Abstract
Background
Obesity causes several health complications along with disruption of the reproductive system. The aim of the current study was to determine how long-term intake of very high fat diet (VHFD) changes the hormonal milieu, affecting the cellular morphology and reproductive cycle in female mice.
Material/Methods
Mice were fed on normal diet (ND) and VHFD for 2 weeks, 12 weeks, and 25–27 weeks. We assessed changes in body weight, food consumption, energy intake, cellular and tissue morphology, hormonal levels (leptin, insulin, and estradiol), and vaginal smears were performed at various time points to determine the length and cellularity at each stage of the estrous cycle.
Results
Mice fed on VHFD showed a significant increase in weight gain, reduction in food intake, and increase in energy intake compared to animals fed on ND, indicating that the caloric density of the diet is responsible for the differences in weight gain. Hormonal analysis showed hyperleptinemia, hyperinsulinemia, and increases in estrogen levels, along with increases in size of the islet of Langerhans and adipocytes. After 25–27 weeks, all animals fed on VHFD showed complete acyclicity; elongation of phases (e.g., diestrous), skipping of phases (e.g., metestrous), or a combination of both, indicating disruption in the reproductive cycle. Quantitative analysis showed that in the diestrous phase there was a 70% increase in cell count in VHFD compared to animals fed on ND.
Conclusions
The above results show that morphological and hormonal changes caused by VHFD probably act via negative feedback to the hypothalamic-pituitary axis to shut down reproduction, which has a direct effect on the estrous cycle, causing acyclicity in mice.
MeSH Keywords: Diet, High-Fat; Estradiol; Estrous Cycle; Insulin; Leptin
Background
Obesity has become a worldwide epidemic [1,2]. The World Health Organization (WHO) estimated that approximately 1.1 billion people globally are overweight and 320 million are obese [3]. Obesity is not limited to adults; childhood obesity has been increasing exponentially and has become a major concern in most developed countries [4,5]. Obesity is associated with many health complications and diseases, including type 2 diabetes, stroke, heart disease, hypertension, and cancer [6]. The primary cause of obesity is energy intake greater than energy expenditure [7,8]. Energy intake can be increased by consuming larger portion sizes, and energy-dense foods (foods high in sodium, fat, and sugar) [9]. There are also several studies that have shown that diet composition, particularity high dietary fat intake, promotes obesity in humans [10,11] and mice [7].
Recent studies have shown that obesity can cause disruptions in the reproductive system [12–14]. Childhood obesity causes increased risk of menstrual disorders at adolescence [15]. Women with obesity are more likely to have menstrual irregularities and higher risk of miscarriages [12,16]. Distinct changes in circulating hormones like leptin and insulin appear to underlie these abnormalities and are associated with disorders related to feeding and reproduction. Leptin, a hormone secreted from adipose tissue, inhibits food intake, regulates reproduction, and controls homeostasis [17–19]. In the absence of an active form of leptin, rodents are usually hyperphagic, obese, have overactive neuropeptide Y (NPY) neurons, and are infertile [20–22]. Similar maladies are seen in women with chronically low circulating leptin levels, including menstrual abnormalities and reproductive failure [23]. Lower leptin levels are also seen in anovulatory and normal-weight amenorrheic females [24]. Similar to leptin, insulin, a hormone secreted by the pancreas, is also associated with obesity and reproduction [25–27]. Insulin resistance causes reproductive infertility [28,29].
The role of the hypothalamic-pituitary-gonadal (hpg) axis in the development and regulation of the reproductive system is tremendous. Gonadotropin-releasing hormone (GnRH), a hormone released from the hypothalamus, travels down to the anterior pituitary to regulate the production of 2 hormones: luteinizing hormone (LH) and follicle-stimulating hormone (FSH). LH and FSH then act on the ovaries to produce estrogen and progesterone, which are used to regulate the menstrual and ovarian cycles [30,31]. Increases in body weight lead to changes in the release of gonadotropins and gonadal hormones [13,32].
Females go through a menstrual cycle that lasts 28±2 days but for mice an estrous cycle is typically 4–5 days in length. The estrous cycle is distinguished by 4 different phases: proestrous, estrous, metestrous, and diestrous. Each phase is characterized by the presence of different types of cells (nucleated, cornified, leukocytes, or a combination of all 3 cells). Vaginal smears can be used to monitor the stages of the estrous cycle [33].
The current study aimed to determine whether prolonged use of VHFD, which affects the body weight, energy consumption, cellular, and tissue morphology and hormonal profile, also results in changes in the reproductive cycle in female mice. This would help to understand whether prolonged intake of a HF diet can lead to reproductive disorders in women.
