these findings support that ERK1/2 phosphorylation has an antiapoptotic role in ischemia-reperfusion injury. Of course, ERK1/2 may participate in other mechanisms beyond inhibition of apoptosis, such as secretion of various neurotrophic factors that promote the regeneration of the myelin sheath, local angiogenesis and vascular remodeling, and improving the axon regeneration microenvironment [20–22]. Thus, intrathecal transplantation of MSCs activates pERK1/2 in the spinal cord following ischemia-reperfusion injury and enhances antiapoptosis in nerve cells, thereby promoting the restoration of spinal cord function.
Conclusions
Intrathecal transplantation of MSCs activates ERK1/2 in the spinal cord following ischemia-reperfusion injury and partially improves nerve function in rats.
Footnotes
Source of support: This research was supported by the Doctoral Fund of Ministry of Education of China, No. 20092104110009; Science and Technology Plan Project of Liaoning
References
- 1.Svensson LG, Crawford ES, Hess KR, et al. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg. 1993;17(2):357–37. [PubMed] [Google Scholar]
- 2.Akurai M, Hayashi T, Abe K, et al. Delayed and selective motor neuron death after transient spinal cord ischemia: A role of apoptosis? J Thorac Cardiovasc Surg. 1998;115(6):1310–15. doi: 10.1016/S0022-5223(98)70213-2. [DOI] [PubMed] [Google Scholar]
- 3.Masaaki H, Satoshi K, Hideo S, et al. Bone marrow stromal cells protect and repair damaged neurons through multiple mechanisms. J Neurosci Res. 2008;86(5):1024–35. doi: 10.1002/jnr.21572. [DOI] [PubMed] [Google Scholar]
- 4.Shi E, Kazui T, Jiang X, et al. Intrathecal injection of bone marrow stromal cells attenuates neurologic injury after spinal cord ischemia. Ann Thorac Surg. 2006;81(6):2227–33. doi: 10.1016/j.athoracsur.2005.12.056. [DOI] [PubMed] [Google Scholar]
- 5.Shi E, Kazui T, Jiang X, et al. Therapeutic benefit of intrathecal injection of marrow stromal cells on ischemia-injured spinal cord. Ann Thorac Surg. 2007;83(4):1484–90. doi: 10.1016/j.athoracsur.2006.11.048. [DOI] [PubMed] [Google Scholar]
- 6.Fang B, Wang H, Ma H, et al. Intrathecal transplantation of bone marrow stromal cells attenuates blood-spinal cord barrier disruption induced by spinal cord ischemia-reperfusion injury in rabbits. J Vasc Surg. 2013;58(4):1043–52. doi: 10.1016/j.jvs.2012.11.087. [DOI] [PubMed] [Google Scholar]
- 7.Wu S, Cui G, Shao H, et al. The cotransplantation of olfactory ensheathing cells with bone marrow mesenchymal stem cells exerts antiapoptotic effects in adult rats after spinal cord injury. Stem Cells International. 2015;2015:516215. doi: 10.1155/2015/516215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meisinger C, Doring A, Schneider A, et al. Serum Gamma-glutamyl-transferase is a predictor of incident coronary events in apparently healthy men from the general population. Atherosclerosis. 2006;189(2):297–302. doi: 10.1016/j.atherosclerosis.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Kwan MW, Mak W, Cheung RT, et al. Ischemic stroke related to intracranial branch atheromatous disease and comparison with large and small artery diseases. J NeurolSci. 2011;303(1–2):80–84. doi: 10.1016/j.jns.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Xu T, Wu X, Chen Q, et al. The anti-apoptotic and cardioprotective effects of salvianolic acid a on rat cardiomyocytes following ischemia/reperfusion by DUSP-mediated regulation of the ERK1/2/JNK pathway. PLoS One. 2014;9(7):e102292. doi: 10.1371/journal.pone.0102292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagata T, Yasukawa H, Kyogoku S, et al. Cardiac-specific SOCS3 deletion prevents in vivo myocardial ischemia reperfusion injury through sustained activation of cardioprotective signaling molecules. PLoS One. 2015;10(5):e0127942. doi: 10.1371/journal.pone.0127942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X-Q, Cao X-Z, Wang J, et al. Sevoflurane preconditioning ameliorates neuronal deficits by inhibiting microglial MMP-9 expression after spinal cord ischemia/reperfusion in rats. Mol Brain. 2014;7:69. doi: 10.1186/s13041-014-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu T, Cao FJ, Xu DD, et al. Upregulated Ras/Raf/ERK1/2 signaling pathway: A new hope in the repair of spinal cord injury. Neural Regen Res. 2015;10(5):792–96. doi: 10.4103/1673-5374.156984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lang-Lazdunski L, Matsushita K, Hirt L, et al. Spinal cord ischemia. Development of a model in the mouse. Stroke. 2000;31:208–13. doi: 10.1161/01.str.31.1.208. [DOI] [PubMed] [Google Scholar]
- 15.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 16.Wang YH, Zhang LC, Zeng YM. Activation of ERK1/2 in spinal cord contributes to the development of acute cystic pain in rabbits. Neurosci Bull. 2006;22(4):216–20. [PubMed] [Google Scholar]
- 17.Yin F, Meng C, Lu R, et al. Bone marrow mesenchymal stem cells repair spinal cord ischemia/reperfusion injury by promoting axonal growth and anti-autophagy. Neural Regen Res. 2014;9(18):1665–71. doi: 10.4103/1673-5374.141801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venkata RD, Daniel G, Spomar, et al. Mesenchymal stem cells from rat bone marrow down regulate caspase-3-mediated apoptotic pathway after spinal cord injury in rats. Neurochem Res. 2007;32:2080–93. doi: 10.1007/s11064-007-9368-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Wang Y, Ye J, et al. bFGF attenuates endoplasmic reticulum stress and mitochondrial injury on myocardial ischaemia/reperfusion via activation of PI3K/Akt/ERK1/2 pathway. J Cell Mol Med. 2015;3(19):595–607. doi: 10.1111/jcmm.12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hyun JK, Kim HW. Clinical and experimental advances in regeneration of spinal cord injury. J Tissue Eng. 2010;2010:650857. doi: 10.4061/2010/650857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong BT, Tu GJ, Han YX, Chen Y. Lithium enhanced cell proliferation and differentiation of mesenchymal stem cells to neural cells in rat spinal cord. Int J Clin Exp Pathol. 2015;8(3):2473–83. [PMC free article] [PubMed] [Google Scholar]
- 22.Lapi D, Vagnani S, Sapio D, et al. Effects of bone marrow mesenchymal stem cells(BM-MSCs) on rat pial microvascular remodeling after transient middle cerebral artery occlusion. Front Cell Neurosci. 2015;9:329. doi: 10.3389/fncel.2015.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]