Abstract
Background
The purpose of our study was to investigate the functional role of microRNA-340 (miR-340) in endometrial carcinoma (EC).
Material/Methods
Human EC cell line RL 95-2 was transfected with miR-340 mimics, inhibitors, or controls. After 48 h of transfection, the cell viability was determined by 3-(4, 5-dimethyl-2- thiazolyl)-2, 5-diphenyl -2-H-tetrazolium bromide (MTT) assay. The BrdU assay and apoptosis assay were performed to determine the effects of miR-340 mimics or inhibitors on cell proliferation and apoptosis, respectively. The underlying mechanisms involved in cell proliferation and apoptosis were explored by measuring the protein levels of cell cycle regulators (p27 kinase inhibition protein (KIP) 1 and p21) and apoptosis-related factors (B-cell lymphoma-2 (Bcl-2), Bax, pro-Caspase 3, and active-Caspase-3).
Results
Overexpression of miR-340 significantly inhibited the cell viability (P<0.05) and cell proliferation (P<0.01) of RL 95-2 cells compared with the control group, but increased the apoptosis (P<0.01). However, suppression of miR-340 had opposite results. Moreover, the protein levels of p27 KIP1, Bax, pro-Caspase 3, and active-Caspase-3 were significantly increased by overexpression of miR-340 but were statistically decreased by suppression of miR-340. Contrary results were found in the protein levels of Bcl-2. However, no significant differences were found in p21 expression.
Conclusions
MiRNA-340 acts as an anti-oncogene in EC cell line RL 95-2 by inhibition of tumor cell proliferation and induction of apoptosis.
MeSH Keywords: Apoptosis, Cell Proliferation, Endometrial Neoplasms, MicroRNAs
Background
Endometrial carcinoma (EC) is the sixth most common malignant tumor in females, with an estimated 320,000 new cases diagnosed worldwide in 2012 [1]. EC is the most common tumor in the United States and many other developed countries [2]. It is a heterogeneous malignancy, with several histological types that exhibit distinct pathogenesis, clinical presentation, and prognosis [3]. Several risk factors have been reported to be involved in EC, such as hypertension, postmenopausal status, infertility, family history of EC, and long-term use of estrogens [4]. In spite of tremendous advances made in the diagnosis and treatment of EC in recent years, the treatment of advanced stages of the disease is still difficult because of the unclear pathological mechanisms. It has been reported that the 5-year survival rate of the advanced stages is 10–29% [5]. Therefore, it is very important to develop novel diagnostic, prognostic, and/or treatment strategies for this disease.
MicroRNAs (miRNAs) are a class of endogenous, small (20–25 nucleotides in length), non-coding single-stranded RNAs molecules that regulate the expression of target genes [6]. MiRNAs are widely expressed in many species and tissues and play significant roles in various biological processes, including cell proliferation, differentiation, apoptosis, metabolism, cancer development, progression, and metastasis [7–9]. They have been identified to act as both tumor suppressors and oncogenes, which is dependent on the role of their target genes [10,11]. Many miRNAs are frequently aberrantly altered in a variety of cancer types, including EC [12–14]. Moreover, it has been reported that miR-340 is downregulated in many kinds of cancers, such as breast cancer, colorectal cancer, and osteosarcoma [15–17]. Imbalance of cell proliferation and apoptosis had been implicated in EC; nevertheless, the functional role of miR-340 in mediating EC proliferation and apoptosis remains largely elusive.
Therefore, in the present study, we investigated the functional role of miR-340 in EC and focused on the effect of miR-340 on EC proliferation and apoptosis, as well as the underlying mechanisms. Here, we describe the role of miR-340 as a novel anti-oncogene miRNA in EC. Our study might provide new targets for EC therapy and uncover the mechanism of the disease.
