Abstract
Background
DcR3 (decoy receptor 3) has been proposed be involved in development and prognosis of female reproductive cancers, including cervical cancer, ovarian cancer, and breast cancer. The purpose of this meta-analysis was to explore the evidence for the correlation between DcR3 and the clinicopathological characteristics, as well as the overall survival time, in female reproductive cancers.
Material/Methods
Relevant studies were searched for in PubMed, Wiley Online Library, Web of Science, Science Direct, Cochrane Central Register of Controlled Trials, Google Scholar, EMBASE, Ovid, LILACS, Chinese CNKI, Chong Qing VIP, Wan Fang, and China Biology Medicine disc up to 30 September 2015. Data on the relationship between DcR3 expression and TNM stage, differentiation, lymph node metastasis, age, and overall survival time were extracted. Pooled odds ratios (ORs) and 95% CIs (confidence intervals) were estimated by forest plot.
Results
Twelve studies with 1127 patients met the inclusion criteria for this meta-analysis. Overexpression of DcR3 was significantly related to the risk of female reproductive cancers (OR=10.69, 95% CI: 6.33–18.05), TNM stage (OR=5.51, 95% CI: 2.83–10.71), differentiation (OR=4.16, 95% CI: 2.28–7.60), lymph node metastasis (OR=5.89, 95% CI: 3.16–10.9), age (OR=0.85, 95% CI: 0.51–1.44), and overall survival time (OR=1.84, 95% CI: 0.58–5.83). Subgroup analyses showed that overexpression of DcR3 in cervical, ovarian, and breast cancer all had similar relationships with these clinicopathological parameters.
Conclusions
Our meta-analysis suggests that overexpression of DcR3 may play vital roles in the tumorigenesis and deterioration of female reproductive cancers. However, the relationship between DcR3 expression and prognosis needs further investigation.
MeSH Keywords: Genes, Neoplasm; Genital Diseases, Female; Meta-Analysis; Prognosis; Receptors, Tumor Necrosis Factor, Member 6b; Survival Analysis
Background
Decoy receptor 3 (DcR3) – also referred to as TR6, M68, or TNFRSF6B – is a family member of the tumor necrosis factor receptor (TNFR) superfamily. DcR3 acts as a binding partner in multiple apoptotic ligands inhibiting apoptosis. DcR3 has been described in many malignant tumors, such as gastric [1], hepatocellular [2], colon [3], lung [4], cervical [5], ovarian [6], and breast cancer [7]. A gastrointestinal cancer meta-analysis [8] reported that overexpression of DcR3 was closely related with clinicopathological features, including TNM stage, grade of differentiation, lymph node metastasis, infiltration degree, and distant metastasis. The present study is the only meta-analysis on the relationship between DcR3 and malignancies.
Female reproductive cancers are among the most common causes of cancer-related death, including breast cancer, ovarian cancer, uterine corpus cancer, and cervical cancer. Some genes and molecules are particularly useful as tracking, identifying, and validating biomarkers for diagnosis and treatment of female reproductive cancers [9–11]. Although some studies have explored the role of DcR3 overexpression in cervical cancer, ovarian cancer, and breast cancer, there is still no summary evidence for the association between DcR3 and overall female reproductive cancers. Therefore, we conducted the present meta-analysis to explore the relation between the level of DcR3, clinicopathological characteristics, and survival of female reproductive cancer patients.
Material and Methods
Search strategy and data extraction
PubMed, Wiley Online Library, Web of Science, Science Direct, Cochrane Central Register of Controlled Trials, Google Scholar, EMBASE, Ovid, LILACS, Chinese CNKI, Chong Qing VIP, Wan Fang, and China Biology Medicine disc were searched up to 30 September 2015 with a random combination of the terms: ‘DcR3 or TR6 or M68 or TNFRSF6B’, ‘cervical or ovarian or ovary or oophoro* or uterine or breast or endometrial or choriocarcinoma or fallopian tube’ and ‘cancer or tumor or carcinoma or neoplas* or malignan*’. All of the relevant literature, including review articles and potential references, were searched for additional pertinent studies. Articles were searched and screened by 2 investigators independently. In the meta-analysis, studies that met the following criteria were eligible: (1) Patients in studies were clearly diagnosed with cervical cancer, ovarian cancer, or breast cancer, as well as other female reproductive cancers; (2) Studies included were case-control studies and evaluated the relationship between DcR3 expression and clinicopathological features or prognosis in female reproductive cancers; (3) The definition of DcR3-positive was tested by immunohistochemistry (IHC) method; (4) Sufficient information of the correlation of DcR3 with clinicopathological features or overall survival time was provided to estimate odds ratio (OR) and hazard ratio (HR); and (5) Articles were written in English or Chinese. Letters, reviews, conference abstracts, and duplicated studies were excluded. Two reviewers screened all information independently to exclude irrelevant studies. Disagreements were resolved by a third reviewer. Two independent reviewers read full texts of all eligible studies and extracted relevant data, including author, year, country, cancer type, patient number, test method, clinicopathological parameters (age, TNM stage, grade of differentiation, and lymph node metastasis), and overall survival time.
