Abstract
Background
Paris saponins have been studied for their anticancer effects in various cancer types, but the mechanisms underlying the cytotoxic effects, especially in EGFR-TKI-resistant cells, are still unclear. We explored the potential mechanism of the antitumor effects of PSI, II, VI, VII in EGFR-TKI-resistant cells and attempted to develop PSI, II, VI, VII as a systemic treatment strategy for EGFR-TKI-resistant lung cancer.
Material/Methods
Growth inhibition was detected by MTT assay. The apoptosis assay was detected using annexin-V/PI and Hoechst staining. The level of PI3K, pAKT, Bax, Bcl-2, caspase-3, and caspase-9 protein expression were detected using Western blot analysis.
Results
The results revealed that PSI, II, VI, VII inhibited the proliferation of PC-9-ZD cells. Furthermore, PSI, II, VI, VII induced significant cell apoptosis. The levels of PI3K, pAKT, Bcl-2 protein decreased, while the Bax, caspase-3, and caspase-9 protein was increased by PSI, II, PSVI, PSVII treatment and resulted in increased sensitivity to gefitinib in PC-9-ZD cells.
Conclusions
The underlying mechanism of Paris saponins may be related to targeting the PI3K/AKT pathways to cause apoptosis. Our results suggest a therapeutic potential of Paris saponins in clinical settings for gefitinib-resistant NSCLC.
MeSH Keywords: Antineoplastic Agents; Apoptosis; Carcinoma, Non-Small-Cell Lung; Phosphatidylinositol 3-Kinases
Background
Lung cancer has become a leading cause of mortality due to malignant tumors around the world. Widespread use of small-molecule tyrosine kinase inhibitors (TKIs) presented a new option for treatment for metastatic non-small cell lung cancer (NSCLC). However, despite dramatic responses of EGFR TKIs in NSCLC patients with EGFR mutation was accompanied by emergence of acquired resistance to TKIs [1]. Salvage chemotherapy showed less efficacy and severe chemotherapy-related adverse effects [2]. Hence, developing new therapeutic agents that could be effectively administered and used in the control of TKI-resistant NSCLC is urgently needed. Natural products are suitable alternatives for development of anticancer drugs.
In recent years, steroidal saponins have attracted scientific attention for their structural diversity and significant bioactivities, including their antitumor, hemostatic, immunotropic, and analgesic properties. Therefore, further pharmacological analyses of the steroidal saponins from Paris polychylia are required to investigate their activity to create a foundation for the molecular design and development of new antitumor medicines. Rhizoma paridis is the root of Paris polychylia Smith var. chinensis (French) Hara. Steroidal saponins, which are glycosides with steroid or triterpenoid attached via C3 and ether, are the main constituents in rhizoma paridis [3–7]. Numerous studies have been conducted to explore the anticancer effects of these steroid saponins [8–12]. In recent decades, the potential antitumor effects of Paris saponins (PSs) have been evaluated by many researchers [13–18]. Among them, Paris saponin I (PSI) has been widely studied for its antitumor action in various cancer types [19–24]. PSI possesses various pharmacological activities and cytotoxic activity related to the malignancies by regulating the expression of proteins such as Bax, cytochrome c, caspases, Bcl-2, and the activity of cleaving poly polymerase and extracellular signal-regulated kinase-1/2 [19–24]. PSI is an effective antitumor agent and a good radiosensitizer of TKI-resistant NSCLC cells [25–26]. Recently, Paris saponin II (PSII), Paris saponin VI (PSVI), and Paris saponin VII (PSVII) have received increasing attention due to their potential antitumor effects [27–31]. In attempting to further indicate the antitumor effects and mechanisms of PSI, II, VI, and VII in TKI-resistant lung cancer, we evaluated their effects on cell proliferation, apoptosis, and PI3K/AKT pathway in gefitinib-resistant cell lines.
