Abstract
Introduction
Adult liver regeneration requires induction and suppression of proliferative activity in multiple types of liver cells. The mechanisms that orchestrate the global changes in gene expression that are required for proliferative activity to change within individual liver cells, and that coordinate proliferative activity among different types of liver cells, are not well understood. Morphogenic signaling pathways that are active during fetal development, including Hedgehog and Hippo/Yes-associated protein 1 (Yap1), regulate liver regeneration in adulthood. Cirrhosis and liver cancer result when these pathways become dysregulated but relatively little is known about the mechanisms that coordinate and control morphogenic signaling during effective liver regeneration.
Methods
We evaluated the hypothesis that the Hedgehog pathway controls Yap1 activation during liver regeneration by studying intact mice and cultured liver cells.
Results
In cultured hepatic stellate cells (HSC), disrupting Hedgehog signaling blocked activation of Yap1, and knocking down Yap1 inhibited induction of both Yap1 and Hedgehog-regulated genes that enable HSC to become myofibroblasts (MF). In mice, disrupting Hedgehog signaling in MF inhibited liver regeneration after PH. Reduced proliferative activity in the liver epithelial compartment resulted from loss of stroma-derived paracrine signals that activate Yap1 and the Hedgehog pathway in hepatocytes. This prevented hepatocytes from up-regulating Yap1- and Hedgehog-regulated transcription factors that normally promote their proliferation.
Conclusion
Morphogenic signaling in HSC is necessary to reprogram hepatocytes to regenerate the liver epithelial compartment after partial hepatectomy. This discovery identifies novel molecules that might be targeted to correct defective repair during cirrhosis and liver cancer.
Keywords: hepatic stellate cells, hepatocytes, morphogens, partial hepatectomy
Introduction
Compared to other vital organs, adult liver has tremendous regenerative capacity. Yet, hepatic regenerative responses are not always successful because liver injury sometimes results in progressive damage that leads to cirrhosis or cancer. Many different types of cells that reside in healthy livers collaborate with each other to orchestrate effective regeneration, and liver re-construction becomes de-railed when these interactions are dysregulated. In order to optimize regeneration in injured livers, more knowledge is needed about mechanisms that control the growth of hepatocytes and other liver cell types in adults.
Both hepatocytes and neighboring hepatic stellate cells (HSCs) are non-proliferative in healthy livers. During liver injury, the proliferative activity of both cell types increases significantly. Proliferative HSCs undergo a fate change that makes them myofibroblastic and fibrogenic (1). The differentiation state of mature hepatocytes might also change as they become proliferative given evidence that HNF4-α (a master transcriptional regulator of hepatocyte differentiation) is suppressed when hepatocytes proliferate in chronically injured livers (2). Proliferative fate changes in both HSC and hepatocytes must be reversed for liver to resume its healthy structure and functions. The mechanisms that orchestrate the global gene expression changes required for fate changes in an individual liver cell, or that coordinate fate changes among different types of liver cells, are poorly understood.
Quiescent (Q)-HSC are stimulated to become proliferative and myofibroblastic (MF) by various soluble factors that accumulate in injured livers (1), as well as injury-related alterations in liver blood flow (3) and the physical characteristics of the liver matrix (4). There is growing evidence that these diverse fibrogenic forces link to a relatively limited number of “driver pathways” that are the proximal mediators of the MF transition (1). Morphogenic signaling pathways that are active during fetal development, including Hedgehog and Hippo/Yes-associated protein 1 (Yap1), are among such fibrogenic “driver pathways” because disrupting Hedgehog signaling (5) or preventing activation of Yap1 (6, 7) is sufficient to prevent HSC from becoming proliferative MF despite ongoing exposure to fibrogenic forces.
Interestingly, the Hedgehog pathway and activated Yap1 also regulate hepatocyte proliferation and liver regeneration (8, 9). Hedgehog and Yap1 activity are barely detectable in healthy adult liver, where signaling is confined to small subpopulations of stromal cells. When the liver is stimulated to regenerate, Hedgehog pathway and Yap1 activities are dramatically up-regulated in both HSC and hepatocytes (10, 11). Hedgehog signaling is critical for liver regeneration because inhibiting the Hedgehog pathway blocks hepatocyte proliferation and liver regeneration after partial hepatectomy (PH) (12). Liver regeneration also requires activated Yap1 as regenerative growth is blocked by preventing Yap1 activation (8). Although both the Hedgehog pathway and Yap1 activity must be induced for injured liver to regenerate, excessive activation of either the Hedgehog pathway or Yap1 results in defective repair that promotes the development of cirrhosis and liver cancer (13). Hence, both Hedgehog signaling and Yap activity must be appropriately constrained for liver regeneration to be effective. The mechanisms that coordinate and control Hedgehog signaling and Yap activity during liver regeneration are not well-understood.
We evaluated the hypothesis that the Hedgehog pathway controls Yap1 activation during liver regeneration by studying intact mice and cultured liver cells. We first focused on hepatic stellate cells (HSC) because they orchestrate liver repair (14). HSC are able to transduce Hedgehog ligand-initiated signals that regulate the activities of the Glioma-associated family of transcription factors (Gli1, Gli2, and Gli3) which control the expression of multiple Hedgehog target-genes that regulate cellular proliferation, viability, and differentiation. HSC fate is also critically regulated by Yap1, a transcription co-factor and terminal effector of the Hippo kinase pathway (7, 14). In HSC the activities of both the Hedgehog pathway and Yap1 change during liver injury, switching from typical low levels of activity during health to high activity during injury. These injury-related changes in signaling change the HSC phenotype from quiescence to myofibroblastic, thereby enabling HSC to become more migratory, proliferative, and resistant to death signals (15). Importantly, simply deregulating the Hedgehog pathway in HSC is sufficient to cause pathology in the entire liver. For example, blocking Hedgehog signaling in HSC-derived MF not only prevents MF accumulation after PH, but also inhibits hepatocyte proliferation, blocks liver regeneration, and causes progressive liver damage in mice (9). The autocrine mechanisms that coordinate the activities of the Hedgehog pathway and Yap1 within HSC themselves are not well-understood. It is also unclear how changing the activities of these master regulators of HSC fate impacts paracrine interactions between HSC and other types of liver cells that are involved in liver regeneration.