Statistics
Stata 12.0 was used for statistical analysis. ORs with 95% confidence intervals (CIs) were calculated with a forest plot. The heterogeneity between the studies was evaluated by I2 test. According to the results of heterogeneity analysis, a random-effects model was used. Potential causes of statistical heterogeneity were explored by subgroup analysis. The software Engauge Digitizer 4.1 was used to extract the survival data from a K-M curve in some articles. Publication bias was analyzed by Begg’s funnel plot test. P<0.05 was considered statistically significant.
Results
Eligible studies
With the aforementioned search strategies, 469 articles were retrieved initially. Of these, 393 were excluded after reviewing the titles and abstracts because these articles described non-human experiments or other cancer types. Then, due to duplication or no report of any relevant outcomes, 26 of the remaining articles were excluded (Figure 1). Finally, a total of 12 eligible studies [5–7,12–20] with 1127 participants were included in this meta-analysis. The characteristics of all 12 articles are summarized in Table 1. The test method of all included studies was only IHC.
Figure 1.
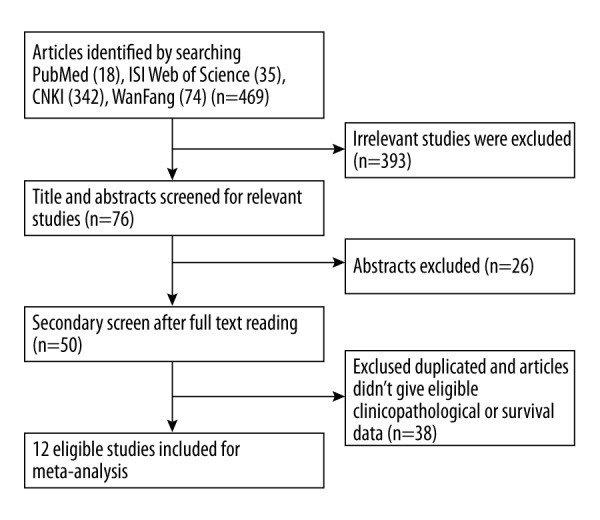
Flow diagram of study identification.
Table 1.
Characteristics of studies included in the meta-analysis.
| No. | Year | Author | Country | Organ | Number | Expression Cancer (+/−) Control (+/−) |
Age Old (+/−) Young (+/−) |
TNM 3,4 (+/−) 1,2 (+/−) |
Differentiation Low (+/−) High (+/−) |
Lymph node metastasis Metastasis (+/−) Non-metastasis (+/−) |
Overall survival time (month) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2013 | Gao SS [5] | China | Cervical | 84 | 36/6 15/27 |
NA | NA | NA | NA | NA |
| 2 | 2012 | Lu JH [12] | China | Cervical | 101 | 27/3 37/34 |
NA | NA | NA | NA | NA |
| 3 | 2011 | Cao YX [13] | China | Cervical | 93 | 42/15 10/26 |
14/3 21/2 |
28/11 8/12 |
15/2 22/18 |
10/0 11/8 |
(+) 66.10±2.78 (−) 77.79±2.22 |
| 4 | 2013 | Wang ZR [14] | China | Cervical | 75 | 29/11 10/25 |
20/24 9/7 |
15/1 14/10 |
12/0 17/11 |
9/1 7/11 |
NA |
| 5 | 2008 | Joseph P [15] | America | Ovarian | 44 | 4/0 18/22 |
NA | NA | 21/19 1/3 |
NA | Median: (+) 31 (−) 36 |
| 6 | 2015 | Cheng L [6] | China | Ovarian | 153 | 77/9 15/52 |
