Abstract
Purpose
Breast cancer diagnosis and treatment are associated with increased inflammatory activity, which can induce sickness symptoms. We examined whether emotional acceptance moderates the association between proinflammatory cytokines and self-reported sickness symptoms in women recently diagnosed with breast cancer.
Methods
Women (N = 136) diagnosed with stage 0-III breast cancer within the previous 6 months provided plasma samples and completed the FACT: Physical Well-Being Scale, as well as the Acceptance of Emotion Scale every 3 months for 2 years. At each time point, we quantified interleukin (IL)-6, IL-8, IL-10, and tumor necrosis factor (TNF)-α using a high sensitivity multiplex assay.
Results
Higher within-subject mean TNF-α across all time-points predicted higher mean sickness symptoms. At individual time-points, higher IL-6 and IL-8 levels were associated with higher sickness symptoms. Mean emotional acceptance across all time-points moderated the relationship between mean IL-8 and sickness symptoms, with sickness symptoms remaining persistently high in women with low emotional acceptance even when IL-8 levels were low. At individual time-points, emotional acceptance positively moderated the correlations of IL-8 and TNF-α with sickness symptoms, such that the associations between higher levels of these proinflammatory cytokines and higher sickness symptoms were attenuated when emotional acceptance was high.
Conclusion
Emotional acceptance was shown for the first time to moderate the associations of cytokines with sickness symptoms in breast cancer patients over time following diagnosis and treatment. The association between emotional acceptance and sickness symptoms was significantly different from zero but relatively small in comparison to the range of sickness symptoms. Results suggest that targeting emotion regulation may help to break the cycle between inflammation and sickness symptoms in women with breast cancer.
Keywords: breast cancer, emotion regulation, proinflammatory cytokines, inflammation, sickness symptoms
1. Introduction
Breast cancer, its treatment, and the associated emotional experiences can influence immune system activity (1). Malignant tumor cells and immune cells at the site of the tumor can secrete proinflammatory and immune activating cytokines, creating the systemic paraneoplastic immune response now documented as a consistent pattern in cancer, including increases in macrophage infiltrating factor, tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-8, IL-10, IL-18, and transforming growth factor (TGF)-β (2). Additionally, cancer treatments such as radiation therapy and chemotherapy can stimulate the immune system to produce proinflammatory cytokines (3). Furthermore, the threats to a woman's goals from breast cancer generate strong and persistent negative emotions, which are associated with inflammatory activity through autonomic and hormonal pathways, as well as behavioral pathways such as poor sleep quality (4-7).
Heightened proinflammatory activity can, in turn, induce symptoms such as feeling physically ill, fatigued, and experiencing pain (8-10). This constellation of symptoms is known as “sickness behavior” and is thought to reflect an adaptive, acute phase state in which the body mounts an organized biological response to defend against a pathogenic threat (9, 11, 12). Sickness behaviors, in turn, can increase inflammatory responses in the body (6), ultimately forming a vicious inflammation-sickness cycle. Although sickness behavior is believed to be an adaptive response to infectious agents, it may be detrimental in cancer patients when activation of the peripheral immune system and/or emotional distress continues unabated and exacerbates the inflammation-sickness cycle, ultimately taxing the person's resources (13). Sickness behavior in animal models parallels symptom expression in cancer patients, including physical symptoms such as pain, nausea, wasting/cachexia, and fatigue (11). Inflammation and symptoms associated with sickness behavior can have profound effects on patients’ lives; interrupting this cycle could improve the quality of life and, potentially, the survival of breast cancer patients (14-17).
The present study focuses on effective emotion regulation as one way to attenuate this vicious cycle. Emotion regulation is the process by which individuals influence which emotions they have, when they have them, and how they experience and express them (18). Emotion regulation can occur before (“antecedent” focused), during, or after (consequent-focused) an emotional response has been generated (19). Effective emotion regulation, whereby individuals regulate their emotions in a way that supports their goals and maintains physiological equilibrium, may buffer against the psychological and physiological consequences of emotional distress related to breast cancer diagnosis and treatment, while ineffective regulation may exacerbate it (20-22).
Emotional acceptance (EA) is an important emotion regulation process that involves a willingness to feel both positive and negative emotions and to allow emotions to develop and dissipate without attempts to control, change, or reject them (23). In breast cancer patients, EA is related to lower distress (23-25), fewer depressive symptoms (26), and increased positive benefit finding (i.e., perceived positive changes and experiences, including, for example, greater purpose in life and closer relationships) (27). Importantly, EA is also associated with increased survival following breast cancer diagnosis (17). Little is known, however, of the role that EA may play in the inflammation-sickness cycle in which inflammatory cytokines contribute to feelings of sickness and fatigue.
The purpose of the current study, therefore, was to conduct a secondary data analysis to examine whether emotional acceptance alters the association between proinflammatory cytokines and sickness symptoms in women with breast cancer. We hypothesized that higher levels of circulating cytokines would be associated with more sickness symptoms, but these associations would be attenuated at high levels of emotional acceptance. Put another way, higher levels of emotional acceptance will moderate the association between inflammation and sickness symptoms in breast cancer patients.
2. Materials and methods
2.1. Participants
Participants included a sample of 136 women (Age = 56 years ± 9.8; Mean time between diagnosis and initial visit = 1.7 months, range = 0.2 – 5.2 months) who were diagnosed with Stage 0 (n = 25), I (n = 55), IIA (n = 31), IIB (n = 15), IIIA (n = 7), or IIIB (n = 1) breast cancer. Additional demographic information is provided in Table 1.
Table 1.
Participant Demographics, Treatment Variables, and Questionnaire Mean Scores
| M | SD | Range | |
|---|---|---|---|
| Age (years) | 55.7 | 9.8 | 27.0 – 83.0 |
| Time between diagnosis and initial visit (months) | 1.7 | 1.1 | 0.2 – 5.2 |
| Number of Comorbid medical conditions | 1.2 | 1.1 | 0 – 5 |
| Sickness Symptoms (FACT-PWB) | |||
| Overall mean 1 | 5.0 | 4.8 | 0.0 – 27.0 |
| Person-specific mean 2 | 5.1 | 3.6 | 0.2 – 21.7 |
| Person-centered | 0.0 | 3.2 | −11.0 – 16.3 |
| Emotional Acceptance score | |||
| Overall mean 1 | 72.8 | 19.3 | 0.0 – 100.0 |
| Person-specific mean 2 | 73.0 | 17.2 | 15.5 – 100.0 |
| Person-centered | 0.0 | 8.7 | −40.4 – 45.5 |
| N | % | (out of N=136) | |
|---|---|---|---|
| Race | |||
| American Indian/Alaskan Native | 1 | 0.7 | |
| Asian | 2 | 1.5 | |
| Black/African American | 1 | 0.7 | |
| White | 132 | 97.1 | |
| Ethnicity | |||
| Hispanic | 17 | 12.5 | |
| Relationship Status | |||
| Cohabiting | 17 | 12.5 | |
| Divorced | 22 | 16.2 | |
| Single | 7 | 5.1 | |
| Widowed | 3 | 2.2 | |
| Married (1st marriage) | 59 | 43.4 | |
| Married (2nd marriage) | 28 | 20.6 | |
| Education | |||
| Some college or higher | 121 | 89 | |
| Less than college | 15 | 11 | |
| Stage of Cancer | |||
| 0 | 25 | 18.4 | |
| I | 55 | 40.5 | |
| IIA | 31 | 22.8 | |
| IIB | 15 | 11.0 | |
| IIIA | 7 | 5.1 | |
| IIIB | 1 | 0.7 | |
| Missing | 2 | 1.5 | |
| Treatment Condition | |||
| Chemotherapy only | 28 | 20.6 | |
| Radiation only | 37 | 27.2 | |
| Chemotherapy and Radiation | 37 | 27.2 | |
| Neither chemotherapy or radiation | 34 | 25.0 |
Note. M = mean; SD = standard deviation.
