Abstract
Background
Early metastasis of osteosarcoma (OS) is highly lethal and responds poorly to drug and radiation therapies. MicroRNAs (miRNAs) are a class of small noncoding RNAs that modulate gene expression at the post-transcriptional level. However, the detailed functions of specific miRNAs are not entirely understood. The aim of the present study was to investigate the role of miR-184 as a mediator of drug resistance in human osteosarcoma.
Material/Methods
qRT-PCR was used to analyze the expression level of miR-184 in OS cell line U-2 OS and MG-63 treated with doxorubicin. MiR-184 agomir or miR-184 antagomir was transferred into cells to regulated miR-184. The target of miR-184 was predicted by TargetScan and confirmed by luciferase reporter assay. Bcl-2-like protein 1 (BCL2L1) expression was detected by Western blot. Cell apoptosis was determined by Annexin V staining and analysis by flow cytometry.
Results
Doxorubicin induced time-dependent expression of miR-184 in OS cell line U-2 OS and MG-63. Luciferase reporter assay identified BCL2L1 as the direct target gene of miR-184. Furthermore, doxorubicin reduced BCL2L1 expression, which was reversed by miR-184 overexpression and further decreased by miR-184 inhibition in OS cells. In addition, miR-184 agomir reduced doxorubicin-induced cell apoptosis, whereas miR-184 antagomir enhanced apoptosis in OS cells, suggesting that up-regulation of miR-184 contributes to chemoresistance of the OS cell line.
Conclusions
Our data show that miR-184 was up-regulated in OS patients treated with doxorubicin therapy and leads to poor response to drug therapy by targeting BCL2L1.
MeSH Keywords: Drug Resistance, MicroRNAs, Osteosarcoma
Background
Osteosarcoma (OS) is one of the most common non-hematological primary bone tumors in children and young adults [1–3]. Although neoadjuvant chemotherapy and improved surgical technology have been the most important treatments to increase the survival rate, the existence of intrinsic or acquired chemoresistance has greatly impeded further improvement in survival rate of osteosarcoma patients with localized tumors and metastatic disease at presentation [4–6]. Earlier studies have shown that tumor chemoresistance is a complex, multistep process characterized by numerous abnormal genes, proteins, microRNAs (miRNAs), and some related signalling pathways [7]. However, the potential molecular mechanisms of chemoresistance in treatment failure remain unclear.
MicroRNAs are a class endogenous small non-coding RNAs that regulate diverse target mRNAs at the level of mRNA degradation or translation in several diseases, including cancers [8–11]. Recently, miR-184 has been reported to be a candidate tumor suppressor gene in breast cancer, glioma, renal cell carcinoma, and other carcinomas [12–15]. Recently, increased attention has been paid to the role of miRNAs in chemoresistance, and their potential role in determining drug sensitivity or resistance has been confirmed. Anomalous miRNAs expression can influence chemoresistance by modulating gene expression of a number of different target genes, such as miRNA-133b, miR-31, miRNA-196a, and miR-506 [16–19]. However, few studies have focused on the involvement of miRNA-184 in the development of doxorubicin resistance in osteosarcoma patients.
In this study, we demonstrated the function of miR-184 in a doxorubicin therapy OS cell line and found that doxorubicin induced time-dependent expression of miR-184 in OS cell lines U-2 OS and MG-63. Luciferase reporter assay identified BCL2L1 as the direct target gene of miR-184. Moreover, doxorubicin inhibited BCL2L1 expression, which was reversed by miR-184 overexpression in OS cells. In addition, miR-184 agomir reduced doxorubicin-induced cell apoptosis, whereas miR-184 antagomir enhanced apoptosis in the doxorubicin-resistant osteosarcoma cell line, suggesting that up-regulation of miR-184 contributed to chemoresistance of the OS cell line.
Material and Methods
Cell culture and transfection
U-2 OS and MG-63 were purchased from the Cell Bank of the Institute of Biochemistry and Cell Biology, China Academy of Sciences (Shanghai, China) and cultured in DMEM (Gibco, CA, USA) supplemented with 10% FBS (Gibco, NY, USA). For transfection, cells were cultured to 80% confluence and transfected with 50 nM miR-184 agomir or 100 nM antagomir (RiboBio Company, Guangzhou, China) according to the manufacturer’s protocol. Further treatment was done several hours after transfection.