49/3 28/6 |
56/2 21/7 |
52/2 25/7 |
48/2 29/7 |
Median: (+) 31 (−) 46 |
| 7 | 2009 | Wang JO [16] | China | Ovarian | 106 | 44/27 7/28 |
6/3 35/14 |
21/3 20/14 |
21/4 20/13 |
NA | NA |
| 8 | 2011 | Gao HH [17] | China | Breast | 82 | 24/17 10/31 |
NA | 7/1 17/16 |
NA | 17/4 7/13 |
NA |
| 9 | 2005 | Wang LP [7] | China | Breast | 53 | 18/11 0/4 |
NA | NA | NA | 14/6 4/5 |
NA |
| 10 | 2007 | Shan GP [18] | China | Breast | 74 | 25/16 0/33 |
11/8 13/8 |
6/2 19/14 |
11/5 14/11 |
20/7 5/9 |
NA |
| 11 | 2010 | Chen G [19] | China | Breast | 136 | 58/21 0/57 |
32/13 26/8 |
51/6 7/15 |
29/1 29/20 |
32/1 26/20 |
NA |
| 12 | 2014 | Wu QW [20] | China | Breast | 126 | 58/5 34/29 |
17/1 41/4 |
5/1 53/4 |
30/2 23/3 |
28/2 30/3 |
NA |
Meta-analysis
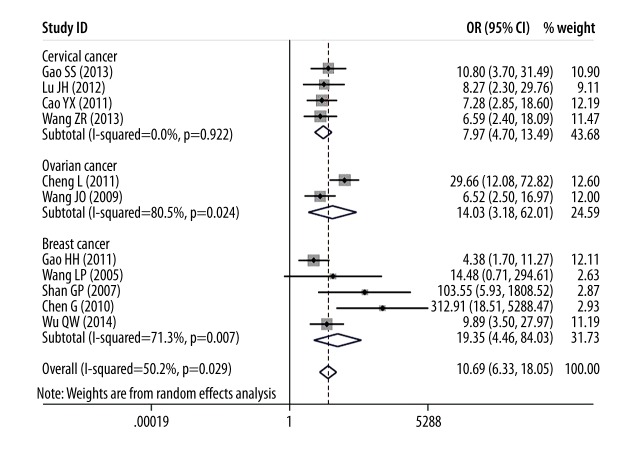
In this meta-analysis, we assessed the correlation between DcR3 expression and cancer risk, clinicopathological features, and overall survival time of patients with female reproductive cancers. As shown in Figure 2, overexpression of DcR3 was associated with female reproductive cancer risk (OR=10.69, 95% CI: 6.33–18.05). Furthermore, subgroup analysis showed a consistent trend; for example, cervical cancer (OR=7.97, 95% CI: 4.70–13.49), ovarian cancer (OR=14.03, 95% CI: 3.16–62.01), and breast cancer (OR=19.35, 95% CI: 4.46–84.03).
Figure 2.
Forest plot of odds ratios (ORs) with corresponding 95% CIs for the association of DcR3 expression with female reproductive cancers risk.
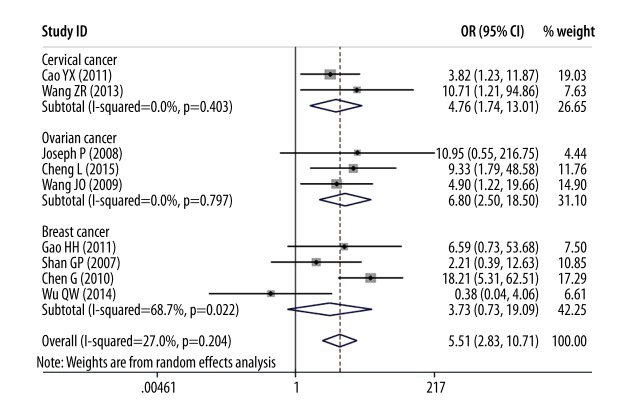
In analysis of female reproductive cancer patients regardless of subtype, 3 clinicopathological parameters were found to be significantly associated with overexpression of DcR3 as compared to controls: advanced TNM stage (OR=5.51, 95% CI: 2.83–10.71), poor grade of differentiation (OR=4.16, 95% CI: 2.28–7.60), and lymph node metastasis (OR=5.89, 95% CI: 3.16–10.96).
In the sub-analysis, a stronger association was found between TNM stage and patients with overexpression of DcR3 in ovarian cancer (OR=6.80, 95% CI: 2.50–18.50) than in cervical cancer (OR=4.76, 95% CI: 1.74–13.01) or breast cancer (OR=3.73, 95% CI: 0.73–19.09, Figure 3).
Figure 3.
Forest plot of odds ratios (ORs) with corresponding 95% CIs for the association of DcR3 expression with TNM stages.
