Abstract
Background
SIRT 1, as a class III histone deacetylase (HDAC), is implicated in the initiation and progression of malignancies. However, the association of SIRT 1 with tumorigenesis or progression of pancreatic ductal adenocarcinoma (PDAC) is not clear.
Material/Methods
In our study we investigated SIRT 1 expression in PDAC samples and evaluated the association of SIRT 1 level with the clinical and pathological characteristics of PDAC patients. We investigated the role of SIRT 1 in the migration and growth of PDAC PANC-1 or BxPC-3 cells using gain-of-function and loss-of-function approach.
Results
We demonstrated that SIRT 1 mRNA level was significantly promoted in intra-tumor tissues compared to peri-tumor tissues of PDAC; and SIRT 1 overexpression was markedly associated with distant or lymph node (LN) metastasis of these PDAC tissues. Moreover, the in vitro wound healing assay demonstrated that SIRT 1 overexpression with lentivirus vector markedly promoted the migration of PANC-1 or BxPC-3 cells, whereas SIRT 1 knockdown using SIRT 1 specific siRNA transfection significantly inhibited the migration of PDAC cells. The colony forming assay confirmed SIRT 1 promotion of the growth of PANC-1 or BxPC-3 cells.
Conclusions
In summary, SIRT 1 overexpression is significantly associated with metastasis of PDAC, and overexpressed SIRT 1 plays an important role in pancreatic cancer cell migration and growth. Our data warrants further studies on SIRT 1 as a novel chemotherapeutic target in PDAC.
MeSH Keywords: SIRT 1, Metastasis, Pancreatic cancer, Migration
Background
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive cancer with a high incidence and mortality rate [1]. The traditional treatment of PDAC is surgical resection, with 5-year survival rate of less than 26% [2]. PDAC is a malignant cancer with lesion extension, metastases to LN or liver, and contiguous organ invasion [3], leading to cancer progression to an advanced stage. Ultrasound and PET-CT are usually used to detect PDCA [4,5], but effective early detection methods are still lacking. In recent years, researchers have explored molecular factors associated with the prognosis of and therapy for metastasis of PDAC. Chemokine (C-C motif) ligand 18 (CCL18) is reported to be a biomarker for prognosis and diagnosis of PDAC, and thought to induce the expression of SNAIL1 (a zinc-finger transcription factor), and promote the epithelial-mesenchymal transition and the migration of cancer cells [6]. TM4SF1 (transmembrane 4 L six family member 1) is a cell surface protein detected in many cancers; it can reduce the migration of PDAC by increased E-cadherin expression [7]. It has been suggested that miRNAs play a key role in prognosis of PDAC; researchers use miRNAs or anti-miRNAs as a biomarker for PDAC therapy [8]. There are other molecular factors associated with the migration of PDAC, such as nicotinic acetylcholine receptor (nAChR) [9], PEBP4 [10], collagen type V [11], and SIRT 1. These biomarkers are implicated in cell migration with different functions, promotions, or suppressions. Exploring the function of biomarkers contributes to the study of tumorigenesis and migration of PDAC.
SIRT 1 is a class III histone deacetylase, a member of the silent information regulator (SIR) 2 family, and modulates various metabolic pathways [12,13]. It is found in many metabolic tissues such as liver, pancreas, skeletal muscle, adipose tissue, and brain. It regulates the protein deacetylation and impacts the pathophysiology of many metabolic diseases [14,15]. It has been reported that SIRT 1 is a potential therapy for non-alcoholic fatty liver disease (NAFLD) [16], amyotrophic lateral sclerosis (ALS) [17], kidney disease [18,19], and pulmonary disease [20]. In recent years, researchers have focused on the function of SIRT 1 in cancers. What is interesting is that the function of SIRT 1 acting as a tumor promoter or tumor suppressor mainly depends on the tumor type. Several studies provide evidence that SIRT 1 may promote genetic stability and suppress tumor formation [21]. Other studies suggest that SIRT 1 takes part in the oncogenic signaling pathway in breast cancer [22]. Moreover, in non-small-cell lung cancer (NSCLC), SIRT 1 was found to inhibited anti-tumor activities by reducing the expression level of tumor suppressor p27 [23]. High expression of SIRT 1 was found to be a negative prognosticator of PDAC [24]. Therefore, SIRT 1 appears to be an essential molecular factor in cancer progression. At present, there are few studies about SIRT 1 and tumor migration; the association of SIRT 1 and cancer migration in PDAC is still equivocal.
