Abstract
The purpose of this study was to evaluate vascular function and activity of Rho-associated kinases in patients with primary aldosteronism. Vascular function, including flow-mediated vasodilation and nitroglycerine-induced vasodilation, and Rho-associated kinase activity in peripheral leukocytes were evaluated in 21 patients with aldosterone-producing adenoma, 23 patients with idiopathic hyperaldosteronism, and 40 age-, gender-, and blood pressure-matched patients with essential hypertension. Flow-mediated vasodilation was significantly lower in the aldosterone-producing adenoma group than in the idiopathic hyperaldosteronism and essential hypertension groups (3.2±2.0% vs. 4.6±2.3% and 4.4±2.2%, P<0.05, respectively), whereas there was no significant difference in flow-mediated vasodilation between the idiopathic hyperaldosteronism and essential hypertension groups. There was no significant difference in nitroglycerine-induced vasodilation in the three groups. Rho-associated kinase activity was higher in the aldosterone-producing adenoma group than in the idiopathic hyperaldosteronism and essential hypertension groups (1.29±0.57 vs. 1.00±0.46 and 0.81±0.36, P<0.05, respectively), whereas there was no significant difference in Rho-associated kinase activity between the idiopathic hyperaldosteronism and essential hypertension groups. Flow-mediated vasodilation correlated with age (r=−0.31, P<0.01), plasma aldosterone concentration (r=−0.35, P<0.01) and aldosterone to renin ratio (r=−0.34, P<0.01). Rho-associated kinase activity correlated with age (r=−0.24, P=0.04), plasma aldosterone concentration (r=0.33, P<0.01) and aldosterone to renin ratio (r=0.46, P<0.01). After adrenalectomy, flow-mediated vasodilation and Rho-associated kinase activity were restored in aldosterone-producing adenoma patients. Aldosterone-producing adenoma was associated with both endothelial dysfunction and increased Rho-associated kinase activity compared with those in idiopathic hyperaldosteronism and essential hypertension. Aldosterone-producing adenoma may have a higher risk of future cardiovascular events.
Keywords: primary aldosteronism, aldosterone, endothelial function, Rho-associated kinases, hypertension
Introduction
Primary aldosteronism (PA) is one of the most common causes of secondary hypertension. The prevalence of cardiovascular events is higher in patients with PA than in patients with essential hypertension (EHT).1,2 Endothelial dysfunction is established in the initial step of atherosclerosis, leading to the development of atherosclerosis.3 In addition, it is well known that endothelial function is an independent predictor of cardiovascular events.4 Hypertension is associated with endothelial dysfunction.5–8 Both nitric oxide (NO) and aldosterone would contribute to the pathogenesis, development, and maintenance of hypertension.9–12
In previous studies, we showed that excess amounts of vasoconstrictors, such as angiotenin II and norepinephrine, markedly impair endothelial function in patients with renovascular hypertension and patients with pheocromocytoma.13,14 Patients with PA also are ideal models for determining how endothelium-dependent and -independent vasodilation is altered in the presence of excess vasoconstricting and pro-atherosclerotic factors. Some studies have shown that PA is associated with endothelial function and that circulating aldosterone levels significantly correlated with endothelial function.15,16 However, there is little information on the relationship between subtype of PA and grade of vascular function. In addition, the prevalence of cardiovascular events in patients with aldosterone-producing adenoma (APA) and patients with idiopathic hyperaldosteronism (IHA) remains unclear.
Rho-associated kinases (ROCKs), one of the first downstream targets of the small GTP-binding protein Rho A, mediate various cellular physiologic functions.17–19 Elevated ROCK activity would play an important pathophysiological role in the development and maintenance of hypertension. It has been reported that an increase in ROCK activity is associated with cardiovascular diseases, including hypertension.20,21 In addition, we have shown that leukocyte ROCK activity may be a new biomarker of cardiovascular events.22 Interestingly, it has been shown that activation of the RhoA/ROCK pathway impairs NO bioavailability through inhibition of endothelial NO synthase (eNOS) mRNA stability and eNOS protein phosphorylation at Ser 1177 via the Akt/PI3K pathway, suggesting the existence of an interaction between the eNOS/NO pathway and ROCK activity.23
The purpose of this study was to evaluate vascular function, endothelium-dependent vasodilation induced by flow-mediated vasodilation (FMD) and endothelium-independent vasodilation induced by sublingual administration of nitroglycerine, and leukocyte ROCK activity before and after adrenalectomy in patients with APA compared with those in patients with IHA and patients with EHT.
Methods
Study protocol 1. Vascular function and ROCK activity in patients with EHT and patients with PA
We studied 21 patients with APA (9 men and 12 women; mean age: 51±14 years), 23 patients with IHA (12 men and 11 women; mean age: 56±10 years), and 40 age-, gender-, and blood pressure-matched patients with EHT (24 men and 16 women; mean age: 53±11 years). Subjects were enrolled from the Hiroshima University Hypertension Database. The ethical committees of our institutions approved the study protocol. Written informed consent for participation in the study was obtained from all of the subjects.
Definition of EHT
Please see the online Data Supplement for additional details.
Definition of PA
PA, including the classification of PA, was defined according to the report of the guidelines for diagnosis and treatment of primary aldosteronism: the Japan Endocrine Society 2009.24 Please see the online Data Supplement for additional details.
Study protocol
Please see the online Data Supplement for additional details.
Study protocol 2. Effect of adrenalectomy on vascular function and ROCK activity in patients with APA
FMD, nitroglycerine-induced vasodilation, and ROCK activity were evaluated in the same manner as that in study 1 before adrenalectomy and at 12 weeks after this procedure in 12 of the 21 patients with APA (3 men and 9 women; mean age: 41±10 years). The surgical approach for adrenalectomy was laparoscopic adrenalectomy in all patients.