Material and Methods
Drugs and reagents
Paris saponin I, II, VI, and VII were purchased from the ZheJiang Institute for Food and Drug Control (batch no. 111590,111591,111592,111593, Hangzhou, China). Gefitinib was obtained from Tocris Bioscience (Cat. No.3000, Avonmouth, Bristol, United Kingdom) The purity was greater than 99% and dissolved in dimethyl sulfoxide (DMSO) then stored at −20°C. The drugs were diluted in Dulbecco’s modified Eagle’s minimum essential medium (DMEM) to achieve the final concentration used for the following experiment. We used DMEM and fetal calf serum (Hyclone Co., Logan, UT, USA); FITC Annexin-V Apoptosis Detection kit (BD Biosciences, NJ, USA); as well as Hoechst (Sigma-Aldrich, St. Louis, MO, USA); rabbit anti-rat PI3 Kinase p110α, AKT antibody, Phospho-Akt Antibody, Bcl-2 Antibody, Bax Antibody, Caspase-3 at 1: 1000 dilution (Cell Signaling Technology, Danvers, MA, USA); and a monoclonal mouse anti-rat caspase-9 at 1:1000 dilution (Cell Signaling Technology, Danvers, MA, USA).
Cell lines and culture
Gefitinib-resistant cell line ‘PC-9-ZD’ obtained from the Laboratory of Biochemistry and Molecular Biology (Tongji University, Shanghai, China) were grown in DMEM (Hyclone, Logan, Utah, USA) with 10% fetal bovine serum, 100 μg/ml penicillin, and 100 μg/ml streptomycin at 37°C in a 5% CO2 humidified atmosphere. PC-9-ZD cells were more resistant to gefitinib than their parental PC-9 cells [30].
Growth inhibition assay
The antiproliferative effects of PSI, II, VI, and VII were assessed using MTT assay. PC-9-ZD cells (100 μl/well, 1×104 cells/ml) were seeded and each group had triplicate treatments. A nontreated group was established as the control, and then treated with different concentrations of PSs (0.5, 1, 2, 3, 4, 5, and 6 μg/ml) for 24, 48, and 72 h. Dose-dependent curves were generated and 50% inhibiting concentration (IC50) was used to evaluate the cytotoxic effects of PSs.
Flow cytometry analysis and Hoechst staining for apoptosis
Annexin-V/PI method and Hoechst staining were used to analyze apoptosis with an Annexin-V FITC apoptosis detection kit and Hoechst 33528. Cells were treated with PSs and gefitinib, then harvested at 48 h and stained following the kit instructions. Cells were incubated with the mixture of Annexin-V FITC and PI in the dark. Apoptosis levels were detected by flow cytometry (Beckman Coulter, Inc., Brea, CA, USA). Cells were collected and stained with Hoechst 33528 (5 μg/ml in PBS), then observed under a fluorescence microscope (Olympus BX-60, Olympus Optical Co., Ltd., Tokyo, Japan).
Western blot analysis
Following treatment, cells were lysed with lysis buffer and equal amounts of protein were electrophoresed using 10% sodium dodecyl sulfate-polyacrylamide gel, then transferred to polyvinylidene fluoride membranes (Thermo Fisher Scientific, Inc., Waltham, MA, USA). The membranes were incubated with the following primary antibodies: Rabbit anti-rat PI3 Kinase p110α, AKT antibody, Phospho-Akt Antibody, Bcl-2 Antibody, Bax Antibody, Caspase-3, and mouse anti-rat GAPDH monoclonal antibody (1: 1,000). We then incubated them with the HRP conjugated goat anti-rabbit IgG secondary antibody (1: 10000). The membranes were visualized using an enhanced chemiluminescence system and X-ray films (Santa Cruz Biotechnology Inc.).
Statistical analyses
Data were statistically analyzed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) and presented as means ±S.D. Groups were compared by one-way ANOVA test and SNK-q test, with P<0.05 as the significance level.
Results
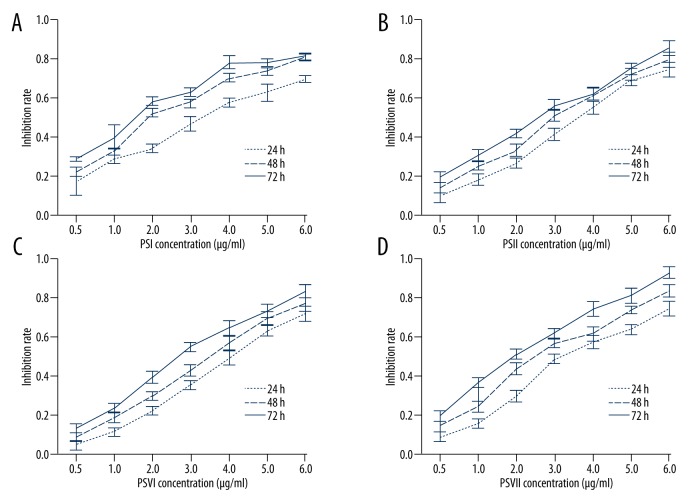
PSI, II, VI, and VII inhibited cell growth in PC-9-ZD cells. PSI, II, VI, and VII inhibited the proliferation of PC-9-ZD cells in a time- and dose-dependent manner, respectively (Figure 1A–1D). The IC50 of PSI, II, VI, and VII for 24, 48, and 72 h is shown in Table 1.