In this study, we focused on SIRT 1 expression in PDAC. We determined the expression level of SIRT 1 in tumor tissues and investigated the role of SIRT 1 in the migration and growth of PDAC PANC-1 or BxPC-3 cells using gain-of-function and loss-of-function approaches. Our results demonstrated the association of SIRT 1 overexpression with cell migration and growth.
Material and Methods
Pancreatic ductal adenocarcinoma tissues and cells culture
In this study, PDAC tissues were collected by surgical resections from 91 PDAC patients, who were registered in the Inner Mongolia People’s Hospital from March 2012 to April 2015. All tissue samples were stored at 80°C before use. Table 1 shows detailed characteristics of the 91 patients. All of the PDAC patients were informed about the study process before the surgical operation, and written consent was obtained for the use of the resected tissues in medical or scientific research. The study was approved by the Institutional Ethics Committee of our hospital. Pancreatic cancer PANC-1 and BxPC-3 cells were purchased from American Type Culture Collection (ATCC) (Rockville, MD, USA), and were cultured in RPMI1640 medium (Gibco, Rockville, MD, USA), and supplemented with 10% FBS (fetal bovine serum, Gibco, Rockville, MD, USA), 100 U/mL of penicillin, and 100 μg/mL of streptomycin (Sigma-Aldrich, St. Louis, MO, USA) at 37°C with 5% CO2.
Table 1.
Correlation of Sirt 1 overexpression with the metastasis of pancreatic ductal adenocarcinoma.
| Characteristics | Cases (n=91) | High Sirt 1* | Low Sirt 1 | P value | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Age (years) | 0.250** | |||||
| <65 | 45 | 22 | 56.41 | 23 | 44.23 | |
| ≥65 | 46 | 17 | 43.59 | 29 | 55.77 | |
| WHO tumor stage | 0.281 | |||||
| I–IIA | 20 | 7 | 17.95 | 13.1 | 25.27 | |
| IIB | 61 | 25 | 64.10 | 36 | 69.23 | |
| III | 6 | 4 | 10.26 | 1.71 | 3.30 | |
| IV | 4 | 3 | 7.69 | 1.14 | 2.20 | |
| Tumor stage | 0.250 | |||||
| T1 | / | / | / | / | / | |
| T2 | 24 | 7 | 17.95 | 17 | 32.69 | |
| T3 | 60 | 28 | 71.79 | 32 | 61.54 | |
| T4 | 7 | 4 | 10.26 | 3 | 5.77 | |
| LN metastasis | 39 | 0.014 | ||||
| Positive | 38 | 22 | 56.41 | 16 | 30.77 | |
| Negative | 53 | 17 | 43.59 | 36 | 69.23 | |
| Distant metastasis | 0.020 | |||||
| Positive | 30 | 18 | 46.15 | 12 | 23.08 | |
| Negative | 61 | 21 | 53.85 | 40 | 76.92 | |
| Grade | 0.006 | |||||
| G1 | 19 | 4 | 10.00 | 15 | 28.85 | |
| G2 | 32 | 11 | 27.50 | 21 | 40.38 | |
| G3 | 41 | 25 | 62.50 | 16 | 30.77 | |
Standard for low or high Sirt 1 expression: Sirt 1 mRNA level was less than two folds of peritumor tissues.
P value was calculated with chi-square test.