Material and Methods
Animals and diet
Fourteen wild-type (C57BL/6J) female mice, age 14–16 weeks, were randomly divided to receive either normal diet (ND, n=6) or VHFD (n=8). The ND consisted of 62% kcal carbohydrate, 25% kcal protein, 13% kcal fat, and 3.07 kcal/g energy density (Rodent Diet 20 5053, Pico Lab). The VHFD consisted of 20% kcal carbohydrate, 20% kcal protein, 60% kcal fat, and 5.24 kcal/gm energy density (D12492, Research Diets). Animals were housed individually in a colony room, with a partially reversed light cycle of 14:10 (lights on at 2300 h and lights off at 1300 h), room temperature 21–22°C (70–72°F), humidity 40–50%, and ad libitum access to food and water. Animals were fed for 25–27 weeks. Body weights were recorded daily with a digital electronic balance for 24 weeks. Food consumption was estimated at the end of 2 weeks, 12 weeks, and 24 weeks by providing pre-weighed food on a specific day and subtracting the weight of the left-over food on that day. Body weights and food consumption were not measured after 24 weeks because animals were decapitated on different days depending on the completion of the estrous cycle. All animal protocols were approved by the Institutional Animal Care and Use Committee at Adelphi University and studies were conducted in accordance with the Guide for the Care and Use of Experimental Animals.
Assessment of estrous cycle
The progression of the estrous cycle was assessed by vaginal smears after female mice were fed on ND or VHFD for 2 weeks, 12 weeks, and 25–27 weeks. Since animals on VHFD showed elongation of estrous cycle, vaginal smears were performed until all animals completed the estrous cycle (starting and ending at the same phase). The entire cycle was repeated to verify the length of the cycle. Since VHFD animals were cycling differently, a 2-week period was required to complete the whole process. Smears were performed at between 11:00 am and 12:00 noon. By inserting the tip into the mouse vagina, vaginal secretions were collected with a plastic pipette filled with 10 μl of normal saline (0.9% NaCl). The process was done carefully so that the pipette is not inserted too deep in the vagina, which could cause cervical stimulation and result in pseudopregnancy [34,35]. Saline was quickly released and immediately drawn back into the syringe.
The vaginal smear containing cells was placed on an untreated glass microscopic slide and viewed at 200× and 400× magnification. The stage within the estrous cycle was determined, number of cells quantified, and images were taken using a Spot Insight camera mounted on a Zeiss microscope (Axioskop, Germany). The Papanicolaou (PAP) staining kit (Thermo Electron Corp., Pittsburg, PA) was used to stain the vaginal smear using the methodology suggested by the manufacturer. An average of the cycles was used to quantify the different cell populations at each stage of the estrous cycle. Ten random fields per slide were viewed and counted for nucleated, leukocytic, and cornified cells.
Leptin, insulin, and estradiol assay
Female mice on ND and VHFD were sacrificed by decapitation at 15:00 h at around 25–27 weeks to assess the levels of leptin, insulin, and estradiol in serum. All animals were sacrificed at the proestrous stage, which is the stage at which the levels of estradiol are lowest [31]. Trunk blood was collected, allowed to clot, and centrifuged, and the resulting serum was stored at −20°C to assay for leptin, insulin, and estrogen. Serum leptin and insulin were assayed using the Radioimmuno Assay kit (Millipore, LIPCO) by New York Obesity Research Center, Hormone & Metabolite Core Laboratory, University Hospital of Columbia University College of Physicians & Surgeons. The β-estradiol concentration in serum was measured using an Enzyme Immuno Assay kit (Enzo Life Sciences). The Inter-assay precision percentage for leptin, insulin, and estradiol was 3–5.7, 8.5–9.4, and 8.3–14.2, respectively, and the intra-assay precision percentage was 2–4.6, 1.4–4.6, and 2.1–5.7, respectively.
Biopsy of different organs
Tissue of appropriate size was dissected out and wrapped in lens paper, marked with a pencil, and placed in formalin overnight. The next day, tissues were placed in cassettes and dipped again in formalin and then sent to Cross Island Laboratories for biopsy. Representative sections were taken from the pancreas (5×3×2 mm) and adipose tissue (1×1×0.5 cm) and photographed at 200×.
Statistical analysis
Statistical analysis was done to compare variables among ND and VHFD animals using ANOVA (JMP software, version 7, SAS Institute Inc., Cary, NC). Results are presented as average ±SEM. Effects were considered significant at p<0.05.
Results
Changes in the body weight
There was an increase in the body weight for both ND and VHFD mice during the time period studied (Figure 1). The weight gain was much greater in VHFD than in ND mice (p<0.0001, 24 weeks). Animals fed on a ND showed an average 8% increase and VHFD mice showed an increase of about 105% of body weight over a period of 24 weeks.
Figure 1.