Figure 1.
Paris saponins-induced growth inhibition of a gefitinib-resistant non-small cell lung cancer cell line following exposure at different concentrations. Inhibition rates were significantly increased in the PSI (A), PSII (B), PSVI (C), and PSVII (D) treatment group compared with those in the control group at the same time-point (P<0.01) and was dose dependent.
Table 1.
The IC50 (μg/ml) of PSs in PC-9-ZD cells.
| 24 h | 48 h | 72 h | |
|---|---|---|---|
| PS I | 2.51 | 2.07 | 1.53 |
| PS II | 3.12 | 2.65 | 2.29 |
| PS VI | 4.21 | 3.68 | 2.72 |
| PS VII | 3.57 | 2.41 | 1.85 |
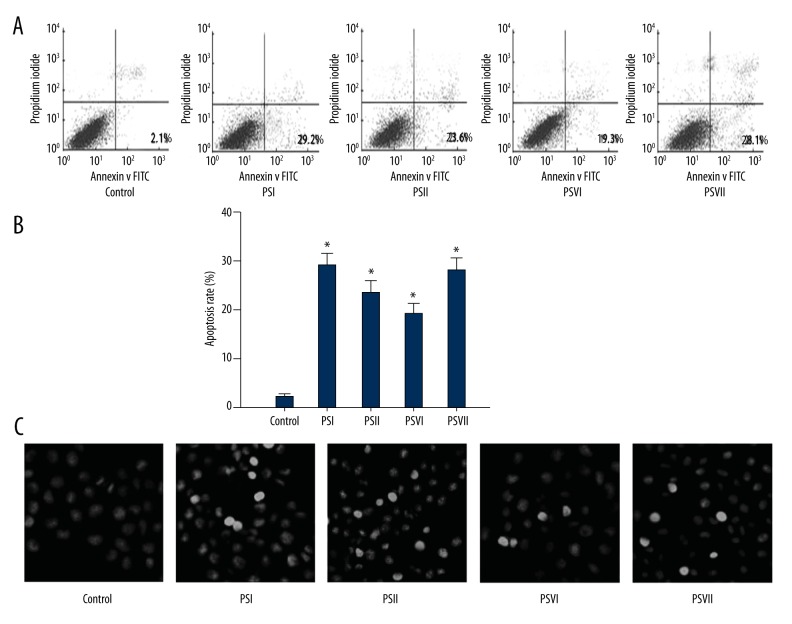
PSI, II, VI, VII induced apoptosis in PC-9-ZD cells. Apoptosis induced by PSI, II, VI, and VII in PC-9-ZD cells was assessed using Annexin-V/PI method and Hoechst staining. PSI, II, VI, and VII (3 μg/ml each) induced a significant apoptosis in PC-9-ZD cells (Figure 2A). The apoptosis rates were 2.1% in the control group, and 29.2%, 23.6%, 19.3%, and 28.1% in the PSI, II, VI, and VII treated groups, respectively, after 48 h (Figure 2B), showing that the control cells were normal in morphology, with regularly shaped nuclei. However, morphological changes such as nuclear shrinkage and chromatin fragmentation were observed in apoptotic cells in the PSI, II, VI, and VII groups (Figure 2C).
Figure 2.
Cells apoptosis induced by Paris saponins. (A) Flow cytometric analysis of apoptosis in the Paris saponins-treated and control groups. (B) Percentage of apoptotic cells at different Paris saponins. * Statistically significant difference (P<0.01) between the treated groups and the control group. (C) Hoechst staining showing features of apoptosis, including nuclear shrinkage, DNA condensation, and chromatin fragmentation in the Paris saponins-treated groups compared with the control group.