Construction of the recombinant plasmid, cell transfection and infection
For the overexpression of SIRT 1 in PANC-1 or BxPC-3 cells, the SIRT 1 coding sequence was amplified and was cloned into pLenti6/TR vector (Invitrogen, Carlsbad, CA, USA). The recombinant virus of pLenti-SIRT 1 or control pLentivirus (pLenti-Con) was produced by cotransfecting 293T cells with pLenti-SIRT 1 or pLenti-Con and ViraSafe™ Lentiviral Packaging System (Cell Biolabs, San Diego, CA, USA). We used pLenti-SIRT 1 or pLenti-Con virus with 5 MOI (multiplicity of infection) to infect PANC-1 or BxPC-3 cells for 0 or 24 hours. To knockdown the SIRT 1 expression in PANC-1 cells, 30 or 60 nM SIRT 1-specific siRNA (siRNA-SIRT 1) or the scramble siRNA (siRNA-Con) (Genscript, Nanjing, China) was transfected with INTERFERin siRNA transfection reagent (Polyplus-Transfection Inc., San Marcos, CA, USA) into the PANC-1 or BxPC-3 cells with more than 85% confluence to abrogate the SIRT 1 expression.
Quantitative analysis of SIRT 1 with RT-qPCR
To investigate the expression level of SIRT 1, we examined the mRNA level of SIRT 1 by RT-qPCR. The pancreatic cancers tissues (intra-tumor tissues and peri-tumor tissues), and PANC-1 or BxPC-3 cells were lysed with Trizol reagent (Life Technologies, Grand Island, NY, USA) according to the manufacturer’s protocol; the mRNA samples were dissolved in RNase-free water and stored at −70°C immediately following isolation and before using. RT-qPCR reaction was performed using SYBR Green OneStep RT-PCR Kit (Takara, Tokyo, Japan) for each sample according to the manufacturer’s manual. After the reaction, the mRNA level of SIRT 1 was calculated and presented as the relative level of SIRT 1 to β-actin (as an internal control) by ΔΔ Ct method.
Cell counting and imaging of cell migration
PANC-1 or BxPC-3 cells were seeded into 24-well plates to 85% confluence, and then infected with 5 MOI pLenti-SIRT 1 or pLenti-Con virus, or transfected with 30 or 60 nM siRNA-SIRT 1 or siRNA-Con. Then cells in each well were scratched with a cell scraper, and observed for 24 or 48 hours. The cells that migrated across the baseline were counted.
Colony formation assay
For the colony formation assay, PANC-1 or BxPC-3 cells (200–500 cells per well) were seeded into 12-well plates, and then infected with 5 MOI pLenti-SIRT 1 or pLenti-Con virus, or transfected with 30 or 60 nM siRNA-SIRT 1 or siRNA-Con. Two days later, cells were stained with 0.5% crystal violet (Sigma-Aldrich, St. Louis, MO, USA). Colonies were counted directly on the plate.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). The characteristics of the pancreatic patients were analyzed by chi-square test. The relative mRNA level of SIRT 1 between intra-tumor tissues and peri-tumor tissues were analyzed by paired t-test. Data on distant metastasis and LN metastasis were analyzed using unpaired t-test between the two groups. The difference in relative SIRT 1 mRNA level between different groups is shown in Table 1 and was calculated with chi-square test. A p value <0.05 was considered statistically significant.
Results
The correlation of SIRT 1 overexpression with the metastasis of PDAC
To study SIRT 1 overexpression with metastasis of PDAC, we collected specimens from 91 patients with PDAC, and analyzed the correlation of SIRT 1 overexpression with different patient characteristics, such as age, WHO tumor stage, LN metastasis, distant metastasis, and grade. The standard for low or high SIRT 1 expression was SIRT 1 mRNA level less than two-fold that of peri-tumor tissues. Result are shown in Table 1. There was no significant difference in the expression level of SIRT 1 by age or tumor stage. However, there was a significant difference in LN metastasis and distant metastasis. The rate of SIRT 1 overexpression was much higher in patients with LN metastasis (p=0.014) or distant metastasis (p=0.020) than those without LN metastasis or distant metastasis. Moreover, the expression level of SIRT 1 was quite different by tumor grade; these results suggest that patients with SIRT 1 overexpression were more likely to have G3 than G2 or G1 tumors (p=0.006). This suggests an association of SIRT 1 overexpression with LN metastasis, distant metastasis, and tumor grade.