Measurement of FMD and nitroglycerine-induced vasodilation
FMD and nitroglycerine-induced vasodilation were measured using ultrasonography with an automated edge tracking system (UNEXEF18G, UNEX) as previously described.25 Please see the online Data Supplement for additional details.
Measurement of ROCK Activity
ROCK activity was assayed in peripheral blood leukocytes as the amount of phospho-Thr853 in the myosin-binding subunit of myosin light chain phosphatase as previously described.21,22 Please see the online Data Supplement for additional details.
Statistical analysis
Results are presented as mean±SD. All reported probability values were 2-sided, and a probability value of <0.05 was considered statistically significant. Categorical variables were compared by means of chi-square test. Continuous variables were compared by using ANOVA for multiple groups. Relations between variables were determined by Spearman correlation coefficients analysis. Multivariate regression analyses were performed to identify factors associated with FMD and ROCK activity in risk factors and laboratory data. The data were processed using the software package Stata version 9 (Stata Co., College Station, Texas, USA).
Results
Study protocol 1. Vascular function and ROCK activity in patients with EHT and patients with PA
The baseline clinical characteristics of the 40 patients with EHT, 21 patients with APA, and 23 patients with IHA are summarized in Table 1. PAC was significantly higher in patients with APA than in patients with IHA or EHT. There was no significant difference in plasma aldosterone concentration (PAC) between patients with IHA and patients with EHT. Plasma renin activity (PRA) was significantly lower in patients with APA or IHA than in patients with EHT. There was no significant difference in PRA between patients with APA and patients with IHA. Aldosterone to renin ration (ARR) was significantly higher in patients with APA or IHA than in patients with EHT and was higher in patients with APA than in patients with IHA. Serum potassium concentration was significantly lower in patients with APA than in patients with IHA or EHT. There was no significant difference in serum potassium concentration between patients with IHA and patients with EHT. The other parameters were similar in the groups.
Table 1.
Clinical Characteristics of the Subjects
| EHT | IHA | APA | |
|---|---|---|---|
|
|
|||
| Variables | (n=40) | (n=23) | (n=21) |
| Male, n (%) | 24 (60.0) | 12 (52.2) | 9 (42.9) |
| Age, y | 53±11 | 56±10 | 51±14 |
| Body mass index, kg/m2 | 25.3±3.6 | 26.1±4.9 | 24.4±3.4 |
| Systolic blood pressure, mm Hg | 144.0±20.3 | 141.6±19.5 | 141.2±16.2 |
| Diastolic blood pressure, mm Hg | 85.9±12.3 | 81.3±12.3 | 85.8±9.4 |
| Heart rate, bpm | 71.4±12.6 | 71.3±11.4 | 74.2±11.4 |
| Total cholesterol, mg/dL | 206.0±33.0 | 206.3±35.5 | 194.9±37.2 |
| Triglycerides, mg/dL | 170.4±81.8 | 154.0±75.9 | 136.4±68.5 |
| High-density lipoprotein cholesterol, mg/dL | 57.9±13.8 | 58.9±13.6 | 51.9±11.6 |
| Low-density lipoprotein cholesterol, mg/dL | 123.0±32.4 | 126.5±31.7 | 123.4±33.9 |
| Serum potassium, mg/dL | 4.1±0.4 | 3.9±0.3 | 3.4±0.7*† |
| Glucose, mg/dL | 112.4±30.8 | 106.5±19.4 | 103.5±20.5 |
| HbA1c, % | 5.9±0.7 | 5.9±0.7 | 5.8±0.8 |
| Plasma aldosterone concentration, ng/dL | 13.7±5.4 | 15.6±5.8 | 35.3±28.3*† |
| Plasma renin activity, ng/mL/hr | 2.0±1.8 | 0.5±0.2† | 0.4±0.2† |
| Aldosterone to renin ratio | 10.6±5.9 | 36.1±8.4‡ | 114.9±77.8*† |
| Medical history, n (%) | |||
| Hypertension | 40 (100) | 23 (100) | 21 (100) |
| Dyslipidemia | 20 (50.0) | 10 (43.5) | 11 (52.4) |
| Diabetes mellitus | 7 (17.5) | 6 (26.1) | 5 (23.8) |
| Current smoker | 18 (46.2) | 10 (43.5) | 7 (33.3) |
| Medications, n (%) | |||
| Calcium channel blockers | 20 (50.0) | 16 (70.0) | 14 (66.7) |
| Renin angiotensin system inhibitors | 10 (25.0) | 4 (17.4) | 3 (14.3) |
| Beta blockers | 2 (5.0) | 2 (8.7) | 3 (14.3) |
| Alpha blockers | 2 (5.0) | 2 (8.7) | 2 (9.5) |
| Statins | 7 (17.5) | 3 (13.0) | 3 (14.3) |
| Duration of hypertension, y | 8.4±7.4 | 8.2±7.1 | 8.7±9.3 |
EHT indicates essential hypertension; IHA, idiopathic hyperaldosteronism; APA, aldosterone-producing adenoma. All results are presented as mean±SD.
P<0.01 vs. IHA.
P<0.01 vs. EHT.
P<0.05 vs. EHT.
FMD was significantly lower in the APA group than in the IHA group and EHT group (3.2±2.0% vs. 4.6±2.3% and 4.4±2.2%, P<0.05, respectively), whereas there was no significant difference in FMD between the IHA group and EHT group (Figure 1A). There was no significant difference in nitroglycerine-induced vasodilation in the three groups (Figure 1B). FMD correlated with age (r=−0.29, P=0.007), PAC (r=−0.34, P=0.002; Supplementary Figure S1A) and ARR (r=−0.35, P=0.001; Supplementary Figure S1B). Nitroglycerine-induced vasodilation did not correlate with PAC (r=−0.19, P=0.11; Supplementary Figure S1C) or ARR (r=−0.13, P=0.24; Supplementary Figure S1D) or with any of the other parameters. Multivariate analysis revealed that age, PAC and ARR were independent predictors of FMD (Table 2).