As shown in Figure 4, for each type of cancer, a concordant relationship was also observed between DcR3 expression and grade of differentiation: cervical cancer (OR=7.70, 95% CI: 1.89–31.38), ovarian cancer (OR=4.32, 95% CI: 1.71–10.92), and breast cancer (OR=3.56, 95% CI: 0.79–16.00).
Figure 4.
Forest plot of odds ratios (ORs) with corresponding 95% CIs for the association of DcR3 expression with differentiation.
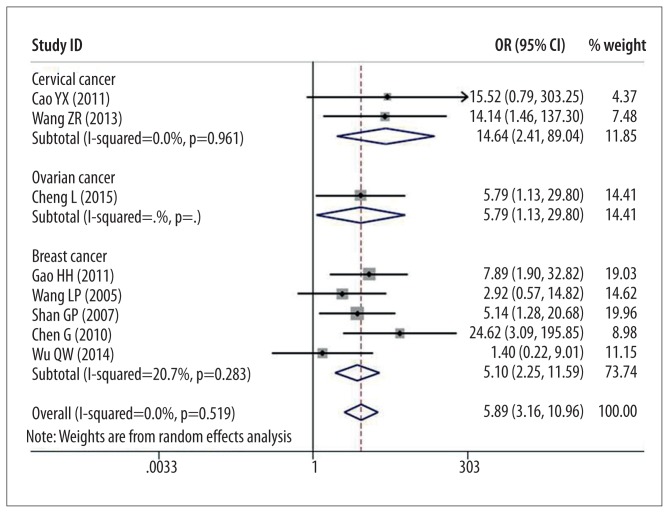
In sub-analysis of association between patients with different cancers with overexpression of DcR3 and lymph node metastasis, cervical cancer had the highest rank (OR=14.64, 95% CI: 2.41–89.04), ovarian cancer was second (OR=5.79, 95% CI: 1.13–29.80), and breast cancer was third (OR=5.10, 95% CI: 2.25–11.59) (Figure 5) (all P<0.05).
Figure 5.
Forest plot of odds ratios (ORs) with corresponding 95% CIs for the association of DcR3 expression with lymph node metastasis.
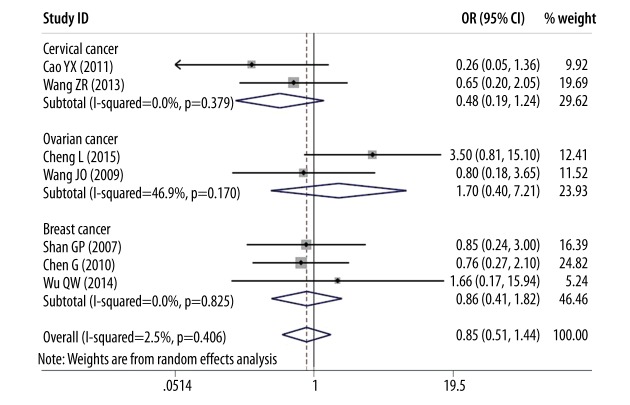
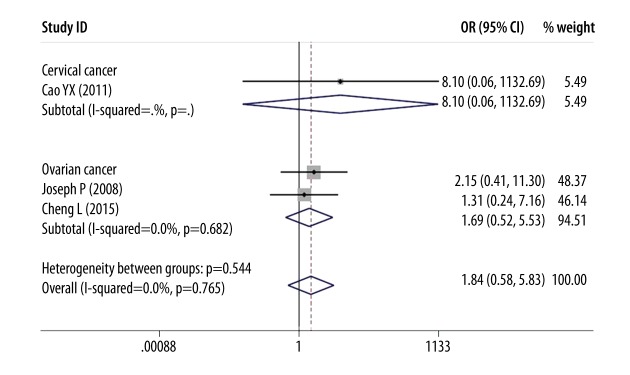
However, as shown in Figures 6 and 7, there was no association between DcR3 overexpression and the age of female reproductive cancer patients (OR=0.85, 95% CI: 0.51–1.44, P=0.554) or overall survival time (OR=1.84, 95% CI: 0.58–5.83, P=0.300).
Figure 6.
Forest plot of odds ratios (ORs) with corresponding 95% CIs for the association of DcR3 expression with age.
Figure 7.
Forest plot of odds ratios (ORs) with corresponding 95% CIs for the association of DcR3 expression with overall survival time.