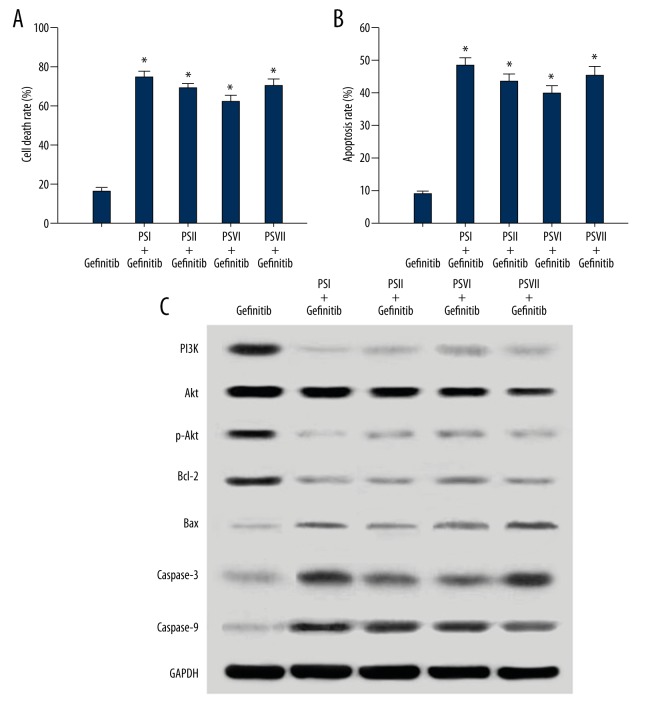
PSI, II, VI, VII inhibited the proliferation and induced apoptosis through the PI3K/AKT pathway in PC-9-ZD cells. The PC-9-ZD cells displayed resistance to gefitinib-induced inhibition of cell growth. We treated cells with PSI, II, VI, and VII (1 μg/ml each) and gefitinib (500 nmol/L) for 48 h. The PC-9-ZD cells were more sensitive to the combined treatment, with inhibition rates of 74.7%, 69.3%, 62.6%, and 70.9% than to that of gefitinib-only treatment, with an inhibition rate of 16.5% (Figure 3A). PSI, II, VI, and VII (1 μg/ml each) with gefitinib (500 nmol/L) induced a significant apoptosis in PC-9-ZD cells with apoptosis rates of 48.4%, 43.7%, 39.9%, and 45.2%, respectively, compare to the gefitinib-only treatment of 9.1% after 48 h (Figure 3B). We then examined a series of protein levels of the PI3K/AKT pathway by Western blot analysis. The level of PI3K, pAKT, and Bcl-2 protein decreased more in PSI, II, VI, and VII combined gefitinib treatment than in gefitinib-only treatment, and the level of Bax, Caspase-3, and Caspase-9 protein increased more in PSI, II, VI, and VII combined gefitinib treatment than in gefitinib-only treatment (Figure 3C).
Figure 3.
(A) Cell death rate after 48 h of gefitinib treatment combined with or without PSI, II, VI, and VII in PC-9-ZD cells. (B) Percentage of apoptotic cells in different groups. * Statistically significant difference (P<0.01) between the gefitinib combined with PSI, II, VI, and VII groups and the gefitinib group. (C) Effect of gefitinib treatment combined with or without PSI, II, VI, and VII on levels of PI3K, AKT, pAKT, Bcl-2, Bax, caspase-3, and caspase-9 protein expression in PC-9-ZD cells. Protein levels were detected using Western blot analysis.
Discussion
Among all NSCLC patients, about 25% are estimated to harbor “activating mutations” in sequences encoding the epidermal growth factor receptor (EGFR), which causes a constitutive activation of the EGFR signaling pathway. In order to target this abnormal hyperactivation, selective agents such as EGFR tyrosine kinase inhibitors (TKIs) have been developed, including gefitinib and erlotinib. Gefitinib has shown measurable efficacy at early stages of treatment, but patients become insensitive to this drug after 6 to 9 months, which finally leads to treatment failure. Some studies have shown that a secondary mutation in EGFR (T790M) is important for acquiring resistance to gefitinib. T790M mutation can alter the conformational space and form a stereo-specific blockade of stable binding between gefitinib and EGFR, resulting in the continued activation of the PI3K/AKT signaling pathway [33–35]. Amplification of the protooncogene Met is also an important mechanism of acquired resistance to gefitinib. MET overexpression drives ErBb3, rather than EGFR, which activates the PI3K/AKT signaling pathway [36,37]. Moreover, the results of these studies suggest that PI3K/AKT inhibitors may block these events, and thereby overcome acquired TKI resistance. The PI3K/Akt pathway has been shown to be a good therapeutic target in tumors. The activation of PI3K also increases the expression of NK-κB and Bcl2, and down-regulates the activity of forkhead transcription factors, caspase 9, MDM2, and Bax, which together influence cell apoptosis [38].