Scores averaged across all women and time points
Scores averaged over (up to) 9 time points for each woman
2.2. Procedures
Participants were recruited from the Multidisciplinary Breast Oncology Clinic at the Arizona Cancer Center. Research staff identified consecutive (within scheduling constraints), potentially eligible patients via medical records, and informed consent was obtained in accordance with procedures approved by the Human Subjects Protection Committee of the University of Arizona prior to any data collection. Eligibility criteria were: new diagnosis or first recurrence/second primary of invasive breast cancer (Stage 0-3), study entry session within six months following cancer diagnosis, and English literacy. Any standard medical treatment for cancer was allowed, as was additional medication. Exclusion criteria were: younger than 21 years; inability to provide informed consent.
Self-report questionnaires and plasma samples were collected at the initial visit and then again approximately every 3 months for 2 years, for up to 9 data collection time points (mean number of time points = 7.6). Collection time points were not anchored to key points in the treatment trajectory. However, at each time point/visit, participants reported whether or not they had received chemotherapy and/or radiation treatment sometime during the 3 months prior (i.e., since the last time point assessed). Breast cancer treatment information and comorbid medical diagnoses were obtained from medical chart review at the end of data collection for each subject. Supplemental material presented in Table 3 describes additional information regarding the clinical sample and characteristics of breast cancer treatment.
2.3. Measures
We incorporated both a between-person and within-person perspective to assess how, on average, cytokines and emotional acceptance were associated with sickness symptoms, as well as how women's fluctuations over time in cytokines and emotional acceptance, relative to their own means, were associated with their sickness symptoms (28-30). Thus, for each woman, we calculated her mean level of cytokines and emotional acceptance by averaging her values for that variable across all of her time points. We also calculated person-centered values of each woman's cytokines and emotional acceptance by subtracting her mean value for that variable from each of her individual observations (at each time point) for that variable. Thus, any individual time point lower than the woman's mean value would have a person-centered value less than zero, and any time point higher than the woman's mean value would have a person-centered value greater than zero.
2.3.1. Sickness symptoms
Functional Assessment of Cancer Therapy: Physical Well-Being Scale (FACT-PWB) assessed sickness symptoms and their effects on physical functioning (31). The scale includes the following 7 items: “I have a lack of energy”, “I have nausea”, “I feel sick”, “Because of my physical condition, I have trouble meeting the needs of my family”, “I have pain”, “I am bothered by the side effects of treatment”, and “I am forced to spend time in bed.” Responses ranged from 0 for “not at all” to 4 for “very much.” Higher scores (sum of 7 responses, range 0-28) indicate more sickness symptoms, and a change of ≥ 1.8-points in the overall level of this scale (i.e., not in the person-centered context) is considered clinically significant (32). Previous research has demonstrated that the sickness behavior cluster, including symptoms addressed by this scale (e.g., pain, lack of energy, etc.), can be used as a framework to explain many of the symptoms associated with cancer and cancer treatment (11, 16, 33). Nevertheless, sickness behavior is a broadly defined term that includes symptoms, experiences, and impact on functioning; the current study focused more specifically on symptoms associated with physical functioning. In the current study, the internal reliability of the total sickness symptoms scores over time (indicative of reliability between participants) was high ( coefficient = 0.89). Internal reliability of the person-centered sickness symptoms scores over time (indicative of reliability within participants) was also good ( coefficient = 0.79).
2.3.2. Emotional acceptance
Emotional acceptance was assessed with the Acceptance of Emotion Scale (AES) (17, 23). This scale assesses the extent to which participants are accepting, friendly, and nurturing toward their own feelings in general, as opposed to their emotional acceptance specifically toward cancer-related emotions. Nevertheless, given the context of the study, we expect variation in emotional acceptance to be substantially impacted by cancer-related experiences. Thirteen items include statements such as, “I naturally and easily attend to my feelings,” “I allow myself to be in touch with my feelings because it is very good for me”, and “Knowing they are ‘not perfect’, I am comfortable with my feelings as they are.” Participants indicate the percentage of time they believe the statement is true for themselves, ranging from 0 for “never” to 100 for “almost always.” The total score is the average for the 13 items (range 0 – 100) and higher scores indicate more emotional acceptance. In the current study, the internal reliability of the mean emotional acceptance scores over time (indicative of reliability between participants) was high ( coefficient = 0.97). Internal reliability of the person-centered emotional acceptance scores over time (indicative of reliability within participants) was also high ( coefficient = 0.87).
2.3.3. Inflammatory cytokines
Plasma samples were collected from EDTA-treated whole blood at each study visit, and frozen at −80°C until assayed; all time points for an individual subject were assayed together in the same 96-well plate to minimize the effects of inter-assay variability. As previously described (34), circulating levels of interleukin (IL)-1β, IL-2, IL-6, IL-8, IL-10, TNF-α, and IFN-γ were assayed with a high sensitivity bead-based multiplex assay (R&D Systems) with a Bio-Plex 200 (Luminex) Instrument, Bio-Plex software v4.1, and a 5-parameter logistic curve fit. All multiplex assays were performed on plasma samples diluted 2-fold according to the manufacturer's protocol, and all calculated concentrations generated by the BioPlex Manager software were included in data analyses. This R&D Systems multiplex assay has been shown to have excellent intra- and inter-assay reproducibility in a recent temporal stability study of circulating cytokine levels (35) and very strong correlations (r ≥ .94) across a wide range of concentrations with high sensitivity ELISA kits from the same manufacturer (36). Due to the strength of the parent study design, which utilized up to nine repeated measures of cytokine values for each subject, each time point was evaluated in a single determination. The lower limit of detection was defined as the lowest calculated value obtained on any sample (0.4 and 0.5 pg/mL for IL-8 and TNF-α, respectively, and 0.1 pg/mL for all others); calculated concentrations <0.1 pg/mL were considered below the limit of detection (35). Due to the low percentage of IFN-γ (47%), IL-2 (75%), and IL-1β (77%) with detectable levels, as well as the low IL-2 and IL-1β concentrations of the samples that were detectable, these biomarkers were excluded from the data analyses. Table 2 presents detectability information and descriptive statistics on cytokines included in analyses (i.e., IL-8, TNF-α, IL-6, and IL-10). Samples with undetectable values for IL-6 and IL-10 were substituted with one-half the concentration of the minimum calculated value per analyte, per plate. Depending on the plate, substituted values ranged from 0.08 – 0.27 pg/mL for IL-6 (2% of values) and from 0.05 – 0.12 pg/mL for IL-10 (10% of values). No samples were undetectable for TNF-α and IL-8. Using these value substitutions is more appropriate than treating the samples with undetectable values as missing at random (37). Immune variables were natural log-transformed before analyses. For tables and figures, immune data have been back-transformed to original values and, in the case of person-centered immune data, expressed as a difference from the mean.