Publication bias
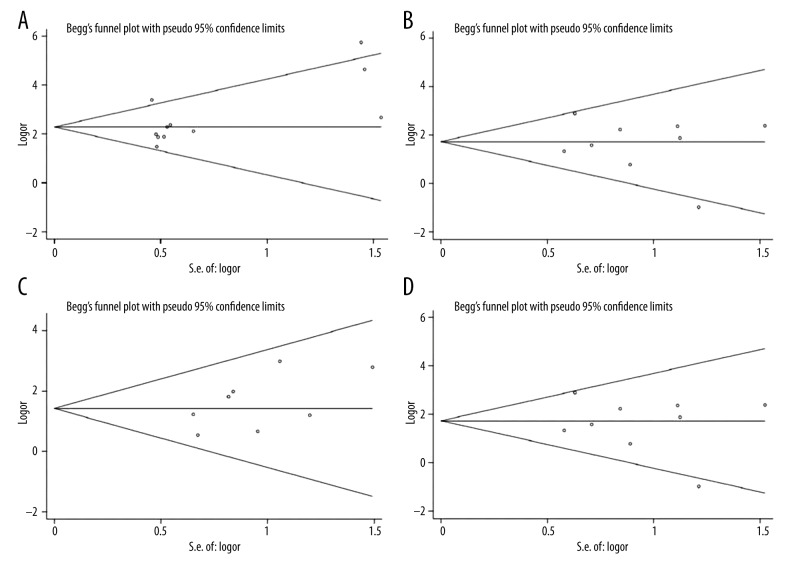
As shown in Figure 8, Begg’s test suggested that there was no publication bias for risk of female reproductive cancers (P=0.097), TNM stage (P=0.559), grade of differentiation (P=0.156), lymph node metastasis (P=0.345), age (P=0.685), or overall survival time (P=0.394).
Figure 8.
(A) Begg’s publication bias plot of publication bias for studies regarding overexpressed DcR3 and female reproductive cancers risk in the meta-analysis. (B) Begg’s publication bias plot of publication bias for studies regarding overexpressed DcR3 and TNM stage in the meta-analysis. (C) Begg’s publication bias plot of publication bias for studies regarding overexpressed DcR3 and differentiation in the meta-analysis. (D) Begg’s publication bias plot of publication bias for studies regarding overexpressed DcR3 and lymph node metastasis in the meta-analysis.
Discussion
Overexpression of DcR3 has been found in many malignant tumors, but, as reported, DcR3 overexpression could not be detected in non-tumor tissues. Studies have shown that DcR3 regulates the activity and differentiation of immune cells, and regulates apoptosis. Therefore, DcR3 may be involved in invasion and metastasis of tumor cells [18]. There are at least 5 possible specific mechanisms involved. First, DcR3 can act as a functional FasL decoy receptor that can bind to Fas ligand (FasL) and inhibit tumor cell killing [21,22]. Second, DcR3 might promote tumor growth by attenuating the Th1 response and suppressing cell-mediated immunity [23–25]. Third, DcR3.Fc (DcR3 fusion protein with immunoglobulin Fc) is able to modulate the expression of a few macrophage markers, including CD14, CD16, CD64, and human leukocyte antigen-DR, suggesting that DcR3 Fc might have potent, suppressive effects in down-regulating the host-immune system [26–28]. Fourth, DcR3 can inhibit stromal cell-derived factor 1 chemotaxis of T lymphocytes, thus reducing the organization of CD4+ and CD8+ T lymphocyte infiltration [29]. Fifth, DcR3 induces the apoptosis of dendritic cell (DC) via activating PKC-delta and JNK, then up-regulates DR5 to recruit Fas-associated death domain (FADD) to propagate the apoptotic signals [30,31]. The functions and mechanisms might not be completely the same in these 3 types of female reproductive cancers.
In breast cancer, DcR3 may be regarded as a negative regulator of cancer aggressiveness during development and progression of certain types of breast cancer by using anti-DcR3 monoclonal antibody and anti-DcR3 hammerhead ribozyme transgenes in breast cancer cells [32,33]. Studies have reported that lymphatic microvessel density (LMVD) was elevated in the cancer tissue and lymph node with metastasis, and DcR3 may promote lymph node metastasis of breast cancer by inducing the formation of new lymphatic vessels and increasing opportunities for lymph node metastasis [17,20]. Wang et al. [7] reported that after the treatment of DcR3 neutralized with DcR3 antibody, the effect on proliferation of breast cancer cell line was decreased, while addition of Fas-L and DcR3 enhanced proliferation. One of the main mechanisms may be that DcR3 blocks the Fas-L-induced apoptosis.
In cervical cancer, Peyre et al. [34] reported that the number of T cells was significantly lower in peripheral blood of cervical cancer patients, and there was an obvious negative correlation between DCR3 expression and CD3+, CD4+/CD8+T in peripheral blood. Therefore, they presumed that DCR3 is related to tumor immune escape in patients with cervical cancer. The expression and distribution of T cells in local tissues of cervical cancer can reflect immune status in the body.