Overexpression of SIRT 1 in pancreatic cancers associating with cancer migration
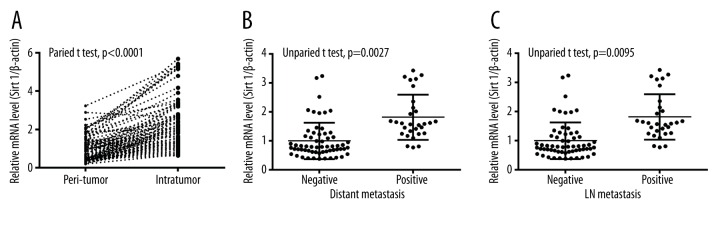
To investigate the association of SIRT 1 overexpression with pancreatic cancer migration, we measured the mRNA level of SIRT 1 between intra-tumor tissues and peri-tumor tissues of pancreatic cancer specimens. The mRNA level of SIRT 1 in intra-tumor tissues was much higher than in peri-tumor tissues, with β-actin as internal control (Figure 1A p<0.0001). In addition, mRNA level in intra-tumor tissues with or without distant metastasis was measured (Figure 1B); the express level of SIRT 1 in the positive group was significant higher than the negative group (p=0.0027). In addition, the mRNA expression level was statistical different between positive and negative LN metastasis (p=0.0095, Figure 1C). Thus, SIRT 1 overexpression was positively associated with cancer migration.
Figure 1.
Overexpression of SIRT 1 in PDACs, associating with cancer migration. (A) Upregulated SIRT 1 mRNA level in intra-tumor tissues vs. peri-tumor tissues of PDACs. (B, C) Relative SIRT 1 mRNA level in PDAC tissues (intra-tumor) with or without distant metastasis (B), or with or without LN metastasis (C); Statistical significance was considered when p<0.05 or less.
SIRT 1 overexpression promotes the migration of pancreatic cancer cells
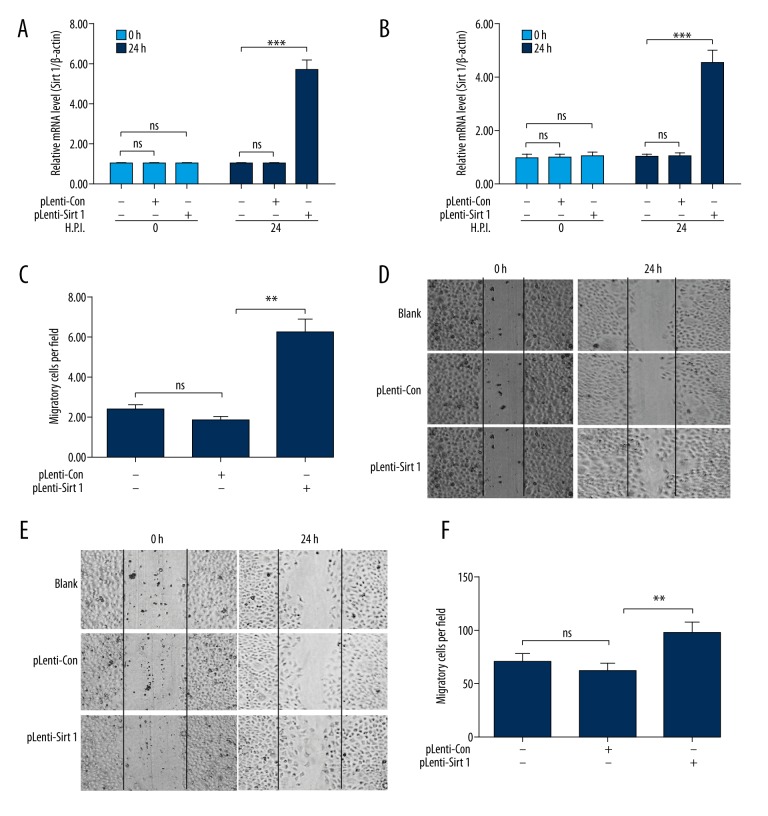
To investigate whether overexpressed SIRT 1 promotes the growth or migration of pancreatic cancer cells, gain-of-function and loss-of-function strategies were used for the cell migration assay and colony forming assay in pancreatic cancer PANC-1 or BxPC-3 cells. The PANC-1 or BxPC-3 cells were infected with one MOI pLenti-SIRT 1 or pLenti-Con virus for 24 hours; we examined the mRNA level of SIRT 1 at 0 and 24 hours. The results indicated that the relative (with β-actin as internal control) mRNA level of SIRT 1 in PANC-1 cells (Figure 2A) or BxPC-3 cells (Figure 2B) was significantly upregulated by infection for 24 hours, with one MOI pLenti-SIRT 1 virus compared to pLenti-Con virus (p<0.001). However, mRNA levels of SIRT 1 was not significantly different among the groups at 0 hours post-infection. The migration assay results demonstrated that there were more migrated cells across baseline in the PANC-1 cells post-infection with one MOI pLenti-SIRT 1 than for post-infection with one MOI pLenti-Con virus for 24 hours (p<0.01, Figure 2C, 2D). The result in BxPC-3 cells also demonstrated that infection with pLenti-SIRT 1 virus promoted cell migration (p<0.01, Figure 2E, 2F). These result demonstrate that SIRT 1 overexpression promotes the migration of pancreatic cancer cells.