Figure 1.
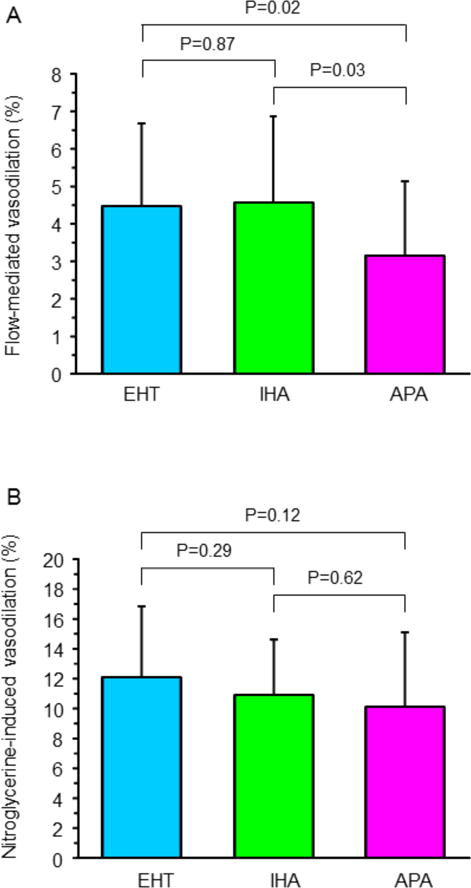
Flow-mediated vasodilation (A) and nitroglycerine-induced vasodilation (B) in patients with essential hypertension (EHT), patients with aldosterone-producing adenoma (APA), and patients with idiopathic hyperaldosteronism (IHA).
Table 2.
Multivariate Analysis of Flow-mediated Vasodilation and Rho-associated Kinases Activity With Clinical Variables
| Flow-mediated vasodilation | |||
|---|---|---|---|
| Variables | β | t value | P value |
| Age, y | −0.26 | −2.65 | 0.01 |
| Male | 0.01 | 0.09 | 0.93 |
| Systolic blood pressure, mm Hg | 0.05 | 0.43 | 0.68 |
| Plasma aldosterone concentration, ng/dL | −0.33 | −3.17 | 0.002 |
|
| |||
| Flow-mediated vasodilation | |||
| Variables | β | t value | P value |
|
| |||
| Age, y | −0.32 | −3.11 | 0.003 |
| Male | −0.05 | −0.51 | 0.61 |
| Systolic blood pressure, mm Hg | 0.05 | 0.45 | 0.66 |
| Aldosterone to renin ratio | −0.38 | −3.71 | 0.0004 |
|
| |||
| Rho-associated kinases activity | |||
| Variables | β | t value | P value |
|
| |||
| Age, y | −0.14 | −1.28 | 0.20 |
| Male | −0.08 | −0.73 | 0.47 |
| Serum potassium, mEq/L | −0.14 | −1.19 | 0.24 |
| Plasma aldosterone concentration, ng/dL | 0.30 | 2.65 | 0.01 |
|
| |||
| Rho-associated kinases activity | |||
| Variables | β | t value | P value |
|
| |||
| Age, y | −0.10 | −0.98 | 0.33 |
| Male | −0.04 | −0.35 | 0.73 |
| Serum potassium, mEq/L | −0.04 | −0.35 | 0.73 |
| Aldosterone to renin ratio | 0.43 | 3.76 | 0.0004 |
ROCK activity was significantly higher in the APA group than in the IHA group and EHT group (1.29±0.57 vs. 1.00±0.46 and 0.81±0.36, P<0.001 and P=0.04, respectively), whereas there was no significant difference in ROCK activity between the IHA group and EHT group (Figure 2). ROCK activity correlated with PAC (r=0.33, P=0.004; Supplementary Figure S2A), ARR (r=0.46, P=0.001; Supplementary Figure S2B), and serum potassium concentration (r=−0.25, P=0.03). Multivariate analysis revealed that PAC and ARR were independent predictors of ROCK activity (Table 2).
Figure 2.
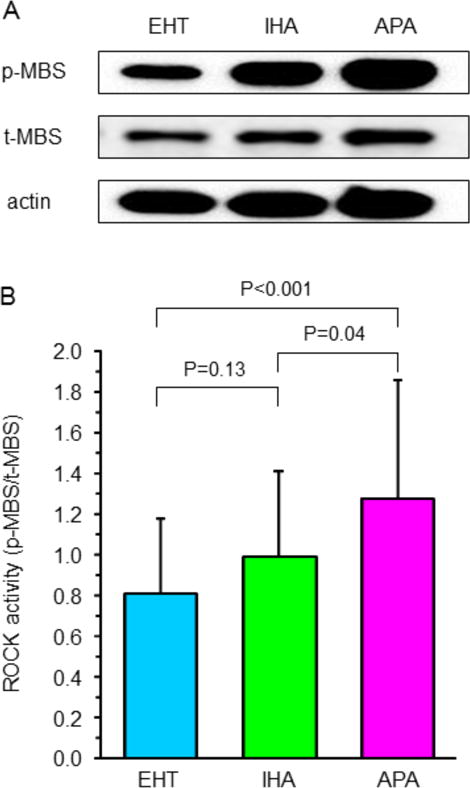
Representative western blot analysis for phospho myosin-binding subunit (p-MBS), total myosin-binding subunit (t-MBS), and actin in peripheral blood leukocytes in patients with essential hypertension (EHT), patients with aldosterone-producing adenoma (APA), and patients with idiopathic hyperaldosteronism (IHA). (A). Rho-associated kinase activity in peripheral blood leukocytes in patients with EHT, patients with APA, and patients with IHA (B).
Study protocol 2. Effect of adrenalectomy on vascular function and ROCK activity in patients with APA
The baseline clinical characteristics before and after adrenalectomy of the 12 patients with APA are summarized in Table 3. Adrenalectomy significantly decreased systolic blood pressure and PAC and significantly increased PRA, ARR, and serum potassium concentration.