In ovarian cancer, Lin et al. [35] reported that the proliferation of CAOV3 cells was significantly decreased by DcR3 siRNA in comparison with the normal control group and negative control group, indicating that DcR3 siRNA can inhibit the proliferation of ovarian cancer cell line CAOV3 by recognizing and degrading DcR3 mRNA. It has been reported that DcR3 is expressed by epithelial ovarian cancers, concentrated in ascites, and ovarian cancer with high levels of DcR3 is associated with Fas-L-induced apoptosis and platinum resistance [15,36].
Several clinicopathological characteristics are known to be associated with poor prognosis, including large tumor size, advanced tumor stage, poor differentiation, deep invasion, lymph node metastasis, perineural invasion, and lymphatic and vascular invasion [37–41]. A previous meta-analysis [8] reported that overexpression of DcR3 was closely related with some clinicopathological features, including TNM stage, grade of differentiation, lymph node metastasis, infiltration degree, and metastasis in gastrointestinal cancer. Some researchers have reported close correlations between DcR3 expression and prognosis of female reproductive cancer, but no clear evidence was provided. Our analysis suggests that overexpression of DcR3 is related to risk of female reproductive cancer, advanced TNM stage, poor differentiation, and worse lymph node metastasis. After subgroup analysis, overexpression of DcR3 in cervical cancer, ovarian cancer, and breast cancer all had a consistent relationship with these clinicopathological parameters.
To the best of our knowledge, the present meta-analysis is the first to explore the potential relationship between DcR3 expression and female reproductive cancers. However, our study has some limitations. First, we only included 12 studies (including 1127 female reproductive cancers patients), in which just 3 studies (including 290 female reproductive cancers patients) investigated the correlation between DcR3 expression and overall survival time. Therefore, the association of DcR3 expression with overall survival time still needs to be studied in a larger number of samples. Second, there could be potential country bias in our meta-analysis, because only 1 study was performed in the USA, and the patients in the other 11 studies were Chinese. Third, although we aimed to study the relationships between DcR3 expression and all female reproductive cancers, only 3 types of female reproductive cancers were recently eligible: cervical, ovarian, and breast cancer. We will continue to study the relationship between DcR3 expression and other female reproductive cancer types, such as ovary, endometrial, choriocarcinoma, and fallopian tube cancer, and we will update our meta-analysis accordingly.
Conclusions
Our meta-analysis indicates a positive association between the overexpression of DcR3 and carcinogenesis, deterioration, and progression of female reproductive cancers. In conclusion, female productive cancer risk is strongly dependent on overexpression of DcR3, and DcR3 may be used as a biomarker to predict unfavorable cancer prognosis. In addition, our group has previously proved that DcR3 neutralizing antibodies can suppress cell growth and induce apoptosis in glioma cells [42,43], which suggests that DcR3 could be a target in molecular therapy in cancers, including female reproductive cancers.
Footnotes
Source of support: This study was supported partly by the Fund of Guangxi Natural Scientific Research (No. 2015GXNSFAA139187) and Research Fund of Guangxi Bureau of Health (Z2014057)
Statement
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Takahama Y, Yamada Y, Emoto K, et al. The prognostic significance of overexpression of the decoy receptor for Fas ligand (DcR3) in patients with gastric carcinomas. Gastric Cancer. 2002;5:61–68. doi: 10.1007/s101200200011. [DOI] [PubMed] [Google Scholar]
- 2.Chen G, Luo DZ, Wang Y, et al. Relationship between expression of decoy receptor 3 and apoptosis in hepatocellular carcinoma. Zhonghua Bing Li Xue Za Zhi. 2007;36:113–17. [PubMed] [Google Scholar]