Methods
Animal Experiments
Adult (8–10 weeks old) male, C57BL/6 wild type (WT) and Smotm2Amc/J (SMO- flox) mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Smotm2Amc/J (SMO-flox) mice were crossed with αSMA-Cre-ERT2 transgenic mice, which express tamoxifen (TMX)-regulated Cre recombinase under control of the αSMA promoter (5). Double transgenic (DTG) αSMA-Cre × SMO/flox homozygote control mice were bred by crossing SMO/flox homozygote, αSMA-Cre-ERT2 hemizygous mice with SMO/flox homozygote mice. Treatment with tamoxifen (TMX) sends Cre recombinase into the nucleus to delete the floxed SMO gene, thereby inhibiting Hedgehog (Hh) signaling selectively in αSMA-expressing cells and their progeny. We confirmed the absence of detectable transgene rearrangement in vehicle-treated DTG mice, and showed that TMX-treated mice exhibit significant loss of the floxed SMO allele and accumulation of the deleted allele only after liver injury, when αSMA is up-regulated (5, 9).
Initial studies were conducted to evaluate the expression of YAP1 during liver regeneration. To this end, 70% partial hepatectomy (PH) was performed on C57BL/6 (WT) mice (n= 60) under isofluorane anesthesia, according to the method of Higgins and Anderson (16). Mice were operated on between 8 and 11 am, and resected (quiescent) livers were formalin-fixed or snap frozen in liquid nitrogen for subsequent analysis. All animals resumed normal activities following recovery from anesthesia and were sacrificed at 15 min (n= 6), 30 min (n= 6), 60 min (n=6), 3h (n=6), 6h (n=6), 12h (n=6), 24h (n=6), 48h (n=6), 72h (n=6) or 96h (n=6) after PH. In this study, and all subsequent PH experiments, regenerating liver remnants were harvested and formalin- fixed or snap frozen in liquid nitrogen for subsequent analysis. Results in regenerating livers were compared to those in the quiescent liver samples resected at the time of PH (0 hour).
In the second experiment, αSMA-Cre-ERT2, SMOflox/flox DTG mice (n = 60) were divided into groups that received either vehicle (VEH) or tamoxifen (TMX), and then sacrificed at 12 h (VEH n =6, TMX n=6), 24 h (VEH n =6, TMX n=6), 48 h (VEH n = 6, TMX=6), 72 h (VEH n =6, TMX n=6), or 96 h (VEH n =6, TMX n = 6) after PH. Smoflox/flox STG (single transgenic) mice (n = 50) were also treated with VEH or TMX, subjected to PH, and sacrificed at the same time points (5 per group). TMX (Sigma-Aldrich, 10 mg/kg body weight) was first administered on day −4 prior to surgery, and then on alternate days until the day of sacrifice. An equivalent amount of olive oil alone was injected as vehicle control. Because pilot studies demonstrated that Smo mRNA levels in TMX- and VEH-treated mice were similar until 24 h after PH, all studies compared vehicle and TMX-treated mice from 24–96 h after PH. Because results in all three control groups (i.e., VEH-treated DTG mice, VEH- and TMX-treated STG mice) were similar, data in TMX-treated DTG mice are displayed relative to VEH-treated control DTG.
In the third experiment, a further 30 WT mice were subjected to PH, treated with either vehicle (olive oil) or Cyclopamine (Cym) i.p., and then sacrificed at 12 h (VEH n = 5, CYM n = 5), 24 h (VEH n = 5, CYM n = 5) or 48 h (VEH n =5, CYM n =5) after PH. Cym (Toronto Research Chemicals Inc, Toronto, Canada), an inhibitor of Hh-signaling, was administered at a dose of 15 mg mg/kg/mouse/day (200 μl volume), according to an established in vivo protocol (12). The first injection of Cym was given 24 hours prior to PH. This treatment protocol effectively inhibited expression of Gli1 and Gli2 mRNAs at 48 hours, which is the time point for maximal hepatocyte replication following PH. Cym treatment significantly reduces survival following PH, with increased mortality evident as early as 48 hours post-PH and only ~10% Cym-treated mice surviving as long as 3 days post-PH. Thus, all mice were sacrificed by 48 h in this study.
Hepatocyte and hepatic stellate cell isolation methods, immunohistochemistry with quantification, real-time RT-PCR and western blot analysis are described in Supplemental Materials and Methods.
Results
Yap1 is activated by Hedgehog and is a downstream effector of the Hedgehog pathway
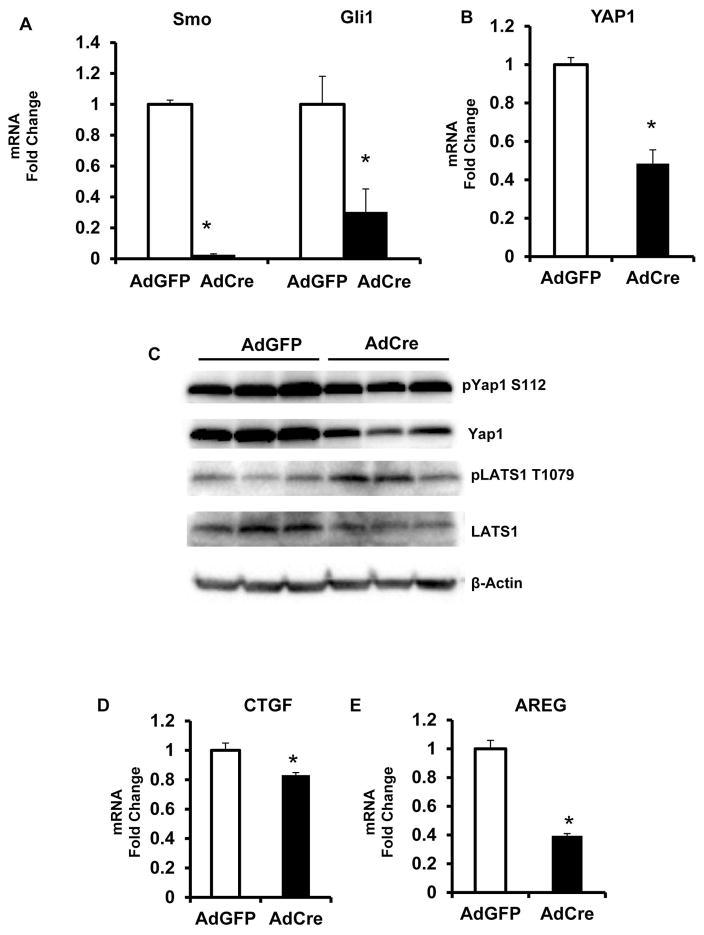
HSC orchestrate liver repair and hence, are prototypical targets for morphogenic signaling pathways that control tissue construction, including Hedgehog (11, 14). To determine how changing Hedgehog signaling influenced Yap1 activity in HSC, we isolated HSC from Smoothened (Smo)flox/flox mice, and cultured the cells in the presence of adenoviral vectors harboring either Cre recombinase (Ad-Cre) or a control construct (Ad-GFP). Treatment with Ad-Cre deletes floxed Smo alleles (Fig 1A). Since Smo is an obligate component of the Hedgehog signaling pathway, Hedgehog activity is blocked when Smo is depleted. This is demonstrated by reduced expression of Hedgehog-regulated genes, such as Gioma (Gli)-1 (Fig 1A). Using this approach, we discovered that blocking Hedgehog signaling decreased expression of Yap1 mRNA (Fig 1B) and protein (Fig 1C) and increased the activity of the Yap-inhibitory Hippo kinase cascade (Fig 1C). Consequently, net Yap1 activity was reduced by blocking Hedgehog signaling in HSC.