Figure 2.
SIRT 1 overexpression promotes the migration of PDAC PANC-1 or BxPC-3 cells. (A, B) Relative SIRT 1 mRNA level in PANC-1 (A) or BxPC-3 (B) cells post-infection with one MOI pLenti-SIRT 1 or pLenti-Con virus for 0 or 24 hours. (C) Representative imaging of PANC-1 cell migration post-infection with one MOI pLenti-SIRT 1 or pLenti-Con virus for 0 or 24 hours. (D) Counting of migratory PANC-1 cells in blank, pLenti-SIRT 1, or pLenti-Con group; Blank: PANC-1 cells without infection with pLenti-SIRT 1 or pLenti-Con virus. E,F: Representative imaging (E) and counting (F) of migratory BxPC-3 cells in blank, pLenti-SIRT 1 or pLenti-Con group. Data was averaged for triple independent results. ** p<0.01, *** p<0.001, ns: not significant.
SIRT 1 knockdown inhibits the migration of pancreatic cancer PANC-1 cells
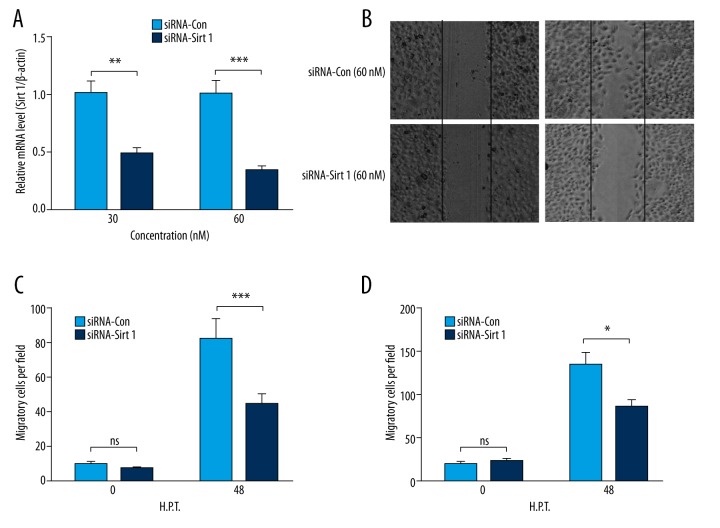
To reconfirm the regulation of SIRT 1 on PANC-1 cell migration, we knocked down SIRT 1 by transfected with SIRT 1-specific siRNA, using siRNA-Con as a control via transfection with 30 or 60 nM siRNA-SIRT 1 or siRNA-Con for 24 hours. Figure 3A demonstrated that the mRNA level of SIRT 1 in the PANC-1 cells was markedly reduced by transfection with siRNA-SIRT 1 compared to siRNA-Con with either 30 or 60 nM (p<0.01 and p<0.001, respectively). Moreover, as indicated by the imaging of the cell migration in Figure 3B, the transfection with siRNA-SIRT 1 significantly reduced the migration of PANC-1 cells (p<0.01, Figure 3C). As such, siRNA-SIRT 1-mediated migration reduction was reconfirmed in BxPC-3 cells (p<0.05, Figure 3D).