Table 3.
Effect of 12 Weeks of Treatment With Adrenalectomy in Aldosterone-producing Adenoma Group
| Variables | Before Adrenalectomy (n=12) |
After Adrenalectomy (n=12) |
|---|---|---|
| Body mass index, kg/m2 | 23.9±3.1 | 23.5±2.9 |
| Systolic blood pressure, mm Hg | 140.8±17.4 | 127.6±8.1* |
| Diastolic blood pressure, mm Hg | 88.4±8.5 | 82.6±9.0 |
| Heart rate, bpm | 75.7±13.3 | 75.4±10.6 |
| High-density lipoprotein cholesterol, mg/dL | 51.8±9.4 | 54.7±11.3 |
| Low-density lipoprotein cholesterol, mg/dL | 127.1±34.9 | 110.9±22.6 |
| Glucose, mg/dL | 107.8±20.7 | 117.4±36.3 |
| Plasma aldosterone concentration, ng/dL | 32.8±22.8 | 14.7±10.9* |
| Plasma renin activity, ng/mL/hr | 0.3±0.2 | 1.4±1.5* |
| Aldosterone to renin ratio | 122.0±79.5 | 22.7±25.4† |
| Serum potassium, mEq/L | 3.3±0.8 | 4.2±0.3† |
All results are presented as mean±SD.
P<0.05 vs. Before.
P<0.01 vs. Before.
After adrenalectomy, FMD was enhanced from 3.6±2.0% to 5.0±2.5% (P=0.005), while nitroglycerine-induced vasodilation was not significantly altered from 12.1±4.8% to 14.6±5.8% (P=0.15) (Figure 3A). The increase in FMD correlated significantly with the decrease in PAC (r=−0.42, P=0.04; Figure 4A) and the decrease in ARR (r=−0.46, P=0.02; Figure 4B). No significant correlation was found between the increase in FMD and changes in blood pressure or variables, such as lipid, glucose, and insulin concentrations, or between these variables and the increase in nitroglycerine-induced vasodilation.
Figure 3.
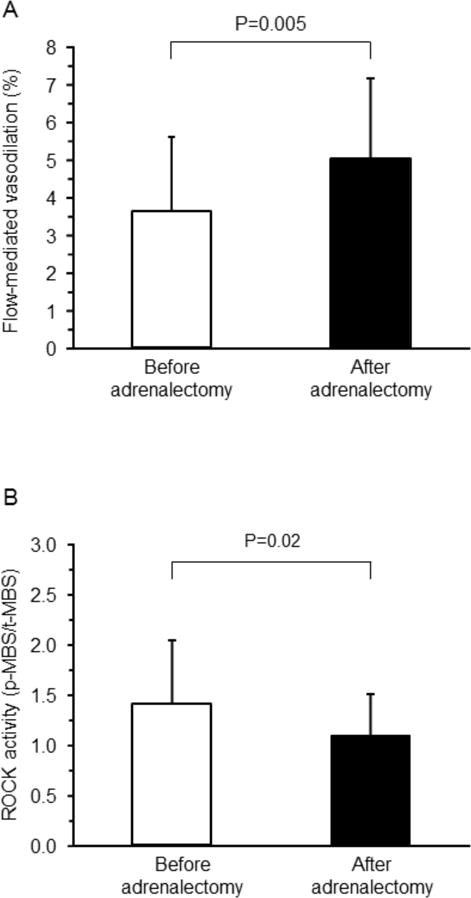
Flow-mediated vasodilation (A) and Rho-associated kinases (ROCK) activity (B) before and after adrenalectomy in patients with aldosterone-producing adenoma.
Figure 4.
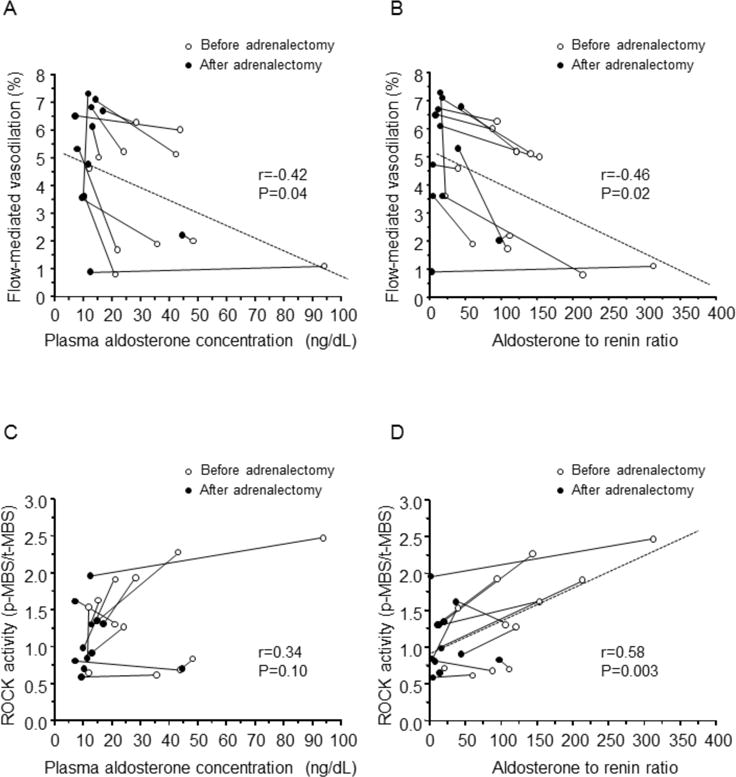
Relationships between flow-mediated vasodilation and plasma aldosterone concentration (A) and the aldosterone to renin ratio (B) and relationship between Rho-associated kinases (ROCK) and plasma aldosterone concentration (C) and the aldosterone to renin ratio (D) before and after adrenolectomy in patients with aldosterone-producing adenoma.