Figure 1. Hedgehog signaling regulates Yap1 activity.
HSC were isolated from Smoflox/flox mice, and treated for 3 days with adenovirus bearing Cre recombinase (Ad-Cre) or adenovirus expressing green fluorescent protein (Ad-GFP, control). Cells were harvested for mRNA and protein analysis by qRT-PCR or western blot respectively. (A) Smo and Gli1 mRNA. (B) Yap1 mRNA. (C) Representative western blot showing pYap1 S112 Yap1, pLats1 T1079, Lats1, and beta-actin. (D) CTGF mRNA. (E) AREG mRNA. Results were expressed as fold change relative to control (Ad-GFP) treated HSC; mean ± SEM were graphed. *p<0.05 vs. control
More specifically, qRT PCR analysis demonstrated that Smoflox/flox HSC expressed less Yap1 mRNA when they were treated with Ad-Cre to delete Smo and block Hedgehog signaling (Fig 1B). Western blot analysis confirmed that decreased Yap1 protein content accompanied the suppression of Yap1 mRNA (Fig 1C). It also showed that Smo-depleted HSC accumulated phospho-LATS1 (Fig 1C). LATS1 is the terminal kinase in the Hippo cascade. Increased phospho-LATS1 indicates that Hippo kinases were activated by inhibiting Hedgehog signaling. LATS1 phosphorylates Yap1, and we observed increases in the ratio of phospho-Yap1 to total Yap1 when HSC were treated with Ad-Cre to inhibit the Hedgehog pathway (Fig 1C). Since phospho-Yap1 cannot accumulate in the nucleus to regulate gene expression, this increase in phospho-Yap1 suggests that Yap1 activity is suppressed when Hedgehog signaling is inhibited. Indeed, qRT PCR analysis confirmed that expression of the Yap1-target genes, connective tissue growth factor (CTGF) and amphiregulin (Areg), was reduced when phospho-Yap1 accumulated due to Hedgehog pathway inhibition (Fig 1D–E). These results reveal that Hedgehog signaling activates Yap1 in HSC and suggest that Yap1 is a down-stream effector of the Hedgehog pathway.
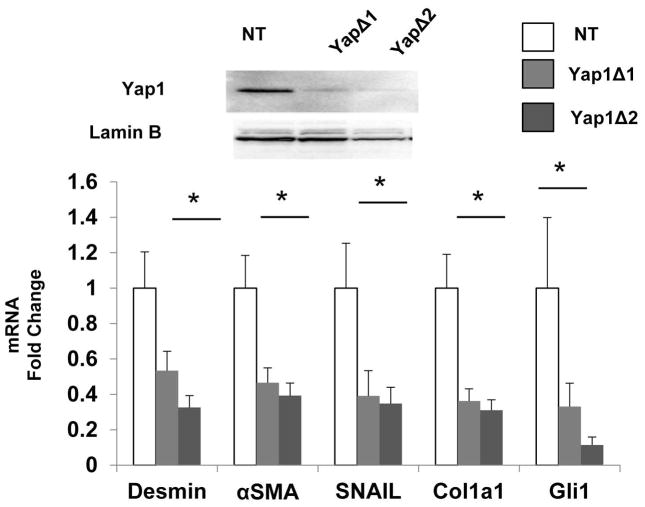
To further assess the significance of Yap1 as a mediator of Hedgehog signaling, we treated wild type HSC with shRNA lentiviral constructs to knockdown Yap1 expression. Knocking down Yap1 was sufficient to suppress HSC expression of various myofibroblast markers, demonstrating that Yap1 and Hedgehog exert similar stimulatory effects on myofibroblastic gene expression in HSC. Importantly, Yap1 deletion also suppressed HSC expression of Gli1 (Fig 2). Because gli1 is a direct transcriptional target of Hedgehog signaling (14), this finding confirms that Yap1 is a downstream effector of the Hedgehog pathway in HSC and identifies a novel positive feedback loop whereby Hedgehog and Yap1 interact to reenforce the myofibroblastic phenotype in HSC.
Figure 2. Yap1 is a downstream effector of the Hedgehog pathway.
HSC were treated with lentiviral vectors harboring distinct shRNA sequences for Yap1(YAP1D1, YAP1D2) or non-targeting control vectors (NT) and harvested for western blot and qRT-PCR analysis. Top: western blot showing Yap1 and Lamin B (nuclear protein loading control). Bottom: composite panel showing Desmin, αSMA, Snail, Collagen 1a1, Gli1 mRNA. Results were expressed as fold change relative to NT treated HSC; mean ± SEM were graphed; *p<0.05 vs. NT HSC.
