Abstract
Fecal samples obtained from wild boar habitats are useful for the surveillance of diseases in wild boar populations; however, it is difficult to determine the species of origin of feces collected in natural habitats. In this study, a fecal IgA ELISA was evaluated as a method for identifying the porcine species from fecal samples. Both domestic pigs (Sus scrofa domestica) and wild boars (Sus scrofa coreanus) showed significantly higher levels of fecal IgA than other animal species. Additionally, age dependent changes in the level of Ig A in wild boars and domestic pigs were identified; Titers of Ig A were highest in suckling period and lowest in weanling period.
Keywords: ELISA, Immunoglobulin A, Feces, Habitats, Wild boar
INTRODUCTION
Wild boars can be a reservoir of agents that are infectious to domestic pigs and other livestock (1). The disease status of wild boars has been surveyed using blood samples collected from animals shot during the hunting season (2,3). However, seroprevalence analysis in hunted wild boars is difficult owing to the limited hunting area, the restricted hunting season, and a biased age distribution (2,4). Testing feces obtained from the habitat of wild boars is an alternative to testing serum samples, since fecal samples are easy to collect in large quantities and can also be used for the direct detection of bacteria, viruses, and parasites (4).
Before testing fecal samples for a specific disease, gross examination is commonly used as a method to screen the species of origin. Additionally, the sequence of cytochrome b or chromosomes carrying endogenous retroviruses has been used for scientific proof of the animals' identification (5,6). However, gross examination is not accurate, and the gene sequencing method is complicated and costly, whereas ELISA can be used to screen a large number of samples simultaneously. In the present study, secretory fecal IgA was tested by ELISA as an alternative method for distinguishing the species origin of fecal samples from wild boars (Sus scrofa coreanus) and domestic pigs (Sus scrofa domestica). IgA was selected as a target protein owing to its presence in large amounts in feces (7) and its conserved domains that allow for comparison across a broad range of species (8,9).
MATERIALS AND METHODS
Sample collection
Fecal samples from 22 different animals in Korea, including wild boars and domestic pigs, were tested (Table I) for their cross-reactivity with the porcine fecal IgA antibody. Forty fresh fecal samples of farmed wild boars from one farm were collected to evaluate IgA differences in wild boars of different ages. In addition, 50 fecal samples were collected from domestic pigs of different age groups on a commercial pig farm. The samples were transported and stored at 4℃ until analyzed.
Table I. Biological classification of the 22 different animals, the fecal samples of which were used in this study.
| Order | Family | Subfamily | Genus | Scientific name | Common name |
|---|---|---|---|---|---|
| Artiodactyla | Bovidae | Caprinae | Capra | Capra hircus coreanae | Korean native goats |
| Caprinae | Ovis | Ovis aries | Sheep | ||
| Cervidae | Odocoileinae | Capreolus | Capreolus pygargus tianschanicus | Siberian roe deer | |
| Cervinae | Cervus | Cervus elaphus | Red deer | ||
| Cervus nippon hortulorum | Manchurian sika deer | ||||
| Cervus nippon nippon | Japanese sika deer | ||||
| Cervus nippon yesoensis | Yeso sika dear | ||||
| Hydropotinae | Hydropotes | Hydropotes inermis argyropus | Korean water deer | ||
| Suidae | Sus | Sus scrofa coreanus | Korean wild boar | ||
| Sus scrofa domestica | Domestic pig | ||||
| Carnivora | Canidae | Caninae | Canis | Canis lupus familiaris | Sapsaree |
| Canis lupus familiaris | Poongsan dog | ||||
| Canis lupus chanco | Korean grey wolf | ||||
| Nyctereutes | Nyctereutes procyonoides | Raccoon dog | |||
| Vulpes | Vulpes vulpes peculiosa | Fox | |||
| Mustelidae | Lutrinae | Lutra | Lutra lutra lutra | Eurasian otter | |
| Mustelinae | Martes | Martes flavigula | Yellow throated marten | ||
| Melinae | Meles | Meles leucurus | Asian badger | ||
| Felidae | Felinae | Prionailurus | Prionailurus bengalensis manchuria | Manchuria leopard cat | |
| Ursidae | Ursinae | Ursus | Ursus arctos lasiotus | Brown bear | |
| Ursus thibetanususs uricus | Asiatic black bear | ||||
| Rodentia | Myocastoridae | Myocastorinae | Myocastor | Myocastor coypus | Nutria |
Quantitative ELISA
Each fecal sample was diluted to a concentration of 0.5 g/mL with phosphate-buffered saline containing 0.05% Tween 20 and 1% BSA, vortexed, and centrifuged at 1,500 g for 20 min at 4℃. The supernatants were centrifuged at 16,000 g for 15 min to remove large solid particles and then transferred into Eppendorf tubes. Fecal samples from the wild boars and domestic pigs were diluted 200-fold (final dilution) and tested in triplicate using the Pig IgA ELISA Quantitation Set (Bethyl Laboratories, Montgomery, TX, USA). The IgA concentrations were quantified according to the following age groups: 1 day~1 month, 1~3 months, 3~6 months, 6 months~1 year, and >1 year (Table II). The average optical density at 450 nm (OD450) of 1% BSA as a negative control was subtracted from the average OD450 values of all other fecal samples. Twelve fecal samples were collected <1 h after excretion from four different individuals of 20 different animals (3 orders, 8 families, 15 genera, 16 species, 19 subspecies) in Seoul Grand Park and analyzed in the same way as described above. Data are presented as the mean and standard deviation (SD).