Table 2.
Circulating Levels of Immune System Cytokines
| Cytokine | Overall Detectabilitya | Overall Mean (SD)a, pg/mL | Person-Specific Mean (SD)b, pg/mL | 25th, 75th percentile Person-Centeredc, pg/mL |
|---|---|---|---|---|
| IL-1β | 77% | NId | NId | NId |
| IL-2 | 75% | NId | NId | NId |
| IL-6 | 98% | 3.4 (2.8) | 2.5 (2.0) | −0.2, 0.3 |
| IL-8 | 100% | 6.6 (9.8) | 5.1 (1.7) | −0.2, 0.2 |
| IL-10 | 90% | 1.2 (2.7) | 0.5 (2.7) | −0.2, 0.3 |
| TNF-α | 100% | 8.5 (3.6) | 7.9 (1.4) | −0.1, 0.1 |
| IFN-γ | 47% | NId | NId | NId |
Overall percent of samples with detectable levels of cytokine, and mean (standard deviation) cytokine levels from all women with cytokine data across all time points (n = 97, total samples = 710)
Mean (standard deviation) cytokine levels from all women with cytokine data averaged over (up to) 9 time points for each woman
Within each woman, person-centered values for each cytokine were calculated by subtracting her mean cytokine values (averaged across all her time points) from each of her corresponding cytokine observations; 25th and 75th percentiles of person-centered cytokine values are shown for all women with cytokine data
NI = not included in analyses due to low detectability
2.3.4. Comorbid medical conditions
The Functional Comorbidity Index (FCI) (38) quantifies the number of chronic medical conditions for each subject. It was developed and validated for use to adjust for comorbid disease in epidemiologic studies in which physical function is an outcome measure. The index quantifies the total number of the following sixteen categories of medical conditions: 1) arthritis (rheumatoid and osteoarthritis); 2) osteoporosis; 3) asthma; 4) chronic obstructive pulmonary disease, acquired respiratory distress syndrome, or emphysema; 5) angina; 6) congestive heart failure (or heart disease); 7) heart attack; 8) neurological disease (such as multiple sclerosis or Parkinson's); 9) stroke or TIA; 10) peripheral vascular disease; 11) diabetes types I and II; 12) upper gastrointestinal disease (ulcer, hernia, reflux); 13) visual impairment (cataract, glaucoma, macular degeneration); 14) hearing impairment (very hard of hearing, even with hearing aids); 15) degenerative disc disease; and 16) obesity (body mass index >30). Other medical conditions associated with inflammation not captured in this index for one or more of the participants in the current study (as indicated) included: Sjogren's syndrome, psoriasis (n=2), inflammatory bowel disease, sarcoidosis, and iritis.
2.3.5. Demographic characteristics
Age, education, ethnicity, race, and marital status were assessed (Table 1). Marital status was characterized according to first versus subsequent marriage, in addition to the usual categories. This is based on recent findings that individuals who experienced relationship disruption, such as a divorce, reported worse health including 20% more chronic conditions compared to individuals who remained in their first marriage (39); Although remarriage was better for health than other types of relationship statuses (e.g. remaining unmarried), the authors concluded that “those who married once and remained married are consistently, strongly, and broadly advantaged” ((39)(39)(39)p. 356).
2.4. Statistical Analyses
Tables 1 and 2 present descriptive information for all study variables. To test our hypothesis, we used multilevel models to account for the repeated measures nested within individuals. We implemented the models with the lme function from the nlme package (version 3.1-118) in R (version 3.0.3). A maximum likelihood estimation procedure and unstructured matrix for the random components were specified in all of the models. Within-person residuals were treated as identical and independently distributed. Analyses first established the functional form over time of the dependent variable (sickness symptoms) and identified related covariates of sickness symptoms. Next, we included the between-person predictors (mean cytokines and mean emotional acceptance) and the within-person predictors (person-centered cytokines and person-centered emotional acceptance) in the models as fixed effects.
2.4.1. Functional form
To establish the functional form across time for the dependent variable (sickness symptoms), we started with a model that included the intercept, linear, quadratic, and cubic time as both fixed and random effects, but the model did not converge. Removing the random effect of cubic time allowed for convergence, thus, in the final model it was only included as a fixed effect. Next, we examined demographic and treatment variables (age, ethnicity, education, relationship status, race, comorbidity, current disease stage, cancer treatment type, time-varying chemotherapy and radiation treatment occasions, and time between diagnosis and initial assessment) in separate models as predictors of sickness symptoms. Those that either had main effects, or interacted with linear, quadratic, or cubic time were included as controls in subsequent models.
2.4.2. Predictive models
After determining the functional form, we estimated separate models to test associations of mean or person-centered cytokines (IL-6, IL-8, IL-10, or TNF-α values), predicting sickness symptoms over time, moderated by mean or person-centered emotional acceptance. All between-person models (i.e., mean level predictors) also controlled for person-centered levels of cytokines and emotional acceptance, and all within-person models (i.e., person-centered predictors) also controlled for mean levels of cytokines and emotional acceptance.
The Level 1 equation for the predictive model is represented below.1 The “pc” variables refer to the person-centered (or within-person) versions of the variables. The “mean” variables refer to the mean (or between-person) versions of the variables.
The Level 2 equation for the predictive model is presented below. The intercept, linear time, and quadratic time were estimated as random effects and improved model fit.
3. Results
3.1. Missing Data
Missingness at any time point (i.e., visits 1-9) was not predicted by cancer treatment, cancer stage, age, nor mean levels of EA, interleukin (IL)-8, IL-10, or tumor necrosis factor (TNF)-α. However, missingness was predicted by mean levels of sickness symptoms (i.e., a one-unit increase in self-reported sickness symptoms was associated with 17% increase in the odds of being missing), and by mean levels of IL-6 (i.e., a one-unit increase in natural-log transfored-IL-6, or a 2.72 increase in IL-6 pg/mL, was associated with a 94% increase in the odds of being missing). Rather than biasing results in an undesirable way, this pattern of missingness makes it less likely for us to find support for our hypotheses, due to the restricted range on the key variables.