Yap1 and Yap1-target genes are upregulated after PH
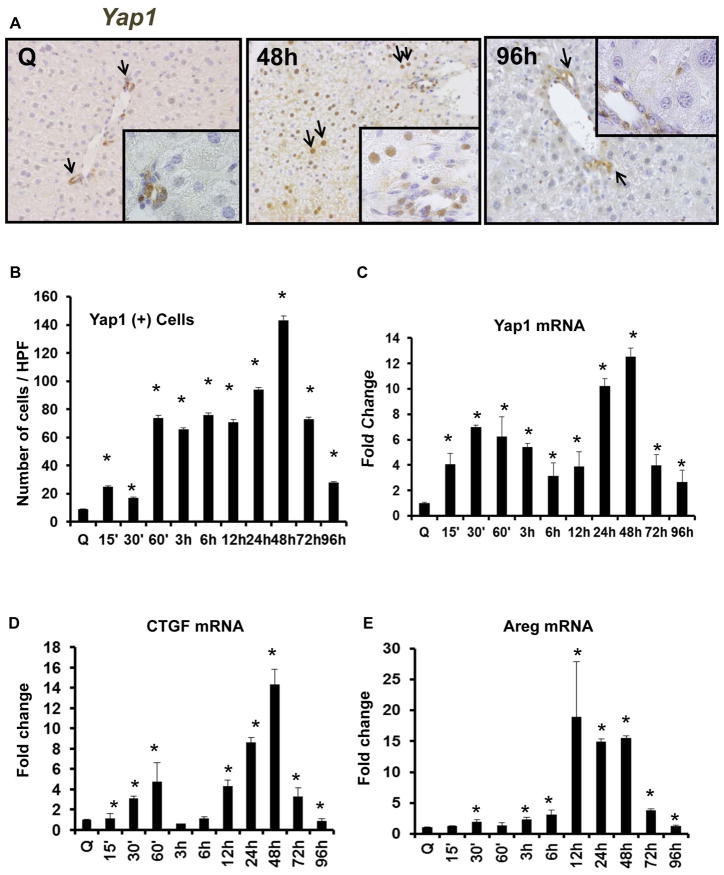
Having shown that HSC use Hedgehog and Yap1 to auto-regulate their fate, we next asked how activating Hedgehog and Yap1 in HSC impacted the ability of HSC to regulate other liver cells involved in liver regeneration, such as hepatocytes. Before addressing this question, however, it was necessary to clarify how Yap1 activity changes during liver regeneration after partial hepatectomy (PH). Other investigators have reported that over-activating Yap1 in the liver causes uncontrolled liver growth (17 – 19). Suppression of the Hippo kinase cascade, accumulation of Yap in hepatocyte nuclei, and increased whole liver expression of Yap1 mRNA, have also been noted 1 day after PH in rodents and shown to subside by the time liver returned to normal size (8) However, detailed information about how endogenous Yap1 activity varies in various liver cell populations during the progression and regression of a normal regenerative response was lacking. Therefore, we subjected wild type mice to PH, harvested liver remnants at various times during the pre-replicative, replicative, and post-replicative periods, and evaluated changes in the expression of Yap1 and Yap1 target genes. We found that PH transiently activated Yap1. Expression of Yap1 and Yap1 target genes was very low before PH but increased within 15 minutes after PH, peaking at 24–48 h, the time when hepatocyte replication is maximal. Thereafter, Yap1 gradually declined, falling to baseline levels by 96 h (4 days) after PH (Fig 3).
Figure 3. Yap1 and Yap1-target genes are upregulated after PH.
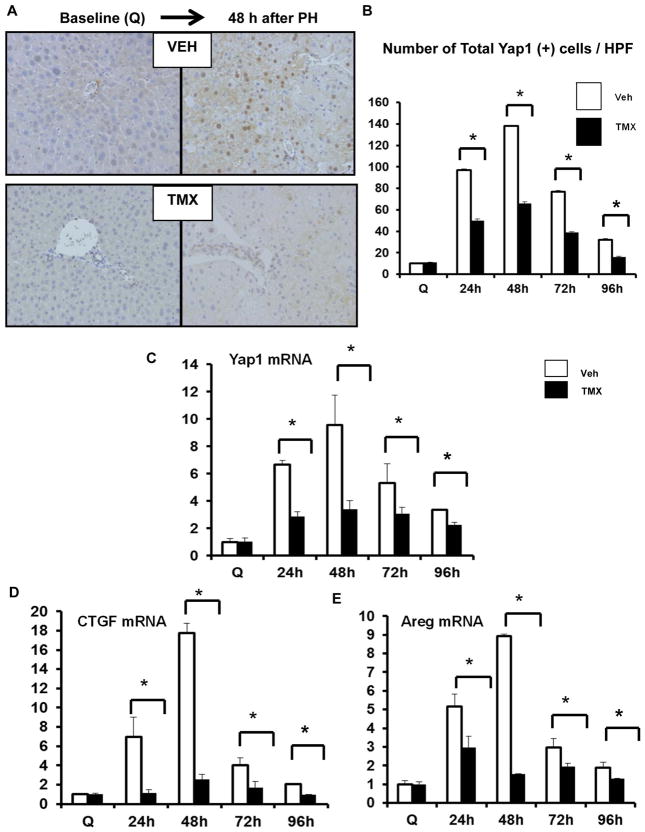
Wild type (WT) mice underwent PH. Whole livers were harvested for immunohistochemistry and qRT-PCR. (A) Representative Yap1 immunostaining in quiescent (Q) liver and at 48 and 96 h post-PH. Black arrows refer to Yap1 (+) cells. Inserts show YAP1 (+) ductular and hepatocytic cells. (B) Yap1 quantification. Results were expressed as mean ± SEM number of cells per high-powered field (HPF). (C) YAP1 mRNA, (D) CTGF mRNA, and (E) AREG mRNA; results were expressed as fold change relative to quiescent (time 0) livers; mean ± SEM were graphed. *p<0.05 vs. time 0.
Yap1 (+) cells are Hedgehog-responsive MF-HSC
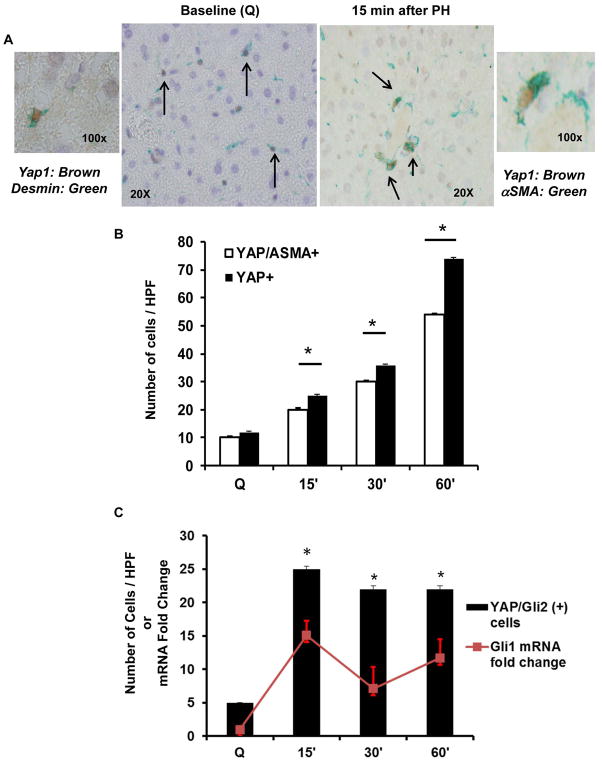
Interestingly, the analysis also revealed that the earliest accumulating Yap1 (+) cells after PH were Hedgehog responsive MF-HSC (Fig 4). At baseline, virtually all of the Yap1 (+) cells co-expressed the myofibroblast marker αSMA. These Yap1 (+) cells also expressed desmin, a marker of HSC, suggesting that Yap1 activity in healthy liver is mainly confined to myofibroblastic HSC. The numbers of Yap1 (+) MF increased 5 fold within the first hour after PH (Fig 4B). During that time period, the Yap1 (+) cells accumulated Gli2 and significantly up-regulated mRNA expression of the Gli2 target gene, Gli1 (Fig 4C), indicating that Hedgehog signaling and Yap1 are co-activated as HSC become MF very early after PH. This finding, in turn, supports our initial studies in cultured HSC which showed that interaction of Hedgehog and Yap1 drives HSC to become MF (Figs 1–3).
