Abstract
Objective
The objective of this prospective pilot study was to assess the clinical and histologic effects of topical imiquimod therapy on recurrent extramammary Paget's disease of the vulva.
Methods
Patients with biopsy-proven recurrent extramammary Paget's disease presenting to the gynecology outpatient services at two participating institutions were recruited for conservative treatment with 5% imiquimod cream from 2007 to 2011. The topical cream was to be applied 3 times per week for 12 weeks. Punch biopsy and photography were performed at baseline and at the 12-week time point.
Results
Eight patients from two institutions were enrolled. Complete clinical and histologic response was achieved in 6 (75%) patients by the 12-week follow-up appointment. Of the two remaining patients, one had a complete clinical response but no significant histologic response; the other patient was removed from the study protocol secondary to intolerable local irritation. Two patients continue to have no evidence of disease after a median follow-up of 35 months. Five are alive with disease. No patients progressed to invasive cancer while receiving therapy.
Conclusion
Topical 5% imiquimod cream is a safe and feasible option for women suffering from recurrent extramammary Paget's disease of the vulva, and should be considered as a viable alternative to surgical management. Given the rare nature of this disease, additional multi-institutional prospective studies should be conducted to explore the efficacy of this treatment regime.
Introduction
Extramammary Paget's disease (EMPD) of the vulva is particularly rare, accounting for approximately 1% of all vulvar neoplasias. The diagnosis is confirmed by the histological identification of unique intraepithelial neoplastic cells showing glandular differentiation [1]. Surgical excision is the standard treatment for EMPD. However, relapse is common. Multiple studies have demonstrated recurrence rates greater than 30%, regardless of whether or not surgical margins were positive for disease at the time of primary excision [2-4]. Many of these patients undergo re-excision. Vulvar surgeries can be associated with significant psychosocial morbidity and decreased quality of life [5]. Given these negative sequelae and the high rate of recurrence, a more conservative approach may be of benefit when there is no evidence of an underlying adenocarcinoma.
Topical therapies including 5-fluorouracil (5-FU), bleomycin, and imiquimod have been used to treat EMPD. Unfortunately, 5-FU and bleomycin are associated with poor response rates and toxic side effects [1]. Imiquimod appears to modify the biologic response to the tumor cells, enhancing immune function, and multiple case reports have shown that it confers a complete clinical response in vulvar EMPD [1, 6-8]. However, there are no prospective clinical trials evaluating EMPD and imiquimod therapy [9]. The objective of this prospective pilot study was to assess the clinical and histologic effects of topical imiquimod therapy on recurrent extramammary Paget's disease of the vulva.
Materials and Methods
All patients presenting to the gynecology outpatient services at Memorial Sloan Kettering Cancer Center (MSKCC) and Ohio State University Medical Center (OSUMC), who were 18 years of age or older and diagnosed with biopsy-proven recurrent extramammary Paget's disease, were recruited for study inclusion from 2007 to 2011. Women were excluded if they had a known hypersensitivity to imiquimod, were pregnant or nursing, or if biopsy of the lesion demonstrated an underlying adenocarcinoma or urothelial carcinoma. Approximately 7 patients from MSKCC and 2 patients from OSUMC are diagnosed with EMPD each year. Based on this, the study accrual goal was 20 patients: 5-8 women per year for 3 years. However, the study was closed after 4 years due to poor accrual.
The pre-therapy evaluation included histologic confirmation of recurrent EMPD, a history and physical examination as per standard clinical work-up at the participating site for a vulvar lesion, and a photograph of each lesion. Patients were seen in clinic every 6 weeks during treatment for examination. Compliance with therapy was monitored with a patient diary, in which date and quantification of residual medication was recorded. These data were collected at completion of the study.
In each photograph obtained by the treating physician, millimeter scales were included to ensure spatial standardization. Given the subjective nature of determining “significant” changes in the appearance of these skin lesions, we used a method similar to that employed in a 2006 study evaluating the effects of imiquimod on dysplastic nevi [10]. Clinical response was assessed by comparing pairs of clinical photographs obtained at baseline and study completion. The pairs of photographs were graded as follows:
Complete response (CR) = reversion to clinically normal-appearing skin.
Partial response (PR) = 50% or greater reduction in diameter of affected skin.
Progression of disease (POD) = 50% or greater increase in diameter of affected skin.