It was demonstrated by numerous studies that Paris saponins possess potential to inhibit malignant tumor cell proliferation and migration, and induce cell apoptosis [19–31]. PSI had definite anticancer effects on various cancer cells [19–26]. PSII, VI, and VII and PSI have similar chemical structural. Further studies needed to explore the efficacy and mechanisms of these in lung cancer treatment, especially in EGFR-TKI-resistant cells.
In the present study, we explored the anticancer effects and underlying mechanism of PSI, II, VI, and VII in EGFR-TKI resistance lung cancer cells and attempted to develop PSI, II, VI, and VII as a systemic treatment strategy for EGFR-TKI-resistant lung cancer. Here, we showed that PSI, II, VI, VII inhibited the proliferation of PC-9-ZD cells in a time- and dose-dependent manner and increased the apoptosis rate in PC-9-ZD cells. The apoptosis rates were 2.1% in the control group, and 29.2%, 23.6%, 19.3%, and 28.1% in the PSI, II, VI, and VII (3 μg/ml each) treated groups, respectively, after 48 h. This was further verified by Hoechst staining, which showed apoptotic changes in morphology. Furthermore, we found that the PC-9-ZD cells were more sensitive to the Paris saponins (1 μg/ml each) and gefitinib (500 nmol/L) combined treatment with inhibition rates of 74.7%,69.3%,62.6%, and 70.9% than to that of gefitinib-only treatment with inhibition rate of 16.5%. Also, Paris saponins (1 μg/ml each) with gefitinib (500 nmol/L) induced significant apoptosis in PC-9-ZD cells, with apoptosis rates of 48.4%, 43.7%, and 39.9%, 45.2%, respectively, compare to the gefitinib-only treatment of 9.1%. We found that the level of PI3K, pAKT, and Bcl-2 protein expression decreased, while the level of Bax, caspase-3, and caspase-9 protein expression increased after combined treatment with Paris saponins (1 μg/ml each) and gefitinib (500 nmol/L). Caspases are critical mediators in apoptosis response. Among them, caspase-3 and caspase-9 were the key proteins to activate death protease, which catalyze the specific cleavage. The most important anti-apoptotic and pro-apoptotic members, like Bcl-2 and Bax, are the main controllers and mediators in apoptosis response. Hence, PSI, II, PSVI, and PSVII can target PI3K/AKT to activate the apoptotic pathway in gefitinib-resistant cell lines.
Conclusions
The underlying mechanism of Paris saponins may be related to targeting the PI3K/AKT pathways to cause apoptosis in PC-9-ZD cells with acquired resistance to gefitinib. These results suggest the therapeutic potential of Paris saponins in clinical settings for gefitinib-resistant NSCLC.
Footnotes
Source of support: This study was supported by a grant from the National Natural Science Foundation of China (grant no. 81303274 and 81202947)
References
- 1.Lin L, Bivona TG. Mechanisms of resistance to epidermal growth factor receptor inhibitors and novel therapeutic strategies to overcome resistance in NSCLC patients. Chemother Res Pract. 2012;2012:817297. doi: 10.1155/2012/817297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramalingam S, Sandler AB. Salvage therapy for advanced non-small cell lung cancer: factors influencing treatment selection. Oncologist. 2006;11:655–65. doi: 10.1634/theoncologist.11-6-655. [DOI] [PubMed] [Google Scholar]
- 3.Yan L, Gao W, Zhang Y, Wang Y. A new phenylpropanoid glycosides from Paris polychylia var.yunnanensis. Fitoterapia. 2008;79:306–7. doi: 10.1016/j.fitote.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 4.Negi JS, Bisht VK, Bhandari AK, et al. Paris polychylia: Chemical and biological prospectives. Anticancer Agents Med Chem. 2014;14:833–39. doi: 10.2174/1871520614666140611101040. [DOI] [PubMed] [Google Scholar]