Figure 4. Yap1 (+) cells are Hedgehog-responsive MF-HSC.
WT livers were harvested as described in Figure legend 3. (A) Representative Yap1 (brown)/Desmin (green) double immunostaining in the quiescent (Q) liver (black arrows; 40x on right, 100x on left); Representative Yap1 (brown)/αSMA (green) double immunostaining 15 min after PH (black arrows; 40x on left, 100x on right). (B) Quantification of Yap1/αSMA double (+) cells. Results were expressed as mean ± SEM number of cells/high-powered field (HPF). (C) Yap1/Gli2 double (+) cells (solid columns) and whole liver Gli1 mRNA (red line) accumulate after PH. Results were expressed as fold change relative to quiescent livers; mean ± SEM. p<0.05 vs. quiescent livers or otherwise indicated.
To determine if Hedgehog-responsive MF played a role in activating Yap1 in other liver cell types, we performed PH on αSMA-CreERT2SMOflox/flox double transgenic (DTG) mice that were treated with tamoxifen to conditionally delete Smo in MF-HSC. Results in these mice with Hedgehog-deficient MF were compared to three groups of controls with intact Hedgehog signaling in MF, i.e., DTG mice that were treated with vehicle and SMOflox/flox single transgenic (STG) mice that were treated with either vehicle or tamoxifen. The results show that turning off Hedgehog (and hence, Yap1) in MF-HSC reduced Yap1 induction in other types of liver cells. For example, hepatocyte nuclei stained strongly for Yap1 at 48 h after PH in DTG mice that were treated with vehicle. However, significantly fewer Yap1 (+) hepatocytes accumulated in DTG mice that had been treated with tamoxifen to delete Smo and abrogate Hedgehog signaling in MF (Fig 5A, B). Consistent with that result, we found that turning off Hedgehog and Yap1 in MF prevented the striking induction of Yap1-target genes that typically occurs in the liver during the period of peak hepatocyte replication (Fig 5C–E). Similar changes were observed in all of these parameters when Smo was inhibited by systemic administration of cyclopamine (Supplemental Figure 1).
Figure 5. Hedgehog-responsive MF-HSC regulate Yap1 expression in total liver.
In separate experiments, αSMA-CreERt2/SMOflox/flox double transgenic (DTG) mice were treated with either vehicle (VEH) or TMX, and then sacrificed at 24, 48, 72, and 96h post-PH. Livers were harvested and analysed by immunohistochemistry. (A) Representative Yap1 immunostaining from VEH- and TMX-treated groups at time 0, and 48 h post-PH. (B) Yap1 quantification at time points after PH. Results were expressed as total number of Yap1 (+) cells per HPF; mean ± SEM; white columns represent VEH-treated DTG; black columns represent TMX-treated DTG. Liver RNA was analysed by qRT-PCR. (C) YAP1 mRNA. (D) CTGF mRNA. (E) AREG mRNA. Results were expressed as fold change relative to quiescent (time 0) livers; mean ± SEM were graphed; *p<0.05 vs time-matched VEH-treated DTG.
Hedgehog signaling/Yap1 activation in αSMA (+) cells promotes hepatocyte dedifferentiation and proliferation
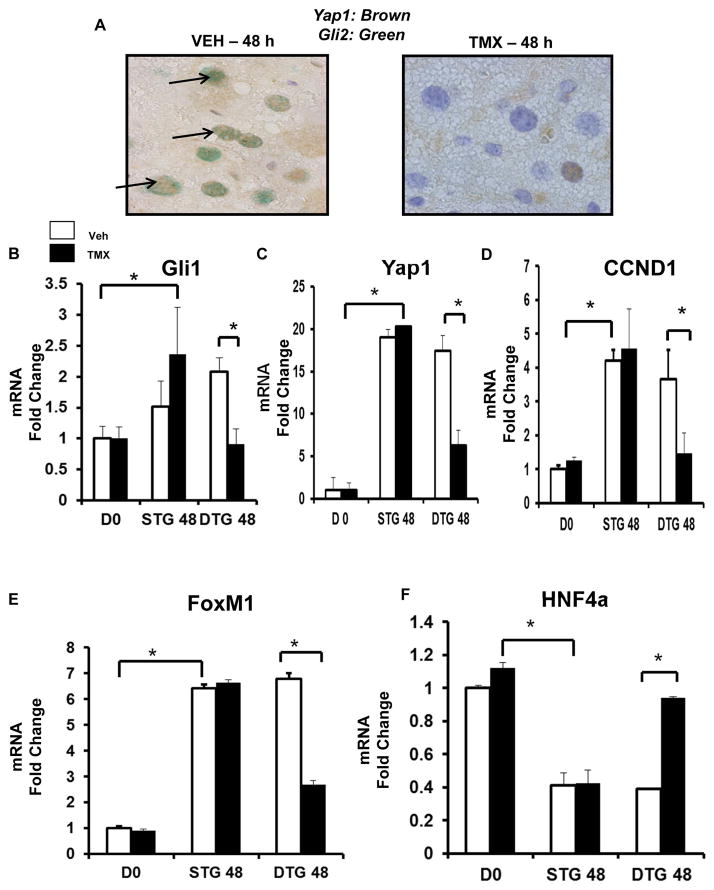
The aggregate data therefore demonstrate that blocking Hedgehog from activating Yap1 in HSC not only alters HSC fate, but also impacts paracrine interactions between HSC and other liver cell types, resulting in impaired induction of Yap1 in those in those other cells. This may be important because our other new data show that Yap1 is an effector of the Hedgehog pathway (Fig 2) and both Hedgehog and Yap1 are known to promote hepatocyte proliferation (20, 21). For example, Hedgehog and Yap1 activate factors, such as cyclin D1 and FoxM1, which are required for hepatocyte cell cycle progression. Proliferating hepatocytes in injured livers down-regulate master transcriptional regulators that control hepatocyte differentiation, such as HNF-4α (22, 23). Therefore, activating Yap1 in hepatocytes is critical for inducing hepatocyte proliferation and de-differentiation after liver injury. To determine if these responses were impacted by turning off Hedgehog and Yap1 in MF, we isolated hepatocytes from tamoxifen-treated DTG mice and the various groups of control transgenic mice at 48 h after PH, the time of maximal hepatocyte proliferation in the controls. We found that turning off Hedgehog and Yap1 in MF blocked activation of Hedgehog and Yap1 in hepatocytes at 48 h after PH (Fig 6A). This was demonstrated by significant reductions in nuclear Gli2/Yap1 co-staining in hepatocytes of tamoxifen-treated DTG mice compared to vehicle-treated DTG controls. Hepatocytes isolated from mice with Hedgehog/Yap1-deficient MF also exhibited significant suppression of Gli1 and Yap1 mRNA expression relative to that of hepatocytes from the various control groups (Fig 6B, C). Primary hepatocytes from mice with Hedgehog/Yap1-deficient MF also failed to up-regulate cyclin D1 and FoxM1 (Fig 6, E), indicating that they were less proliferative. Consistent with that finding, the numbers of mitotic hepatocytes were reduced by more than 80 % in livers of mice with Hedgehog/Yap1-deficient MF (Supplemental Figure 2). Further, hepatocytes from these mice also had significantly higher expression of the hepatocyte differentiation factor, HNF-4α (Fig 6G). The aggregate data show that disrupting Hedgehog signaling/Yap1 activation in MF blocked the induction of hepatocyte proliferation and de-differentiation that typically follows PH in mice. These results demonstrate that MF have a key role in the regenerative reprogramming of hepatic epithelial cells during liver repair, and identify Hedgehog and Yap1 as important mediators of this process.
