Abstract
Many studies have found that education is associated with better health, but the causal basis of this association is unclear. The current study used a co-twin control design to examine if differences in years of education within twin pairs predict allostatic load. The strength of this design is that it controls for genetic and other familial confounds shared between twins. The sample consisted of 381 twins (with 292 twins from 146 complete pairs; mean age=57; 61% female) who participated in the biomarker project of the Midlife Development in the United States (MIDUS) study. Individual-level analyses showed a significant, negative association between years of education and allostatic load, but this association was explained entirely by familial influences shared between twins. The results of this study suggest that schooling does not itself protect against allostatic load.
Keywords: education, allostatic load, health, discordant twin, co-twin control
1. Introduction
A large and growing literature has examined the relationship between education and health (Albouy & Lequien, 2009; Amin et al., 2015; Arendt, 2005; Behrman et al., 2011; Buckles et al., 2013; Clark & Royer, 2013; Fonseca & Zheng, 2011; Fujiwara & Kawachi, 2009; Gruenewald et al., 2012; Jürges et al., 2013; Lleras-Muney, 2005; Lundborg, 2013; Lundborg et al., 2012; Madsen et al., 2014; Manor et al., 2004; Mazumder, 2008; Meghir et al., 2012; Rosengren et al., 2009; Spasojevic, 2010; Strand & Tverdal, 2004; van Kippersluis et al., 2011; Webbink et al., 2010). Findings include that education is associated with better self-reported health (Amin et al., 2015; Fujiwara & Kawachi, 2009; Lundborg, 2013), lower mortality (Buckles et al., 2013; Lleras-Muney, 2005; Manor et al., 2004; van Kippersluis et al., 2011), greater longevity (Lundborg et al., 2012), lower odds of hypertension and diabetes (Fonseca & Zheng, 2011), reduced risk for acute myocardial infarction (Rosengren et al., 2009), and fewer chronic conditions (Lundborg, 2013).
An important index of health that has also been found to correlate with education is allostatic load. This term captures the cumulative toll of dysregulation across major physiological systems, including the cardiovascular, endocrine, metabolic, hypothalamic-pituitary-adrenal (HPA), sympathetic, and immune systems (McEwen, 2000; Taylor et al., 2011). Allostatic load is based on the concept of allostasis, which refers to bodily changes in response to environmental demands. An example of allostasis is the fact that bears prepare for winter by eating larger amounts of food and gaining body fat. Allostasis thus represents an effort to adapt to the environment. But when environmental stressors are chronic or persistent, ongoing adaptational efforts can lead to physiological dysregulation. Allostatic load captures the cumulative burden of this dysregulation, which is “the price the body pays” for adaptation (McEwen, 2000; p. 110).
Of relevance to the current study, low education is a marker of socioeonomic stress and may thus take a toll on physiological functioning (e.g., Gruenewald et al., 2012). A few studies have specifically examined the association between education and allostatic load (e.g., Gruenewald et al., 2012; Kubzansky et al., 1999), and additional investigations have documented socioeconomic gradients in specific health indices that can be considered measures of allostatic load (e.g., markers of inflammation; Loucks et al., 2010; Pollitt et al., 2008). Collectively, these studies indicate that higher levels of education are associated with a lower allostatic load, denoting a healthier profile. The findings are consistent with the rest of the literature in showing that higher levels of education are related to better health.
Several explanations exist for the association between education and better health, including that (1) more educated individuals are more health-literate and health-conscious (Buckles et al., 2013), (2) more educated individuals are better positioned to access health resources, including high-quality medical care (Buckles et al., 2013; Lundborg, 2013), (3) low education is a marker of socioeconomic adversity, which has been proposed to up-regulate pro-inflammatory genes and down-regulate antiviral genes, increasing risk for disease (Cole, 2013), and (4) unobserved factors, such as one’s genetic endowment and familial upbringing, account for the association between education and health (Amin et al., 2015; Madsen et al., 2014). Methodologically, it is challenging to adjudicate between these various causal and non-causal hypotheses. Several studies have used sophisticated methodologies, such as instrumental variables or twin designs, to interrogate causal claims. Whereas some of these studies have found enduring evidence for an effect of education on health after taking steps to control for confounding influences (Buckles et al., 2013; Fonseca & Zheng, 2011; Lundborg, 2013; Lundborg et al., 2012; Spasojevic, 2010; van Kippersluis et al., 2011), others have concluded that evidence for a causal link is limited and not particularly compelling (Albouy & Lequien, 2009; Amin et al., 2015; Behrman et al., 2011; Clark & Royer, 2013; Fujiwara & Kawachi, 2009; Madsen et al., 2014; Mazumder, 2008).
The discordant twin design is especially useful for investigating causality in observational research (McGue et al., 2010). This design examines if differences in an exposure variable (e.g., education) within twin pairs are associated with an outcome of interest (e.g., health). The strength of the design is that it controls for all familial contributions to the exposure. Thus, in the case of monozygotic (MZ) twins who are genetically identical, the design would control for all confounds related to the rearing environment as well as all genetic confounds. In the case of dizygotic (DZ) twins who share about 50% of their genes on average, the discordant twin design would partially control for genetic factors while fully controlling for other familial factors. Seven discordant twin studies have examined the association between education and health so far. Results are mixed overall, with four studies (Amin et al., 2015; Behrman et al., 2011; Fujiwara & Kawachi, 2009; Madsen et al., 2014) indicating that shared familial factors largely account for the association between education and health, two studies (Lundborg 2013; Lundborg et al., 2012) finding a residual causal effect, and one study (Webbink et al., 2010) suggesting the causal effect is evident only in men. In general, existing studies find that education may result in better self-rated global health (i.e., how participants rate their health overall; Amin et al., 2015; Fujiwara & Kawachi, 2009; Lundborg, 2013). Evidence for a causal effect of education on more specific measures of health (e.g., body-mass index or cardiovascular disease) or health-related behaviors (e.g., smoking) is much more limited.