Material and Methods
Cell culture
Human endometrial carcinoma cell line RL 95-2 was purchased from the American Type Culture Collection (ATCC). The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM)/F12 (1: 1, Invitrogen) supplemented with 10 mm 4-(2-hydroxyethyl)-1-piperazineëthanesulfonic acid (HEPES, Beyotime Institute of Biotechnology), 5 μg/ml insulin (Gibco BRL, Gaithersburg, MD, USA), and 10% fetal calf serum (FCS, Sigma Chemical Co., St. Louis, MO) at 37°C in a humidified 5% CO2 incubator. The cells were allowed to grow to 70–80% confluence before further treatment. Our study was approved by the Institutional Ethics Committee of the First Affiliated Hospital of Guangxi Medical University.
Transfection with miRNA mimics and inhibitors
MiR-340 mimics, inhibitors, and controls were obtained from GenePharma (Shanghai, China). For the transfection, the cells (2×105/well) were seeded in 96-well plates overnight and then transiently transfected with either miR-340 mimics or inhibitors according to the manufacturer’s instructions. MiR-340 mimics or inhibitors were re-suspended in 50 μl Lipofectamine 2000/Opti-MEM (Invitrogen). The cells were incubated at 37°C for 4 h.
Cell viability
The cell viability was determined by 3-(4, 5-dimethyl-2- thiazolyl)-2, 5-diphenyl -2-H-tetrazolium bromide (MTT) assay. Briefly, the cells (1×105 cells/cm2) were seeded in 96-well plates and incubated for 12 h. At 48 h after transfection with miR-340 mimics or inhibitors, 5 mg/ml MTT solution (20 μl, Sigma Chemical Co., St. Louis, MO) was added to each plate and incubated for 4 h at 37°C. To dissolve the reduced formazan crystals, the medium was replaced with dimethylsulfoxide (DMSO, Sigma Chemical Co., St. Louis, MO). Subsequently, the plate was read in an enzyme-linked immunosorbent microplate-reader (Bio-Rad 2550, Bio-Rad, Hercules, CA, USA) at 590 nm. Each experiment was carried out in triplicate.
BrdU assay
The BrdU assay was performed to determine the effects of miR-340 mimics or inhibitors on cell proliferation. Briefly, the cells were seeded in 6-well plates (2×104 cells/well) on sterilized coverslips. After 48 h of transfection with miR-340 mimics or inhibitors, 10 μM BrdU (Sigma-Aldrich, St. Louis, MO) was added to each plate and incubated at 37°C for 5 h. Thereafter, the cells were washed 3 times with phosphate-buffered saline (PBS) and fixed in cold 70% ethanol for 10 minutes. The cells were incubated with 1.5 M hydrogen chloride (HCl) for 30 minutes and were neutralized by incubating with 0.1 M borate buffer for 10 minutes at room temperature. After washing 3 times with PBS, immunofluorescence was performed to visualize incorporated BrdU by using a mouse anti-BrdU antibody (Santa Cruz Biotechnology) according to the manufacture’s protocol. Thereafter, the cells were incubated with secondary anti-mouse secondary antibody (Santa Cruz Biotechnology) for 1 h at room temperature and mounted in VECTASHIELD mounting medium with DAPI (Vector Laboratories, Burlingame, CA). An automated microscope (DMI6000B) was used to automatically visualize images of the cells.
Apoptosis assay
After 48 h of transfection with miR-340 mimics or inhibitors, the cells were harvested by trypsinization and washed with PBS. The cells were then fixed with ice-cold methanol at −20°C overnight, incubated with 1× binding buffer containing 10 μl Annexin V-FITC and 5 μl propidium iodide (PI) for 20 min in the dark at room temperature. Apoptotic cells were read by a FACScan flow cytometer (Becton Dickinson, NJ, USA) and the results were analyzed by CellQuest® software (BD Biosciences, USA). Annexin V-positive cells were considered as early apoptotic cells, and both Annexin V- and PI-positive cells were recorded as late apoptotic cells. Each condition was repeated at least 3 times.