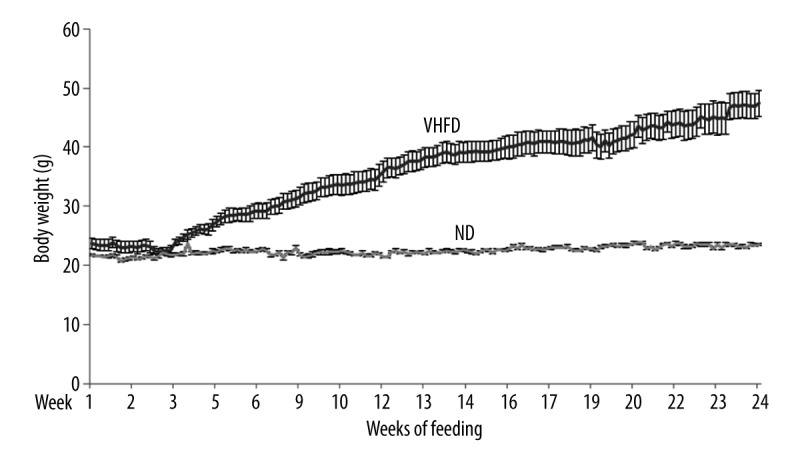
Changes in the body weight when mice were fed on Normal diet (ND) and Very high-fat diet (VHFD) for 24 weeks. Values are average ±SEM.
Food consumption and energy intake
VHFD animals showed lower food intake when compared to ND animals (Table 1). However, total energy intake by animals on VHFD was significantly higher than in ND mice at both 12 and 24 weeks. The total protein energy intakes in VHFD at all time points were similar. However, the intake of energy from fat was significantly higher in VHFD animals. The energy intake from carbohydrates was significantly higher in ND than in VHFD mice (Table 1).
Table 1.
Food intake by mice on Normal diet (ND) and Very high fat diet (VHFD) at various time points.
| Time | Diet | Food consumed (g) | Total energy intake (kcal) | Total fat (kcal) | Total protein (kcal) | Total carbohydrates (kcal) |
|---|---|---|---|---|---|---|
| 2 Weeks | ND | 35±3.1 | 103±5.1 | 14±0.6 | 26±1.1 | 67±2.9* |
| VHFD | 21±1.9** | 123±10.0 | 66±5.5** | 22±1.8 | 22±1.8 | |
| 12 Weeks | ND | 31±1.8 | 97±6.8 | 12±0.5 | 23±0.8 | 60±2.2* |
| VHFD | 21±1.3** | 112±19* | 66±2.2** | 22±0.8 | 22±0.8 | |
| 24 Weeks | ND | 29±1.2 | 96±0.0 | 12±0.5 | 24±0.6 | 56±1.3* |
| VHFD | 23±1.2** | 120±7.3** | 71±2.4** | 22±0.8 | 24±0.8 |
Values are average ±SEM. Statistical analysis using ANOVA showed the following level of significance between animals fed on ND and corresponding VHFD. Level of significance
p<0.0001 and
p<0.001.
Leptin, insulin, and estradiol levels in different mouse models
Serum leptin, insulin, and estradiol levels were measured at 25–27 weeks of age. As shown in Table 2, leptin, insulin, and estrogen levels were significantly higher in VHFD mice compared to ND mice. The increases were about 8-, 10-, and 2-fold for leptin, insulin and, estradiol levels, respectively, in VHFD fed animals compared to ND.
Table 2.
Hormonal levels of mice fed on Normal diet (ND) and Very high fat diet (VHFD) for 25–27 weeks.
| Hormones | Leptin (ng/ml) | Insulin (pg/ml) | Estradiol (ng/ml) |
|---|---|---|---|
| ND | 2.2±0.2 | 0.25±0.0 | 0.3±0.0 |
| VHFD | 16.7±0.7** | 2.5±0.4** | 0.5±0.0** |
| p value showing significance between ND and VHFD | p<0.0001 | p< 0.0003 | p<0.0007 |
Values are average ±SEM. Statistical analysis using ANOVA showed the following level of significance between animals fed on ND and corresponding VHFD. Level of significance
p<0.0001 and
p<0.001.