Leukocyte ROCK activity was significantly lower after adrenalectomy than before adrenalectomy (1.09±0.41 vs. 1.42±0.62, P=0.02; Figure 3B). The decrease in ROCK activity correlated significantly with the decrease in ARR (r=0.58, P=0.003; Figure 4D) but not with the decrease in PAC (r=0.34, P=0.10; Figure 4C). No significant correlation was found between the decrease in ROCK activity and changes in blood pressure or variables, such as lipid, glucose, insulin, and potassium concentrations.
Discussion
In the present study, we demonstrated for the first time that 1) endothelial function was impaired in patients with APA compared with that in patients with IHA or EHT, while endothelial function was similar in patients with IHA and patients with EHT and it was impaired to a greater extent in relation to levels of PAC or ARR, 2) leukocyte ROCK activity was increased in patients with APA compared with that in patients with IHA or EHT, while leukocyte ROCK activity was similar in patients with IHA and patients with EHT and it was increased to a greater extent in relation to levels of PAC or ARR, and 3) surgical resection of APA improved endothelial function and inhibited leukocyte ROCK activity in patients with APA. There were significant correlations between improvement of FMD and ROCK activity and decrease in PAC or ARR.
Endothelial function and aldosterone
Patients with PA are ideal models for the study of how endothelial function is altered in the presence of excess vasoconstricting and pro-atherosclerotic factors. In the present study, excess aldosterone blunted FMD as an index of endothelium-dependent vasodilation in APA patients, while nitroglycerine-induced vasodilation was similar in patients with APA, IHA, and EHT, indicating that endothelial function, but not smooth muscle function, is selectively impaired and is restored after adrenalectomy in patients with APA.
Previous studies showing that PA is associated with endothelial dysfunction and that there is a significant relationship between PAC and endothelial function support our results.15,16 Several investigators have shown the existence of an interaction between NO and the renin-angiotensin-aldosterone system in the normal endothelium.9–16 An imbalance between NO and aldosterone may directly result in conditions associated with endothelial dysfunction in humans. Aldosterone as well as angiotensin II plays an important role in the regulation of vascular function through the NO/endothelial NO synthase (eNOS) pathway.26,27 It has been reported that aldosterone attenuates eNOS activity through increase in reactive oxygen species-induced eNOS uncoupling and protein phosphatase 2A-induced dephosphorylation of p-eNOS in a mineralocorticoid receptor-dependent manner in human umbilical vein endothelial cells.26,27 The mineralocorticoid receptor blocker eplerenone improved endothelial function through enhancement of expression of the eNOS gene in Dahl salt-sensitive rats and two-kidney, one clip rats,28,29 suggesting that eplerenone directly enhances the NO/eNOS pathway. Inhibition of the aldosterone and/or mineralocorticoid receptor also may contribute to decrease in oxidative stress, resulting in improvement of endothelial function through inhibition of NO inactivation.30 Eplerenone also improved FMD in patients with EHT.31 In addition, the non-selective mineralocorticoid receptor blocker spironolactone improved FMD and acetylcholine-induced vasodilation in patients with hyperaldosteronism and in patients with heart failure.32,33 In the present study, there was a significant relationship between PAC and FMD in all patients, and there was a significant relationship between the decrease in PAC and improvement in FMD in patients with APA. These findings suggest that aldosterone per se and/or mineralocorticoid receptor may impair endothelial function through inactivation of the NO/eNOS pathway, including decrease in NO production and increase in NO inactivation.
Endothelial function becomes impaired as blood pressure increases, and the degree of dysfunction is related to the severity of hypertension.34,35 It is expected that endothelial dysfunction will be improved by antihypertensive therapy. However, several experimental and clinical studies have provided conflicting results concerning the relationship between reduction in blood pressure and improvement in endothelial function.36,37 Although adrenalectomy acutely decreased blood pressure in patients with APA in the present study, changes in blood pressure did not correlate with improvement of FMD. In previous studies, we and other investigators have shown that although clinically effective antihypertensive therapy, such as antihypertensive drugs and aerobic exercise, restored resistance artery endothelial function of forearm circulation in patients with EHT, there is no significant correlation between the degree of reduction in blood pressure and the augmentation of endothelial function.38,39 Therefore, it is unlikely that a reduction in blood pressure per se is involved in the restoration of endothelial function in forearm circulation.
ROCK activity and aldosterone
Patients with PA are also ideal models for the study of how ROCK activity is altered in the presence of excess vasoconstricting and pro-atherosclerotic factors. In the present study, leukocyte ROCK activity was increased in patients with APA compared with that in patients with IHA or EHT and was increased to a greater extent in relation to levels of PAC or ARR. The increase in ROCK activity was restored after adrenalectomy in patients with APA. These findings suggest that excess aldosterone plays a critical role in the increase in ROCK activity in humans.
ROCKs are one of the first downstream targets of the small GTP-binding protein Rho A. It is well known that angiotensin II is a potent stimulator of ROCK activity in endothelial cells and/or smooth muscle cells and subsequently modulates cell contraction, proliferation, apoptosis and gene expression via several signaling pathways.17–19 It has been reported that aldosterone also stimulates ROCK activity through binding to the mineralocorticoid receptor in vascular smooth muscle cells and cardiomyocytes.40,41 ROCK activity is elevated in rats with aldosterone-induced hypertension, leading to vascular remodeling and tissue injury in the heart and kidney.29,42 In addition, treatment with mineralocorticoid receptor blockers prevented cardiovascular injury through inhibition of ROCK activity in these animal models.42 We showed that in a previous blind, randomized, parallel group study that the mineralocorticoid receptor blocker eplerenone decreased leukocyte ROCK activity in patients with EHT.31 These findings suggest that aldosterone-induced activation of ROCK activity may be due to classical genomic actions that regulate gene transcription and protein synthesis through binding of aldosterone to the mineralocorticoid receptor. However, we cannot deny the possibility that nongenomic responses contribute to the aldosterone-induced activation of ROCK activity, especially under the condition of excess aldosterone. Future studies are needed to confirm the precise mechanisms by which the aldosterone/mineralocorticoid receptor is associated with activation of ROCK activity in vitro and in vivo and in a clinical setting.