The biopsied lesion was the focus of histologic assessment. All biopsies were routinely sectioned and stained (H&E). Primary histologic evaluation entailed quantification of the number of involved areas /mm2 containing Paget's cells. Histologic responses were graded as follows:
Complete response (CR) = no evidence of Paget's cells
Partial response (PR) = ratio of involved areas/mm2 in post-treatment biopsy specimen/pre-treatment biopsy specimen < 50%
Progression of disease (POD) = ratio of involved areas/mm2 in post-treatment biopsy specimen/ pre-treatment biopsy specimen >150%
Punch biopsy and photography were performed at baseline and 12-week time points. If the lesion was still present after 12 weeks of therapy, the treating physician recommended surgical excision 4 weeks following completion of therapy (week 16). If no lesion was present, or the patient refused surgical excision at week 16, the patient was instructed to return for follow-up exams every 3 months for at least 2 years.
Aldara™ 5% Cream is the brand name for imiquimod, an immune response modifier. Each gram of the 5% cream contains 50 mg of imiquimod in an off-white oil-in-water vanishing cream base. Imiquimod 5% Cream is a Food and Drug Administration (FDA)-approved safe and effective treatment for anogenital warts, superficial basal cell carcinoma, and actinic keratosis [11]. The mechanism of imiquimod in treating these conditions is not fully understood. While imiquimod has no direct antiviral activity in cell culture, mouse skin studies suggest that imiquimod induces cytokines, such as interferon-α. However, the specific clinical relevance of these findings is unknown [12, 13].
The technique for proper dose administration was undertaken in accordance with the manufacturer's instructions, in order to maximize the benefit of topical therapy. Imiquimod 5% Cream is available in single-use packets, each containing sufficient product to cover an area of up to 20 cm2. Patients were instructed to apply a thin layer to the skin and rub it in until the cream was no longer visible. Imiquimod cream was to be applied 3 times per week prior to the patient's normal sleeping hours, and left on the skin for 6-10 hours. Following this, the cream was removed by washing the treated area with mild soap and water. Some local skin reactions at the treatment site, such as erythema, were expected. Local anesthetic cream or non-occlusive dressings such as cotton gauze or cotton underwear were permissible in managing skin reactions. Treatment was allowed to resume once the reaction had subsided.
Patients would have been removed from the study if the treated lesion changed in a clinically worrisome fashion and/or histology demonstrated invasive carcinoma during treatment. Patients who experienced unacceptable toxicities were removed from the study. Serious toxicities included headache, influenza-like symptoms, myalgia, or intolerable local side effects.
Results
Eight patients were enrolled in the study. Six of the 8 patients presented with a first recurrence of EMPD. The median age at enrollment was 71.5 (range 47-78 years). Seven patients were Caucasian and 1 was Asian. All 8 women had been previously treated with a vulvar surgical excision for primary management of their disease. Five had undergone unilateral simple partial vulvectomies, and 1 had a bilateral simple partial vulvectomy. Case 4 had undergone multiple vulvectomies and skin flaps. All of the patients had documented microscopic positive margins after their primary surgery. In Case 4—the patient who enrolled after having had multiple prior re-excisions—the pathologic specimen obtained during her most recent pre-enrollment surgery was significant for positive margins. None of the patients received adjuvant therapy after their primary surgery.
Erythema, anal and clitoral involvement, pruritus, burning, discharge, pain, bleeding, and the presence and location of a visible lesion were assessed and documented upon enrollment. These findings are reported in Table 1. The labia majora was involved in 5 (63%) cases, the posterior fourchette in 2 (25%), the peri-clitoral region in 1 (13%), and the perianal skin in 1 (13%). Three (38%) patients had a history of secondary malignancy. Case 1 had a squamous cell carcinoma of the face and neck and a basal cell carcinoma of the leg, diagnosed 5 years after her EMPD diagnosis. Case 7 had a remote history of basal cell carcinoma of the face, and ductal and lobular breast cancer, diagnosed prior to her EMPD diagnosis. Case 8 had a breast cancer diagnosis prior to her EMPD diagnosis.
Table 1.