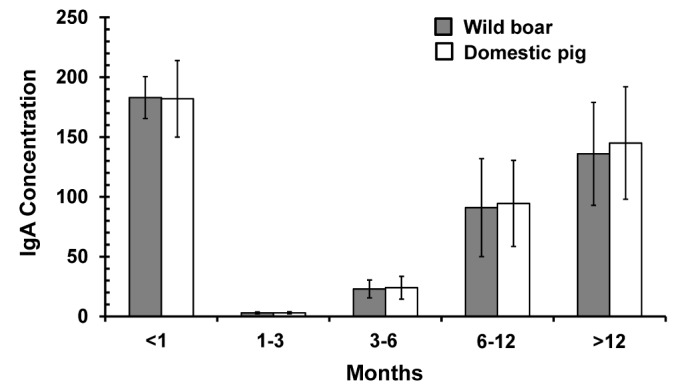
Table 2. Concentration of IgA in different age groups of wild boars and domestic pigs.
| Age of wild boar (months) | Wild boar (Sus scrofa coreanus) | Domestic pig (Sus scrofa domestica) | ||||
|---|---|---|---|---|---|---|
| Number of samples | Mean±Standard deviation | Number of samples | Mean±Standard deviation | |||
| Porcine-IgA ELISA OD450 | Concentration of fecal IgA (µg/g of fresh fecal sample) | Porcine-IgA ELISA (OD450) | Concentration of fecal IgA (µg/g of fresh fecal sample) | |||
| <1 | 6 | 2.8604±0.0788 | 184.81±17.56 | 10 | 2.8404±0.1728 | 183.72±32.37 |
| 1-3 | 8 | 0.0900±0.0300 | 4.07±0.59 | 10 | 0.1453±0.0285 | 5.17±0.58 |
| 3-6 | 9 | 0.8369±0.2640 | 22.97±8.86 | 10 | 0.9425±0.2499 | 24.99±8.67 |
| 6-12 | 7 | 2.0857±0.4843 | 91.83±40.99 | 10 | 2.1579±0.4063 | 95.32±36.92 |
| >12 | 10 | 2.5186±0.3680 | 136.71±44.16 | 10 | 2.5786±0.3680 | 146.08±47.81 |
In the suckling period (<1 month), the fecal IgA concentration peaked, followed by a decrease during the weanling period (1~3 months). The level of IgA began to rise again after the weanling period. No statistically significant differences in OD450 values between wild boars and domestic pigs (p=0.000) of each age group was detected.
Statistical analysis
The two-tailed Student's t-test was used to compare differences in the OD450 values between Sus scrofa and the other animals. The genetic distance based on the cytochrome b peptide sequence from Sus scrofa was calculated for each subspecies using the MEGA 6 software with the Jones–Taylor–Thornton amino acid substitution model with gamma distribution (Table III).
Table 3. Cross-reactivity of porcine IgA with immunoglobulin from 20 animal species in Korea.