Quantitative real-time (qRT-PCR)
Total RNA, including miRNAs, was isolated from the cells using TRIzol reagent (Qiagen, Valencia, CA) according to the manufacturer’s protocol. First-strand complementary DNA (cDNA) was synthesized using the TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) and amplified by TaqMan Universal PCR Master Mix (Applied Biosystems). U6 snRNA was used as a loading control. The reverse transcription and primers of miR-340 were provided by GenePharma (Shanghai, China). Experiments were carried out in triplicate and the data were analyzed using the comparative 2−ΔΔCT methods.
Western blotting
The cells were collected in standard RIPA buffer (Thermal) 48 h after transfection with miR-340 mimics or inhibitors. The cell protein concentrations were evaluated by using a BCA protein assays kit (Novogen, Darmstadt, Germany) according to the manufacturer’s instructions. The protein samples were separated on 10–12% sodium dodecyl sulfate (SDS)-PAGE gels and transferred onto nitrocellulose membrane (Millipore, USA). After blocking with 5% nonfat dry milk for 2 h at room temperature, the membranes were probed with the following primary antibodies overnight at 4°C: anti-p27 kinase inhibition protein (KIP) 1 antibody (ab54563, Abcam), anti-p21 antibody (ab7960, Abcam), anti- B-cell lymphoma (Bcl)-2 antibody (ab7973, Abcam), anti-Bax antibody (2772, Cell Signaling Technology), anti-pro-Caspase 3 antibody (ab32150, Abcam), and anti-active-Caspase-3 antibody (ab2302, Abcam). The membranes were then washed with Tris Buffered Saline with Tween (TBST) and incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies with GAPDH as a lysate loading control. Immunoreactive protein bands were visualized with enhanced chemiluminescence (ECL) reagent (GE Healthcare).
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) software (version 17.0; SPSS, Chicago, IL). Statistical significance was determined by analysis of variance (ANOVA) or t test. Non-parametric tests were performed to compare variables between groups when the assumption of normal distribution was not possible. A statistical significance was defined as P<0.05.
Results
Overexpression of miR-340 reduced cell viability in RL 95-2 cells
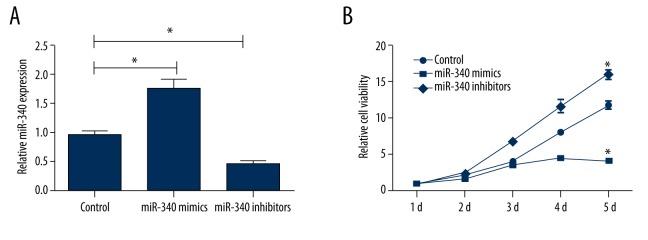
To evaluate the functional role of miR-340 in EC, RL 95-2 cells were transiently transfected with either miR-340 mimics or inhibitors. The expression levels of miR-340 in the cells were determined by using qRT-PCR analysis. As shown in Figure 1A, the results showed that the expression levels of miR-340 were significantly increased by transfection with miR-340 mimics but were significantly decreased by transfection with miR-340 inhibitors (both P<0.05). We further analyzed the effects of miR-340 mimics or inhibitors on cell viability. MTT assay was performed at 1 d, 2 d, 3 d, 4 d, and 5 d of transfection. As shown in Figure 1B, overexpression of miR-340 significantly inhibited the viability of RL 95-2 cells at different time points compared with the control group (P<0.05). However, suppression of miR-340 significantly increased the viability of RL 95-2 cells at different time points compared with the control group (P<0.05). This result indicates that miR-340 might be an important tumor suppressor gene and play a critical role in EC.
Figure 1.
Overexpression of miR-340 reduces cell viability in RL 95-2 cells. (A) Shows that expression levels of miR-340 are significantly increased by transfection with miR-340 mimics but are significantly decreased by transfection with miR-340 inhibitors. (B) Shows that overexpression of miR-340 significantly inhibits the viability of RL 95-2 cells at different time points, but suppression of miR-340 significantly increases the viability of RL 95-2 cells. * P<0.05 compared with the control group.