Pre-treatment symptom assessment
| Symptom | N (%) |
|---|---|
| Visible lesion | 8 (100) |
| Erythema | 6 (75) |
| Pruritus | 5 (63) |
| Burning | 2 (25) |
| Pain | 2 (25) |
| Anal involvement | 1 (13) |
| Bleeding | 0 (0) |
| Clitoral involvement | 0 (0) |
| Discharge | 0 (0) |
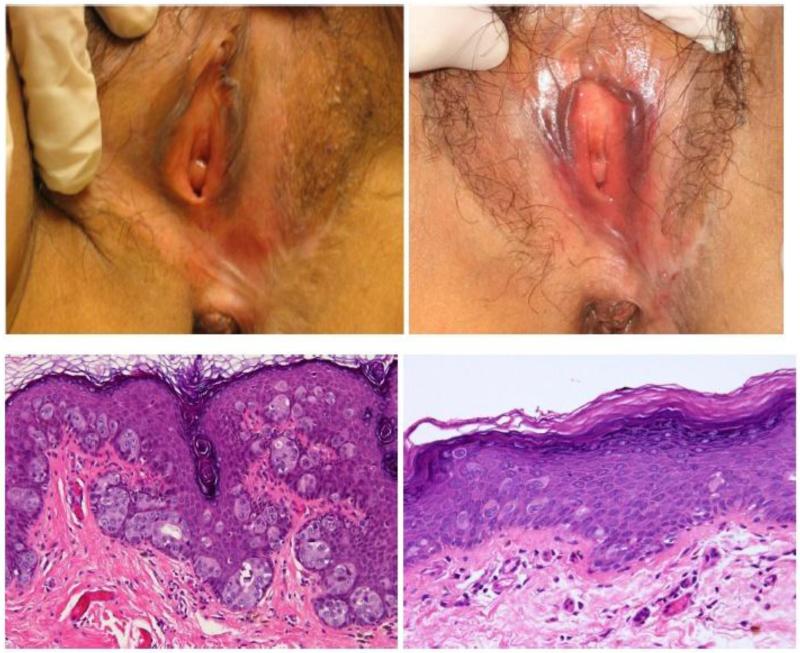
A complete clinical and histologic remission was observed in 6 (75%) women at the 12-week follow-up visit (Figures 1-3). Case 2 was noted to have a complete clinical response to treatment, but persistent disease was identified on follow-up punch biopsy (Figure 2). Overall, topical therapy with imiquimod was well-tolerated. The most commonly reported side effects were erythema and pain/burning. Case 4 was noted to have a partial clinical and histologic response, but did not complete the protocol due to inability to tolerate treatment side effects and was removed from the study. This patient continued use of imiquimod off-protocol for 16 weeks on a modified schedule, and demonstrated a partial clinical response. She never received a third punch biopsy. All patients who completed the protocol also completed patient diaries reporting compliance. Of the 6 patients in whom a complete response was achieved, EMPD recurred in 4 (67%) during a median follow-up of 35 months (range 5 to 72 months) (Table 2). Of the 5 patients currently alive with disease (AWD), 3 have opted to use imiquimod for treatment of their recurrence and 2 have declined further treatment. All 5 were offered re-excision; however, none opted for further surgery. None of the patients progressed to an invasive adenocarcinoma within the follow-up period.
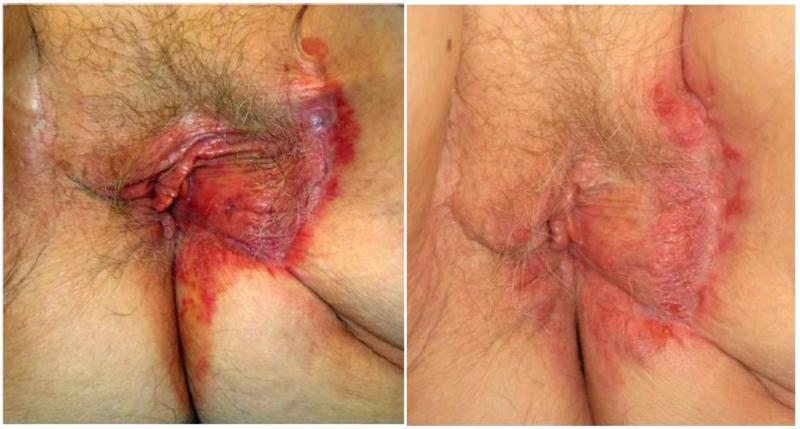
Figure 1.