Pathomorphological changes
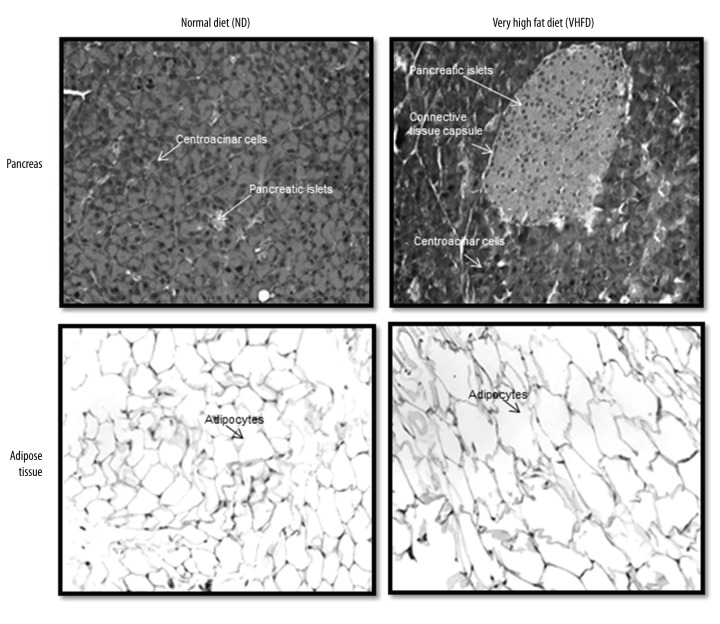
Pancreas: Light microscopic analysis of pancreatic islets from animals on ND showed circular, oval, or elongated shapes with a thin connective sheath surrounding each islets. No inflammation was observed in animals fed on ND for 25–27 weeks. The islets of VHFD showed marked morphological changes with significant increase in the size (hypertrophy) compared to ND within 25–27 weeks (Figure 2).
Adipose: There was increase in the size of adipocytes in VHFD over a period of 25–27 weeks. Microscopic analysis showed adipocytes with nucleus on the side. In VHFD the size of the cell increased significantly with the change in the shape of the cell (Figure 2).
Figure 2.
Pancreatic islets and adipocytes stained with hematoxylin-eosin stain: Representative pancreatic islets and adipocytes show increase in size in animals fed on VHFD at 25–27 weeks. Microphotographs taken at 200×.
Changes in the cellular morphology in estrous cycle: qualitative and quantitative assessment
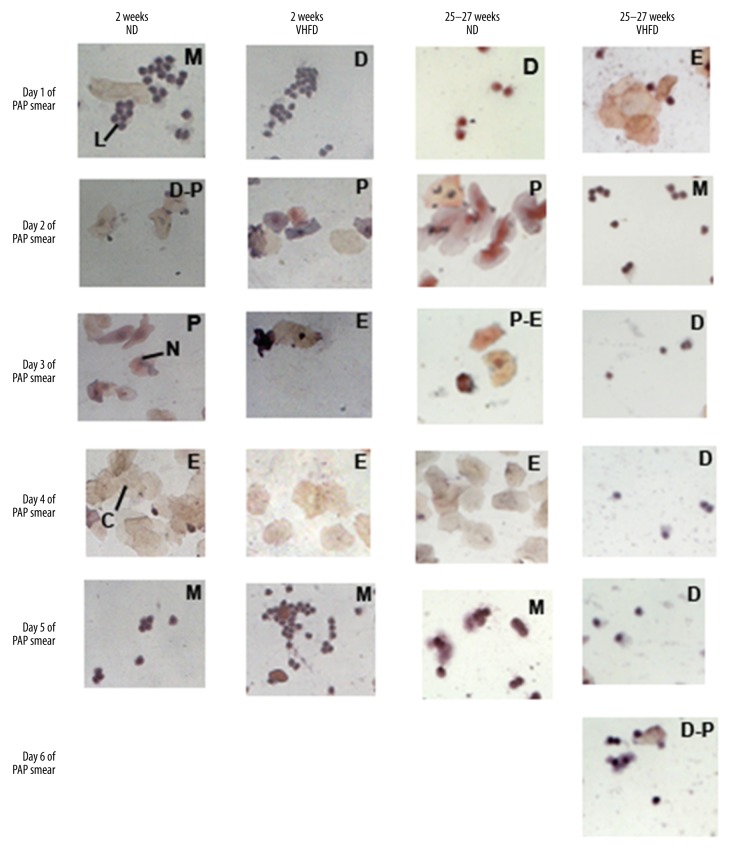
Mouse fed on ND for 2 weeks, 12 weeks and 25–27 weeks showed 4–5 day cycles (proestrous, estrous, metestrous, diestrous) with proestrous phase showing nucleated cells stained dark purple, granulated nuclei with pink or blue cytoplasm surrounded with a blue rim, estrous phase; pale orange and pink stained cornified cells and leukocytic cells stained blue were visible, metestrous phase; dark blue stained leukocytic cells were usually seen in clusters, diestrous phase; similar profile as that of metestrous but with fewer leukocytic cells (Figure 3).
Figure 3.
Representative animals showing cellular changes across the estrous cycle on ND and VHFD for 2 weeks (1st and 2nd column), and 25–27 weeks (3rd and 4th column). Stages of estrous Cycle, metestrous – M, diestrous – D, proestrous – P, and estrous – E. Cells in the different phases of the estrous cycle are denoted by nucleated cells – N, cornified cells – C, and leukocytic cells – L at 400× magnification. Elongation of phases in VHFD animals and repeated diestrous phase are indicative of the disruption of estrous cycle.