Overexpression of miR-340 induced cell growth arrest through p27 KIP1
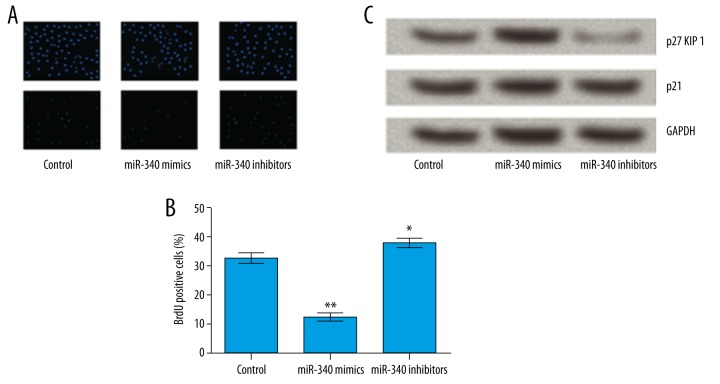
We then determined the effect of miR-340 on cell proliferation by using BrdU assay. As indicated in Figure 2A, 2B, the results showed that overexpression of miR-340 significantly decreased the number of BrdU-positive cells compared with the control group (P<0.01). However, suppression of miR-340 markedly increased the number of BrdU-positive cells compared with the control group (P<0.05). To further understand the mechanisms of miR-340-induced cell growth inhibition, we analyzed the effect of miR-340 on the expression of cell cycle regulators (p27 KIP1 and p21) in RL 95-2 cells. We observed that the protein expression of p27 KIP1 was significantly increased by overexpression of miR-340 but was significantly decreased by suppression of miR-340 compared to the control group. However, no significant differences were found in the effect of abnormal expression of miR-340 on p21 expression (Figure 2C). The results demonstrated that overexpression of miR-340-induced cell growth arrest was correlated with the accumulation of p27 KIP1.
Figure 2.
Overexpression of miR-340 induces cell growth arrest through p27 KIP1. (A, B) Show that overexpression of miR-340 significantly decreases the number of positive cells, while suppression of miR-340 markedly increases the number of positive cells. (C) Shows that the protein levels of p27 KIP1 are significantly increased by overexpression of miR-340 but are significantly decreased by suppression of miR-340. No significant differences were found in p21 expression. KIP – kinase inhibition protein. * P<0.05 compared with the control group; ** P<0.01 compared with the control group.
Overexpression of miR-340 induced apoptosis in RL 95-2 cells
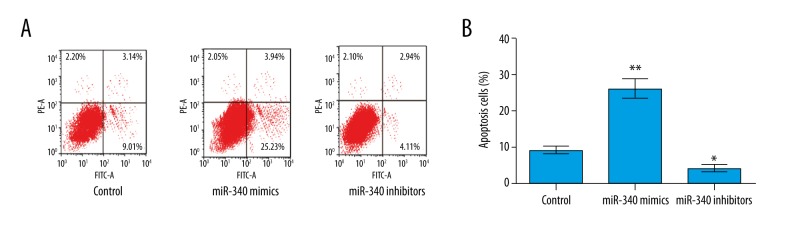
Subsequently, the effect of miR-340 on cell apoptosis was investigated. After transfection with miR-340 mimics or inhibitors, the apoptosis cells were significantly upregulated by overexpression of miR-340 compared to the control group (P<0.01), but the apoptosis cells were significantly downregulated by suppression of miR-340 compared to the control group (P<0.05) (Figure 3A, 3B). The results indicate that overexpression of miR-340 induced apoptosis of RL 95-2 cells.
Figure 3.
Overexpression of miR-340 induces apoptosis in RL 95-2 cells. (A, B) Show that the apoptosis cells were significantly upregulated by overexpression of miR-340, but were significantly downregulated by suppression of miR-340. * P<0.05 compared with the control group; ** P<0.01 compared with the control group.