Case 1 baseline photo on the left, post-treatment on the right
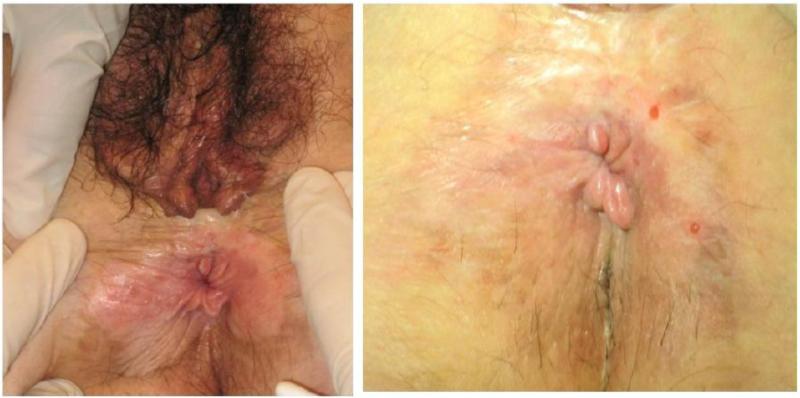
Figure 3.
Case 6 baseline photo on the left, post-treatment on the right
Figure 2.
Case 2 baseline photo on the left, post-treatment on the right (photo and H&E slide)
Table 2.
Patient outcomes and follow-up
| Case No. |
Age at Enrollment |
Outcome | Completed Protocol Requirements |
Recurred after Study |
Recurrence Free Interval (months) |
Current Status |
Follow-up (months)* |
|---|---|---|---|---|---|---|---|
| 1 | 78 | Complete Response | Yes | Yes | 10 | AWD | 72 |
| 2 | 53 | Clinical response, histologic persistence | Lost to Follow-up | Unknown | N/A | Unknown | 5 |
| 3 | 65 | Complete Response | Yes | No | N/A | NED | 68 |
| 4 | 74 | Partial clinical response, histologic persistence | Removed from study due to intolerable side effects | Yes | N/A | AWD | 35 |
| 5 | 74 | Complete Response | Yes | Yes | 4 | AWD | 35 |
| 6 | 47 | Complete Response | Yes | Yes | 4 | AWD | 20 |
| 7 | 78 | Complete Response | Yes | Yes | N/A | NED | 29 |
| 8 | 69 | Complete Response | Yes | Yes | 7 | AWD | 38 |
Months from completion of or removal from study to last date of known follow-up
Discussion
Surgical excision has long been recommended as the standard treatment strategy for primary or recurrent EMPD. Historically, these procedures have ranged from the extensive and aggressive radical vulvectomy with skin grafting for reconstruction, to the more conservative (and common) wide local excision [9]. Regardless of approach, the data demonstrate high recurrence rates ranging from 30-60%, likely due to the presence of subclinical multifocal microscopic disease [1, 9, 14]. These recurrences may lead to secondary, tertiary, and even quaternary surgical procedures. Vulvar surgery, regardless of how minor, often has long-lasting deleterious effects on sexual function and overall quality of life, especially in an older patient population [5, 15].
A topical therapeutic approach with minimal side effects and similar or improved recurrence rates could shift the treatment paradigm for this disease away from surgery, decreasing the negative impact on patients’ quality of life. Some studies have investigated the effects of conservative topical regimens such as bleomycin, 5-FU, and imiquimod. A 1978 case series of 7 women with recurrent vulvar EMPD treated with topical bleomycin, reported a clinical response in 4 patients. However, there has been minimal investigation of this specific treatment approach [16]. Topical bleomycin has demonstrated efficacy in the treatment of other dermatologic neoplasias, with minimal adverse effects [17]. There are few studies exploring 5-FU as a topical monotherapy for EMPD. Patients treated with topical 5-FU have demonstrated clinical resolution, but biopsy specimens show histologic persistence of disease [18-20].
Imiquimod is an anti-tumor immune response-modifying agent that activates toll-like receptor 7 (TLR7) and results in cytokine release and activated CD8 (+) T cells [21]. Imiquimod was initially approved by the FDA in 1997 for the treatment of external genital warts, but had previously been used off-label for numerous intraepithelial neoplasms. It has been widely studied for other such applications [22]. Topical imiquimod 5% cream was first reported as a treatment for primary and recurrent EMPD of the vulva in 2002 and 2003, respectively, and has since been documented in over 60 reports [6-8, 23]. Machida and colleagues recently conducted a systematic review on the effects of imiquimod on primary and recurrent vulvar EMPD. They recommended that imiquimod be considered for patients with recurrent disease who have undergone multiple surgical resections or are poor candidates for surgery. Imiquimod 5% cream can also be used for tertiary and quaternary recurrences [6].