Most studies have relied on survey data, hospital records, or registries in inferring health status (e.g., Amin et al., 2015; Behrman et al., 2011; Fujiwara & Kawachi, 2009; Lundborg, 2013; Lundborg et al., 2012; Madsen et al., 2014; Webbink et al., 2010), and no twin study so far has included direct biological measures of health. The current study builds on the extant literature by examining the relationship between years of education and a direct, multi-system measure of allostatic load that captures dysregulation across the cardiovascular, inflammation, metabolic, HPA, sympathetic, and parasympathetic systems. No prior studies of education and allostatic load have used a discordant twin design. Thus, ours is the first discordant twin study to investigate the causal nature of the relationship between education and health using directly measured biomarkers from multiple regulatory systems.
2. Methods
2.1. Participants
Data come from the MIDUS study, which examines physical health, psychological wellbeing, and social responsibility throughout midlife. The MIDUS sample is representative of non-institutionalized English-speaking adults living in the United States. Participants were recruited through random-digit dialing in 1995-1996 and were assessed via a 30-45 minute telephone interview and two self-administered questionnaires that were mailed to individuals. Available participants were re-assessed in 2004-2006. At this second wave of assessment, participants were invited to take part in additional MIDUS projects, including a biomarker project. Data in the current study come primarily from the biomarker project, which directly assesses biological indicators of health via blood, urine, and saliva collections, physical exams, and psychophysiological assessments. A total of 1255 participants, including 388 twins, completed this biomarker assessment. The twin participants were the focus of the current study.
Twin recruitment took place as part of the original MIDUS sample recruitment in 1995-1996 and involved screening 50,000 nationally representative households for the presence of twins. Approximately 15% of respondents identified a twin in the family, and 60% of those respondents gave the research team permission to contact the twin. More information on twin recruitment can be found in Kendler et al. (2000). Zygosity was determined by querying twins about the extent to which they resemble each other (e.g., the similarity of their eye and hair color and the degree to which others have difficulty telling them apart). This approach has been shown to classify over 90% of twins accurately (Krueger & Johnson, 2002; Lykken et al., 1990).
Among the 388 twins who completed the biomarker project, four twins (2 twin pairs) came from families with more than one twin pair per family. These four twins were excluded from the sample to achieve independence of observations between families. Another two twins (1 twin pair) had insufficient biomarker data to allow construction of an allostatic load index, and one other twin was missing information on educational attainment, so these three individuals were also not included in the current analyses. These exclusions resulted in a sample size of 381 twins, with 292 twins from 146 complete twin pairs (i.e., there were 89 singletons). This final sample of 146 pairs included 81 MZ pairs, 37 same-sex DZ pairs, 27 opposite-sex DZ pairs, and one pair of indeterminate zygosity (this last pair was included in analyses that pooled MZ and DZ pairs but was not included in analyses carried out separately in MZ pairs and DZ pairs). Mean age in this sample was 57 years (SD=11, range=37-86), and 61% of participants were female.
2.2. Procedures
The allostatic load index used in the current study was based on biomarkers collected as part of the MIDUS biomarker project. Participants completed all project procedures during an overnight stay at one of three University General Clinical Research Centers (GCRCs; University of California Los Angeles, Georgetown University, University of Wisconsin-Madison). During their stay, subjects provided urine and blood samples, underwent heart rate variability assessments, and completed cardiovascular testing. Urine was collected during a 12-hour protocol that began at 7:00 pm and ended at 7:00 am. Because participants provided urine samples overnight while resting in an inpatient research center, their physical activity levels were very low. The urine collection protocol thus minimized potential effects of physical activity on urinary hormone levels. Blood was collected in the morning while participants were fasting. Fresh whole blood samples were subsequently assayed for glycosylated hemoglobin (hemoglobin A1c), and frozen serum samples were assayed for cholesterol biomarkers, inflammatory biomarkers, and serum dehydroepiandrosterone sulfate. Most samples were collected over a 5-year period and were assayed in batches on an approximately annual basis. As a result, any given batch contained samples that were frozen a year or more before the assay as well as samples that were frozen only a month or two before the assay. This was the protocol for the C-reactive protein (CRP), intercellular adhesion molecule-1 (ICAM-1), e-Selectin, and fibrinogen assays, which were conducted at the University of Vermont. The interleukin 6 (IL-6) assays were conducted at the Biocore lab in Madison in larger batches with longer lags between the assays.
Participants completed heart rate variability assessments in the morning as part of a psychophysiology protocol. Their electrocardiographic (ECG) activity was monitored via electrodes for 11 minutes while they were seated. GCRC medical staff took participants’ cardiovascular measurements, including their systolic blood pressure, heart rate, and pulse pressure, and also measured participant height, weight, and waist and hip circumference.
2.3. Measures
Allostatic Load
Allostatic load was computed as the sum of seven physiological risk indices, each of which was based on 2-5 directly measured biomarkers. Table 1 lists the seven physiological systems contributing to our allostatic load index, as well as the specific biomarkers included in each system. For each biomarker, the research team determined which individuals fell into a “high-risk quartile,” corresponding to either the top quartile or the bottom quartile depending on whether high or low values of the biomarker typically confer risk for health problems. Table 2 provides descriptive statistics for each biomarker, the “high-risk” cutpoint in the MIDUS sample, and, when available, “high-risk” or “borderline” cutpoints generally used in clinical practice. As can be seen from the table, our high-risk cutpoints tended to correspond fairly closely to the typical clinical cutpoints.
Table 1.