RNA extraction and quantitative real-time PCR
Total RNA, including miRNA, was extracted from the cell lines and tissue samples using TRIzol reagent (Invitrogen, USA). RNA was synthesized into cDNA using a reverse transcriptase kit (Takara, Dalian, China). Amplification of cDNA was performed according to the manufacturer’s protocol using SYBR®Premix Ex Taq™ (Takara, Dalian, China). PCR was carried out in triplicate and analyzed using the ABI 7500 Fast Realtime PCR system (Applied Biosystems, Life Technologies, USA). The relative gene expression level was calculated by the comparative CT method 2−ΔΔCt using U6 as an internal reference. The RT and PCR primers for miR-184 and U6 were purchased from RiBoBio (Guangzhou, China).
Luciferase assay
The wide-type (WT) or mutant (MT) 3′UTR of BCL2L1 was cloned into the Renilla luciferase gene. A mutant 3′UTR of BCL2L1 was cloned by use of GeneChem (Shanghai GeneChem Co., Ltd, Shanghai, China). The U-2 OS cells were cotransfected with the vectors carrying WT 3′UTR or MT 3′UTR and miR-184 agomir or antagomir. Cells were collected 48 h after transfection and analyzed using the Dual-Luciferase Reporter Assay System (Promega Corporation, Fitchburg, USA). Luciferase activity values were normalized relative to that of the Renilla luciferase internal control.
Western blot analysis
Protein quantification was performed according to the Bradford method using the Bio-Rad protein assay kit (Bio-Rad Laboratories Inc., Hercules, CA, USA). A total of 30 μg of samples were run on 10% SDS-PAGE. The proteins were then transferred to NC filter membranes. The membranes were blocked overnight in 5% skim milk in Tris-buffered saline. For immunoblotting, the membranes were incubated at 4°C overnight with the BCL2L1 antibody (1:500; ProteinTech Group, Chicago, USA) or GAPDH antibody (1:5000; Beyotime Biotechnology, Haimen, China), followed by the incubation with the appropriate IRDye 800CW-conjugated secondary antibody (1:5000; Li-Cor Bioscience, USA). The infrared fluorescence image was obtained using the Odyssey infrared imaging system (Li-Cor Bioscience, USA).
Apoptosis analysis
After 48 h of transfection in 6-well plates, cells were exposed to the freshly prepared medium containing different concentrations of 0.5 nM doxorubicin for 24 h. Cells were then harvested and washed with ice-cold phosphate-buffered saline (PBS) and then subjected to Annexin V Apoptosis Detection kit (BD Pharmingen, USA) for staining, which was followed by flow cytometry analysis. The test was repeated 3 times per experiment.
Statistical analysis
Statistical evaluation was performed using the Student’s t-test to differentiate the means of different groups. A p-value of <0.05 was considered statistically significant. SPSS 17.0 software was used to analyze all data.
Results
Doxorubicin induced time-dependent expression of miR-184 in OS cell lines
We first assessed the effects of doxorubicin on the expression of miR-184. As shown in Figure 1A, exposing MG-63 to 0.5 nM or 1.0 nM doxorubicin significantly promoted time-dependent expression of miR-184. After being treated with 0.5 nM doxorubicin for 12 h, the expression of miR-184 reached a peak and then declined. Moreover, 1.0 nM doxorubicin-induced miR-184 expression mimicked the effect of 0.5 nM doxorubicin. Consistently, the effect of doxorubicin on expression of miR-184 in U-2 OS was further confirmed (Figure 1B). These data suggest the key role of miR-184 in chemoresistance of OS cells.
Figure 1.
The effect of doxorubicin on the expression of miR-184 in OS cell lines. (A). mRNA expression of miR-184 in MG-63 exposed to 0.5 nM or 1.0 nM doxorubicin for the indicated time. (B) mRNA expression of miR-184 in U-2 OS after being exposed to 0.5 nM or 1.0 nM doxorubicin for the indicated time. * p<0.05, ** p<0.01 vs. 0 h; Dox – doxorubicin.
BCL2L1 was a direct target of miR-184
BCL2L1 was predicted to be target gene of miR-184 by TargetScan. To verify the prediction, we constructed the luciferase reporter vector containing wild-type or mutant 3′UTR of BCL2L1. As shown in Figure 2A, miR-184 distinctly induced the luciferase activity of vector carrying wild-type 3′UTR of BCL2L1 compared to that carrying mutant 3′UTR of BCL2L1. Next, we assayed the effect of doxorubicin on the expression of BCL2L1. As shown in Figure 2B, doxorubicin inhibited BCL2L1 expression in a time-dependent manner. To clarify the relation between BCL2L1 and miR-184, we further assayed the effect of miR184 on the expression of BCL2L1 in cells treated with or without doxorubicin. We found that up-regulated miR-184 led to a marked increase in the level of BCL2L1 when U-2 OS was treated with doxorubicin, whereas down-regulated miR-184 suppressed BCL2L1 expression (Figure 2C).