Elevated ROCK activity would play an important pathophysiological role in the development and maintenance of hypertension. Hypertension is associated with activation of the Rho/ROCK pathway.19–21,31,43,44 Therefore, it is expected that increased ROCK activity will be improved by antihypertensive therapy. However, changes in blood pressure did not correlate with decrease in ROCK activity in patients with APA. In previous studies, we showed that although clinically effective antihypertensive therapy using antihypertensive drugs, such as eplerenone and the calcium channel blocker nifedipine, decreased ROCK activity in patients with EHT, there was no significant correlation between degree of reduction in blood pressure and decrease in ROCK activity. Therefore, it is unlikely that a reduction in blood pressure per se is involved in the restoration of leukocyte ROCK activity.
Some studies showed that either APA or IHA had a higher risk for target organ damage of the heart, brain, and kidneys than did EHT.1,2 However, unfortunately, there have been no large clinical trials in which differences in cardiovascular events between patients with APA and IHA were evaluated. Several lines of evidence have shown that endothelial function is not only the initial step of atherosclerosis but also a predictor of cardiovascular events.3,4 In addition, we have recently shown that leukocyte ROCK activity is an independent predictor of cardiovascular events.22 In the present study, patients with APA had vascular dysfunction and an increase in ROCK activity compared with those in patients with IHA, suggesting that the prevalence of future cardiovascular events may be higher in patients with APA than in patients with IHA.
Study limitations
In the present study, the number of patients with PA, especially patients with APA, was relatively small. Nonetheless, we observed a marked augmentation of FMD and reduction in ROCK activity after adrenalectomy in patients with APA and significant relationships between both increase in FMD and decrease in ROCK activity and decrease in PAC or ARR.
It is well known that various vasoconstricting factors other than aldosterone affect vascular function in humans. We confirmed in a preliminary study that circulating levels of endothelin-1 were normal and did not change after adrenalectomy (2.1±0.3 to 2.0±0.4 pg/mL) in 10 patients with APA (4 men and 6 women; mean age: 53±12 years). However, we cannot deny the possibility that other vasoconstrictors contribute to endothelial function and ROCK activity and were restored after adrenalectomy in patients with APA.
In a previous study, we confirmed that eplerenone improved endothelial function and decreased ROCK activity independently of blood pressure reduction in patients with EHT.31 Evaluation of whether there are benefits beyond blood pressure control for medical interventions using maximum tolerated doses of mineralocorticoid receptor blockers or adrenalectomy in patients with APA will enable more specific conclusions concerning the role of aldosterone in vascular function and ROCK activity to be drawn. These findings may help to know the indication for adrenalectomy in elderly patients with APA.
Perspectives
Endothelial function was impaired in patients with APA compared with that in patients with IHA or EHT, while endothelial function was similar in patients with IHA and patients with EHT and it was impaired to a greater extent in relation to levels of PAC or ARR. Leukocyte ROCK activity was increased in patients with APA compared with that in patients with IHA or EHT, while leukocyte ROCK activity was similar in patients with IHA and patients with EHT and it was increased to a greater extent in relation to levels of PAC or ARR. Surgical resection of APA improved endothelial function and inhibited leukocyte ROCK activity in patients with APA. There were significant correlations between improvement of FMD and ROCK activity and decrease in PAC or ARR. APA may have a higher risk of future cardiovascular events.
Supplementary Material
Novelty and Significance.
1) What is new?
The present study is the first study showing that increased secretion of aldosterone impairs vascular function and increases ROCK activity, that patients with APA had impairment of endothelial function and increased ROCK activity compared with those in patients with IHA or EHT, and that resection of an aldosterone-secreting tumor restores vascular function and ROCK activity in patients with APA.
2) What is Relevant?
APA may have a higher risk of future cardiovascular events.
3) Summary
The results of this study showed for the first time the relationships between PAC or ARR and vascular function and ROCK activity in patients with EHT, APA and IHA.
Acknowledgments
We thank Megumi Wakisaka, Ki-ichiro Kawano, and Satoko Michiyama for their excellent secretarial assistance.
Sources of Funding
This study was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (1859081500 and 21590898) and the National Institutes of Health (HL052233).
Footnotes
Financial Disclosures
Dr. James K. Liao is a consultant for Asahi-Kasei Pharmaceutical, Inc.