VHFD fed animals showed all 3 stages in sequence similar to ND fed animals during the first 2 weeks indicating no change. Gradually animals started showing abnormalities in the phases. By 25–27 weeks, ND fed animals still showed the 3 phases but VHFD fed mice showed abnormalities in phases; elongation of phases (e.g. diestrous for 4 consecutive days), skipping of phases (e.g. metestrous), or a combination of both indicating disruption in the reproductive cycle (Figure 3). Quantitative cellular counts showed significant elongation of diestrous phase (70% increase in cell count between animals fed on ND and VHFD for 25–27 weeks). By 25–27 weeks the cycles were 7–8 days in VHFD animals compared to the 4–5 days seen in normal cycling animals (Figures 3, 4).
Figure 4.
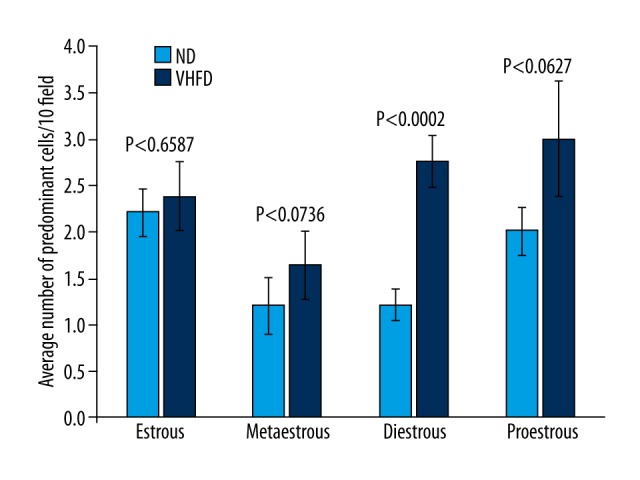
Quantification of cells at various phases of the estrous cycle at 25–27 weeks. Statistical analysis using ANOVA showed level of significance, as indicated. Phases with different cell counts were proestrous, predominantly nucleated cells; estrous, cornified cells; metestrous, leukocytic cells; diestrous, fewer leukocytic cells than metestrous. Data were an average of 10 random fields under 20× magnification. Values are average ±SEM. Significant increase in cellular count in diestrous phase was about 70% that of ND animals.
Discussion
This study was undertaken to observe the effects of long term (2 weeks, 12 weeks and 25–27 weeks) use of VHFD on reproductive cycle in a rodent model which was equivalent to human age; 1.25 years (childhood), 7 years (pre-teen) and 17.5 years (teen). The result addresses 3 major points: 1. In female mice, diet-induced obesity (DIO) results in an increase in body weight related to the energy content and not the quantity of food consumed. Reduced amount of food intake in VHFD animals was not able to completely compensate for the increased energy density, so little change in energy intake was observed; 2. Significant increases in the leptin and insulin release was related to the cellular morphology and size (islets of Langerhans and adipose tissue) in VHFD animals; 3. Prolonged feeding of VHFD to female mice results in disruption of the estrous cycle.
The regulation of body weight involves diet and genetic make-up [36]. High Fat Diet (HFD) induces an energy surplus that causes obesity [6]. Results indicate that the increase in body weight was not due to the quantity of food intake but due to the high energy content of the food. Mice on HFD showed a broad range of body fat with increases in the leptin levels. The decrease in food consumption in VHFD animals compared to animals on ND could be due to increased leptin and insulin levels [37,38]. However, the energy intake was not affected by the increases in the hormonal level. It appears that leptin suppresses the amount of food intake but has little effect on the caloric intake.
The reproductive cycles in humans or the estrous cycle in mice are both under the influence of the hpg axis [39,40]. The effects are most drastic during puberty. The increase in estrogen in VHFD mice could be due to an increase in the adipose tissue, which produces aromatase and thereby increases the conversion of testosterone to estradiol [41]. Long-term obesity leads to decreased hepatic inactivation by estrogen 2-hydroxylation, which also causes increases in estradiol levels in blood. Moreover, the adipoinsular axis may be affected by the increases in size of adipose and islet size, which leads to increases in the release of insulin and leptin, causing lowering of sex hormone-binding protein levels, resulting in increased bio-availability of estrogen in blood [42,43]. In rats, DIO results in lack of LH surge [44] along with reduced LH levels after treatment with HF for 180 days. It has also been reported that the levels of progesterone are increased when rats are treated with HFD for 180 days [45]. Thus, it appears that decreases in the LH levels with reduced LH surge in conjunction with increased progesterone and estradiol levels results in the elongation of estrous cycle and acyclicity, leading to reduced reproductive function. Once these changes have occurred, even reversing the diet from HF to ND does not change the reproductive capacity, although several metabolic parameters may return to normal levels [46]. This shows that the adverse effects of HF are more severe and irreparable in reproductive tissues than any other affected tissues.