Overexpression of miR-340 induced apoptosis by regulating Bcl-2, Bax, and Caspase-3 in RL 95-2 cells
To further clarify the mechanisms underlying the effect of miR-340 on cell apoptosis, we measured the protein expression of Bcl-2, Bax, and Caspase-3. After transfection with miR-340 mimics or inhibitors, we found that the expression of Bcl-2 was significantly decreased by overexpression of miR-340 but was significantly increased by suppression of miR-340 compared to the control group. However, the expression of Bax, pro-Caspase-3, and active-Caspase-3 had the opposite results. The protein expression levels of Bax, pro-Caspase-3, and active-Caspase-3 were significantly elevated by overexpression of miR-340 but were significantly decreased by suppression of miR-340 compared to the control group (Figure 4). The results demonstrate that overexpression of miR-340-induced apoptosis occurred by regulating the expression of Bcl-2, Bax, and Caspase-3 in RL 95-2 cells.
Figure 4.
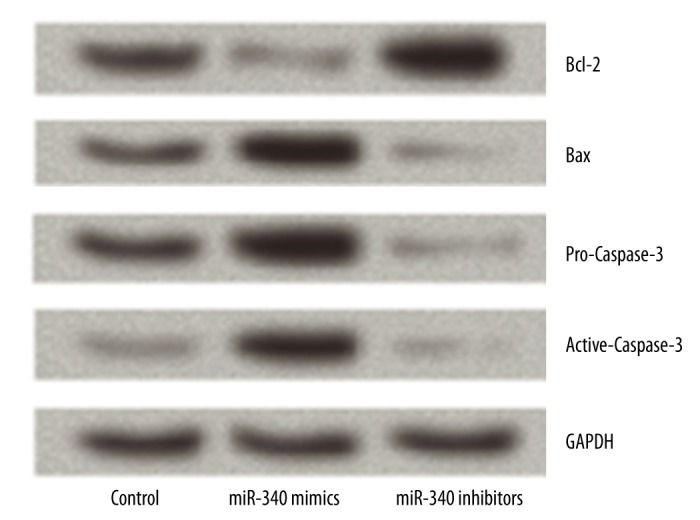
Overexpression of miR-340 induces apoptosis by regulating the expression of Bcl-2, Bax, and Caspase-3. Overexpression of miR-340 significantly increases the protein levels of Bax, pro-Caspase-3, and active-Caspase-3, but significantly decreases the levels of Bcl-2. Contrary results were found by suppression of miR-340. Bcl-2 – B-cell lymphoma-2.
Discussion
In the present study, we found that miRNA-340 acts as an anti-oncogene in EC cell line RL 95-2. Overexpression of miR-340 significantly inhibits EC cell proliferation and induces EC apoptosis. Inhibition of EC cell proliferation is through regulating the expression of p27 KIP1, and induction of EC cell apoptosis occurs by modulating the expression of apoptosis-related factors Bcl-2, Bax, pro-Caspase 3, and active-Caspase-3. MiR-340 may play an important role in EC, suggesting that miR-340 should be further evaluated as a novel biomarker for EC proliferation and apoptosis, and potentially a therapeutic target.
It has been demonstrated that miRNAs are responsible for the development, progression, and prognosis of tumors [18,19]. Among numerous mechanisms, cell proliferation and apoptosis are both well-known to be regulated by miRNAs [20,21]. Recently, several miRNAs have been reported to either promote or suppress EC cell proliferation and apoptosis, such as miR-125b [22], miR-101 [23], miR-199a-3p [24], and miR-205 [25]. Similarly, our results confirmed that miR-340 plays an essential role in EC proliferation and apoptosis. We first altered the expression of miR-340 by transfection with miR-340 mimics or inhibitors. The effect of miR-340 on cell viability, cell proliferation, and cell apoptosis were investigated. The results showed that overexpression of miR-340 inhibited EC cell viability and proliferation and induced cell apoptosis. However, suppression of miR-340 reversed the results. We further explored the underlying mechanisms.