Figure 2.
BCL2L1 is a direct target of miR-184 in osteosarcoma cells. (A) Luciferase reporter assay in U-2 OS cells co-transfected with miR-184 or NC and wide-type (WT) or mutant-type (MT) BCL2L1 3′UTR. (B) Expression of BCL2L1 in U-2 OS after exposure to 0.5 nM doxorubicin for the indicated time. (C) The effects of miR-184 agomir and miR-184 antagomir on BCL2L1 expression in cells treated with or without doxorubicin. * p<0.05, ** p<0.01; NC – negative control; ago – miR-184 agomir; antago – miR-184 antagomir.
MiR-184 agomir reduced doxorubicin-induced cell apoptosis but miR-184 antagomir enhanced apoptosis in OS cell lines
As shown in Figure 3A, up-regulated miR-184 significantly inhibited the cell apoptosis induced by doxorubicin, whereas down-regulated miR-184 facilitated doxorubicin-induced cell apoptosis in U-2 OS. Similar results were also found in MG-63 (Figure 3B). These data suggest that miR-184 overexpression promotes resistance of OS cells to doxorubicin.
Figure 3.
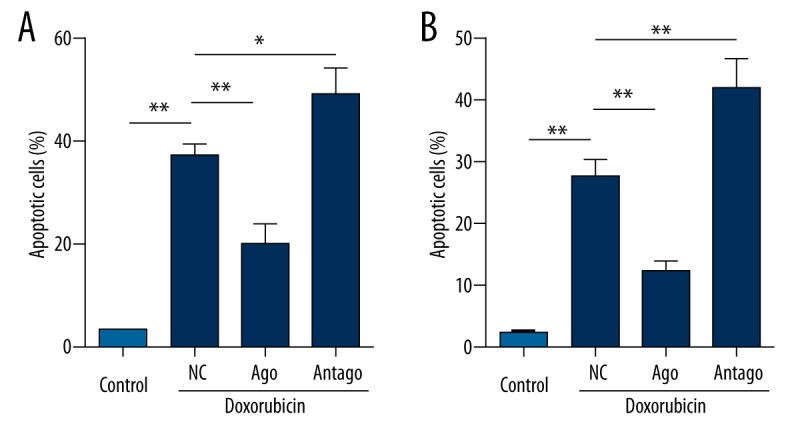
miR-184 contributes to the apoptosis induced by doxorubicin. (A) The effect of miR-184 agomir and miR-184 antagomir on apoptosis of U-2 OS treated with or without 0.5 nM doxorubicin. (B) The effect of miR-184 agomir and miR-184 antagomir on apoptosis of MG-63 treated with or without 0.5 nM doxorubicin. * p<0.05, ** p<0.01; NC – negative control; ago – miR-184 agomir; antago – miR-184 antagomir.
Discussion
Osteosarcoma (OS) is an important cause of cancer-correlated deaths worldwide. OS progression is a complex, multistep process involving numerous abnormal genes and proteins [20,21]. The combination of local radiotherapy/chemotherapy and surgery is currently the main anti-tumor therapy, but treatment fails in many patients, in part due to resistance to chemotherapeutic agents [22,23]. Accumulating studies have recently demonstrated that microRNAs play a key role in cancer chemotherapeutic resistance [16,22,24,25]. MicroRNA-184 functions as a tumor suppressor in carcinomas such as malignant gliomas and non-small cell lung cancers [26,27] and all these antitumor functions are involved in cell proliferation and metastasis [13,14,28]. However, little is known about the contribution of miR-184 to chemoresistance of cancers. In this study, we demonstrated that doxorubicin induced time-dependent expression of miR-184 in OS cells, which suggests that miR-184 is critical for chemoresistance in OS.