| Scientific name | Common name | Genetic distance from Sus scrofaa | Mean±Standard deviation | |
|---|---|---|---|---|
| Porcine-IgA ELISA (OD450)b | Concentration of porcine IgA-like molecules (µg/g fresh fecal sample) | |||
| Canis lupus chanco | Korean grey wolf | 0.149 | 0.0060±0.0058 | 0.8613±0.1178 |
| Canis lupus familiaris | Sapsaree | 0.139 | 0.0055±0.0036 | 0.8507±0.0727 |
| Canis lupus familiaris | Poongsan dog | NA | 0.0075±0.0032 | 0.8912±0.0633 |
| Capra hircus coreanae | Korean native goats | 0.138 | 0.0115±0.0022 | 0.9690±0.0440 |
| Capreolus pygargus tianschanicus | Siberian roe deer | 0.115 | 0.0090±0.0051 | 0.9204±0.1019 |
| Cervus elaphus | Red deer | 0.108 | 0.0063±0.0044 | 0.8669±0.0874 |
| Cervus nippon hortulorum | Manchurian sika deer | 0.102 | 0.0063±0.0041 | 0.8677±0.0814 |
| Cervus nippon nippon | Japanese sika deer | 0.125 | 0.0082±0.0028 | 0.9033±0.0562 |
| Cervus nippon yesoensis | Yeso sika dear | 0.108 | 0.0090±0.0037 | 0.9204±0.0739 |
| Hydropotes inermis argyropus | Korean water deer | 0.114 | 0.0020±0.0048 | 0.7836±0.0946 |
| Lutra lutra lutra | Eurasian otter | 0.153 | 0.0077±0.0040 | 0.8944±0.0780 |
| Martes flavigula | Yellow throated marten | 0.163 | 0.0143±0.0116 | 1.0232±0.2279 |
| Meles leucurus | Asian badger | 0.137 | 0.0145±0.0048 | 1.0268±0.0941 |
| Myocastor coypus | Nutria | 0.207 | 0.0128±0.0133 | 0.9933±0.2611 |
| Nyctereutes procyonoides | Raccoon dog | 0.155 | 0.0090±0.0024 | 0.9203±0.0477 |
| Ovis aries | Sheep | 0.130 | 0.0095±0.0039 | 0.9301±0.0773 |
| Prionailurus bengalensis manchuria | Manchuria leopard cat | 0.151 | 0.1197±0.0444 | 4.1388±0.9125 |
| Ursus arctos lasiotus | Brown bear | 0.143 | 0.0075±0.0040 | 0.0889±0.0810 |
| Ursus thibetanususs uricus | Asiatic black bear | 0.153 | 0.0096±0.0092 | 0.9312±0.1803 |
| Vulpes vulpes peculiosa | Fox | 0.159 | 0.0084±0.0055 | 0.9073±0.1073 |
aCytochrome b sequences were downloaded from GenBank under the following accession numbers: Canis lupus chanco (NC_010340), Canis lupus familiaris breed Sapsaree (AY656755), Capra hircus (EU259132), Capreolus pygargus tianschanicus (EF139144), Cervus elaphus (JF489133), Cervus Nippon hortulorum (GU377266), Cervus Nippon Nippon (AB021093), Cervus Nippon yesoensis (AB160860), Hydropotes inermis argyropus (EF139155), Lutra lutra (EF689067), Martes flavigula (EF987749), Meles leucurus (HQ711951), Myocastor coypus (AF422919), Nyctereutes procyonoides (NC_013700), Ovis aries (DQ903227), Prionailurus bengalensis (AB210238), Sus scrofa coreanus (AY692029), Ursus arctos (NC_003427), Ursus thibetanususs uricus (AY522430), Vulpes vulpes (AY928669). NA, Not available.
bTwelve samples were tested from each species. OD450, Optical density at 450 nm.
RESULTS AND DISCUSSION
There were no differences in OD450 values between wild boars and domestic pigs (p=0.000) in each age group. Except for the Manchurian leopard cat (Prionailurus bengalensis Manchuria), all fecal samples from the other animal species had low reactivity with porcine IgA antibodies, showing statistically significant difference with that of wild boar in all age groups (p<0.001). Despite its similar genetic distance from Sus scrofa, the OD450 of the Manchurian leopard cat was 10 times higher than that of the other animal species (Table III). This result contradicts the finding of the previous report (10) showing the significant correlation between cytochrome b sequence and cross-reactivity with dolphin Ig G antibodies. However, there has been no known report that the structure of Ig A is evolutionarily related with cytochrome b sequence. Therefore, to elucidate the high affinity of pig Ig A antibodies with immunoglobulin-like molecules of Manchurian leopard cat, further studies about the genetic relationship between Ig A and cytochrome b sequence will be needed.
The fecal IgA concentrations in the suckling period were high, whereas they were lower in weanling pigs (1~3 months old) and higher again in pigs older than 6 months (Fig. 1, Table II), which agrees with previous reports of lower porcine secretory fecal IgA during the weanling period (7). Additionally, it was identified that the OD450 of the weanling pigs did not show a statistical difference with that of Manchurian leopard cat (Table I, p=0.0652). For this reason, in case of a fecal sample not showing statistically significant difference in OD450 with that of weanling pigs, we cannot convince it as droppings from wild boar. However, the IgA concentration in fecal samples of wild boars of all ages, except those 1~3 months old, was distinguishable from that of all wild animal species used for comparison in this paper, which means that the porcine IgA ELISA could be a useful method for differentiating wild boar feces from the feces of other wild animal species.