- 3.Wu Y, Han B, Sheng H, et al. Clinical significance of detecting elevated serum DcR3/TR6/M68 in malignant tumor patients. Int J Cancer. 2003;105:724–32. doi: 10.1002/ijc.11138. [DOI] [PubMed] [Google Scholar]
- 4.Pitti RM, Marsters SA, Lawrence DA, et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396:699–703. doi: 10.1038/25387. [DOI] [PubMed] [Google Scholar]
- 5.Gao SS. Expression of DcR3 and micro vessel density marked with CDl05 in cervical carcinoma and its significance. China Modern Doctor. 2013;26:32–34. 161. [Google Scholar]
- 6.Cheng L, Yang YH, Zhou CR, et al. Expression of decoy receptor 3 in epithelia ovarian carcinoma and its clinical significance. J Mod Oncol. 2015;1:116–19. [Google Scholar]
- 7.Wang LP, Chen J, Ning HY, et al. Function, expression and significance of TR6 in breast cancer. Chinese J Diagn Pathol. 2005;2:57–60. 97. [Google Scholar]
- 8.Tong J, Ao R, Wang Y, et al. Prognostic and clinicopathological of DcR3 in gastrointestinal cancer: evidence from meta-analysis. Int J Clin Exp Med. 2014;7:3096–105. [PMC free article] [PubMed] [Google Scholar]
- 9.Ayed ME, Bonnel D, Longuespee R, et al. MALDI imaging mass spectrometry in ovarian cancer for tracking, identifying, and validating biomarkers. Med Sci Monit. 2010;16(8):BR233–45. [PubMed] [Google Scholar]
- 10.Balakan O, Kalender ME, Suner A, et al. The relationship between Urotensin II and its receptor and the clinicopathological parameters of breast cancer. Med Sci Monit. 2014;20:1419–25. doi: 10.12659/MSM.890459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan DH, Wei KL, Ling YX, et al. The prognostic role of Ki-67/MIB-1 in cervical cancer: a systematic review with meta-analysis. Med Sci Monit. 2015;21:882–89. doi: 10.12659/MSM.892807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu JH, Xiong M, Shu HM, et al. Expression and clinical significance of DcR3 in cervical intraepithelial neoplasia and cervical cancer. Chin J Obstet Cynecol. 2012;47:150–52. [Google Scholar]
- 13.Cao YX. DcR3 expression and clinical significance of research in cervical cancer. Central South University. 2011 [Google Scholar]
- 14.Wang ZR, Xing LY, Liu NX, et al. Expression and clinical significance of DcR3 in cervical cancer. J Xi’an Jiaotong University (Medical Sciences) 2013;2:224–28. [Google Scholar]
- 15.Joseph P, Connor JP, Felder M. Ascites from epithelial ovarian cancer contain high levels of functional decoy receptor 3 (DcR3) and is associated with platinum resistance. Gynecol Oncol. 2008;111:300–5. doi: 10.1016/j.ygyno.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Wang JO, Lv QJ, Jiang WG, et al. Expression of DcR3 in ovarian cancer and its significance. J Oncol. 2009;11:988–89. [Google Scholar]
- 17.Gao HH. The investigation about the correlation of DcR3 with lymphangiogenesis and lymphatic Metastasis in breast cancer. Fujian Medical University. 2011 [Google Scholar]
- 18.Shan GP, Xu MR, Lv QS, Chi MM. Expression of DcR3 in breast cancer and its clinical significance. Chin J Clin Oncol Rehabil. 2007;2:97–100. [Google Scholar]
- 19.Chen G, Dang YW, Yang MSZ, Luo DZ. Tumor necrosis factor receptor 6 expression and clinical significance in breast cancer. J Practical Med. 2010;19:3539–41. [Google Scholar]
- 20.Wu Q, Zheng Y, Chen D, et al. Aberrant expression of decoy receptor 3 in human breast cancer: relevance to lymphangiogensis. J Surq Res. 2014;188:459–65. doi: 10.1016/j.jss.2014.01.058. [DOI] [PubMed] [Google Scholar]
- 21.Connolly K, Cho YH, Duan R, et al. In vivo inhibition of Fas ligand-mediated killing by TR6, a Fas ligand decoy receptor. J Pharmacol Exp Ther. 2001;298:25–33. [PubMed] [Google Scholar]
- 22.Pitti RM, Marsters SA, Lawrence DA, et al. Genomic amplification of a decoy receptor for Fas ligand in lung and colon cancer. Nature. 1998;396:699–703. doi: 10.1038/25387. [DOI] [PubMed] [Google Scholar]
- 23.Migone TS, Zhang J, Luo X, et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell contimulator. Immunity. 2002;16:479–92. doi: 10.1016/s1074-7613(02)00283-2. [DOI] [PubMed] [Google Scholar]