Figure 3.
SIRT 1 knockdown inhibits the migration of PDAC PANC-1 or BxPC-3 cells. (A) Relative SIRT 1 mRNA level in PANC-1 cells post-transfection with 30 or 60 nM siRNA-Con or siRNA-SIRT 1 for 24 hours. (B) Representative imaging of migrating PANC-1 cells post-transfection with 60 nM siRNA-Con or siRNA-SIRT 1 for 48 hours, post scratching. (C, D) Counting of migratory PANC-1 (C) or BxPC-3 (D) cells post-transfection with siRNA-Con- or siRNA-SIRT 1. Experiments were performed independently in triplicate. * p<0.05, ** p<0.01, or *** p<0.001, ns: not significant.
Therefore, knockdown SIRT 1 could significantly inhibit the migration of pancreatic cancer cells.
SIRT 1 promotes the growth of pancreatic cancer cells
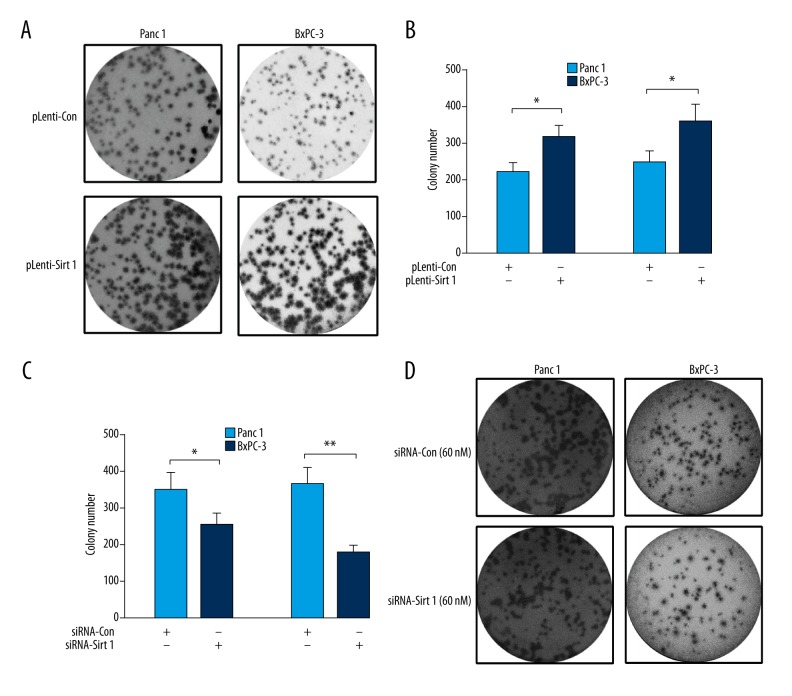
In addition, we evaluated the influence of SIRT 1 on the growth of pancreatic cancer cells with colony formation assay. As shown in Figure 4A, there were more colonies formed by PANC-1 or BxPC-3 cells post-infection with one MOI pLenti-SIRT 1 compared to one MOI pLenti-Con (p<0.05, Figure 4B). On the other hand, there were fewer colonies formed by either PANC-1 or BxPC-3 cells transfected with 60 nM siRNA-SIRT 1 compared to 60 nM siRNA-Con (p<0.05 and p<0.01, respectively, Figure 4C, 4D). Thus, we confirmed the promotion by SIRT 1 of the growth of pancreatic cancer cells.
Figure 4.
Colony forming assay for PDAC Panc-1 or BxPC-3 cells post the up- or downregulation of SIRT 1. (A, B) Representative imaging (A) or counting (B) of the colonies formed by PANC-1 or BxPC-3 cells post-infection with one MOI pLenti-SIRT 1 or pLenti-Con virus for 48 hours. (C, D) Representative imaging (C) or counting (D) of the colonies formed by PANC-1 or BxPC-3 cells post-transfection with 60 nM siRNA-SIRT 1 or with 60 nM siRNA-Con for 48 hours; The experiments were performed independently in triplicate. Statistical significance was shown as * p<0.05, ** p<0.01.