To our knowledge, this is the first prospective pilot trial of topical imiquimod 5% cream in women with recurrent vulvar EMPD. We were able to demonstrate not only safety and feasibility, but also a significant rate of complete clinical and histologic response in these patients. Recurrence rates were comparable to those reported in the published literature [9]. It is noteworthy that even the patients who eventually recurred after treatment with imiquimod preferred to use the topical cream again rather than undergo another surgical excision.
Given the rarity of EMPD, we were unable to accrue a larger number of patients despite using a multi-institutional approach. This is an obvious limitation. However, our study also has numerous strengths: it is prospective in nature; it utilizes photographic and histologic documentation of disease progression/regression at multiple time points; and it has good long-term follow-up. A prospective safety and efficacy trial in the Netherlands (NCT02385188) is currently recruiting patients with primary and recurrent vulvar EMPD [24]. The results of that study, and others, will likely validate our findings and contribute to the growing body of knowledge regarding conservative management of this disease.
In summary, we recommend that imiquimod 5% cream be considered as a treatment option for patients with recurrent EMPD of the vulva, after any underlying or co-existent malignancy is ruled out. There may be some benefit to a longer course of treatment. Dosing regimens can be modified to accommodate for associated local toxicity. In the future, larger multi-center, prospective, randomized trials will help to further establish the optimal use of imiquimod 5% cream for treatment of patients with recurrent EMPD.
Highlights.
Conservative therapy with a topical agent for recurrent extramammary Paget's disease (EMPD) of the vulva is proposed.
Imiquimod is an anti-tumor immune response modifying agent that can be used off-label for EMPD.
Complete clinical and histologic responses can be achieved using topical imiquimod 5% cream on recurrent EMPD.
The most commonly reported side effects are erythema and pain/burning at site of application.
Acknowledgments
This study was funded in part through the NIH/NCI Support Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
The authors have nothing to disclose.
References
- 1.Lam C, Funaro D. Extramammary Paget's disease: summary of current knowledge. Dermatol Clin. 2010;28:807–26. doi: 10.1016/j.det.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Fanning J, Lambert HC, Hale TM, Morris PC, Schuerch C. Paget's disease of the vulva: prevalence of associated vulvar adenocarcinoma, invasive Paget's disease, and recurrence after surgical excision. Am J Obstet Gynecol. 1999;180:24–7. doi: 10.1016/s0002-9378(99)70143-2. [DOI] [PubMed] [Google Scholar]
- 3.Parker LP, Parker JR, Bodurka-Bevers D, Deavers M, Bevers MW, Shen-Gunther J, et al. Paget's disease of the vulva: pathology, pattern of involvement, and prognosis. Gynecol Oncol. 2000;77:183–9. doi: 10.1006/gyno.2000.5741. [DOI] [PubMed] [Google Scholar]
- 4.Black D, Tornos C, Soslow RA, Awtry CS, Barakat RR, Chi DS. The outcomes of patients with positive margins after excision for intraepithelial Paget's disease of the vulva. Gynecol Oncol. 2007;104:547–50. doi: 10.1016/j.ygyno.2006.09.017. [DOI] [PubMed] [Google Scholar]
- 5.McFadden K, Sharp L, Cruickshank M. The prospective management of women with newly diagnosed vulval intraepithelial neoplasia: clinical outcome and quality of life. J Obstet Gynaecol. 2009;29:749–53. doi: 10.3109/01443610903191285. [DOI] [PubMed] [Google Scholar]
- 6.Machida H, Moeini A, Roman LD, Matsuo K. Effects of imiquimod on vulvar Paget's disease: A systematic review of literature. Gynecol Oncol. 2015;139:165–71. doi: 10.1016/j.ygyno.2015.07.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang LC, Blanchard A, Judge DE, Lorincz AA, Medenica MM, Busbey S. Successful treatment of recurrent extramammary Paget's disease of the vulva with topical imiquimod 5% cream. J Am Acad Dermatol. 2003;49:769–71. doi: 10.1067/s0190-9622(03)02107-8. [DOI] [PubMed] [Google Scholar]
- 8.Zampogna JC, Flowers FP, Roth WI, Hassenein AM. Treatment of primary limited cutaneous extramammary Paget's disease with topical imiquimod monotherapy: two case reports. J Am Acad Dermatol. 2002;47:S229–35. doi: 10.1067/mjd.2002.126584. [DOI] [PubMed] [Google Scholar]
- 9.Edey KA, Allan E, Murdoch JB, Cooper S, Bryant A. Interventions for the treatment of Paget's disease of the vulva. Cochrane Database Syst Rev. 2013;10:CD009245. doi: 10.1002/14651858.CD009245.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Dusza SW, Delgado R, Busam KJ, Marghoob AA, Halpern AC. Treatment of dysplastic nevi with 5% imiquimod cream, a pilot study. J Drugs Dermatol: JDD. 2006;5:56–62. [PubMed] [Google Scholar]
- 11.U.S. Department of Health and Human Services. FDA U.S. Food and Drug Administration Highlights of Prescribing Information. [March 14, 2016];Aldara® (imiquimod) Cream, 5% Revised: October 2010]; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020723s022lbl.pdf.