Leptin acts as a metabolic factor linking fat with reproduction. Leptin has been linked with multiple factors that lead to pulsatile release of GnRH [47]. When the levels of leptin are chronically high, the actions of leptin on reproduction are probably disrupted. This may be due to impaired flow of leptin through the blood-brain barrier [48] or perhaps the lower expression of leptin receptor or leptin itself causes ‘leptin-induced leptin resistance’. All of the above factors reduce the availability of leptin in the CNS, affecting the hypothalamus, which in turn affects the release of GnRH, damping the cyclicity. Our previous research [39,49] has shown that ob/ob mice lacking leptin and Agouti mice with chronically high leptin levels are acyclic, and our current study shows that animals fed with VHFD develop chronically high leptin levels and are also acyclic. These results suggest that leptin is required in optimal concentrations but is not the sole determining factor for reproduction.
Leptin and insulin together have been implicated in GnRH secretion [50,51]. We therefore speculate that when insulin is produced in chronically high amounts (hyperinsulinemia), it probably causes GnRH to release tonically or causes an increase in pulsatility, both of which can disrupt the reproductive cycle. Further, chronic hyperstimulation causes a decreased biosynthetic capacity, which also can result in acyclicity and disruption of the estrous cycle.
Conclusions
Taken together, it seems that mice on an HF diet undergo changes in the adipose and islet morphology and size, which results in release of chronically high levels of insulin and leptin. This results in changes in the hpg and adipoinsular axis, causing disruption in the LH surge and resulting in changes in the secretion of gonadal hormones. These changes in the hormonal milieu have a direct effect on the estrous cycle, causing disruption of the cycle. Thus, we can conclude that prolonged intake of VHFD changes several biochemical parameters and has a drastic effect on the reproductive cycle, affecting fertility. The effects of a high-fat diet on reproductive tissues are irreparable and irreversible.
Acknowledgement
The authors acknowledge KaYan Ng for her technical help in the study.
Footnotes
Source of support: Departmental sources
References
- 1.Caballero B. The global epidemic of obesity: An overview. Epidemiol Rev. 2007;29:1–5. doi: 10.1093/epirev/mxm012. [DOI] [PubMed] [Google Scholar]
- 2.Ross SE, Flynn JI, Pate RR. What is really causing the obesity epidemic? A review of reviews in children and adults. J Sports Sci. 2015;34:1–6. doi: 10.1080/02640414.2015.1093650. [DOI] [PubMed] [Google Scholar]
- 3.Deitel M. Overweight and obesity worldwide now estimated to involve 1.7 billion people. Obes Surg. 2003;13:329–30. doi: 10.1381/096089203765887598. [DOI] [PubMed] [Google Scholar]
- 4.Sahoo K, Sahoo B, Choudhury AK, et al. Childhood obesity: Causes and consequences. J Family Med Prim Care. 2015;4:187–92. doi: 10.4103/2249-4863.154628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lobstein T, Jackson-Leach R, Moodie ML, et al. Child and adolescent obesity: part of a bigger picture. Lancet. 2015;385:2510–20. doi: 10.1016/S0140-6736(14)61746-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab. 2004;89:2583–89. doi: 10.1210/jc.2004-0535. [DOI] [PubMed] [Google Scholar]
- 7.Bourgeois F, Alexiu A, Lemonnier D. Dietary-induced obesity: Effect of dietary fats on adipose tissue cellularity in mice. Br J Nutr. 1983;49:17–26. doi: 10.1079/bjn19830006. [DOI] [PubMed] [Google Scholar]
- 8.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 9.Hensrud DD. Diet and obesity. Curr Opin Gastroenterol. 2004;20:119–24. doi: 10.1097/00001574-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Boozer CN, Schoenbach G, Atkinson RL. Dietary fat and adiposity: A dose-response relationship in adult male rats fed isocalorically. Am J Physiol. 1995;268:546–50. doi: 10.1152/ajpendo.1995.268.4.E546. [DOI] [PubMed] [Google Scholar]
- 11.Ghibaudi L, Cook J, Farley C, et al. Fat intake affects adiposity, comorbidity factors, and energy metabolism of sprague-dawley rats. Obes Res. 2002;10:956–63. doi: 10.1038/oby.2002.130. [DOI] [PubMed] [Google Scholar]