To determine the role of miR-184 in chemoresistance of OS, the target of miR-184 was predicted by TargetScan and luciferase reporter assay was performed to confirm the potential target of miR-184. The results showed that miR-184 directly targeted the Bcl-2-like protein 1 (BCL2L1) in U-2 OS cells. Recently, changes in survival and apoptotic pathways such as AKT, BCL-2, and NF-κB pathways have been shown to have a major role in determining the effectiveness of chemotherapeutic agents [22,29]. It is also reported that genomic alterations in BCL2L1 contribute to drug sensitivity in gastric cancer [30]. In addition, the BCL2 family members are dual agents, regulating autophagy and apoptosis in drug resistance [31]. We hypothesized that elevated levels of miR-184 suppressed BCL2L1 expression, allowing OS cells to withstand the toxicity of doxorubicin. We next analyzed the effects of miR-184 agomir and antagomir on BCL2L1 expression in OS cells treated with or without doxorubicin. Our data show that doxorubicin reduced BCL2L1 expression, which was reversed by miR-184 overexpression and was further decreased by miR-184 inhibition.
Given these data, it was likely that miR-184 promotes BCL2L1 expression, which might be related to the doxorubicin-induced up-regulation of miR-184. Then, it was necessary to confirm whether miR-184 amplified BCL2L1 expression to protect OS cells from doxorubicin-induced apoptosis. Thus, we next examined the effects of up-regulated or down-regulated miR-184 on cell death in the presence or absence of doxorubicin. The data showed that miR-184 significantly reduced the number of apoptotic cells from doxorubicin-induced cell death, but down-regulated miR-184 promoted doxorubicin-induced cell apoptosis. These findings suggest that up-regulation of miR-184 made OS cells resistant to doxorubicin.
Conclusions
We found that miR-184 was up-regulated in doxorubicin-treated OS and leads to poor response to drug therapy by targeting BCL2L1. Our data suggest that miR-184 might be a potential biomarker for doxorubicin resistance in OS. For other chemotherapy drugs, more studies need to be conducted.
Footnotes
Source of support: Departmental sources
Conflict of interest
None of the authors have any possible conflicts of interest.
Reference
- 1.Heare T, Hensley MA, Dell’Orfano S. Bone tumors: Osteosarcoma and Ewing’s sarcoma. Curr Opin Pediatr. 2009;21:365–72. doi: 10.1097/MOP.0b013e32832b1111. [DOI] [PubMed] [Google Scholar]
- 2.Moore DD, Luu HH. Osteosarcoma. Cancer Treat Res. 2014;162:65–92. doi: 10.1007/978-3-319-07323-1_4. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Teng Z, Wang Y, et al. Prognostic significance of survivin expression in osteosarcoma patients: A meta-analysis. Med Sci Monit. 2015;21:2877–85. doi: 10.12659/MSM.894448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mankin HJ, Hornicek FJ, Rosenberg AE, et al. Survival data for 648 patients with osteosarcoma treated at one institution. Clin Orthop Relat Res. 2004;(429):286–91. doi: 10.1097/01.blo.0000145991.65770.e6. [DOI] [PubMed] [Google Scholar]
- 5.Bacci G, Briccoli A, Rocca M, et al. Neoadjuvant chemotherapy for osteosarcoma of the extremities with metastases at presentation: recent experience at the Rizzoli Institute in 57 patients treated with cisplatin, doxorubicin, and a high dose of methotrexate and ifosfamide. Ann Oncol. 2003;14:1126–34. doi: 10.1093/annonc/mdg286. [DOI] [PubMed] [Google Scholar]
- 6.Luetke A, Meyers PA, Lewis I, Juergens H. Osteosarcoma treatment – where do we stand? A state of the art review. Cancer Treat Rev. 2014;40:523–32. doi: 10.1016/j.ctrv.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Gillet JP, Gottesman MM. Mechanisms of multidrug resistance in cancer. Methods Mol Biol. 2010;596:47–76. doi: 10.1007/978-1-60761-416-6_4. [DOI] [PubMed] [Google Scholar]
- 8.Cipolla GA. A non-canonical landscape of the microRNA system. Front Genet. 2014;5:337. doi: 10.3389/fgene.2014.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ameres SL, Zamore PD. Diversifying microRNA sequence and function. Nat Rev Mol Cell Biol. 2013;14:475–88. doi: 10.1038/nrm3611. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki H, Maruyama R, Yamamoto E, Kai M. DNA methylation and microRNA dysregulation in cancer. Mol Oncol. 2012;6:567–78. doi: 10.1016/j.molonc.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang JT, Fang JY. MicroRNA regulatory network in human colorectal cancer. Mini Rev Med Chem. 2009;9:921–26. doi: 10.2174/138955709788681672. [DOI] [PubMed] [Google Scholar]