Description of Each Physiological System Contributing to the Allostatic Load Index
| Physiological Systems | Biomarkers |
|---|---|
| 1. Sympathetic system | Based on urinary epinephrine and norepinephrine |
| 2. Parasympathetic system | Based on heart rate variability measures, including the
standard deviation of heartbeat-to- heartbeat intervals (SDRR), the root mean square of successive differences (RMSSD), and low- frequency and high-frequency heart rate variability |
|
3. Hypothalamic–pituitary–
adrenal (HPA) axis |
Based on urinary cortisol and serum dehydroepiandrosterone sulfate (DHEA-S) |
| 4. Inflammation | Based on fibrinogen, plasma C-reactive protein (CRP),
serum interleukin 6 (IL-6), e-Selectin, and the intercellular adhesion molecule-1 (ICAM-1) |
| 5. Cardiovascular system | Based on resting systolic blood pressure, resting heart rate, and pulse pressure |
| 6. Metabolic system—glucose | Based on hemoglobin A1c (HbA1c), fasting glucose, and
insulin resistance quantified through the homeostatic model assessment (HOMA-IR) |
| 7. Metabolic system—lipids | Based on body-mass index (BMI), waist-hip ratio (WHR),
triglycerides, high density lipoprotein (HDL) cholesterol, and low density lipoprotein (LDL) cholesterol |
Table 2. Descriptive Statistics and High-risk Cutpoint for Biomarkers Within Each Physiological System.
| Physiological system and representative biomarkers |
N | M | SD | High-risk cutpoint | Clinical cutpoint |
|---|---|---|---|---|---|
| Sympathetic system | |||||
| Urine epinephrine (ug/g) | 288 | 2.06 | 1.29 | ≥ 2.54 | |
| Urine norepinephrine (ug/g) | 290 | 27.94 | 13.09 | ≥ 33.33 | |
| Parasympathetic system | |||||
| SDRR (msec) | 274 | 35.40 | 16.02 | ≤ 23.54 | |
| RMSSD | 274 | 21.88 | 15.94 | ≤ 11.83 | |
| Low-frequency HRV | 274 | 401.34 | 440.52 | ≤ 113.96 | |
| High-frequency HRV | 274 | 263.37 | 430.78 | ≤ 54.16 | |
| HPA axis | |||||
| Urine cortisol (ug/g) | 292 | 18.52 | 19.11 | ≥ 21.00 | |
| Blood DHEA-S (ug/dL) | 292 | 100.54 | 71.20 | ≤ 51.00 | |
| Inflammation | |||||
| Fibrinogen (mg/dL) | 289 | 348.17 | 83.74 | ≥ 390.00 | |
| CRP(mg/L) | 289 | 2.78 | 3.92 | ≥ 3.18 | > 3 |
| IL6 (pg/mL) | 292 | 2.65 | 2.63 | ≥ 3.18 | |
| E-Selectin (ng/Ml) | 292 | 43.05 | 21.56 | ≥ 50.58 | |
| ICAM-1 (ng/Ml) | 292 | 275.67 | 99.48 | ≥ 329.7 | |
| Cardiovascular | |||||
| Resting SBP (mmHg) | 292 | 130.29 | 17.58 | ≥143.00 | ≥140 (≥120) |
| Resting Heart rate (bpm) | 291 | 70.36 | 11.26 | ≥77.00 | > 90 (>80) |
| Pulse pressure (SBP-DBP) | 292 | 55.69 | 14.84 | ≥ 65 | |
| Metabolic- Glucose | |||||
| Hemoglobin A1c % | 291 | 5.93 | .85 | ≥ 6.10 | ≥7 (> 6.4) |
| Fasting Glucose | 292 | 99.59 | 27.38 | ≥ 105 | |
| HOMA-IR | 292 | 3.20 | 4.02 | ≥ 4.05 | |
| Metabolic-Lipids | |||||
| BMI | 292 | 28.42 | 5.61 | ≥ 32.31 | ≥25, ≥30 |
| WHR | 292 | .88 | .09 | > .97 | >1 (>.85, >.9) |
| Triglycerides (mg/dL) | 292 | 125.91 | 76.48 | ≥ 160.00 | ≥200 (≥150) |
| HDL Cholesterol (mg/dL) | 292 | 56.96 | 17.90 | ≤ 41.37 | <40 |
| LDL Cholesterol (mg/dL) | 292 | 108.05 | 35.52 | ≥ 128.00 | ≥160 (≥130) |
Note: N=sample size; M=mean; SD=standard deviation; SDRR=standard deviation of heartbeat-to-heartbeat intervals; RMSSD=root mean square of successive differences; HRV=heart rate variability; HPA=Hypothalamic-pituitary-adrenal; DHEAS=dehydroepiandrosterone sulfate; CRP=C-reactive protein; IL6=interleukin 6; ICAM-1=intercellular adhesion molecule-1; SBP=systolic blood pressure; DBP=diastolic blood pressure; HOMA-IR=homeostatic model assessment of insulin resistance; BMI=body-mass index; WHR=waist-hip ratio; HDL=high density lipoprotein; LDL=low density lipoprotein. Urine epinephrine, norepinephrine, and cortisol levels are divided by urine creatinine levels to adjust for body size. Levels of urine epinephrine, norepinephrine, and cortisol (ug) are reported per level of creatinine (g).
A risk index was computed for each of the seven physiological systems, with scores computed only for individuals with valid data on at least half of the biomarkers in the physiological system. Each risk index was calculated as the proportion of biomarkers in the physiological system that fell into the high-risk range for a given individual. Scores on the risk indices ranged from 0 to 1, indicating that, for any given participant, somewhere between 0% and 100% of relevant biomarkers fell into the high-risk range. As mentioned previously, allostatic load was equal to the sum of the seven risk indices, and it was computed only for individuals with data on at least 6 of the 7 physiological risk indices. In the event of a missing risk index, imputation occurred, generally at the level of the risk index. For more information on the original construction of the allostatic load index, see Gruenewald et al. (2012).
The average allostatic load value was 1.66 (SD=1.12, range=0-5.03) in the current sample, with a possible (but unobserved) maximum value of 7. Because it had a right-skewed distribution (skew=.78), allostatic load was natural-log transformed, which reduced the skew to −.08. After transformation, all observations fell well within 3 standard deviations of the mean. Allostatic load was subsequently standardized (to have a mean of 0 and a standard deviation of 1) for ease of interpretation.