References
- 1.Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Savard S, Amar L, Plouin PF, Steichen O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension. 2013;62:331–336. doi: 10.1161/HYPERTENSIONAHA.113.01060. [DOI] [PubMed] [Google Scholar]
- 3.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 4.Lerman A, Zeiher AM. Endothelial function: cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 5.Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 6.Linder L, Kiowski W, Buhler FR, Lüscher TF. Indirect evidence for release of endothelium-derived relaxing factor in human forearm circulation in vivo: blunted response in essential hypertension. Circulation. 1990;81:1762–1767. doi: 10.1161/01.cir.81.6.1762. [DOI] [PubMed] [Google Scholar]
- 7.Taddei S, Agostino V, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation. 1995;91:1981–1987. doi: 10.1161/01.cir.91.7.1981. [DOI] [PubMed] [Google Scholar]
- 8.Higashi Y, Sasaki S, Kurisu S, Yoshimizu A, Sasaki N, Matsuura H, Kajiyama G, Oshima T. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects - role of endothelium-derived nitric oxide - Circulation. 1999;100:1194–1202. doi: 10.1161/01.cir.100.11.1194. [DOI] [PubMed] [Google Scholar]
- 9.Furchgott RF. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983;53:557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- 10.Lüscher TF. Imbalance of endothelium-derived relaxing and contracting factors. Am J Hypertens. 1990;3:317–330. doi: 10.1093/ajh/3.4.317. [DOI] [PubMed] [Google Scholar]
- 11.Vanhoutte PM. Endothelium and control of vascular function. Hypertension. 1989;13:658–667. doi: 10.1161/01.hyp.13.6.658. [DOI] [PubMed] [Google Scholar]
- 12.Fuller PJ, Young MJ. Mechanisms of mineralocorticoid action. Hypertension. 2005;46:1227–1235. doi: 10.1161/01.HYP.0000193502.77417.17. [DOI] [PubMed] [Google Scholar]
- 13.Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Oshima T, Chayama K. Endothelial function and oxidative stress in renovascular hypertension. N Engl J Med. 2002;346:1954–1962. doi: 10.1056/NEJMoa013591. [DOI] [PubMed] [Google Scholar]
- 14.Higashi Y, Sasaki S, Nakagawa K, Kimura M, Noma K, Sasaki S, Hara K, Matsuura H, Goto C, Oshima T, Chayama K. Excess norepinephrine impairs both endothelium-dependent and -independent vasodilation in patients with pheochromocytoma: a comparison before and after adrenalectomy. Hypertension. 2002;39:513–158. doi: 10.1161/hy02t2.102820. [DOI] [PubMed] [Google Scholar]
- 15.Nishizaka MK, Zaman MA, Green SA, Renfroe KY, Calhoun DA. Impaired endothelium-dependent flow-mediated vasodilation in hypertensive subjects with hyperaldosteronism. Circulation. 2004;109:2857–2861. doi: 10.1161/01.CIR.0000129307.26791.8E. [DOI] [PubMed] [Google Scholar]
- 16.Duffy SJ, Biegelsen ES, Eberhardt RT, Kahn DF, Kingwell BA, Vita JA. Low-renin hypertension with relative aldosterone excess is associated with impaired NO-mediated vasodilation. Hypertension. 2005;46:707–713. doi: 10.1161/01.HYP.0000184231.84465.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 18.Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- 19.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 20.Masumoto A, Hirooka Y, Shimokawa H, Hironaga K, Setoguchi S, Takeshita A. Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension. 2001;38:1307–1310. doi: 10.1161/hy1201.096541. [DOI] [PubMed] [Google Scholar]
- 21.Soga J, Hata T, Hidaka T, Fujii Y, Idei N, Fujimura N, Mikami S, Maruhashi T, Kihara Y, Chayama K, Kato H, Noma K, Liao JK, Higashi Y, for ROCK investigator group Rho-associated kinase (ROCK) activity, endothelial function and cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2011;31:2353–2359. doi: 10.1161/ATVBAHA.111.227892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kajikawa M, Maruhashi T, Mikami S, Iwamoto Y, Iwamoto A, Matsumoto T, Hidaka T, Kihara Y, Chayama K, Nakashima A, Goto C, Noma K, Liao JK, Higashi Y. Rho-associated kinase is a predictor of cardiovascular outcomes. Hypertension. 2014;63:856–864. doi: 10.1161/HYPERTENSIONAHA.113.02296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolfrum S, Dendorfer A, Rikitake Y, Stalker TJ, Gong Y, Scalia R, Dominiak P, Liao JK. Inhibition of Rho-kinase leads to rapid activation of phosphatidylinositol 3-kinase/protein kinase Akt and cardiovascular protection. Arterioscler Thromb Vasc Biol. 2004;24:1842–1847. doi: 10.1161/01.ATV.0000142813.33538.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nishikawa T, Omura M, Satoh F, Shibata H, Takahashi K, Tamura N, Tanabe A, Task Force Committee on Primary Aldosteronism, The Japan Endocrine Society Guidelines for the diagnosis and treatment of primary aldosteronism–the Japan Endocrine Society 2009. Endocr J. 2011;58:711–721. doi: 10.1507/endocrj.ej11-0133. [DOI] [PubMed] [Google Scholar]
- 25.Maruhashi T, Soga J, Idei N, Fujimura N, Mikami S, Iwamoto Y, Kajikawa M, Matsumoto T, Hidaka T, Kihara Y, Chayama K, Noma K, Nakashima A, Goto C, Higashi Y. Nitroglycerine-induced Vasodilation for Assessment of Vascular Function: A Comparison with Flow-mediated Vasodilation. Arterioscler Thromb Vasc Biol. 2013;33:1401–1408. doi: 10.1161/ATVBAHA.112.300934. [DOI] [PubMed] [Google Scholar]
- 26.Hashikabe Y, Suzuki K, Jojima T, Uchida K, Hattori Y. Aldosterone impairs vascular endothelial cell function. J Cardiovasc Pharmacol. 2006;47:609–613. doi: 10.1097/01.fjc.0000211738.63207.c3. [DOI] [PubMed] [Google Scholar]