- 5.He H, Zheng L, Sun YP, et al. Steroidal Saponins from Paris polychylia Suppress Adhesion, Migration and Invasion of Human Lung Cancer A549 Cells Via Down-Regulating MMP-2 and MMP-9. Asian Pac J Cancer Prev. 2014;15:10911–16. doi: 10.7314/apjcp.2014.15.24.10911. [DOI] [PubMed] [Google Scholar]
- 6.Cheng ZX, Liu BR, Qian XP, et al. Proteomic analysis of anti-tumor effects by Rhizoma Paridis total saponin treatment in HepG2 cells. J Ethnopharmacol. 2008;120:129–37. doi: 10.1016/j.jep.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 7.Man S, Gao W, Zhang Y, et al. Antitumor and antimetastatic activities of Rhizoma Paridis saponins. Steroids. 2009;74:1051–56. doi: 10.1016/j.steroids.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Sun J, Liu BR, Hu WJ, et al. In vitro anticancer activity of aqueous extracts and ethanol extracts of fifteen traditional Chinese medicines on human digestive tumor cell lines. Phytother Res. 2007;21:1102–4. doi: 10.1002/ptr.2196. [DOI] [PubMed] [Google Scholar]
- 9.Lee MS, Yuet-Wa JC, Kong SK, et al. Effects of polyphyllin D, a steroidal saponin in Paris polyphylla, in growth inhibition of human breast cancer cells and in xenograft. Cancer Biol Ther. 2005;4:1248–54. doi: 10.4161/cbt.4.11.2136. [DOI] [PubMed] [Google Scholar]
- 10.Chiang HC, Wang JJ, Wu RT. Immunomodulating effects of the hydrolysis products of formosanin C and betaecdysone from Paris formosana Hayata. Anticancer Res. 1992;12:1475–78. [PubMed] [Google Scholar]
- 11.Wu RT, Chiang HC, Fu WC, et al. Formosanin-C, an immunomodulator with antitumor activity. Int J Immunopharmacol. 1990;12:777–86. doi: 10.1016/0192-0561(90)90042-l. [DOI] [PubMed] [Google Scholar]
- 12.Lee JC, Su CL, Chen LL, Won SJ. Formosanin C-induced apoptosis requires activation of caspase-2 and change of mitochondrial membrane potential. Cancer Sci. 2009;100:503–13. doi: 10.1111/j.1349-7006.2008.01057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma DD, Lu HX, Xu LS, Xiao W. Polyphyllin D exerts potent anti-tumour effects on Lewis cancer cells under hypoxic conditions. J Int Med Res. 2009;37:631–40. doi: 10.1177/147323000903700305. [DOI] [PubMed] [Google Scholar]
- 14.Shuli M, Wenyuan G, Yanjun Z, et al. Paridis saponins inhibiting carcinoma growth and metastasis in vitro and in vivo. Arch Pharm Res. 2011;34:43–50. doi: 10.1007/s12272-011-0105-4. [DOI] [PubMed] [Google Scholar]
- 15.GuangLie C, WeiShi G, GaiLing H, JianPing C. Effect of Paris saponin on antitumor and immune function in U14 tumor-bearing mice. Afr J Tradit Complement Altern Med. 2013;10:503–7. doi: 10.4314/ajtcam.v10i3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wen F, Yin H, Chen C, et al. Chemical characteristics of saponins from Paris fargesii var. brevipetala and cytotoxic activity of its main ingredient, paris saponin H. Fitoterapia. 2012;83:627–35. doi: 10.1016/j.fitote.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Zhao Y, Kang LP, Liu YX, et al. Steroidal saponins from the rhizome of Paris polychylia and their cytotoxic activities. Planta Med. 2009;75:356–63. doi: 10.1055/s-0028-1088380. [DOI] [PubMed] [Google Scholar]
- 18.Xiao X, Bai P, Bui Nguyen TM, et al. The antitumoral effect of Paris Saponin I associated with the induction of apoptosis through the mitochondrial pathway. Mol Cancer Ther. 2009;8:1179–88. doi: 10.1158/1535-7163.MCT-08-0939. [DOI] [PubMed] [Google Scholar]
- 19.Xiao M, Dai X, He X, et al. Paris saponin I induces G2/M cell cycle arrest and apoptosis in human gastric carcinoma SGC7901 cells. J Huazhong Univ Sci Technolog Med Sci. 2011;31:768–72. doi: 10.1007/s11596-011-0674-y. [DOI] [PubMed] [Google Scholar]