Educational Attainment
Education was assessed in a phone interview conducted during the second wave of MIDUS assessment in 2004-2006. This variable was measured as the amount of schooling participants had completed out of 12 possible levels, with the lowest level equal to “No school/some grade school (1-6)” and the highest level equal to “Ph.D., Ed.D., M.D., D.D.S., LL.B., LL.D., J.D., or other professional degree.” Education was recoded to measure approximate years of education. The average number of years of education in the current sample was 14.75 (SD=2.53, range=6-20). Because one observation fell just over 3 standard deviations below the mean, analyses were run in two ways, including (1) retaining this observation in the sample and (2) winsorizing the observation (i.e., replacing it with the nearest observation that is within 3 standard deviations of the mean). Winsorization did not alter any of our results (i.e., estimated regression coefficients for education differed by no more than .002 units, and inferences about statistical significance were unchanged). Presented in this article are results for the original (i.e., unwinsorized) data with robust standard errors. Additional results are available from the first author upon request.
3. Analytic Plan
We first examined the association between education and allostatic load in an individual-level regression analysis. This analysis yields the individual-level effect of exposure (i.e., education) on outcome (i.e., allostatic load), without controlling for genetic or other familial confounding. Next, we applied a co-twin control (CTC) design that has been previously employed to strengthen causal inference in observational twin research (e.g., Burt et al., 2010; Huibregtse et al., 2011; Irons et al., 2015; McGue et al., 2010). This design investigates if differences in education within twin pairs predict allostatic load. The rationale is that if education directly protects against allostatic load, then within twin pairs discordant in years of education, the more educated twin should have lower allostatic load than his or her less educated co-twin. The power of this design lies in its ability to control for all genetic and environmental factors shared by members of a twin pair.
In technical terms, the CTC design models the exposure variable (i.e., education) in terms of a “within-twin pair” regression coefficient (βW) and a “between-twin pair” regression coefficient (βB):
where Yij is the allostatic load outcome for individual j within the ith twin pair, xij is education for individual j within the ith twin pair, and is mean education for the ith twin pair. The “between-pair” coefficient provides an approximation of the individual-level effect in a standard regression analysis. The “within-pair” coefficient directly estimates the effect of exposure (i.e., education) on outcome (i.e., allostatic load) within twin pairs, and this effect controls for genetic and other familial confounding.
Analyses were run in SPSS software, using generalized estimating equations (GEE) with robust standard errors. Based on our sample of 146 twin pairs and a two-tailed alpha of .05, we had power of at least 80% to detect an average within-pair effect accounting for 4.4% or more of variance. We had power of at least 70% to detect an average within-pair effect accounting for as little as 3.2% of variance.
4. Results
4.1. Descriptive Statistics
Table 3 presents demographic statistics for the current sample, as well as for the total biomarker sample. As can be seen from the table, participants included in the current study were representative of the larger biomarker sample from which they derived in terms of age, sex, education, and allostatic load.
Table 3.
Demographic Statistics for Current Sample and Total Biomarker Sample
| Current Sample (n=292) |
Total Biomarker Sample
(n=1255) |
|
|---|---|---|
| Sex | 61% female | 57% female |
| Age | ||
| Mean | 56.85 | 57.32 |
| Standard Deviation | SD=11.17 | SD=11.55 |
| Range | (37-86) | (35-86) |
| Years of Education | ||
| Mean | 14.75 | 14.83 |
| Standard Deviation | SD=2.53 | SD=2.51 |
| Range | (6-20) | (6-20) |
| Allostatic Load | ||
| Mean | 1.66 | 1.77 |
| Standard Deviation | SD=1.12 | SD=1.07 |
| Range | (0-5.03) | (0-5.03) |
4.2. Preliminary Analyses
Preliminary correlational analyses show that our composite, biologically based measure of allostatic load was moderately correlated with various self-report measures of health (e.g., it correlated .37 with extent of health limitations on vigorous activity, −.25 with self-rated physical health, −.27 with self-rated overall health compared to others of same age, .28 with suspected or confirmed heart trouble, and .29 with diagnosed high blood pressure; all ps<.001), indicating that our allostatic load variable captures information in common with self-reported health without being entirely redundant with self-reported health.
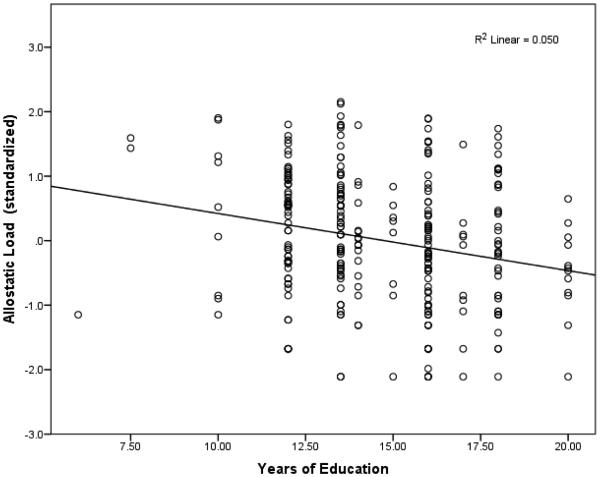
Results from an individual-level regression analysis of allostatic load on education show that a 1-year increase in educational attainment is associated with a reduction of .089 standard deviation units in allostatic load (p=.001). Figure 1 depicts the bivariate association between education and allostatic load. This effect corresponds to a zero-order correlation of −.22. When age and sex were included as controls, the effect of education on allostatic load was somewhat reduced but still remained significant (see Table 4).
Figure 1. Association Between Allostatic Load and Years of Education.
Allostatic Load was natural-log transformed and standardized to have a mean of 0 and a standard deviation of 1.
Table 4. Individual-level Regression Analysis of Allostatic Load on Education.
| Allostatic Load | |
|---|---|
| (n=292 individuals) | |
| B | |
| (SE) | |
| p | |
| Sex | .081 |
| (.110) | |
| .462 | |
| Age | .042 |
| (.004) | |
| <.001 | |
| Education | −.056 |
| (.023) | |
| .015 |
Note: Shown are the regression coefficient, standard error, and p-value for each predictor. Allostatic load was log-transformed to normalize its distribution and was subsequently standardized for ease of interpretation. Sex was coded 1 for men and 2 for women. Generalized Estimating Equations were used to account for the family structure.