Figure 6. Hedgehog pathway activity in αSMA (+) cells promotes proliferation and de-differentiation in hepatocytes.
αSMACreERt2/Smoflox/flox (DTG) mice were treated as described in Figure legend 5. Livers were analyzed by immunohistochemistry. (A) Yap1 (brown)/Gli2 (green) double immunostaining in VEH- and TMX-treated DTG 48 h post-PH (black arrows; 100x). In separate experiments, hepatocytes were isolated from VEH or TMX-treated DTG (n =10) and STG (n = 10) 48 h post-PH, and at time 0 (i.e. DO), and analyzed by qRT-PCR. (B) Gli1 mRNA. (C) YAP1 mRNA. (D – E) Proliferation markers: CCND1 (D) and FoxM1 (E) mRNA. (F) Hepatocyte marker, HNF4a mRNA. Results were expressed as fold change relative to DO hepatocytes; mean ± SEM were graphed; *p<0.05 versus DO or VEH-treated DTG hepatocytes.
Discussion
Reconstructing healthy liver tissue in adulthood requires the collaborative efforts of diverse types of resident liver cells that survive the initial insult (24). It is becoming evident that various morphogens help to regulate this regenerative process (14) but it is unclear how the activities of their respective signaling pathways are coordinated to achieve effective tissue repair. Clarifying the mechanisms that orchestrate effective regeneration is important as such knowledge may identify novel therapeutic targets to correct defective repair and prevent cirrhosis. Here we show for the first time that the Hedgehog pathway controls Yap1 activation during liver regeneration after partial hepatectomy (PH). Our studies reveal both cell-autonomous (i.e., autocrine) and non-cell-autonomous (i.e., paracrine) mechanisms that are involved and prove that these responses are necessary for an optimal regenerative response.
Specifically, we demonstrate for the first time that HSC auto-regulate Hedgehog and Hippo/Yap1 signaling to control their own fate. This discovery is important because there is considerable evidence that transient accumulation of HSC-derived myofibroblasts (MF) is necessary for the liver to regenerate effectively after PH (9, 25), whereas protracted accumulation of MF is a hallmark of defective liver repair that results in cirrhosis (11, 26). The new results show that disrupting Hedgehog signaling by deleting the Hedgehog co-receptor, Smoothened (Smo), down-regulates expression of Yap1 mRNA and protein and stimulates the Hippo kinase cascade to block post-translational activation of Yap1. This combined inhibition of Yap1 expression and Yap1 activity effectively suppresses Yap1’s actions on gene transcription and alters expression of Yap1-regulated mRNAs. These findings reveal novel mechanisms that control Yap1 activity in HSC. The data are also generally noteworthy because they validate the one earlier report which linked the Hedgehog- and Hippo/Yap- pathways in mammalian cells. That study showed that Hedgehog signaling promoted accumulation of Yap1 mRNA, inhibited LATS1, and activated Yap1 in a neural progenitor cell line (27). Thus, the aggregate findings in HSC and neural progenitors indicate that the Hedgehog pathway mobilizes complementary pre- and post-translational mechanisms that re-enforce Yap1 activation. An important objective of future research will be characterizing the Hedgehog-dependent mechanisms that control Yap1 mRNA levels. Studies of cell types other than HSC have identified two factors that regulate Yap1 transcription (28) and one microRNA that controls Yap mRNA stability (27). Such work has broad implications because we also demonstrated that directly knocking down Yap1 inhibits induction of Hedgehog-regulated genes, such as Gli1, that enable HSC to become MF. These findings provide novel evidence that Yap1 is an effector of the Hedgehog signaling pathway in HSC and prove that HSC fate is controlled by cross-talk between the Hedgehog and Hippo/Yap1 pathways. Activation of Hedgehog and Yap1 promotes and maintains myofibroblastic trans-differentiation of HSC.
Importantly, the findings in cultured HSC are pertinent to HSC biology in regenerating liver tissue because selectively disrupting Hedgehog signaling in MF inhibited MF accumulation and blocked liver regeneration after PH (9). Systematic analysis of the Hedgehog pathway and Yap1 in various liver cell populations from 15 min to 4 days after PH showed that liver regeneration starts with activation of Hedgehog and Yap1 in the stromal compartment. Before PH, we found that nuclear Yap1 mainly co-localized with desmin and αSMA, indicating that Yap1 is mainly expressed by small populations of myofibroblastic HSC in healthy adult mice. These Yap1 (+) MF also demonstrated nuclear immunoreactivity for Gli2 protein, suggesting that they were Hedgehog-responsive, and supporting our studies in cultured HSC which showed that Hedgehog signaling activates Yap1 and that activated Yap1 is a down-stream effector of the Hedgehog pathway. Indeed, whole liver mRNA expression of the Hedgehog target gene Gli1 increased very rapidly after PH and paralleled accumulation of MF with nuclear Gli2 and Yap1 immunoreactivity. These cells began to accumulate within minutes of PH and increased 5 fold by one hour after PH. Thereafter, other types of αSMA-negative liver cells with nuclear Yap1 gradually emerged. Hepatocytes and ductal cells were among the non-myofibroblastic cell types with “delayed” Yap1 activation. These Yap1-positive liver epithelial cells also co-expressed nuclear Gli2 and accumulated along with the Yap1/Gli2-positive MF during the first couple of days after PH. Thereafter, all types of Yap1-positive liver cells progressively dissipated, returning to nearly undetectable levels by 4 days post-PH. Thus, Hedgehog signaling and Yap1 activation occurs in the stromal compartment almost immediately after PH and persists throughout the peak period of liver epithelial proliferation, by which time many hepatocytes and ductal cells also exhibit evidence of Hedgehog and Yap1 activity.