- 12.Pharmaceuticals M. Product Information: Imiquimod(R), imiquimod 5% cream. St. Paul, MN.: 2004. [Google Scholar]
- 13.Beutner KR, Geisse JK, Helman D, Fox TL, Ginkel A, Owens ML. Therapeutic response of basal cell carcinoma to the immune response modifier imiquimod 5% cream. J Am Acad Dermatol. 1999;41:1002–7. doi: 10.1016/s0190-9622(99)70261-6. [DOI] [PubMed] [Google Scholar]
- 14.Gunn RA, Gallager HS. Vulvar Paget's disease. A topographic study. Cancer. 1980;46:590–4. doi: 10.1002/1097-0142(19800801)46:3<590::aid-cncr2820460327>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 15.Forner DM, Dakhil R, Lampe B. B. Quality of life and sexual function after surgery in early stage vulvar cancer. Eur J Surg Oncol (EJSO) 2015;41:40–5. doi: 10.1016/j.ejso.2014.10.050. [DOI] [PubMed] [Google Scholar]
- 16.Watring WG, Roberts JA, Lagasse LD, Berman ML, Ballon SC, Moore JG, et al. Treatment of recurrent Paget's disease of the vulva with topical bleomycin. Cancer. 1978;41:10–1. doi: 10.1002/1097-0142(197801)41:1<10::aid-cncr2820410103>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 17.Chiu HY, Tsai TF. Topical use of systemic drugs in dermatology: a comprehensive review. J Am Acad Dermatol. 2011;65:1048.e1–1048.e22. doi: 10.1016/j.jaad.2010.08.034. [DOI] [PubMed] [Google Scholar]
- 18.Del Castillo LF, Garcia C, Schoendorff C, Garcia JF, Torres LM, Garcia Almagro D. Spontaneous apparent clinical resolution with histologic persistence of a case of extramammary Paget's disease: response to topical 5-fluorouracil. Cutis. 2000;65:331–3. [PubMed] [Google Scholar]
- 19.Brown RS, McCormack M, Lankester KJ, Spittle MF. Spontaneous apparent clinical resolution with histologic persistence of a case of extramammary Paget's disease: response to topical 5-fluorouracil. Cutis. 2000;66:454–5. [PubMed] [Google Scholar]
- 20.Kawatsu T, Miki Y. Triple extramammary Paget's disease. Arch Dermatol. 1971;104:316–9. [PubMed] [Google Scholar]
- 21.Fehres CM, Bruijns SC, van Beelen AJ, Kalay H, Ambrosini M, Hooijberg E, et al. Topical rather than intradermal application of the TLR7 ligand imiquimod leads to human dermal dendritic cell maturation and CD8+ T-cell cross-priming. Eur Journal Immunol. 2014;44:2415–24. doi: 10.1002/eji.201344094. [DOI] [PubMed] [Google Scholar]
- 22.Tyring S, Rosen T. Beyond a decade of 5% imiquimod topical therapy. J Drugs Dermatol: JDD. 2009;8:467–74. [PubMed] [Google Scholar]
- 23.Flowers F. Imiquimod in the treatment of actinic keratoses and other intraepithelial neoplasms. Int J Dermatol. 2002;41(suppl 1):12–5. doi: 10.1111/j.1365-4632.2002.00019.x. [DOI] [PubMed] [Google Scholar]
- 24.Topical 5% Imiquimod Cream for Vulvar Paget's Disease. [2/9/2016];ClinicalTrials.gov Identifier: NCT02385188. Available from: https://clinicaltrials.gov/ct2/show/NCT02385188.