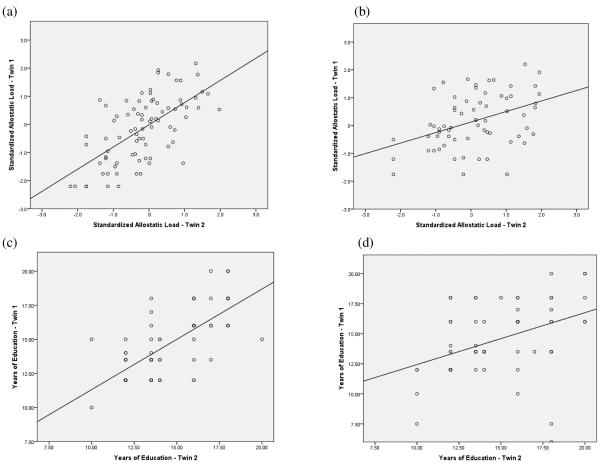
The twin correlation in allostatic load was .52 (MZ correlation: .59, DZ correlation: .39), and the twin correlation in education was .53 (MZ correlation: .72, DZ correlation: .38). Figure 2 shows the degree of twin resemblance with respect to allostatic load and education. The fact that the MZ twin correlation exceeds the DZ twin correlation for allostatic load and education indicates that both are genetically influenced and that the association between them could potentially reflect common genetic factors.
Figure 2. (a) MZ Correlation in Allostatic Load, (b) DZ Correlation in Allostatic Load, (c) MZ Correlation in Years of Education, (d) DZ Correlation in Years of Education.
MZ=monozygotic, DZ=dizygotic.
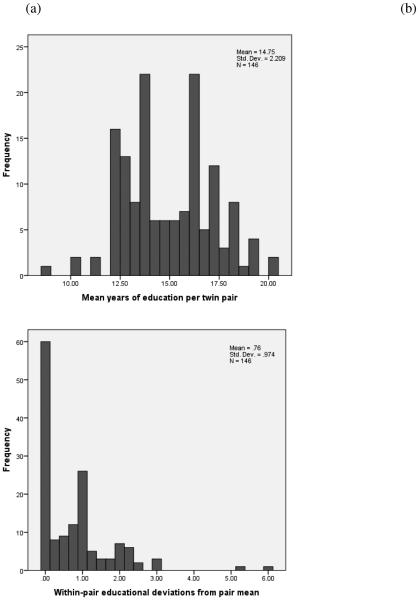
For the CTC analysis, education was decomposed into two terms: (a) the mean years of education per twin pair and (b) each twin’s deviation from the pair mean. Figure 3 shows the distribution of these two variables for one twin per pair. The figure makes clear that there is a non-trivial amount of within-pair variation in education. Of the 146 twin pairs included in the analysis, 60 pairs were concordant for years of education (36 MZ pairs, 11 same-sex DZ pairs, 12 opposite-sex DZ pairs, and 1 pair of indeterminate zygosity) and 86 pairs were discordant for years of education (45 MZ pairs, 26 same-sex DZ pairs, 15 opposite-sex DZ pairs). On average, twins from discordant pairs differed by 2.56 years of education (SD=1.93). Of note, twins from discordant pairs did not differ in average years of education from twins from concordant pairs (discordant: M=14.86, SD=2.64; concordant: M=14.59, SD=2.36, p=.367).
Figure 3. Distribution of Years of Education Between and Within Twin Pairs.
Figure (3a) shows the mean years of education per twin pair and Figure (3b) shows Twin 1’s deviation from the pair mean in absolute terms.
4.3. Co-Twin Control Analysis
Table 5 shows the results of the CTC analysis. The between-pair effect for education negatively predicted allostatic load, consistent with more education being associated with lower allostatic load. The within-pair effect for education was essentially zero. Neither the within-pair effect nor the between-pair effect varied significantly by zygosity (p>.05). Still, for completeness, Table 5 presents results for MZ pairs and DZ pairs separately.
Table 5. Co-Twin Control Analyses for Association Between Education and Allostatic Load.
| Allostatic Load | Allostatic Load | Allostatic Load | |
|---|---|---|---|
| (146 pairs) | (64 DZ pairs) | (81 MZ pairs) | |
| B | B | B | |
| (SE) | (SE) | (SE) | |
| p | p | p | |
| Sex | .054 | −.071 | .129 |
| (.110) | (.159) | (.156) | |
| .621 | .656 | .407 | |
| Age | .041 | .034 | .047 |
| (.004) | (.006) | (.007) | |
| <.001 | <.001 | <.001 | |
|
Education
between-pair |
−.075 | −.081 | −.066 |
| (.026) | (.035) | (.039) | |
| .004 | .021 | .089 | |
|
Education
within-pair |
.000 | .007 | −.002 |
| (.038) | (.047) | (.057) | |
| .990 | .886 | .969 |
Note: MZ=monozygotic; DZ=dizygotic.
Shown are the regression coefficient, standard error, and p-value for each predictor. Allostatic load was log-transformed to normalize its distribution and was subsequently standardized for ease of interpretation. Sex was coded 1 for men and 2 for women. One twin pair of indeterminate zygosity was included in the full sample but not in the separate MZ or DZ analyses. Generalized Estimating Equations were used to account for the family structure.
To test the robustness of our results, we ran additional CTC analyses in three alternate ways. First, we ran the analysis with a modified measure of allostatic load that reclassified individuals currently using medications for health conditions to a “high-risk” level on the affected biomarker (e.g., reclassifying individuals using cholesterol-lowering drugs to “high-risk” on low density lipoprotein [LDL] cholesterol). This modified variable reclassified the risk level of 122 participants out of the sample of 292 individuals. The analysis conducted with this new variable produced very similar results to the ones found for the original allostatic load measure (between-pair effect of education=−.071, p=.008; within-pair effect of education=.020, p=.598). Second, we ran the CTC analysis with the original (i.e., non-recoded) education variable, which measured level of education on a 12-point scale ranging from “No school/some grade school (1-6)” to “Ph.D., Ed.D., M.D., D.D.S., LL.B., LL.D., J.D., or other professional degree.” Results were very similar (between-pair effect=−.074, p=.007; within-pair effect=−.010, p=.795). Third, we ran the CTC analysis with all twins (i.e., including the 89 singletons who participated without their co-twin). For singletons in these analyses, mean education per twin pair was equal to the singleton’s years of educational attainment, and the deviation from the pair mean was equal to zero. This analysis produced essentially the same results (between-pair effect of education=−.073, p<.001; within-pair effect of education=.000, p=.999). Complete results are available from the first author upon request.