Previously, we reported that the loss of stroma-derived signals significantly decreased whole liver expression of hepatocyte growth factor and IL6, inhibited hepatocyte accumulation of the S phase marker Ki67, suppressed recovery of liver mass, and caused progressive liver damage after PH (9). Here we confirmed that disrupting Hedgehog signaling in MF inhibited liver regeneration by showing that this significantly reduced hepatocyte mitoses after PH. We examined immunostained liver sections and primary hepatocytes isolated 48 h after PH from controls and mice with Hedgehog-depleted MF to identify mechanisms that might explain how MF direct regenerative responses in hepatocytes. Immunohistochemistry demonstrated that disrupting Hedgehog signaling in MF significantly reduced nuclear accumulation of Gli2 and Yap1 proteins in hepatocytes, resulting in significantly fewer numbers of Gli2/Yap1-positive hepatocytes at 48 h after PH. Hepatocyte proliferation was maximal at 48 h after PH in control mice and hepatocytes from these groups consistently demonstrated activation of Hedgehog and Yap (evidenced by increased mRNA expression of Gli1 and Yap1), as well as striking induction of cyclin D1 and FoxM1. Cyclin D1 and FoxM1 are Hedgehog and Yap1-regulated factors that promote cell cycle progression and each is necessary for the liver to regenerate normally after PH (29, 30). Yap1, Gli1, cyclin D1 and FoxM1 were all significantly reduced in primary hepatocytes from mice with disrupted Hedgehog signaling in MF.
Expression of HNF4-α inversely correlated with that of proliferation markers (cyclin D1 and Fox M1) in all mice, consistent with evidence that proliferating hepatocytes typically down-regulate expression of this master transcriptional regulator of hepatocyte differentiation (23). Hence, hepatocytes in mice with Hedgehog-depleted MF were not only less proliferative; they expressed significantly higher levels of HNF4-α, suggesting that they were also more differentiated. Levels of HNF4-α mRNA and Yap accumulation in hepatocyte nuclei were also inversely correlated. This suggests a mechanism for the hepatocyte de-differentiation that occurs after PH because ectopic expression of activated Yap1 promoted nuclear accumulation of Yap1 in hepatocytes and significantly decreased HNF4-α occupancy at adult-specific enhancers and increased expression levels of various genes associated with embryonic liver (22).
In summary, this study demonstrates that Hedgehog-responsive MF play a critical role in reprogramming hepatocytes to become regenerative during an effective repair response. Further, the data provide novel evidence that the mechanism driving such regenerative reprogramming is likely to involve paracrine stromal-to-epithelial signaling that activates Hedgehog and Yap1 in the epithelial compartment. More research is needed to characterize these mediators, to examine other HSC-initiated mechanisms that might also activate epithelial reprogramming in damaged livers, and to clarify why the process occurs only transiently when regeneration is effective. None-the-less, the present findings suggest a working model that can be used as a starting point for future studies. According to the new model, liver injury triggers activation of Yap1 in HSC via mechanisms that depend upon activation of the signaling-competent Hedgehog co-receptor, Smoothened (Smo). Yap1 activation in HSC is mediated via Smo-dependent increases in Yap1 expression, as well as Smo-dependent inhibition of the Hippo kinase that normally phosphorylates Yap to constrain cellular Yap1 activity. Activated Yap1 transduces many of the down-stream actions of the Hedgehog pathway that operate in HSC-derived MF. The latter include the ability of HSC-derived MF to produce Yap-sensitive factors that regulate their own growth (e.g., Areg, CTGF). At least one of these HSC-derived factors (e.g., Areg) is also a primary mitogen for hepatocytes (31) and can induce Yap1 in hepatocytes (32). Activating Yap1 in hepatocytes promotes expression of cyclin D1 and FoxM1, Yap-regulated factors that stimulate cell cycle progression to enable hepatocyte proliferation. Yap1 activation also suppresses hepatocyte differentiation by moving HNF4-α off adult-specific enhancer elements and onto embryonic-associated enhancer elements. This reduces expression levels of genes associated with fully differentiated, functional hepatocytes (e.g., HNF4-α). By extension, the model predicts that failure to constrain activation of Yap1in HSC would increase the risk for both cirrhosis and liver cancer.
Supplementary Material
Acknowledgments
Funding:
This work was supported by the following grants: R37 AA010154, R56 DK106633 and R01 DK077794 (to A.M. Diehl).
Footnotes
Conflict of Interest:
All authors declare no conflict of interest in relation to this study
Author contributions:
M.S. designed and performed experiments, analyzed data, and wrote the manuscript; G.X., G.A.M., and M.L.J. performed experiments; W.K.S., G.A.M., and R.T.P. wrote the manuscript; A.M.D. designed experiments, supervised research, analyzed data, and wrote the manuscript.