3.2. Effects of Time and Demographic/Medical Variables on Sickness Symptoms
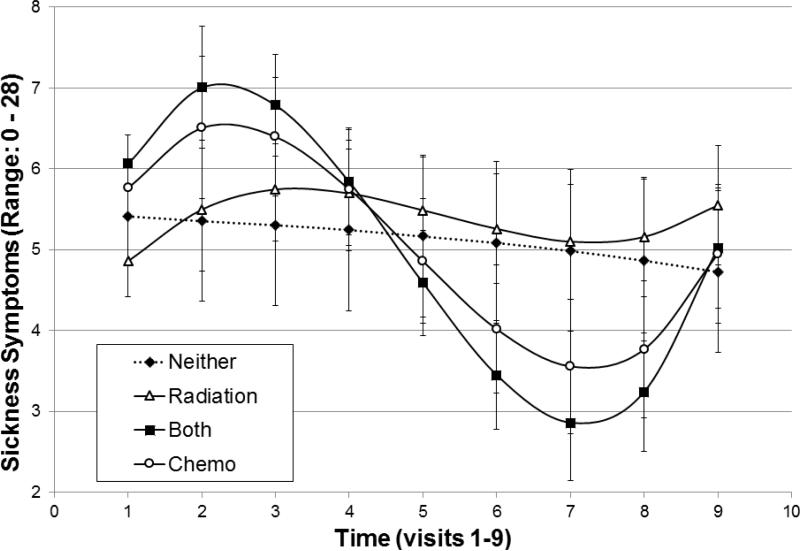
The fixed effects of linear (b=1.01, 95% CI [0.378, 1.633], t(850) =3.14, p =.002), quadratic (b = −0.43, 95% CI [−0.621, −0.245], t(850)= −4.51, p<.001), and cubic (b =0.04, 95% CI [0.022, 0.053], t(850)=4.66, p<.001) time, centered around the initial visit, were all significant main effect predictors of sickness symptoms. Cancer treatment type (i.e., chemotherapy, radiation, both, or neither) interacting with linear time (F(3, 841)=3.99, p=.008) and cubic time (F(3, 841)=3.97, p=.008) was a significant predictor of sickness symptoms (Figure 1). Additionally, time-varying chemotherapy (but not radiation) treatment occasions predicted sickness symptoms (b=2.88, t(844)=8.20, p=.000). Other predictors of sickness symptoms were age (b= −0.06, t(134)=2.04, p=.043), the number of medical comorbidities (b=1.68, t(134)=6.98, p=.000), and relationship status (F(5,840) =3.86, p=.002; i.e., married women in their first marriage reported significantly lower sickness symptoms than divorced women). No other demographic and treatment variables were significantly associated with sickness symptoms.2 Continuous variables were centered around their respective means for inclusion in models.
Figure 1.
Pattern of Sickness Symptoms over time (visits 1-9) by cancer treatment group. Error bars represent standard errors. Time scale begins at diagnosis and the interval between visits was 3 months.
3.3. Effects of Cytokines and Emotional Acceptance on Sickness Symptoms3
3.3.1. Cytokines: Mean levels across time and person centered levels
Overall, IL-8 increased over time (linear: b=0.15, 95% CI [0.024, 0.291], t(610)=2.31, p=.021), and TNF-α showed significant cubic growth over time (cubic: b=0.002, 95% CI [0.001, 0.004], t(610)=2.96, p=.003). Women with higher average TNF-α across all time points reported higher mean sickness symptoms across time (b=1.66, 95% CI [0.067, 3.255], t(84)=2.03, p=.046). There were no other significant effects of mean level cytokines predicting sickness symptoms.
For a given woman, time-points with higher than her average IL-6 and IL-8 values were associated with more self-reported sickness symptoms for that woman, compared to her average sickness symptoms: IL-6 (b=0.44, 95% CI [0.003, 0.882], t(556)=1.93, p=.053), and IL-8 (b=1.01, 95% CI [0.339, 1.678], t(556) =2.92, p=.004).
3.3.2. Emotional Acceptance: Mean levels across time and person centered levels
There was no significant change in EA over time, however there was variability in EA both between participants and from time to time (intra-class correlation: 0.76). Mean level EA did not significantly predict sickness symptoms (b = 0.00, 95% CI [−0.027, 0.032], t(125) = 0.16, p = .87), but person-centered EA did (b = −0.02, 95% CI [−0.047, −0.001], t(815) = −2.06, p = .039). For a given woman, time-points with higher than average EA values were associated with lower sickness symptoms for that woman, compared to her average sickness symptoms.
3.4. Moderation Effect of Emotional Acceptance on Associations Between Mean or Person-Centered Time-Varying Cytokines and Sickness Symptoms
To provide a sense of relative magnitude of effects, we report both unstandardized (b) and standardized betas (B) for the simple slopes involved in significant interactions and provide interpretations in original scale units. All significant interactions were probed using the 25th percentile for low levels of the variables and 75th percentile for the high levels of the variables, following methods outlined by Aiken and West (40).
3.4.1. Mean emotional acceptance and cytokines
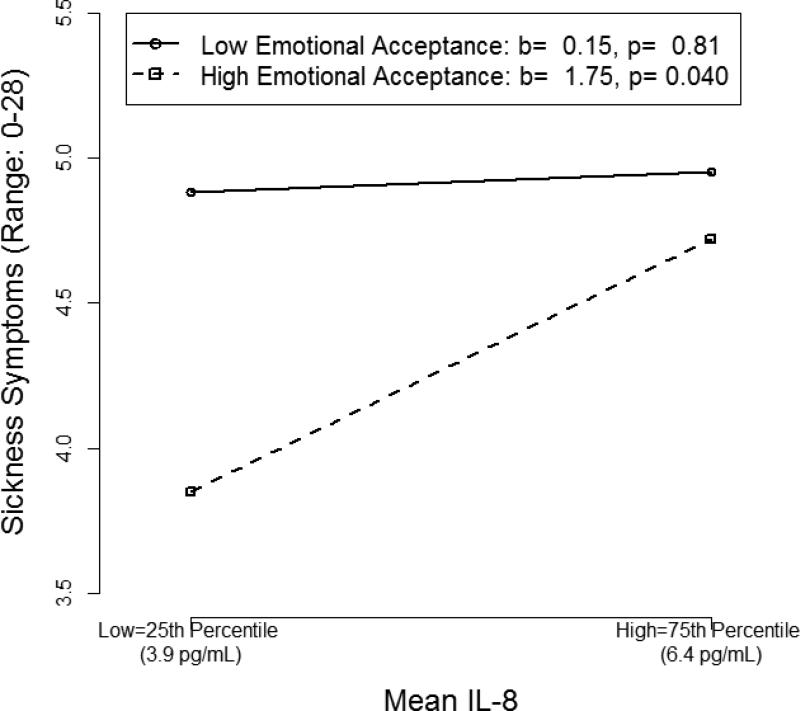
Mean EA significantly moderated the association between mean IL-8 and sickness symptoms (b=.06, 95% CI [0.020, 0.103], t(83) = 2.90, p= .005). As shown in Figure 2, when average EA was high, overall IL-8 levels correlated with sickness symptoms (b = 1.75, B= .19, p= .040). A 1 SD decrease in mean IL-8 was associated with a 0.9 unit decrease (18%, given the 5-point scale) in sickness symptoms for women reporting high mean EA. In contrast, women reporting low mean EA had persistently high sickness symptoms regardless of mean IL-8 levels (b = .15, B= .02, p= .81).