4.4. Follow-up Analyses
We followed up the analysis displayed in Table 5 to examine if childhood socioeconomic disadvantage mediates the between-pair effect of education on allostatic load. Our measure of childhood socioeconomic disadvantage aggregated welfare status, relative poverty, and low parental education. Follow-up analysis revealed that childhood socioeconomic disadvantage did not significantly predict allostatic load (B=.021, p=.819), beyond age, sex, between-pair education, and within-pair education. Additionally, the between-pair effect of education was relatively unchanged after the addition of childhood socioeconomic disadvantage (between-pair effect of education=−.073, p=.005).
Because allostatic load is a composite variable, we also ran follow-up analyses for each of the 7 physiological indices that contribute to allostatic load. For the purpose of these analyses, we retained the continuous distribution of the biomarkers instead of dichotomizing them. Specifically, we standardized all biomarkers, aggregated them within the 7 separate physiological systems, and then standardized each of the 7 system-level variables. Our analyses revealed that years of education was significantly associated with reduced risk for 2 of the 7 system-level variables (inflammation and poor lipid metabolism, p<.05), after accounting for the effects of age and sex. Co-twin control analyses showed that the between-pair effect of education accounted for the reduced risk (between-pair effect on inflammation=−.102, p<.001; between-pair effect on lipid metabolism=−.113, p<.001). The within-pair effect on inflammation did not reach statistical significance, but there was a significant within-pair effect on lipid metabolism (B=.072, p=.008), with additional years of education being associated with increased risk.
5. Discussion
The existing literature indicates that education is positively associated with health, though it is unclear whether this relationship is causal. Several studies have used sophisticated methodologies, such as instrumental variables or twin designs, to investigate causality. They have produced rather conflicting findings, with some supporting a causal relationship and others failing to do so. A few studies have found that education may result in better self-rated global health (Amin et al., 2015; Fujiwara & Kawachi, 2009; Lundborg, 2013), but evidence for an effect on more specific measures of health and health behaviors is much more limited. The current study made use of a discordant twin design to control for familial confounds in examining the association between education and a direct, biologically based measure of allostatic load that captures physiological dysregulation across multiple, major regulatory systems. Our initial individual-level analysis showed a significant, negative relationship between education and allostatic load, but the subsequent co-twin control analysis revealed that this association is explained by familial influences that are shared between twins. Moreover, we found that these familial influences are separate from childhood socioeconomic disadvantage. Our findings align with those of most, though not all, previous twin studies in indicating that the relationship between education and objective health is not likely to be causal (Amin et al., 2015; Behrman et al., 2011; Fujiwara & Kawachi, 2009; Madsen et al., 2014).
We chose a multisystem measure of allostatic load as the outcome because we wanted a comprehensive indicator of health, and a previous study had already established the existence of an association between education and the current measure of allostatic load in the MIDUS sample (see Gruenewald et al., 2012). Nevertheless, because allostatic load is a composite variable, we ran follow-up analyses for each of the 7 physiological indices that contribute to allostatic load. Our analyses showed that years of education was significantly associated with reduced risk for inflammation and poor lipid metabolism (p<.05), after accounting for the effects of age and sex. Co-twin control analyses revealed that the reduction in risk was due to familial factors that are shared between twins, consistent with our overall findings for allostatic load.
The current study has several strengths relative to other discordant twin analyses. For example, a major contribution of this study is its use of a direct, biologically based, and comprehensive measure of health that is able to pick up on subtle problems that may not have been reported otherwise, as well as more serious conditions. Moreover, concerns about measurement error are reduced in this study. A common critique of discordant twin analyses is that they exacerbate concerns about measurement error given that error in the exposure variable is expected to attenuate within-pair associations more than individual-level associations (Boardman & Fletcher, 2015; McGue et al., 2010). There are two reasons, however, why concerns about measurement error are minimized in the current study. First, education was assessed at two waves spaced 9-10 years apart, and the two variables correlated about .9 in twins who completed the biomarker assessment, notwithstanding the fact that some twins acquired additional schooling between the two waves. Second, measurement error is an unlikely explanation for the particular pattern of results observed in this study. Specifically, measurement error would be expected to attenuate the MZ within-pair effect to a greater degree than the DZ within-pair effect given that the MZ twin correlation is higher than the DZ twin correlation (McGue et al., 2010). In fact, we found that the MZ and DZ within-pair effects were roughly the same and both equal to zero. Another criticism that has been leveled against discordant twin analyses is that discordant pairs constitute a proportionally small and potentially unrepresentative subsample of all twin pairs, and they tend to exhibit restricted within-pair variation in the exposure measure (Boardman & Fletcher, 2015). Our sample, however, included 86 discordant pairs out of a total of 146 pairs, so analyses were not based on a proportionally small and unrepresentative sample. Additionally, discordant pairs in our study did not differ from concordant pairs in terms of average years of education, indicating that they were representative in that respect, too. As is evident in Figure 3, there was also a sufficient amount of within-pair educational variation.