Conflict of Interests:
None
References
- 1.Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010 Aug;7(8):425–36. doi: 10.1038/nrgastro.2010.97. [DOI] [PubMed] [Google Scholar]
- 2.Nishikawa T, Bell A, Brooks JM, Setoyama K, Melis M, Han B, et al. Resetting the transcription factor network reverses terminal chronic hepatic failure. J Clin Invest. 2015 Apr;125(4):1533–44. doi: 10.1172/JCI73137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeLeve LD. Liver sinusoidal endothelial cells in hepatic fibrosis. Hepatology. 2015 May;61(5):1740–6. doi: 10.1002/hep.27376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perepelyuk M, Chin L, Cao X, van Oosten A, Shenoy VB, Janmey PA, et al. Normal and Fibrotic Rat Livers Demonstrate Shear Strain Softening and Compression Stiffening: A Model for Soft Tissue Mechanics. PLoS One. 2016 Jan 6;11(1):e0146588. doi: 10.1371/journal.pone.0146588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michelotti GA, Xie G, Swiderska M, Choi SS, Karaca G, Krüger L, et al. Smoothened is a master regulator of adult liver repair. J Clin Invest. 2013;123:2380–94. doi: 10.1172/JCI66904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bai H, Zhang N, Xu Y, Chen Q, Khan M, Potter JJ, et al. Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology. 2012 Sep;56(3):1097–107. doi: 10.1002/hep.25769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mannaerts I, Leite SB, Verhulst S, Claerhout S, Eysackers N, Thoen LF, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol. 2015 Sep;63(3):679–88. doi: 10.1016/j.jhep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Grijalva JL, Huizenga M, Mueller K, Rodriguez S, Brazzo J, Camargo F, et al. Dynamic alterations in Hippo signaling pathway and YAP activation during liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2014 Jul 15;307(2):G196–204. doi: 10.1152/ajpgi.00077.2014. [DOI] [PubMed] [Google Scholar]
- 9.Swiderska-Syn M, Syn WK, Xie G, Krüger L, Machado MV, Karaca G, et al. Myofibroblastic cells function as progenitors to regenerate murine livers after partial hepatectomy. Gut. 2014;63:1333–44. doi: 10.1136/gutjnl-2013-305962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ray K. Developmental biology: On the origin of liver regeneration. Nat Rev Gastroenterol Hepatol. 2015;12(10):549. doi: 10.1038/nrgastro.2015.147. [DOI] [PubMed] [Google Scholar]
- 11.Machado MV, Diehl AM. Liver renewal: detecting misrepair and optimizing regeneration. Mayo Clin Proc. 2014;89:120–30. doi: 10.1016/j.mayocp.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Ochoa B, Syn WK, Delgado I, Karaca GF, Jung Y, Wang J, et al. Hedgehog signaling is critical for normal liver regeneration after partial hepatectomy in mice. Hepatology. 2010;51:1712–23. doi: 10.1002/hep.23525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen Q, Anders RA, Alpini G, Bai H. Yes-associated protein in the liver: Regulation of hepatic development, repair, cell fate determination and tumorigenesis. Dig Liver Dis. 2015 Oct;47(10):826–35. doi: 10.1016/j.dld.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Fung E, Tsukamoto H. Morphogen-related therapeutic targets for liver fibrosis. Clin Res Hepatol Gastroenterol. 2015 Sep;39(Suppl 1):S69–74. doi: 10.1016/j.clinre.2015.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hellerbrand C. Hepatic stellate cells--the pericytes in the liver. Pflugers Arch. 2013 Jun;465(6):775–8. doi: 10.1007/s00424-012-1209-5. [DOI] [PubMed] [Google Scholar]
- 16.Higgins GMARM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol. 1931;12:186–202. [Google Scholar]
- 17.Lee KP, Lee JH, Kim TS, Park HD, Byun JS, Kim MC, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:8248–53. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yimlamai D, Fowl BH, Camargo FD. Emerging evidence on the role of the Hippo/YAP pathway in liver physiology and cancer. J Hepatol. 2015;63(6):1491–501. doi: 10.1016/j.jhep.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen Q, Anders RA, Alpini G, Bai H. Yes-associated protein in the liver: Regulation of hepatic development, repair, cell fate determination and tumorigenesis. Dig Liver Dis. 2015;47(10):826–35. doi: 10.1016/j.dld.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Shao DD, Xue W, Krall EB, Bhutkar A, Piccionie F, Wang X, et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171–84. doi: 10.1016/j.cell.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katoh Y, Katoh M. Hedgehog target genes: mechanisms of carcinogenesis induced by aberrant hedgehog signaling activation. Curr Mol Med. 2009 Sep;9(7):873–86. doi: 10.2174/156652409789105570. [DOI] [PubMed] [Google Scholar]
- 22.Alder O, Cullum R, Lee S, Kan AC, Wei W, Yi Y, et al. Hippo signaling influences HNF4A and FOXA2 enhancer switching during hepatocyte differentiation. Cell Rep. 2014 Oct 9;9(1):261–71. doi: 10.1016/j.celrep.2014.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saha SK, Parachoniak CA, Ghanta KS, Fitmant J, Ross KN, Najem MS, et al. Mutant IDH inhibits HNF-4α to block hepatocyte differentiation and promote biliary cancer. Nature. 2014 Sep 4;513(7516):110–4. doi: 10.1038/nature13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michalopoulos GK. Principles of liver regeneration and growth homeostasis. Compr Physiol. 2013;3:485–513. doi: 10.1002/cphy.c120014. [DOI] [PubMed] [Google Scholar]
- 25.Kordes C, Sawitza I, Gotze S, Herebian D, Haussinger D. Hepatic stellate cells contribute to progenitor cells and liver regeneration. J Clin Invest. 2014;124:5503–15. doi: 10.1172/JCI74119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trautwein C, Friedman SL, Schuppan D, Pinzani M. Hepatic fibrosis: Concept to treatment. J Hepatol. 2015;62:S15–24. doi: 10.1016/j.jhep.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-L A, Northcott PA, Dalton J, Fraga C, Ellison D, Angers S, et al. YAP1 is amplified and up-regulated in hedgehog-associated medulloblastomas and mediates Sonic hedgehog-driven neural precursor proliferation. Genes Dev. 2009 Dec 1;23(23):2729–41. doi: 10.1101/gad.1824509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Danovi SA, Rossi M, Gudmundsdottir K, Yuan M, Melino G, Basu S. Yes-associated protein (YAP) is a critical mediator of c-Jun-dependent apoptosis. Cell Death Differ. 2008 Jan;15(1):217–9. doi: 10.1038/sj.cdd.4402226. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Kiyokawa H, Dennewitz MB, Costa RH. The Forkhead Box m1b transcription factor is essential for hepatocyte DNA replication and mitosis during mouse liver regeneration. Proc Natl Acad Sci U S A. 2002 Dec 24;99(26):16881–6. doi: 10.1073/pnas.252570299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Albrecht JH, Hoffman JS, Kren BT, Steer CJ. Cyclin and cyclin-dependent kinase 1 mRNA expression in models of regenerating liver and human liver diseases. Am J Physiol. 1993 Nov;265(5 Pt 1):G857–64. doi: 10.1152/ajpgi.1993.265.5.G857. [DOI] [PubMed] [Google Scholar]
- 31.Berasain C, García-Trevijano ER, Castillo J, Erroba E, Lee DC, Prieto J, et al. Amphiregulin: an early trigger of liver regeneration in mice. Gastroenterology. 2005 Feb;128(2):424–32. doi: 10.1053/j.gastro.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Urtasun R, Latasa MU, Demartis MI, Balzani S, Goni S, Garcia-Irigoyen O, et al. Connective tissue growth factor autocriny in human hepatocellular carcinoma: oncogenic role and regulation by epidermal growth factor receptor/yes-associated protein-mediated activation. Hepatology. 2011 Dec;54(6):2149–58. doi: 10.1002/hep.24587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.