Figure 2.
Simple slopes depicting the two-way interaction between mean interleukin(IL)-8 and mean emotional acceptance.
3.4.2. Person-centered time-varying emotional acceptance and cytokines
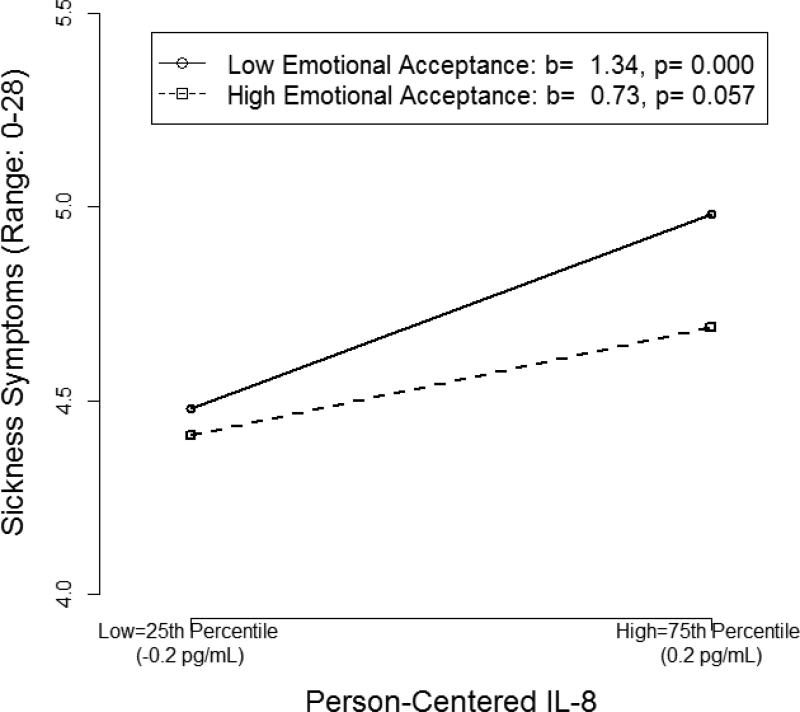
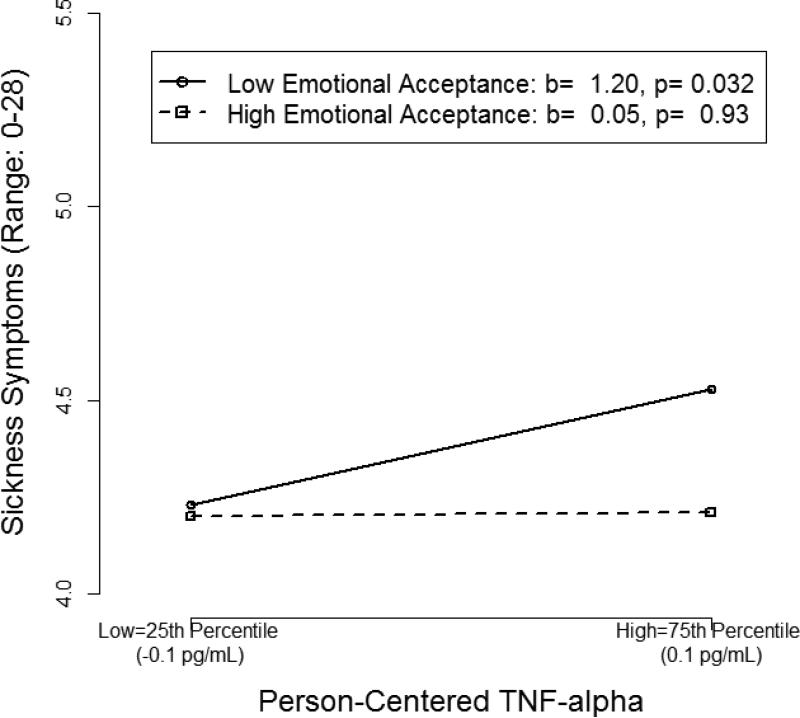
At individual time-points, person-centered EA significantly moderated the associations between IL-8 (b = −0.05, 95% CI [−0.101, −0.006], t(555) = −2.14, p= .033) and TNF-α (b = −0.13, 95% CI [−0.242, −0.015], t(555)= −2.18, p= .030) and sickness symptoms. For a given woman, higher than average IL-8 (Figure 3) and TNF-α (Figure 4), relative to her own cytokine means, were associated with higher than average sickness symptoms at times when her emotional acceptance was lower than average (IL-8: b = 1.34, B=.10, p= .000; TNF-α: b = 1.20, B= .06, p = .032). However, cytokines and sickness symptoms were not associated at times when her emotional acceptance was higher than average. In terms of magnitude of effects, when women reported lower than their own average EA, a 1 SD increase in their cytokine level was associated with a 0.5 unit increase (9%, given the 5-point scale) in sickness symptoms for IL-8, and 0.3 unit increase (6% given the 5-point scale) in sickness symptoms for TNF-α. When women reported higher than average EA, sickness symptoms were not significantly associated with person-centered IL-8 (b=.73, B=.054, p=.057), nor TNF-α (b= .05, B= .003, p= .93); a 1 SD increase in the person-centered cytokine was associated with a 0.3 unit change (5% given the 5-point scale) in sickness symptoms for IL-8, but only a 0.01 unit change (0.3% given the 5-point scale) in sickness symptoms for TNF-α.
Figure 3.
Simple slopes depicting the two-way interaction between person-centered interleukin (IL)-8 and person-centered emotional acceptance.
Figure 4.
Simple slopes depicting the two-way interaction between person-centered tumor necrosis factor (TNF)-α and person-centered emotional acceptance.