- 12.Phua YW, Nguyen A, Roden DL, et al. MicroRNA profiling of the pubertal mouse mammary gland identifies miR-184 as a candidate breast tumour suppressor gene. Breast Cancer Res. 2015;17:83. doi: 10.1186/s13058-015-0593-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng Z, Wang HZ, Li X, et al. MicroRNA-184 inhibits cell proliferation and invasion, and specifically targets TNFAIP2 in Glioma. J Exp Clin Cancer Res. 2015;34:27. doi: 10.1186/s13046-015-0142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Su Z, Chen D, Li Y, et al. microRNA-184 functions as tumor suppressor in renal cell carcinoma. Exp Ther Med. 2015;9:961–66. doi: 10.3892/etm.2015.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tivnan A, Foley NH, Tracey L, et al. MicroRNA-184-mediated inhibition of tumour growth in an orthotopic murine model of neuroblastoma. Anticancer Res. 2010;30:4391–95. [PMC free article] [PubMed] [Google Scholar]
- 16.Chen S, Jiao JW, Sun KX, et al. MicroRNA-133b targets glutathione S-transferase pi expression to increase ovarian cancer cell sensitivity to chemotherapy drugs. Drug Des Devel Ther. 2015;9:5225–35. doi: 10.2147/DDDT.S87526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuel P, Pink RC, Caley DP, et al. Over-expression of miR-31 or loss of KCNMA1 leads to increased cisplatin resistance in ovarian cancer cells. Tumour Biol. 2015 doi: 10.1007/s13277-015-4081-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 18.Li JH, Luo N, Zhong MZ, et al. Inhibition of microRNA-196a might reverse cisplatin resistance of A549/DDP non-small-cell lung cancer cell line. Tumour Biol. 2015 doi: 10.1007/s13277-015-4017-7. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Liu G, Xue F, Zhang W. miR-506: a regulator of chemo-sensitivity through suppression of the RAD51-homologous recombination axis. Chin J Cancer. 2015;34:44. doi: 10.1186/s40880-015-0049-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu S, Chen Z. The functional role of PMP22 gene in the proliferation and invasion of osteosarcoma. Med Sci Monit. 2015;21:1976–82. doi: 10.12659/MSM.893430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu C, Deng Z, Zhang Y, et al. The prognostic significance of Src and p-Src expression in patients with osteosarcoma. Med Sci Monit. 2015;21:638–45. doi: 10.12659/MSM.892803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dehghanzadeh R, Jadidi-Niaragh F, Gharibi T, Yousefi M. MicroRNA-induced drug resistance in gastric cancer. Biomed Pharmacother. 2015;74:191–99. doi: 10.1016/j.biopha.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–26. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 24.Liu R, Liu X, Zheng Y, et al. MicroRNA-7 sensitizes non-small cell lung cancer cells to paclitaxel. Oncol Lett. 2014;8:2193–200. doi: 10.3892/ol.2014.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Pickard K, Jenei V, et al. miR-153 supports colorectal cancer progression via pleiotropic effects that enhance invasion and chemotherapeutic resistance. Cancer Res. 2013;73:6435–47. doi: 10.1158/0008-5472.CAN-12-3308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emdad L, Janjic A, Alzubi MA, et al. Suppression of miR-184 in malignant gliomas upregulates SND1 and promotes tumor aggressiveness. Neuro Oncol. 2015;17:419–29. doi: 10.1093/neuonc/nou220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Z, Mai C, Yang H, et al. Candidate tumour suppressor CCDC19 regulates miR-184 direct targeting of C-Myc thereby suppressing cell growth in non-small cell lung cancers. J Cell Mol Med. 2014;18:1667–79. doi: 10.1111/jcmm.12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foley NH, Bray IM, Tivnan A, et al. MicroRNA-184 inhibits neuroblastoma cell survival through targeting the serine/threonine kinase AKT2. Mol Cancer. 2010;9:83. doi: 10.1186/1476-4598-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fulda S. Targeting apoptosis for anticancer therapy. Semin Cancer Biol. 2015;31:84–88. doi: 10.1016/j.semcancer.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Park H, Cho SY, Kim H, et al. Genomic alterations in BCL2L1 and DLC1 contribute to drug sensitivity in gastric cancer. Proc Natl Acad Sci USA. 2015;112:12492–97. doi: 10.1073/pnas.1507491112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai Y, Grant S. BCL2L11/Bim as a dual-agent regulating autophagy and apoptosis in drug resistance. Autophagy. 2015;11:416–18. doi: 10.1080/15548627.2014.998892. [DOI] [PMC free article] [PubMed] [Google Scholar]