This study also has a number of limitations. First, our sample size was modest, meaning that we may have had relatively lower power to detect effects. Still, the fact that the within-pair effect on allostatic load was essentially zero in both MZ pairs and DZ pairs (see Table 5) indicates that there likely is no causal influence of education on allostatic load. Second, there may have been selection biases related to study participation, such that older participants were healthier than the typical person their age. This would have resulted in an unrepresentative sample of older individuals, especially since participants were included in the current analyses only if their co-twin participated as well. The seriousness of this concern is diminished by the fact that participants in the biomarker project were comparable to the larger MIDUS cohort in terms of demographic characteristics including age, race/ethnicity, marital status, and income as well as health characteristics such as self-rated health, number of health conditions, and impairments in activities of daily living (Gruenewald et al., 2012). Additionally, we observed a significant positive association between age and allostatic load, suggesting that older individuals in our study had a higher allostatic load, as would be expected. Third, the MIDUS sample and the biomarker subsample were relatively well educated, which may have restricted variance in the exposure. Though we did observe a fair amount of variation in education—even within discordant pairs—it is possible that our findings would have differed in a less educated sample. Still, our results are consistent with what most previous twin studies have found (Amin et al., 2015; Behrman et al., 2011; Fujiwara & Kawachi, 2009; Madsen et al., 2014). Fourth, allostatic load is affected by a myriad of factors—including stressful life experiences, substance use, and lifestyle choices like diet and exercise—for which we did not control directly. Had our analyses suggested a potentially causal effect of education on allostatic load, it would have been important to examine whether any of these factors may mediate the effect of education. Because we found that familial influences mediate the association between education and allostatic load, it is less informative to control for these mostly non-familial confounds.
Of relevance to the current study, Frisell et al. (2012) noted that within-twin pair regression coefficients (βW) in discordant sibling designs are less biased than regular individual-level regression coefficients (β) only when siblings resemble each other more with respect to the full set of confounders than to the exposure variable. For this reason, we include here a discussion of the degree to which the exposure (i.e., education) and the confounders (e.g., cognitive functioning, income, parental education) are likely to be shared by family members. We reported previously that the overall twin correlation in education is about .5 (without adjusting for age or sex). Whereas some confounders may be less shared between twins (e.g., adult income and lifestyle factors), most confounders are likely to be more familial (e.g., cognitive functioning), with many confounders being entirely familial (e.g., childhood school system, parental education). Moreover, the pattern of results that we observed in this study (i.e., βW closer to the null than β) is not what would have been expected had the confounders been less shared between twins compared to the exposure, especially given that inverse confounding is unlikely in this case (i.e., confounders are expected to relate to the outcome in the same direction as the exposure).
In sum, the main finding of the current study is that the association between education and an objective, multisystem measure of allostatic load is not causal. Rather, familial factors of a genetic or environmental nature simultaneously influence education and allostatic load; these factors are separate from childhood socioeconomic disadvantage. Our study expands on the existing literature by using directly measured biomarkers of health instead of relying on self-report. The major contribution of the current study is its use of a sophisticated, multifaceted, and biologically based assessment of allostatic load combined with an elegant twin design to clarify causality. The implication of our finding is that education may not directly lower allostatic load. As a result, policies aimed at increasing schooling may not directly result in better observed health outcomes.
Highlights.
Education is negatively associated with allostatic load, but it is unclear if this relationship is causal.
Studies of discordant twins are especially useful for investigating causality because they control for all unobserved factors shared between twins.
The current discordant twin study found a significant, negative association between years of education and allostatic load, but this association was explained entirely by familial influences shared between twins.
Results of this study suggest that schooling does not itself protect against allostatic load.
Acknowledgments
The MIDUS study was supported by the John D. and Catherine T. MacArthur Foundation Research Network on Successful Midlife Development and by the National Institute on Aging Grant AG20166.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nayla R. Hamdi, Department of Psychology, University of Minnesota, 75 E River Parkway, Minneapolis, MN 55455, USA, hamdi002@umn.edu
Susan C. South, Department of Psychology, Purdue University, 703 Third Street, West Lafayette, IN 47907, USA, ssouth@psych.purdue.edu
Robert F. Krueger, Department of Psychology, University of Minnesota, 75 E River Parkway, Minneapolis, MN 55455, USA, krueg038@umn.edu
References
- Albouy V, Lequien L. Does compulsory education lower mortality? Journal of Health Economics. 2009;28:155–168. doi: 10.1016/j.jhealeco.2008.09.003. [DOI] [PubMed] [Google Scholar]
- Amin V, Behrman JR, Kohler H-P. Schooling has smaller or insignificant effects on adult health in the US than suggested by cross-sectional associations: New estimates using relatively large samples of identical twins. Social Science & Medicine. 2015;127:181–189. doi: 10.1016/j.socscimed.2014.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt JN. Does education cause better health? A panel data analysis using school reforms for identification. Economics of Education Review. 2005;24:149–160. [Google Scholar]
- Behrman JR, Kohler H-P, Jensen VM, Pedersen D, Petersen I, Bingley P, Christensen K. Does schooling reduce hospitalization and delay mortality? New evidence based on Danish twins. Demography. 2011;48:1347–1375. doi: 10.1007/s13524-011-0052-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman JD, Fletcher JM. To cause or not to cause? That is the question, but identical twins might not have all of the answers. Social Science & Medicine. 2015;127:198–200. doi: 10.1016/j.socscimed.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckles K, Hagemann A, Ofer M, Morril MS, Wozniak AK. The effect of college education on health (NBER working paper number 19222) National Bureau of Economic Research; Cambridge, MA: 2013. [Google Scholar]