- 20.Jiang H, Su D, Ma SL. [The effect of Chonglou Saponin I on proliferation and apoptosis in lung adenocarcinoma cell line PC9]. J Chin Oncol. 2012;18:166–69. [in Chinese] [Google Scholar]
- 21.Jiang H, Zhao PJ, Ma SL. [The effect of Paris saponin I on apoptosis associating with PI3K/Akt pathway in pancreatic carcinoma cell line PANC-1]. J Chin Oncol. 2014;20:127–30. [in Chinese] [Google Scholar]
- 22.Jiang H, Zhao PJ, Feng JG, et al. [Radiosensitivity of Paris saponin I on pancreatic carcinoma cell line PANC-1 in vitro]. J Chin Oncol. 2014;20:483–87. [in Chinese] [Google Scholar]
- 23.Xiao X, Zou J, Bui-Nguyen TM, et al. Paris saponin II of Rhizoma Paridis – a novel inducer of apoptosis in human ovarian cancer cells. Biosci Trends. 2012;6:201–11. doi: 10.5582/bst.2012.v6.4.201. [DOI] [PubMed] [Google Scholar]
- 24.Zhao P, Jiang H, Su D, et al. Inhibition of cell proliferation by mild hyperthermia at 43°C with Paris saponin I in the lung adenocarcinoma cell line PC-9. Mol Med Rep. 2015;11:327–32. doi: 10.3892/mmr.2014.2655. [DOI] [PubMed] [Google Scholar]
- 25.Jiang H, Zhao P, Feng J, et al. Effect of Paris saponin I on radiosensitivity in a gefitinib-resistant lung adenocarcinoma cell line. Oncol Lett. 2014;7:2059–64. doi: 10.3892/ol.2014.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang H, Zhao PJ, Su D, et al. Paris saponin I induces apoptosis via increasing the Bax/Bcl-2 ratio and caspase-3 expression in gefitinib-resistant non-small cell lung cancer in vitro and in vivo. Mol Med Rep. 2014;9:2265–72. doi: 10.3892/mmr.2014.2108. [DOI] [PubMed] [Google Scholar]
- 27.Xiao X, Yang M, Xiao J, et al. Paris saponin II suppresses the growth of human ovarian cancer xenografts via modulating VEGF-mediated angiogenesis and tumor cell migration. Cancer Chemother Pharmacol. 2014;73:807–18. doi: 10.1007/s00280-014-2408-x. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Sun Y, Fan L, et al. Paris saponin VII inhibits growth of colorectal cancer cells through Ras signaling pathway. Biochem Pharmacol. 2014;88:150–57. doi: 10.1016/j.bcp.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Fan L, Li Y, Sun Y, et al. Paris saponin VII inhibits metastasis by modulating matrix metalloproteinases in colorectal cancer cells. Mol Med Rep. 2015;11:705–11. doi: 10.3892/mmr.2014.2728. [DOI] [PubMed] [Google Scholar]
- 30.Zhao PJ, Song SC, Du LW, et al. Paris Saponins enhance radiosensitivity in a gefitinib resistant lung adenocarcinoma cell line by inducing apoptosis and G2/M cell cycle phase arrest. Mol Med Rep. 2016;13:2878–84. doi: 10.3892/mmr.2016.4865. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J, Yang Y, Lei L, Tian M. Rhizoma Paridis saponins induces cell cycle arrest and apoptosis in non-small cell lung carcinoma A549 cells. Med Sci Monit. 2015;21:2535–41. doi: 10.12659/MSM.895084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ji Y, Ma SL, Zhang YP, et al. Combined treatment with TNF-alpha/gefitinib alleviates the resistance to gefitinib in PC-9 cells. Anticancer Drugs. 2009;20:832–37. doi: 10.1097/CAD.0b013e32832f4b64. [DOI] [PubMed] [Google Scholar]
- 33.Shih JY, Gow CH, Yang PC. EGFR mutation conferring primary resistance to gefitinib in non-small-cell lung cancer. N Engl J Med. 2005;353:207–8. doi: 10.1056/NEJM200507143530217. [DOI] [PubMed] [Google Scholar]
- 34.Pao W, Miller VA, Politi KA, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci USA. 2008;105:2070–75. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 37.Bean J, Brennan C, Shih JY, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc Natl Acad Sci USA. 2007;104:20932–37. doi: 10.1073/pnas.0710370104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]