Dysregulation of cell proliferation is one of the most important features of human cancers, and excessive cell proliferation is one of the hallmarks of cancers [26]. Inhibition of cell proliferation is one of the anticancer strategies to inhibit tumor growth and metastasis. Also, inhibition of cell proliferation is associated with enhanced apoptosis [27]. Clearly, there are numerous mechanisms involved in the cell proliferation, but it is usually inhibited by induction of cell cycle arrest. Cyclin-dependent kinases (CDKs) or CDK inhibitors (CDKI) are involved in deregulation of cell cycle progression during tumorigenesis. P27 KIP1 is an important member of the Cip/Kip family of CDKI, and p27 KIP1 negatively controls the cell cycle progression from G 1 to S phase by binding to CDK2 and cyclin E complexes [28]. The levels of p27 have shown prognostic value in several types of cancer. For example, reduced expression of p27 KIP1 has been reported to be associated with tumor size and poor prognosis of renal cell carcinoma and progression and lymph node metastasis of gastric carcinoma [29,30]. Although no association was found between p27 KIP1 and stage, age, histology, or prognosis for survival in advanced EC, there may be a trend associated with increased p27 KIP1 with advanced EC grades [31]. Moreover, recent studies have shown that p27 KIP1 is key target of miR-221/222 in many types of cancers [32–35]. In our study, we found that miR-340 regulated cell growth arrest by up-regulation of p27 KIP1 expression, but not p21, in RL 95-2 cells.
Apoptosis is another important target for therapeutic intervention in all tumors. Apoptosis is modulated partially by the Bcl-2 family, including apoptosis-inhibiting genes (e.g., Bcl-2) and apoptosis-accelerating genes (e.g., Bax) [36]. In addition to the Bcl-2 family, members of the Caspase family are also crucial mediators of apoptosis [37]. Caspase 3, one of the primary actuators of apoptosis, is required for the cleavage of many proteins, DNA fragmentation, apoptosis-associated chromatin margination, and nuclear collapse during apoptosis [38]. Activation of pro-Caspase-3 is the core element in the process of apoptosis and seems to function as the convergence point of all apoptotic pathways [39]. Active-Caspase-3 is an important actuating caspase, playing a critical role in different apoptotic signaling pathways [40]. In our study, we found that overexpression of miR-340 significantly increased the protein levels of Bax, pro-Caspase 3, and active-Caspase 3, but decreased the protein levels of Bcl-2 in RL 95-2 cells, indicating that overexpression of miR-340 induced EC apoptosis. However, considering the results of the present study, some limitations should be noted. We only focused on an EC cell line RL 95-2, so more EC cell lines, animal research, or clinical research should be performed to confirm the results.
Conclusions
miRNA-340 functions as an anti-oncogene in EC cell line RL 95-2. Overexpression of miR-340 inhibits EC cell proliferation and induces EC apoptosis. Overexpression of miR-340 might be a target treatment for EC.
Footnotes
Source of support: This study was supported by Guangxi Planning of National Excellent Doctoral Dissertations (No. YCBZ2014033), and The Star of the Future Academic Foundation of Guangxi Medical University (No. WLXSZX16071)
Conflict of interests
There are no conflicts of interests.