4. Discussion
This study confirms previous reports that biomarkers of increased inflammation, specifically proinflammatory cytokines TNF-α and IL-6 and the inflammation-associated chemokine IL-8, are associated with sickness behaviors (e.g., feeling physically ill) in women with breast cancer (1, 3). More importantly for the hypotheses of this study, these data provide the first evidence that emotional acceptance attenuates these associations; at time points when a given woman increases her acceptance of emotion as compared to her usual functioning, the association between inflammatory biomarkers and sickness behaviors is no longer present. Consistent with our hypothesis, variation in EA moderated the linkage of IL-8 and TNF-α with sickness symptoms at certain times. In other words, when emotional acceptance dropped below average for a given woman, higher levels of IL-8 and TNF-α at that time point were associated with more self-reported sickness symptoms. In contrast, when emotional acceptance increased above average, there was no association between IL-8 or TNF-α and sickness symptoms. This variability in the coupling of IL-8 and TNF-α and sickness symptoms suggests a novel mechanism through which a feedback loop of stress-inducing sickness behaviors and inflammation might be interrupted.
The within-person results (i.e., variation of the inflammatory biomarker/sickness symptom linkage at different time points) reflect changes from a woman's average level of EA. However, these results occurred in the context of a different pattern of associations at the average levels of IL-8. Average sickness symptoms were high in subjects whose EA was persistently low, regardless of their average levels of IL-8. These results are consistent with the well-documented association of low EA and negative mood (22), which is known to perpetuate sickness symptoms (41). Low average EA perpetuated sickness symptoms after accounting for the linked covariation of IL-8 and symptoms, but this linkage of low average EA with higher average symptoms seems to have remained suppressed by the stronger influence of average TNF-α on average symptom levels. These results should be interpreted with caution, however, because mean levels of EA were not associated with sickness symptoms overall and the association of sickness symptoms with the mean level of TNF-α was not moderated by EA. Additional research is needed to compare and contrast the influences of emotion regulation and proinflammatory cytokines, and their interaction, on sickness symptoms.
Overall, IL-8 and TNF-α, but not IL-6 increased over the year following breast cancer diagnosis in this sample. For TNF-α and IL-8, these results and the parallel increases of their average levels with average sickness symptoms are consistent with previous reports (42, 43). Additionally, breast cancer tumor cells produce IL-8 (2). IL-8 is an activator and chemoattractant for neutrophils and activated neutrophils release a cascade of inflammatory cytokines, suggesting a chain of tumor secretion of IL-8 to increased inflammation. The lack of increase in average IL-6 for breast cancer patients is not consistent with the majority of studies, although other null results have been reported (44). The 94% increased likelihood of missing data for subjects with high levels of IL-6 at their sampled time points may have contributed to this outcome.
A number of plausible behavioral, psychological, and physiological mechanisms may help to explain why effective emotion regulation may attenuate this vicious cycle, whereas ineffective regulation could exacerbate it (45). The ability to regulate emotions effectively may contribute to fewer disruptions in goal-directed behavior, including health-promoting behaviors (46, 47). (However, emotion regulation strategies that are typically considered as effective, such as emotional acceptance, may not be as adaptive in circumstances in which they prevent resources from being used that could help to manage a controllable illness.) Additionally, failure to appropriately regulate strong or persistent negative emotions may hinder cognitive-emotional functioning and contribute to depressive symptoms (48) (49), which are known to be associated with greater sickness symptoms. Furthermore, ineffective emotion regulation may also directly disrupt biological functioning, including contributing to decreased parasympathetic nervous system activity, along with heighted activity in the sympathetic nervous system, hypothalamic-pituitary-adrenal axis, and immune system (50-53). The findings from the present study help to clarify the role of emotional acceptance, and emotion regulation more broadly, in potentially mitigating the vicious inflammation-sickness cycle that breast cancer patients may experience.
Lastly, in the present study, we focus on emotional acceptance as an effective emotion regulation strategy to attenuate the effects of inflammation on sickness symptoms. However, inflammation and sickness symptoms may also be antecedents of emotional acceptance and other forms of emotion regulation, thus creating a circular, bi-directional process. Although the present study focused on one part of the cycle in which emotional acceptance is effective in a shorter time frame at downregulating the effects of inflammation on sickness symptoms, we found some support for the circularity of these associations.2 Interventions such as Acceptance and Commitment Therapy (ACT) (54) and Barlow's Unified Protocol for Transdiagnostic Treatment of Emotional Disorders (55) have been demonstrated to increase emotional acceptance in conjunction with decreasing experiential avoidance (56, 57). A recent meta-analysis of ACT found it to be effective for reduction of somatic complaints in an analysis of 15 studies with 683 participants (Hedges’ g = 0.58, SE =0.13, 95% CI: 0.33–0.84, p < 0.001) (58). Experimental medicine interventions to test therapies to increase acceptance of emotion are important future steps for testing the mechanisms by which emotion regulation may be effective in uncoupling the linkage of inflammation and sickness behavior.
4.1. Strengths and Limitations
Strengths of the current study include the within-person design, which is similar to a time-series design used in quasi-experimental studies, only sans manipulation of an independent variable, as well as the use of a multiplex immunoassay. Although enzyme linked immunosorbent assay (ELISA) is the most widely used and best validated method for measuring cytokines, it is limited due to only being able to measure a single protein at a time (59). An important part of the process in the current study was to determine which cytokines were detectable and told a coherent story. For example, IFN-γ, IL-2, and IL-1β were not used in analyses due to a low percentage of detectable values. In a recent study that used the multiplex immunoassay of cytokines in breast cancer patients undergoing chemotherapy, Cheung and colleagues (60) found a substantial portion of IL-2 and IFN-γ concentrations were also below the detection limit. Additionally, a previous study using the same multiplex method found a similar proportion of non-detectable IL-1β values (35). Apart from IFN-γ, IL-2, and IL-1β, we found that almost all of the other cytokines assayed in the multiplex (IL-6, IL-8, and TNF-α) played a coherent role in the story, as hypothesized – either as a main effect (at the mean or person-centered level), or as an interaction effect with emotional acceptance in predicting sickness symptoms. Interestingly, the only cytokine not associated with variables of interest was IL-10, which is predominantly considered an anti-inflammatory cytokine and is reported to exert tumor inhibiting action on breast cancer (61). An important direction for future research will be to take a systems perspective and unpack the associations among multiple cytokines, assessed using multiplex immunoassays, to better understand whether certain cytokines are unique contributors to sickness symptoms in different contexts, or whether other emotion regulatory strategies moderate different cytokines.
Limitations of the current study include the lack of an emotion measure to sufficiently determine if emotional acceptance was effective in decreasing negative affect, as well as focus on a single emotion regulation strategy, emotional acceptance. Other emotion regulation strategies that have been found to be effective in decreasing psychological distress in breast cancer patients include emotional expression (62) and positive reappraisal (26, 27, 63). As such, these strategies may also positively influence the inflammation-sickness cycle, whereas less effective emotion regulation strategies (e.g., suppression, repression, avoidance, cognitive perseveration) may exacerbate it (64-69). However, we did not have measures of other emotion regulation strategies and identify this as a future research direction.