Discussion
SIRT 1 is a key modulator in various metabolic pathways; it is implicated in many diseases, including NAFLD, ALS, and even cancers [25]. SIRT 1 promotes the tumorigenesis of various cancers, such as gastric cancer, lung cancer, and colorectal cancer [26]. Our study confirmed the association of SIRT 1 overexpression with metastasis of PDAC in vivo. Patients with LN metastasis or distant metastasis tended to overexpress SIRT 1 compared to those patients without LN metastasis or distant metastasis. There were fewer patients with SIRT 1 overexpression in G1 than in G2 or G3 tumor groups.
SIRT 1 is involved in protein deacetylation and activation, and involved in activation of various other proteins [16]. The expression level of SIRT 1 is much higher in intra-tumor tissues than in peri-tumor tissues, and higher in patients with distant metastasis or with LN metastasis. SIRT 1 and its ligand modulate the cellular metabolism and further influence the transcription, autophagy DNA damage repair, and apoptosis [25]. Some reports suggest SIRT 1 activates acetylation of FOXO-1, and induces apoptosis in pancreatic cancer [27], but other reports imply that high SIRT 1 expression is a negative prognosticator in PDAC [24]. Our study investigated the regulation by SIRT 1 on migration and growth of PDAC cells in vitro. Results using both gain-of-function and loss-of-function strategies confirmed SIRT 1 promoted the migration and growth of PDAC PANC-1 or BxPC-3 cells. Thus, SIRT 1 is directly associated with the metastasis of PDAC and the expression of SIRT 1 promoted the migration and growth of PDAC cells. However, the mechanisms underlining such a promontory role of SIRT 1 in PDAC cells is not clear.
Conclusions
In summary, this study demonstrated that SIRT 1 was overexpression in PDAC in association with the tumor metastasis. Our study also showed that overexpression of SIRT 1 promoted the migration and growth of PDAC cells. These results suggest that SIRT 1 might be a prognostic biomarker for PDAC, and a novel chemotherapeutic target in PDAC.
Footnotes
Source of support: Departmental sources
References
- 1.Saif MW, Cornfeld D, Modarresifar H, et al. 18F-FDG positron emission tomography CT (FDG PET-CT) in the management of pancreatic cancer: initial experience in 12 patients. J Gastrointestin Liver Dis. 2008;17(2):173–78. [PubMed] [Google Scholar]
- 2.Stark AP, Sacks GD, Rochefort MM, et al. Long-term survival in patients with pancreatic ductal adenocarcinoma. Surgery. 2016 doi: 10.1016/j.surg.2015.12.024. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roldan-Valadez E, Ortega-Lopez N, Valdivieso-Cardenas G, et al. (18)F-FDG PET/CT for discrimination between tumor extension and blood thrombus in pancreatic adenocarcinoma associated with portal vein thrombosis. Rev Esp Med Nucl. 2008;27(1):40–44. doi: 10.1157/13114369. [DOI] [PubMed] [Google Scholar]
- 4.Zins M. [Conventional imaging of pancreatic cancer]. Rev Prat. 2015;65(3):376–78. [PubMed] [Google Scholar]
- 5.Foygel K, Wang H, Machtaler S, et al. Detection of pancreatic ductal adenocarcinoma in mice by ultrasound imaging of thymocyte differentiation antigen 1. Gastroenterology. 2013;145(4):885–94. doi: 10.1053/j.gastro.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng F, Li W, Li C, et al. CCL18 promotes epithelial-mesenchymal transition, invasion and migration of pancreatic cancer cells in pancreatic ductal adenocarcinoma. Int J Oncol. 2015;46(3):1109–20. doi: 10.3892/ijo.2014.2794. [DOI] [PubMed] [Google Scholar]
- 7.Zheng B, Ohuchida K, Cui L, et al. TM4SF1 as a prognostic marker of pancreatic ductal adenocarcinoma is involved in migration and invasion of cancer cells. Int J Oncol. 2015;47(2):490–98. doi: 10.3892/ijo.2015.3022. [DOI] [PubMed] [Google Scholar]