- 24.Hsu TL, Wu YY, Chang YC, et al. Attenuation of Th1 response in decoy receptor 3 transgenic mice. J Immunol. 2005;175:5135–45. doi: 10.4049/jimmunol.175.8.5135. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Salcedo TW, Wan X, et al. Modulation of T-cell responses to alloantigens by TR6/DcR3. J Clin Invest. 2001;107:1359–468. doi: 10.1172/JCI12159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu MJ, Lin WW, Taso WC, et al. Enhanced adhesion of monocytes via reverse signaling triggered by decoy receptor 3. Exp Cell Res. 2004;292:241–51. doi: 10.1016/j.yexcr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Kim S, Fotiadu A, Kotoula V. Increased expression of soluble decoy receptor 3 in acutely inflamed intestinal epithelia. Clin Immunol. 2005;115:286–94. doi: 10.1016/j.clim.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 28.Chang YC, Hsu TL, Lin HH, et al. Modulation of macrophage differentiation and activation by decoy receptor 3. J Leukoc Biol. 2004;75:486–94. doi: 10.1189/jlb.0903448. [DOI] [PubMed] [Google Scholar]
- 29.Shi G, Wu Y, Zhang J, Wu J. Death decoy receptor TR6/DcR3 inhibits T cell chemotaxis in vitro and in vivo. J Immunol. 2003;171:3407–14. doi: 10.4049/jimmunol.171.7.3407. [DOI] [PubMed] [Google Scholar]
- 30.Hsu TL, Chang YC, Chen SJ, et al. Modulation of dendritic cell differentiation and maturation by decoy receptor 3. J Immunol. 2002;168:4846–53. doi: 10.4049/jimmunol.168.10.4846. [DOI] [PubMed] [Google Scholar]
- 31.You RI, Chang YC, Chen PM, et al. Apoptosis dendritic cells induced by decoy receptor 3 (DcR3) Blood. 2008;111:1480–88. doi: 10.1182/blood-2007-09-114850. [DOI] [PubMed] [Google Scholar]
- 32.Yang MSZ, Chen G, Dang YW, Luo DZ. Effect of DcR3 on apoptosis of breast carcinoma cells and expression in sera of breast carcinoma patients. Cancer Research on Prevention and Treatment. 2011;7:784–7. 798. [Google Scholar]
- 33.Ge ZC, Sanders AJ, Ye L, et al. Expression of death decoy receptor-3(DcR3) in human breast cancer and its functional effects on breast cancer cells in vitro. J Exp Ther and Oncol. 2011;9(2):109–18. [PubMed] [Google Scholar]
- 34.Wang FJ, Wang KK, Chen XB, et al. Correlation between DcR3 expression and T cell subgroup in peripheral blood of uterine cervical cancer. J Immunol. 2015;3:246–49. [Google Scholar]
- 35.Lin JX, Peng Y, Yu GF, et al. Effect of DcR3 interference on the proliferation of ovarian cancer cell CAOV3. J Pra Med. 2015;16:2601–4. [Google Scholar]
- 36.Connor JP, Felder M. Ascites from epithelial ovarian cancer contain high levels of functional decoy receptor (DcR3) and is association with platinum resistance. Gynecol Oncol. 2008;111:330–35. doi: 10.1016/j.ygyno.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Peyre CG, Hagen JA, DeMeester ER, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg. 2008;248:549–56. doi: 10.1097/SLA.0b013e318188c474. [DOI] [PubMed] [Google Scholar]
- 38.Wilson M, Rosato EL, Chojnacki KA, et al. Prognostic significance of lymph node metastases and ratio in esophageal cancer. J Surg Res. 2008;146:11–15. doi: 10.1016/j.jss.2007.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Torres CM, Wang HH, Turner JR, et al. Pathologic prognostic factors in esophageal squamous cell carcinoma: a follow-up study of 74 patients with or without preoperative chemoradiation therapy. Mod Pathol. 1999;12:961–68. [PubMed] [Google Scholar]
- 40.Schoppmann SF, Jesch B, Zacherl J, et al. Lymphangiogenesis and lymphovascular invasion diminishes prognosis in esophageal cancer. Surgery. 2013;153:526–34. doi: 10.1016/j.surg.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 41.Davies AR, Pillai A, Sinha P, et al. Factors associated with early recurrence and death after esophagectomy for cancer. J Surg Oncol. 2014;109:459–64. doi: 10.1002/jso.23511. [DOI] [PubMed] [Google Scholar]
- 42.Huang SN, Chen G, Dang YW, Chen LH. Overexpression of DcR3 and its significance on tumor cell differentiation and proliferation in glioma. Scientific World J. 2014;10:1155–62. doi: 10.1155/2014/605236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruan Y, Huang S, He D, et al. Effect of TNFRSF6B neutralization antibody on cell growth suppression and apoptosis induction in glioma cells. Neoplasma. 2015;62(4):574–81. doi: 10.4149/neo_2015_069. [DOI] [PubMed] [Google Scholar]