Sample and study characteristics are also worth mentioning: participants were somewhat ethnically diverse but racially homogenous, which may be due to the study having employed only a single site for assessment. Given the nature of secondary data analysis, other potentially relevant covariates (e.g., socioeconomic status) were not available for inclusion in analyses in the current sample. Additionally, the current study focused on sickness symptoms that were most closely aligned with physical functioning and not representative of other symptoms associated with sickness, such as loss of interest in activities, fever, and sleep changes. Fully capturing “sickness behavior”, given its polymorphous meaning, is a challenge. Nevertheless, effective emotion regulation would likely influence not only functional, but also other symptoms associated with sickness behavior, and future research that takes an integrative approach to measure sickness behavior and its association with emotion regulation is needed.
These findings of increased sickness symptoms in women who received chemotherapy and/or radiation treatment, as compared to those who did not, or on assessment occasions associated with chemotherapy administration, are consistent with reports of increased inflammation associated with Adriamycin and taxanes (70). In depth assessment of inflammatory markers potential mediation of this association is beyond the scope of this investigation but marks an important question that we will examine in future analyses.
Lastly, the observational nature of the study prevents us from establishing causality between higher levels of proinflammatory cytokines, low EA, and more sickness symptoms. Nevertheless, they suggest increases in EA may be beneficial for women with breast cancer, by interrupting the link between inflammatory cytokines and sickness behaviors. Analyses demonstrated relevant demographic characteristics and treatment variables do not account for the associations.
5. Conclusions
The present study extends previous work and demonstrates that proinflammatory cytokines are associated with more sickness symptoms in women with breast cancer. Importantly, however, when controlling for relevant demographic variables, emotional acceptance moderates the associations between cytokines and sickness symptoms such that, for a given woman, higher than average emotional acceptance attenuates the linkage of proinflammatory biomarkers (IL-8 and TNF-α) with sickness symptoms. These data suggest novel directions for emotion regulation and breast cancer research and, if replicated, would support the exploration of emotional acceptance interventions for treating sickness symptoms among women with breast cancer.
Supplementary Material
Highlights.
Inflammation was associated with more sickness symptoms in breast cancer patients.
On average, low emotional acceptance predicted high sickness symptoms across inflammatory levels.
Within-person, higher emotional acceptance attenuated effects of inflammation on sickness.
Acknowledgments
This research was supported by American Cancer Society Grant RSG-13-020-01-CPPB (to EAB), USA Med Research & Material Command, Breast Cancer Research Program Idea Award W81XWH-04-1-0603 (to KLW), National Cancer Institute at the National Institutes of Health 1R01 CA133081 (to KLW) and University of Arizona Cancer Center Support Grant NCI P30CA023074, the Cousins Center for Psychoneuroimmunology at the University of California Los Angeles, and the Frances McClelland Institute for Children, Youth, and Families in the Norton School of Family and Consumer Sciences at the University of Arizona.
Thanks to Allison Stopeck, MD, Robert Livingston, MD, Anna Maria Lopez, MD, Amy Waer, MD, Jan Degan, RN, and Michelle Ley, MD for facilitation of recruitment; to Laura Eparvier, RN, Amanda Brody and Diane Sells, RN for data collection; and to the patients whose participation made this research possible.
Abbreviations
- IFN
interferon
- IL
interleukin
- TNF
tumor necrosis factor
- EA
emotional acceptance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare no conflict of interest.
Section 3.2 explains the choice of variables in this equation.
Cancer disease stage did not significantly predict sickness symptoms, emotional acceptance, nor any cytokines. Cancer disease stage was trending to be associated with IL-8 (F(4,91)=2.46, p=.051) such that Stage I and Stage IIB reported significantly higher levels of IL-8 than Stage 0.
We also ran these analyses in the opposite direction (i.e., predicting emotional acceptance from mean and person-centered cytokines and sickness symptoms). The results from these analyses provide some support for the cyclical associations among inflammation and emotional acceptance. Specifically, mean IL-8 was positively associated with emotional acceptance (b=7.77, 95% CI [1.42, 14.12], t(92) = 2.42, p=.018). Person-centered IL-8 and TNF-α were negatively associated with emotional acceptance (IL-8: b=−3.58, 95% CI [−5.56, −1.60], t(568) = −3.52, p=.001; TNF-α b=−4.23, 95% CI [−6.96, −1.50], t(568) = −3.03, p=.003). Results suggest that in general, women with higher overall IL-8 may have had more of a need to regulate their emotions and thus had higher emotional acceptance, but at individual time points (i.e., within-person), a woman may have had a harder time accepting her emotions (i.e., lower emotional acceptance) when her IL-8 and TNF-α levels were higher than average. Mean and person-centered versions of sickness symptoms did not significantly predict emotional acceptance.