References
- 1.Chou ST, Chang WL, Chang CT, et al. Cinnamomum cassia essential oil inhibits alpha-MSH-induced melanin production and oxidative stress in murine B16 melanoma cells. Int J Mol Sci. 2013;14:19186–201. doi: 10.3390/ijms140919186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim A, Nguyen L, Kalir T, Chuang L. Pelvic recurrence of stage 1a well-differentiated endometrial carcinoma after 13 years: A case report. J Turk Ger Gynecol Assoc. 2016;17(1):51–54. doi: 10.5152/jtgga.2015.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres A, Torres K, Pesci A, et al. Diagnostic and prognostic significance of miRNA signatures in tissues and plasma of endometrioid endometrial carcinoma patients. Int J Cancer. 2013;132:1633–45. doi: 10.1002/ijc.27840. [DOI] [PubMed] [Google Scholar]
- 4.Fong P, Meng LR. Effect of mTOR inhibitors in nude mice with endometrial carcinoma and variable PTEN expression status. Med Sci Monit Basic Res. 2014;20:146–52. doi: 10.12659/MSMBR.892514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansal N, Yendluri V, Wenham RM. The molecular biology of endometrial cancers and the implications for pathogenesis, classification, and targeted therapies. Cancer Control. 2009;16:8–13. doi: 10.1177/107327480901600102. [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441–50. doi: 10.1016/j.devcel.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Baranwal S, Alahari SK. miRNA control of tumor cell invasion and metastasis. Int J Cancer. 2010;126:1283–90. doi: 10.1002/ijc.25014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartels CL, Tsongalis GJ. MicroRNAs: Novel biomarkers for human cancer. Clin Chem. 2009;55:623–31. doi: 10.1373/clinchem.2008.112805. [DOI] [PubMed] [Google Scholar]
- 10.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Dev Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 11.Chen CZ. MicroRNAs as oncogenes and tumor suppressors. N Engl J Med. 2005;353:1768–71. doi: 10.1056/NEJMp058190. [DOI] [PubMed] [Google Scholar]
- 12.Matias-Guiu X, Prat J. Molecular pathology of endometrial carcinoma. Histopathology. 2013;62:111–23. doi: 10.1111/his.12053. [DOI] [PubMed] [Google Scholar]
- 13.Tsukamoto O, Miura K, Mishima H, et al. Identification of endometrioid endometrial carcinoma-associated microRNAs in tissue and plasma. Gynecol Oncol. 2014;132:715–21. doi: 10.1016/j.ygyno.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 14.Lee TS, Jeon HW, Kim YB, et al. Aberrant microRNA expression in endometrial carcinoma using formalin-fixed paraffin-embedded (FFPE) tissues. PLoS One. 2013;8:e81421. doi: 10.1371/journal.pone.0081421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu ZS, Wu Q, Wang CQ, et al. miR-340 inhibition of breast cancer cell migration and invasion through targeting of oncoprotein c-Met. Cancer. 2011;117:2842–52. doi: 10.1002/cncr.25860. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Zhao X, Zhou Y, Hu Y. miR-124, miR-137 and miR-340 regulate colorectal cancer growth via inhibition of the Warburg effect. Oncol Rep. 2012;28:1346–52. doi: 10.3892/or.2012.1958. [DOI] [PubMed] [Google Scholar]
- 17.Zhou X, Wei M, Wang W. MicroRNA-340 suppresses osteosarcoma tumor growth and metastasis by directly targeting ROCK1. Biochem Biophys Res Commun. 2013;437:653–58. doi: 10.1016/j.bbrc.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 18.Calin GA, Croce CM. MicroRNA-cancer connection: the beginning of a new tale. Cancer Res. 2006;66:7390–94. doi: 10.1158/0008-5472.CAN-06-0800. [DOI] [PubMed] [Google Scholar]