- Burt SA, Donnellan MB, Humbad MN, Hicks BM, McGue M, Iacono WG. Does marriage inhibit antisocial behavior? An examination of selection vs causation via a longitudinal twin design. Archives of General Psychiatry. 2010;67:1309–1315. doi: 10.1001/archgenpsychiatry.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D, Royer H. The effect of education on adult mortality and health: Evidence from Britain. American Economic Review. 2013;103:2087–2120. doi: 10.1257/aer.103.6.2087. [DOI] [PubMed] [Google Scholar]
- Cole SW. Social regulation of human gene expression: Mechanisms and implications for public health. American Journal of Public Health (Supplement 1) 2013;103:S84–S92. doi: 10.2105/AJPH.2012.301183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca R, Zheng Y. The effect of education on health: Cross-country evidence (Working paper number WR-864) Rand Corporation; 2011. [Google Scholar]
- Frisell T, Öberg S, Kuja-Halkola R, Sjölander A. Sibling comparison designs: Bias from non-shared confounders and measurement error. Epidemiology. 2012;23:713–720. doi: 10.1097/EDE.0b013e31825fa230. [DOI] [PubMed] [Google Scholar]
- Fujiwara T, Kawachi I. Is education causally related to better health? A twin fixed-effect study in the USA. International Journal of Epidemiology. 2009;38:1310–1322. doi: 10.1093/ije/dyp226. [DOI] [PubMed] [Google Scholar]
- Gruenewald TL, Karlamangla AS, Hu P, Stein-Merkin S, Crandall C, Koretz B, Seeman TE. History of socioeconomic disadvantage and allostatic load in later life. Social Science & Medicine. 2012;74:75–83. doi: 10.1016/j.socscimed.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huibregtse BM, Bornovalova MA, Hicks B, McGue M, Iacono W. Testing the role of adolescent sexual initiation in later-life sexual risk behavior: A longitudinal twin design. Psychological Science. 2011;22:924–933. doi: 10.1177/0956797611410982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons DE, Iacono WG, McGue M. Tests of the effects of adolescent early alcohol exposures on adult outcomes. Addiction. 2015;110:269–278. doi: 10.1111/add.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürges H, Kruk E, Reinhold S. The effect of compulsory schooling on health— evidence from biomarkers. Journal of Population Economics. 2013;26:645–672. [Google Scholar]
- Kendler KS, Thornton LM, Gilman SE, Kessler RC. Sexual orientation in a national sample of twin and sibling pairs. Am. J. Psychiat. 2000;157:1843–1846. doi: 10.1176/appi.ajp.157.11.1843. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Johnson W. The Minnesota Twin Registry: current status and future directions. Twin Res. 2002;5:488–492. doi: 10.1375/136905202320906336. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Kawachi I, Sparrow D. Socioeconomic status, hostility, and risk factor clustering in the normative aging study: any help from the concept of allostatic load? Annals of Behavioral Medicine. 1999;21:330–338. doi: 10.1007/BF02895966. [DOI] [PubMed] [Google Scholar]
- Lleras-Muney A. The relationship between education and adult mortality in the United States. Review of Economic Studies. 2005;72:189–221. [Google Scholar]
- Loucks EB, Pilote L, Lynch JW, Richard H, Almeida ND, Benjamin EJ, et al. Life course socioeconomic position is associated with inflammatory markers: the Framingham Offspring Study. Social Science & Medicine. 2010;71:187–195. doi: 10.1016/j.socscimed.2010.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundborg P. The health returns to schooling–what can we learn from twins? Journal of Population Econonmics. 2013;26:673–701. [Google Scholar]
- Lundborg P, Lyttkens CH, Nysted P. Evidence from 50,000 twins (Working paper number WP12/19) Health, Econometrics and Data Group, University of York; 2012. Human capital and longevity. [Google Scholar]
- Lykken DT, Bouchard TJ, McGue M, Tellegen A. The Minnesota twin family registry: some initial findings. Acta Geneticae Medicae et Gemellologiae. 1990;39:35–70. doi: 10.1017/s0001566000005572. [DOI] [PubMed] [Google Scholar]
- Madsen M, Andersen PK, Gerster M, Andersen A-MN, Christensen K, Osler M. Are the educational differences in incidence of cardiovascular disease explained by underlying familial factors? A twin study. Social Science & Medicine. 2014;118:182–190. doi: 10.1016/j.socscimed.2014.04.016. [DOI] [PubMed] [Google Scholar]
- Manor O, Eisenbach Z, Friedlander Y, Kark JD. Educational differentials in mortality from cardiovascular disease among men and women: The Israel Longitudinal Mortality Study. Annals of Epidemiology. 2004;14:453–460. doi: 10.1016/j.annepidem.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Mazumder B. Does education improve health? A reexamination of the evidence from compulsory schooling laws. Economic Perspectives. 2008;33:2–16. [Google Scholar]
- McEwen BS. Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- McGue M, Osler M, Christensen K. Causal inference and observational research: The utility of twins. Perspectives on Psychological Science. 2010;5:546–556. doi: 10.1177/1745691610383511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meghir C, Palme M, Simeonova E. NBER Working Paper No. 17932. National Bureau of Economic Research; Cambridge, MA: 2012. Education, health and mortality: Evidence from a social experiment. [Google Scholar]
- Pollitt RA, Kaufman JS, Rose KM, Diez-Roux AV, Zeng D, Heiss G. Cumulative life course and adult socioeconomic status and markers of inflammation in adulthood. Journal of Epidemiology and Community Health. 2008;62:484–491. doi: 10.1136/jech.2006.054106. [DOI] [PubMed] [Google Scholar]
- Rosengren A, Subramanian SV, Islam S, Chow CK, Avezum A, Kazmi K, Silwa K, Zubaid M, Rangarajan S, Yusuf S. Education and risk for acute myocardial infarction in 52 high, middle and low-income countries: INTERHEART case-control study. Heart. 2009;95:2014–2022. doi: 10.1136/hrt.2009.182436. [DOI] [PubMed] [Google Scholar]
- Spasojevic J. Effects of education on adult health in Sweden: Results from a natural experiment. In: Slottje D, Tchernis R, editors. Current Issues in Health Economics Contributions to Economic Analysis. Emerald Group Publishing Limited; 2010. pp. 179–199. [Google Scholar]
- Strand BH, Tverdal A. Can cardiovascular risk factors and lifestyle explain the educational inequalities in mortality from ischaemic heart disease and from other heart diseases? 26 year follow up of 50 000 Norwegian men and women. Journal of Epidemiology and Community Health. 2004;58:705–709. doi: 10.1136/jech.2003.014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Seeman TE. Early adversity and adult health outcomes. Development and Psychopathology. 2011;23:939–954. doi: 10.1017/S0954579411000411. [DOI] [PubMed] [Google Scholar]
- van Kippersluis H, O'Donnell O, van Doorslaer E. Long-run returns to education: Does schooling lead to an extended old age? Journal of Human Resources. 2011;46:695–721. [PMC free article] [PubMed] [Google Scholar]
- Webbink D, Martin N, Visscher P. Does education reduce the probability of being overweight? Journal of Health Economics. 2010;29:29–38. doi: 10.1016/j.jhealeco.2009.11.013. [DOI] [PubMed] [Google Scholar]