Figure 1. Fecal IgA level of wild boars and domestic pigs in each age group.
Fecal IgM concentrations are higher than IgA concentrations in weanling pigs (7) and could therefore be more useful than IgA for species identification in pigs and wild boars at 1~6 months of age. Conversely, the low level of fecal IgA in animals at 1~3 months of age could be useful to differentiate feces of the weaning period from those of the adult period. The prevalence of many infectious diseases in wild boar populations depends on the density and abundance of juveniles (11). In this situation, the population structure of weaners, as estimated from the IgA concentration, may contribute to understanding the disease status of wild boars.
ACKNOWLEDGEMENTS
This study was partially supported by a grant from Animal, Plant and Fisheries Quarantine and Inspection Agency (Project Code No. Z-AD14-2011-11-0301).
Footnotes
CONFLICTS OF INTEREST: The authors have no financial conflict of interest.
References
- 1.Meng XJ, Lindsay DS, Sriranganathan N. Wild boars as sources for infectious diseases in livestock and humans. Philos Trans R Soc Lond B Biol Sci. 2009;364:2697–2707. doi: 10.1098/rstb.2009.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albina E, Mesplede A, Chenut G, Le Potier MF, Bourbao G, Le GS, Leforban Y. A serological survey on classical swine fever (CSF), Aujeszky's disease (AD) and porcine reproductive and respiratory syndrome (PRRS) virus infections in French wild boars from 1991 to 1998. Vet Microbiol. 2000;77:43–57. doi: 10.1016/s0378-1135(00)00255-8. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson M, Gerth LM, Holmgren N, Lundeheim N, Fellstrom C. The prevalences of Brachyspira spp. and Lawsonia intracellularis in Swedish piglet producing herds and wild boar population. J Vet Med B Infect Dis Vet Public Health. 2005;52:386–391. doi: 10.1111/j.1439-0450.2005.00865.x. [DOI] [PubMed] [Google Scholar]
- 4.Seo S, Sunwoo S, Hyun B, Lyoo YS. Detection of antibodies against classical swine fever virus in fecal samples from wild boar. Vet Microbiol. 2012;161:218–221. doi: 10.1016/j.vetmic.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh HM, Chiang HL, Tsai LC, Lai SY, Huang NE, Linacre A, Lee JC. Cytochrome b gene for species identification of the conservation animals. Forensic Sci Int. 2001;122:7–18. doi: 10.1016/s0379-0738(01)00403-0. [DOI] [PubMed] [Google Scholar]
- 6.Nikitin SV, Iudin NS, Kniazev SP, Aitnazarov RB, Kobzev VF, Bekenev VA, Savvina MA, Ermolaev VI. Frequency of chromosomes carrying endogenous retroviruses in the populations of domestic pig and wild boar. Genetika. 2008;44:789–797. [PubMed] [Google Scholar]
- 7.Franz J, Corthier G. Measurement of porcine faecal IgA, IgG and IgM levels by a competitive enzyme-linked immunosorbent assay. Clin Exp Immunol. 1981;43:645–649. [PMC free article] [PubMed] [Google Scholar]
- 8.Asada Y, Kawamoto Y, Shotake T, Terao K. Molecular evolution of IgG subclass among nonhuman primates: implication of differences in antigenic determinants among Apes. Primates. 2002;43:343–349. doi: 10.1007/BF02629608. [DOI] [PubMed] [Google Scholar]
- 9.Omatsu T, Ishii Y, Kyuwa S, Milanda EG, Terao K, Yoshikawa Y. Molecular evolution inferred from immunological cross-reactivity of immunoglobulin G among Chiroptera and closely related species. Exp Anim. 2003;52:425–428. doi: 10.1538/expanim.52.425. [DOI] [PubMed] [Google Scholar]
- 10.Nollens HH, Ruiz C, Walsh MT, Gulland FM, Bossart G, Jensen ED, McBain JF, Wellehan JF. Cross-reactivity between immunoglobulin G antibodies of whales and dolphins correlates with evolutionary distance. Clin Vaccine Immunol. 2008;15:1547–1554. doi: 10.1128/CVI.00219-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kern B, Depner KR, Letz W, Rott M, Thalheim S, Nitschke B, Plagemann R, Liess B. Incidence of classical swine fever (CSF) in wild boar in a densely populated area indicating CSF virus persistence as a mechanism for virus perpetuation. Zentralbl Veterinarmed B. 1999;46:63–67. doi: 10.1046/j.1439-0450.1999.00214.x. [DOI] [PubMed] [Google Scholar]