- 27.Nagata D, Takahashi M, Sawai K, Tagami T, Usui T, Shimatsu A, Hirata Y, Naruse M. Molecular mechanism of the inhibitory effect of aldosterone on endothelial NO synthase activity. Hypertension. 2006;48:165–171. doi: 10.1161/01.HYP.0000226054.53527.bb. [DOI] [PubMed] [Google Scholar]
- 28.Hao L, Kanno Y, Fukushima R, Watanabe Y, Ishida Y, Suzuki H. Effects of eplerenone on heart and kidney in two-kidney, one-clip rats. Am J Nephrol. 2004;24:54–60. doi: 10.1159/000075945. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi N, Hara K, Tojo A, Onozato ML, Honda T, Yoshida K, Mita S, Nakano S, Tsubokou Y, Matsuoka H. Eplerenone shows renoprotective effect by reducing LOX-1-mediated adhesion molecule, PKCepsilon-MAPK-p90RSK, and Rho-kinase pathway. Hypertension. 2005;45:538–544. doi: 10.1161/01.HYP.0000157408.43807.5a. [DOI] [PubMed] [Google Scholar]
- 30.Sanz-Rosa D, Oubiña MP, Cediel E, De las Heras N, Aragoncillo P, Balfagón G, Cachofeiro V, Lahera V. Eplerenone reduces oxidative stress and enhances eNOS in SHR: vascular functional and structural consequences. Antioxid Redox Signal. 2005;7:1294–1301. doi: 10.1089/ars.2005.7.1294. [DOI] [PubMed] [Google Scholar]
- 31.Fujimura N, Hata T, Soga J, Hidaka T, Idei N, Fujii Y, Mikami S, Maruhashi T, Iwamoto Y, Kihara Y, Chayama K, Kato H, Noma K, Liao JK, Higashi Y, for ROCK investigator group Selective mineralocorticoid receptor blocker eplerenone improves endothelial function and inhibits Rho-associated kinase activity in patients with essential hypertension. Clin Pharmacol & Therapeut. 2012;91:289–297. doi: 10.1038/clpt.2011.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishizaka MK, Zaman MA, Green SA, Renfroe KY, Calhoun DA. Impaired endothelium-dependent flow-mediated vasodilation in hypertensive subjects with hyperaldosteronism. Circulation. 2004;109:2857–2861. doi: 10.1161/01.CIR.0000129307.26791.8E. [DOI] [PubMed] [Google Scholar]
- 33.Macdonald JE, Kennedy N, Struthers AD. Effects of spironolactone on endothelial function, vascular angiotensin converting enzyme activity, and other prognostic markers in patients with mild heart failure already taking optimal treatment. Heart. 2004;90:765–770. doi: 10.1136/hrt.2003.017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dohi Y, Thiel MA, Buhler FR, Lüscher TF. Activation of endothelial L-arginine pathway in resistance arteries. effect of age and hypertension. Hypertension. 1990;16:170–179. doi: 10.1161/01.hyp.16.2.170. [DOI] [PubMed] [Google Scholar]
- 35.Panza JA, Casino PR, Kilcoyne CM, Quyyumi AA. Role of endothelium-derived nitric oxide in the abnormal endothelium- dependent vascular relaxation of patients with essential hypertension. Circulation. 1993;87:1468–7144. doi: 10.1161/01.cir.87.5.1468. [DOI] [PubMed] [Google Scholar]
- 36.Creager MA, Roddy MA. Effect of captopril and enarapril on endothelial function in hypertensive patients. Hypertension. 1994;24:499–505. doi: 10.1161/01.hyp.24.4.499. [DOI] [PubMed] [Google Scholar]
- 37.Schiffrin EL, Deng LY. Comparison of effects of angiotensin I-converting enzyme inhibition and β-blockade for 2 years on function of small arteries from hypertensive patients. Hypertension. 1995;25:699–703. doi: 10.1161/01.hyp.25.4.699. [DOI] [PubMed] [Google Scholar]
- 38.Iwatubo H, Nagano M, Sakai T, Kumamoto K, Morita R, Higaki J, Ogihara T, Hata T. Converting enzyme inhibitor improves forearm reactive hyperemia in essential hypertension. Hypertension. 1997;29:286–290. doi: 10.1161/01.hyp.29.1.286. [DOI] [PubMed] [Google Scholar]
- 39.Higashi Y, Sasaki S, Nakagawa K, Kurisu S, Yoshimizu A, Matsuura H, Kajiyama G, Oshima T. A comparison of angiotensin-converting enzyme inhibitors, calcium antagonists, beta blockers and diuretic agents on reactive hyperemia in patients with essential hypertension: a multicenter study. J Am Coll Cardiol. 2000;35:284–291. doi: 10.1016/s0735-1097(99)00561-6. [DOI] [PubMed] [Google Scholar]
- 40.Miyata K, Hitomi H, Guo P, Zhang GX, Kimura S, Kiyomoto H, Hosomi N, Kagami S, Kohno M, Nishiyama A. Possible involvement of Rho-kinase in aldosterone-induced vascular smooth muscle cell remodeling. Hypertens Res. 2008;31:1407–1413. doi: 10.1291/hypres.31.1407. [DOI] [PubMed] [Google Scholar]
- 41.Doi T, Sakoda T, Akagami T, Naka T, Mori Y, Tsujino T, Masuyama T, Ohyanagi M. Aldosterone induces interleukin-18 through endothelin-1, angiotensin II, Rho/Rho-kinase, and PPARs in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008;295:H1279–H1287. doi: 10.1152/ajpheart.00148.2008. [DOI] [PubMed] [Google Scholar]
- 42.Sun GP, Kohno M, Guo P, Nagai Y, Miyata K, Fan YY, Kimura S, Kiyomoto H, Ohmori K, Li DT, Abe Y, Nishiyama A. Involvements of Rho-kinase and TGF-beta pathways in aldosterone-induced renal injury. J Am Soc Nephrol. 2006;17:2193–2201. doi: 10.1681/ASN.2005121375. [DOI] [PubMed] [Google Scholar]
- 43.Seasholtz TM, Zhang T, Morissette MR, Howes AL, Yang AH, Brown JH. Increased expression and activity of RhoA are associated with increased DNA synthesis and reduced p27(Kip1) expression in the vasculature of hypertensive rats. Circ Res. 2001;89:488–495. doi: 10.1161/hh1801.096337. [DOI] [PubMed] [Google Scholar]
- 44.Wehrwein EA, Northcott CA, Loberg RD, Watts SW. Rho/Rho kinase and phosphoinositide 3-kinase are parallel pathways in the development of spontaneous arterial tone in deoxycorticosterone acetate-salt hypertension. J Pharmacol Exp Ther. 2004;309:1011–1019. doi: 10.1124/jpet.103.062265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