- 12.Pasquali RPL, Gambineri A. Obesity and infertility. Curr Opin Endocrinol Diabetes Obes. 2007;14:482–87. doi: 10.1097/MED.0b013e3282f1d6cb. [DOI] [PubMed] [Google Scholar]
- 13.Klenov VE, Jungheim ES. Obesity and reproductive function: A review of the evidence. Curr Opin Obstet Gynecol. 2014;26:455–60. doi: 10.1097/GCO.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 14.Aladashvili-Chikvaidze N, Kristesashvili J, Gegechkori M. Types of reproductive disorders in underweight and overweight young females and correlations of respective hormonal changes with BMI. Iran J Reprod Med. 2015;13:135–40. [PMC free article] [PubMed] [Google Scholar]
- 15.Hecht Baldauff N, Arslanian S. Optimal management of polycystic ovary syndrome in adolescence. Arch Dis Child. 2015;100:1076–83. doi: 10.1136/archdischild-2014-306471. [DOI] [PubMed] [Google Scholar]
- 16.Castillo-Martinez L, Lopez-Alvarenga JC, Villa AR, Gonzalez-Barranco J. Menstrual cycle length disorders in 18- to 40-y-old obese women. Nutrition. 2003;19:317–20. doi: 10.1016/s0899-9007(02)00998-x. [DOI] [PubMed] [Google Scholar]
- 17.Blum WF. Leptin: The voice of the adipose tissue. Horm Res. 1997;48(Suppl 4):2–8. doi: 10.1159/000191303. [DOI] [PubMed] [Google Scholar]
- 18.Barash IA, Cheung CC, Weigle DS, et al. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137:3144–47. doi: 10.1210/endo.137.7.8770941. [DOI] [PubMed] [Google Scholar]
- 19.Tena-Sempere M. Keeping puberty on time: Novel signals and mechanisms involved. Curr Top Dev Biol. 2013;105:299–329. doi: 10.1016/B978-0-12-396968-2.00011-7. [DOI] [PubMed] [Google Scholar]
- 20.Williams G, Bing C, Cai XJ, et al. The hypothalamus and the control of energy homeostasis: different circuits, different purposes. Physiol Behav. 2001;74:683–701. doi: 10.1016/s0031-9384(01)00612-6. [DOI] [PubMed] [Google Scholar]
- 21.Legradi G, Emerson CH, Ahima RS, et al. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology. 1997;138:2569–76. doi: 10.1210/endo.138.6.5209. [DOI] [PubMed] [Google Scholar]
- 22.Legradi G, Emerson CH, Ahima RS, et al. Arcuate nucleus ablation prevents fasting-induced suppression of ProTRH mRNA in the hypothalamic paraventricular nucleus. Neuroendocrinology. 1998;68:89–97. doi: 10.1159/000054354. [DOI] [PubMed] [Google Scholar]
- 23.Kopp W, Blum WF, von Prittwitz S, et al. Low leptin levels predict amenorrhea in underweight and eating disordered females. Mol Psychiatry. 1997;2:335–40. doi: 10.1038/sj.mp.4000287. [DOI] [PubMed] [Google Scholar]
- 24.Warren MP, Voussoughian F, Geer EB, et al. Functional hypothalamic amenorrhea: Hypoleptinemia and disordered eating. J Clin Endocrinol Metab. 1999;84:873–77. doi: 10.1210/jcem.84.3.5551. [DOI] [PubMed] [Google Scholar]
- 25.Dunaif A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 26.Legro RS. Polycystic ovary syndrome: The new millenium. Mol Cell Endocrinol. 2001;184:87–93. doi: 10.1016/s0303-7207(01)00640-2. [DOI] [PubMed] [Google Scholar]
- 27.Diamanti-Kandarakis E, Kandarakis HA. Conservative management of gynecologic diseases: insulin sensitizing agents in polycystic ovary syndrome. Ann NY Acad Sci. 2003;997:322–29. doi: 10.1196/annals.1290.035. [DOI] [PubMed] [Google Scholar]
- 28.Kjaer K, Hagen C, Sando SH, Eshoj O. Epidemiology of menarche and menstrual disturbances in an unselected group of women with insulin-dependent diabetes mellitus compared to controls. J Clin Endocrinol Metab. 1992;75:524–29. doi: 10.1210/jcem.75.2.1639955. [DOI] [PubMed] [Google Scholar]
- 29.Zaadstra BM, Seidell JC, Van Noord PA, et al. Fat and female fecundity: Prospective study of effect of body fat distribution on conception rates. BMJ. 1993;306:484–87. doi: 10.1136/bmj.306.6876.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marshall JC, Kelch RP. Gonadotropin-releasing hormone: Role of pulsatile secretion in the regulation of reproduction. N Engl J Med. 1986;315:1459–68. doi: 10.1056/NEJM198612043152306. [DOI] [PubMed] [Google Scholar]
- 31.Wood GA, Fata JE, Watson KL, Khokha R. Circulating hormones and estrous stage predict cellular and stromal remodeling in murine uterus. Reproduction. 2007;133:1035–44. doi: 10.1530/REP-06-0302. [DOI] [PubMed] [Google Scholar]