- 8.Kadera BE, Li L, Toste PA, et al. MicroRNA-21 in pancreatic ductal adenocarcinoma tumor-associated fibroblasts promotes metastasis. PLoS One. 2013;8(8):e71978. doi: 10.1371/journal.pone.0071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaal C, Padmanabhan J, Chellappan S. The role of nAChR and calcium signaling in pancreatic cancer initiation and progression. Cancers. 2015;7(3):1447–71. doi: 10.3390/cancers7030845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang D, Dai Y, Cai Y, et al. PEBP4 promoted the growth and migration of cancer cells in pancreatic ductal adenocarcinoma. Tumour Biol. 2015 doi: 10.1007/s13277-015-3906-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Berchtold S, Grunwald B, Kruger A, et al. Collagen type V promotes the malignant phenotype of pancreatic ductal adenocarcinoma. Cancer Lett. 2015;356(2 Pt B):721–32. doi: 10.1016/j.canlet.2014.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Colak Y, Ozturk O, Senates E, et al. SIRT1 as a potential therapeutic target for treatment of nonalcoholic fatty liver disease. Med Sci Monit. 2011;17(5):HY5–9. doi: 10.12659/MSM.881749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng HL, Mostoslavsky R, Saito S, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci USA. 2003;100(19):10794–99. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Canto C, Gerhart-Hines Z, Feige JN, et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458(7241):1056–60. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carafa V, Nebbioso A, Altucci L. Sirtuins and disease: The road ahead. Front Pharmacol. 2012;3:4. doi: 10.3389/fphar.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colak Y, Yesil A, Mutlu HH, et al. A potential treatment of non-alcoholic fatty liver disease with SIRT1 activators. J Gastrointestin Liver Dis. 2014;23(3):311–19. doi: 10.15403/jgld.2014.1121.233.yck. [DOI] [PubMed] [Google Scholar]
- 17.Pasinetti GM, Bilski AE, Zhao W. Sirtuins as therapeutic targets of ALS. Cell Res. 2013;23(9):1073–74. doi: 10.1038/cr.2013.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong L, Wu H, Zhou W, et al. Sirtuin 1: A target for kidney diseases. Mol Med. 2015;21(1):87–97. doi: 10.2119/molmed.2014.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yacoub R, Lee K, He JC. The role of SIRT1 in diabetic kidney disease. Front Endocrinol (Lausanne) 2014;5:166. doi: 10.3389/fendo.2014.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rahman I, Kinnula VL, Gorbunova V, et al. SIRT1 as a therapeutic target in inflammaging of the pulmonary disease. Prev Med. 2012;54(Suppl):S20–28. doi: 10.1016/j.ypmed.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roth M, Chen WY. Sorting out functions of sirtuins in cancer. Oncogene. 2014;33(13):1609–20. doi: 10.1038/onc.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santolla MF, Avino S, Pellegrino M, et al. SIRT1 is involved in oncogenic signaling mediated by GPER in breast cancer. Cell Death Dis. 2015;6:e1834. doi: 10.1038/cddis.2015.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu L, Chiao CY, Enzer KG, et al. SIRT1 inactivation evokes antitumor activities in NSCLC through the tumor suppressor p27. Mol Cancer Res. 2015;13(1):41–49. doi: 10.1158/1541-7786.MCR-14-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stenzinger A, Endris V, Klauschen F, et al. High SIRT1 expression is a negative prognosticator in pancreatic ductal adenocarcinoma. BMC Cancer. 2013;13:450. doi: 10.1186/1471-2407-13-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simmons GJ, Pruitt WM, Pruitt K. Diverse roles of SIRT1 in cancer biology and lipid metabolism. Int J Mol Sci. 2015;16(1):950–65. doi: 10.3390/ijms16010950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hipps D, Ausania F, Manas DM, et al. Selective Interarterial Radiation Therapy (SIRT) in colorectal liver metastases: How do we monitor response? HPB Surg. 2013;2013:570808. doi: 10.1155/2013/570808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pramanik KC, Fofaria NM, Gupta P, et al. CBP-mediated FOXO-1 acetylation inhibits pancreatic tumor growth by targeting SirT. Mol Cancer Ther. 2014;13(3):687–98. doi: 10.1158/1535-7163.MCT-13-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]