- 19.Visone R, Croce CM. MiRNAs and cancer. Am J Pathol. 2009;174:1131–38. doi: 10.2353/ajpath.2009.080794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gammell P. MicroRNAs: Recently discovered key regulators of proliferation and apoptosis in animal cells: Identification of miRNAs regulating growth and survival. Cytotechnology. 2007;53:55–63. doi: 10.1007/s10616-007-9049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2006;94:776–80. doi: 10.1038/sj.bjc.6603023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang F, Liu T, He Y, et al. MiR-125b promotes proliferation and migration of type II endometrial carcinoma cells through targeting TP53INP1 tumor suppressor in vitro and in vivo. BMC Cancer. 2011;11:425. doi: 10.1186/1471-2407-11-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konno Y, Dong P, Xiong Y, et al. MicroRNA-101 targets EZH2, MCL-1 and FOS to suppress proliferation, invasion and stem cell-like phenotype of aggressive endometrial cancer cells. Oncotarget. 2014;5:6049–62. doi: 10.18632/oncotarget.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu D, Huang HJ, He CN, Wang KY. MicroRNA-199a-3p regulates endometrial cancer cell proliferation by targeting mammalian target of rapamycin (mTOR) Int J Gynecol Cancer. 2013;23:1191–97. doi: 10.1097/IGC.0b013e31829ea779. [DOI] [PubMed] [Google Scholar]
- 25.Su N, Qiu H, Chen Y, et al. miR-205 promotes tumor proliferation and invasion through targeting ESRRG in endometrial carcinoma. Oncol Rep. 2013;29:2297–302. doi: 10.3892/or.2013.2400. [DOI] [PubMed] [Google Scholar]
- 26.Sandal T. Molecular aspects of the mammalian cell cycle and cancer. Oncologist. 2002;7:73–81. doi: 10.1634/theoncologist.7-1-73. [DOI] [PubMed] [Google Scholar]
- 27.de Ruijter AJ, Kemp S, Kramer G, et al. The novel histone deacetylase inhibitor BL1521 inhibits proliferation and induces apoptosis in neuroblastoma cells. Biochem Pharmacol. 2004;68:1279–88. doi: 10.1016/j.bcp.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Koff A. How to decrease p27Kip1 levels during tumor development. Cancer Cell. 2006;9:75–76. doi: 10.1016/j.ccr.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Kim DH, Lee HI, Nam ES, et al. Reduced expression of the cell-cycle inhibitor p27Kip1 is associated with progression and lymph node metastasis of gastric carcinoma. Histopathology. 2000;36:245–51. doi: 10.1046/j.1365-2559.2000.00842.x. [DOI] [PubMed] [Google Scholar]
- 30.Migita T, Oda Y, Naito S, Tsuneyoshi M. Low expression of p27(Kip1) is associated with tumor size and poor prognosis in patients with renal cell carcinoma. Cancer. 2002;94:973–79. [PubMed] [Google Scholar]
- 31.Nycum LR, Smith LM, Farley JH, et al. The role of p27 in endometrial carcinoma. Gynecol Oncol. 2001;81:242–46. doi: 10.1006/gyno.2001.6144. [DOI] [PubMed] [Google Scholar]
- 32.le Sage C, Nagel R, Egan DA, et al. Regulation of the p27(Kip1) tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation. EMBO J. 2007;26:3699–708. doi: 10.1038/sj.emboj.7601790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galardi S, Mercatelli N, Giorda E, et al. miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J Biol Chem. 2007;282:23716–24. doi: 10.1074/jbc.M701805200. [DOI] [PubMed] [Google Scholar]
- 34.Miller TE, Ghoshal K, Ramaswamy B, et al. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J Biol Chem. 2008;283:29897–903. doi: 10.1074/jbc.M804612200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Visone R, Russo L, Pallante P, et al. MicroRNAs (miR)-221 and miR-222, both overexpressed in human thyroid papillary carcinomas, regulate p27Kip1 protein levels and cell cycle. Endocr Relat Cancer. 2007;14:791–98. doi: 10.1677/ERC-07-0129. [DOI] [PubMed] [Google Scholar]
- 36.Kelly PN, Strasser A. The role of Bcl-2 and its pro-survival relatives in tumourigenesis and cancer therapy. Cell Death Differ. 2011;18:1414–24. doi: 10.1038/cdd.2011.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan TJ, Han LH, Cong RS, Liang J. Caspase family proteases and apoptosis. Acta Biochim Biophys Sin (Shanghai) 2005;37:719–27. doi: 10.1111/j.1745-7270.2005.00108.x. [DOI] [PubMed] [Google Scholar]
- 38.Slee EA, Adrain C, Martin SJ. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem. 2001;276:7320–26. doi: 10.1074/jbc.M008363200. [DOI] [PubMed] [Google Scholar]
- 39.Samali A, Cai J, Zhivotovsky B, et al. Presence of a pre-apoptotic complex of pro-caspase-3, Hsp60 and Hsp10 in the mitochondrial fraction of jurkat cells. EMBO J. 1999;18:2040–48. doi: 10.1093/emboj/18.8.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: Structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]