- 32.Michalakis K, Mintziori G, Kaprara A, et al. The complex interaction between obesity, metabolic syndrome and reproductive axis: A narrative review. Metabolism. 2013;62:457–78. doi: 10.1016/j.metabol.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 33.Schreiner P, Siracky J. Estrogenic effect in vaginal smears in cancer of the uterine cervix. Neoplasma. 1978;25:637–39. [PubMed] [Google Scholar]
- 34.Freeman M. The neuroendocrine control of the ovarian cycle of the rat. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. Raven Press; New York: 1994. pp. 613–58. [Google Scholar]
- 35.Hubscher CH, Brooks DL, Johnson JR. A quantitative method for assessing stages of the rat estrous cycle. Biotech Histochem. 2005;80:79–87. doi: 10.1080/10520290500138422. [DOI] [PubMed] [Google Scholar]
- 36.Xia QGS. The genetics of human obesity. Ann NY Acad Sci. 2013;1281:178–90. doi: 10.1111/nyas.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kratz M, von Eckardstein A, Fobker M, et al. The impact of dietary fat composition on serum leptin concentrations in healthy nonobese men and women. J Clin Endocrinol Metab. 2002;87:5008–14. doi: 10.1210/jc.2002-020496. [DOI] [PubMed] [Google Scholar]
- 38.Riccardi G, Giacco R, Rivellese AA. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin Nutr. 2004;23:447–56. doi: 10.1016/j.clnu.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 39.Ng KYYJ, Chakraborty TR. Estrous cycle in ob/ob and ovariectomized female mice and its relation with estrogen and leptin. Physiol Behav. 2010;99:125–30. doi: 10.1016/j.physbeh.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Guimaraes RA, Asth L, Engelberth RC, et al. Spontaneous failure of the estrous cycle induces anxiogenic-related behaviors in middle-aged female mice. Physiol Behav. 2015;147:319–23. doi: 10.1016/j.physbeh.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 41.Jasik CB, Lustig RH. Adolescent obesity and puberty: The “perfect storm”. Ann NY Acad Sci. 2008;1135:265–79. doi: 10.1196/annals.1429.009. [DOI] [PubMed] [Google Scholar]
- 42.Kieffer TJ, Habener JF. The adipoinsular axis: Effects of leptin on pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2000;278:E1–14. doi: 10.1152/ajpendo.2000.278.1.E1. [DOI] [PubMed] [Google Scholar]
- 43.Cerf ME. High fat diet modulation of glucose sensing in the beta-cell. Med Sci Monit. 2007;13(1):RA12–17. [PubMed] [Google Scholar]
- 44.Balasubramanian P, Jagannathan L, Mahaley RE, et al. High fat diet affects reproductive functions in female diet-induced obese and dietary resistant rats. J Neuroendocrinol. 2012;24:748–55. doi: 10.1111/j.1365-2826.2011.02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akamine EH, Marcal AC, Camporez JP, et al. Obesity induced by high-fat diet promotes insulin resistance in the ovary. J Endocrinol. 2010;206:65–74. doi: 10.1677/JOE-09-0461. [DOI] [PubMed] [Google Scholar]
- 46.Reynolds KA, Boudoures AL, Chi MM, et al. Adverse effects of obesity and/or high-fat diet on oocyte quality and metabolism are not reversible with resumption of regular diet in mice. Reprod Fertil Dev. 2015;27:716–27. doi: 10.1071/RD14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheung CC, Thornton JE, Kuijper JL, et al. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology. 1997;138:855–58. doi: 10.1210/endo.138.2.5054. [DOI] [PubMed] [Google Scholar]
- 48.Banks WA. Blood-brain barrier and energy balance. Obesity (Silver Spring) 2006;14(Suppl 5):234S–37S. doi: 10.1038/oby.2006.315. [DOI] [PubMed] [Google Scholar]
- 49.Chakraborty S, Sachdev A, Salton SR, Chakraborty TR. Stereological analysis of estrogen receptor expression in the hypothalamic arcuate nucleus of ob/ob and agouti mice. Brain Res. 2008;1217:86–95. doi: 10.1016/j.brainres.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 50.Chehab FF. 20 years of leptin: Leptin and reproduction: Past milestones, present undertakings, and future endeavors. J Endocrinol. 2014;223:T37–48. doi: 10.1530/JOE-14-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Powell-Braxton L, Hollingshead P, Giltinan D, et al. Inactivation of the IGF-I gene in mice results in perinatal lethality. Ann NY Acad Sci. 1993;692:300–1. doi: 10.1111/j.1749-6632.1993.tb26240.x. [DOI] [PubMed] [Google Scholar]