References
- 1.Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nature Reviews Cancer. 2008;8:887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- 2.Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. The lancet oncology. 2013;14:218–228. doi: 10.1016/S1470-2045(12)70582-X. [DOI] [PubMed] [Google Scholar]
- 3.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bower JE, Ganz PA, Aziz N, Olmstead R, Irwin MR, Cole SW. Inflammatory responses to psychological stress in fatigued breast cancer survivors: Relationship to glucocorticoids. Brain, Behavior, and Immunity. 2007;21:251–258. doi: 10.1016/j.bbi.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: Implications for health. Nature Reviews Immunology. 2005;5:243–251. doi: 10.1038/nri1571. [DOI] [PubMed] [Google Scholar]
- 6.Segerstrom SC, Miller GE. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychological Bulletin. 2004;130:601–630. doi: 10.1037/0033-2909.130.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson J, Campisi J, Sharkey C, Kennedy S, Nickerson M, Greenwood B, Fleshner M. Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience. 2005;135:1295–1307. doi: 10.1016/j.neuroscience.2005.06.090. [DOI] [PubMed] [Google Scholar]
- 8.Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in long-term breast carcinoma survivors. Cancer. 2006;106:751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 9.Dantzer R. Cytokine-induced sickness behavior: Where do we stand? Brain, Behavior, and Immunity. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- 10.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. Journal of Clinical Oncology. 2008;26:971–982. doi: 10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleeland CS, Bennett GJ, Dantzer R, Dougherty PM, Dunn AJ, Meyers CA, Wang XS. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? Cancer. 2003;97:2919–2925. doi: 10.1002/cncr.11382. [DOI] [PubMed] [Google Scholar]
- 12.Kelley KW, Bluthé R-M, Dantzer R, Zhou J-H, Shen W-H, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain, Behavior, and Immunity. 2003;17:112–118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 13.Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain, Behavior, and Immunity. 2007;21:153–160. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: Occurrence, correlates, and impact on quality of life. Journal of Clinical Oncology. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 15.Miller AH. Cytokines and sickness behavior: Implications for cancer care and control. Brain, Behavior, and Immunity. 2003;17:132–134. doi: 10.1016/s0889-1591(02)00080-6. [DOI] [PubMed] [Google Scholar]
- 16.Myers JS. Proinflammatory cytokines and sickness behavior: Implications for depression and cancer-related symptoms. Oncology Nursing Forum. 2008;35:802–807. doi: 10.1188/08.ONF.802-807. [DOI] [PubMed] [Google Scholar]
- 17.Weihs KL, Enright TM, Simmens SJ. Close relationships and emotional processing predict decreased mortality in women with breast cancer: Preliminary evidence. Psychosomatic Medicine. 2008;70:117–124. doi: 10.1097/PSY.0b013e31815c25cf. [DOI] [PubMed] [Google Scholar]
- 18.Gross JJ. The emerging field of emotion regulation: An integrative review. Review of General Psychology. 1998;2:271–299. [Google Scholar]
- 19.Gross JJ, Thompson RA. Gross JJ, editor. Emotion regulation: Conceptual foundations. Handbook of Emotion Regulation New York Guilford. 2007 [Google Scholar]
- 20.Campbell-Sills L, Barlow DH, Brown TA, Hofmann SG. Acceptability and suppression of negative emotion in anxiety and mood disorders. Emotion. 2006;6:587–595. doi: 10.1037/1528-3542.6.4.587. [DOI] [PubMed] [Google Scholar]
- 21.Gross JJ, Muñoz RF. Emotion regulation and mental health. Clinical Psychology: Science and Practice. 1995;2:151–164. [Google Scholar]
- 22.Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- 23.Politi MC, Enright TM, Weihs KL. The effects of age and emotional acceptance on distress among breast cancer patients. Supportive Care in Cancer. 2007;15:73–79. doi: 10.1007/s00520-006-0098-6. [DOI] [PubMed] [Google Scholar]
- 24.Jensen CG, Elsass P, Neustrup L, Bihal T, Flyger H, Kay SM, Würtzen H. What to listen for in the consultation: Breast cancer patients’ own focus on talking about acceptance-based psychological coping predicts decreased psychological distress and depression. Patient Education and Counseling. 2014;97:165–172. doi: 10.1016/j.pec.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 25.Stanton AL, Danoffburg S, Huggins ME. The first year after breast cancer diagnosis: Hope and coping strategies as predictors of adjustment. Psycho-Oncology. 2002;11:93–102. doi: 10.1002/pon.574. [DOI] [PubMed] [Google Scholar]
- 26.Wang Y, Yi J, He J, Chen G, Li L, Yang Y, Zhu X. Cognitive emotion regulation strategies as predictors of depressive symptoms in women newly diagnosed with breast cancer. Psycho-Oncology. 2014;23:93–99. doi: 10.1002/pon.3376. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y, Zhu X, Yang Y, Yi J, Tang L, He J, Yang Y. What factors are predictive of benefit finding in women treated for non-metastatic breast cancer? A prospective study. Psycho-Oncology. 2014;24:533–539. doi: 10.1002/pon.3685. [DOI] [PubMed] [Google Scholar]
- 28.Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2002;1 [Google Scholar]
- 29.Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press; New York, NY: 2003. [Google Scholar]
- 30.Hoffman L, Stawski RS. Persons as contexts: Evaluating between-person and within-person effects in longitudinal analysis. Research in Human Development. 2009;6:97–120. [Google Scholar]
- 31.Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Shiomoto G. Reliability and validity of the Functional Assessment of Cancer Therapy-Breast quality-of-life instrument. Journal of Clinical Oncology. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 32.Cella D, Eton DT, Lai J-S, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. Journal of pain and symptom management. 2002;24:547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 33.Lee B-N, Dantzer R, Langley KE, Bennett GJ, Dougherty PM, Dunn AJ, Reuben JM. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation. 2004;11:279–292. doi: 10.1159/000079408. [DOI] [PubMed] [Google Scholar]
- 34.Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI. Sex Differences in Depressive and Socioemotional Responses to an Inflammatory Challenge: Implications for Sex Differences in Depression. Neuropsychopharmacology. 2015;40:1709–1716. doi: 10.1038/npp.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Epstein MM, Breen EC, Magpantay L, Detels R, Lepone L, Penugonda S, Birmann BM. Temporal stability of serum concentrations of cytokines and soluble receptors measured across two years in low-risk HIV-seronegative men. Cancer Epidemiology Biomarkers & Prevention. 2013;22:2009–2015. doi: 10.1158/1055-9965.EPI-13-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Breen E, Perez C, Olmstead R, Eisenberger N, Irwin M. 35. Comparison of multiplex immunoassays and ELISAs for the determination of circulating levels of inflammatory cytokines. Brain, Behavior, and Immunity. 2014;40:e39. [Google Scholar]
- 37.Uh H-W, Hartgers FC, Yazdanbakhsh M, Houwing-Duistermaat JJ. Evaluation of regression methods when immunological measurements are constrained by detection limits. BMC immunology. 2008;9:59–69. doi: 10.1186/1471-2172-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. Journal of clinical epidemiology. 2005;58:595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Hughes ME, Waite LJ. Marital biography and health at mid-life. Journal of health and social behavior. 2009;50:344–358. doi: 10.1177/002214650905000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage; Newbury Park, CA: 1991. [Google Scholar]
- 41.Croicu C, Chwastiak L, Katon W. Approach to the Patient with Multiple Somatic Symptoms. Medical Clinics of North America. 2014;98:1079–1095. doi: 10.1016/j.mcna.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 42.Dantzer R, Meagher MW, Cleeland CS. Translational approaches to treatment-induced symptoms in cancer patients. Nature Reviews Clinical Oncology. 2012;9:414–426. doi: 10.1038/nrclinonc.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Janelsins MC, Mustian KM, Palesh OG, Mohile SG, Peppone LJ, Sprod LK, Williams JP. Differential expression of cytokines in breast cancer patients receiving different chemotherapies: implications for cognitive impairment research. Supportive Care in Cancer. 2012;20:831–839. doi: 10.1007/s00520-011-1158-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vichaya EG, Chiu GS, Krukowski K, Lacourt TE, Kavelaars A, Dantzer R, Walker AK. Mechanisms of chemotherapy-induced behavioral toxicities. Frontiers in neuroscience. 2015;9:1–17. doi: 10.3389/fnins.2015.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Butler EA. Three views of emotion regulation and health. Social and personality psychology compass. 2011;5:563–577. [Google Scholar]
- 46.Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: New perspectives from psychoneuroimmunology. Annual Review of Psychology. 2002;53:83–107. doi: 10.1146/annurev.psych.53.100901.135217. [DOI] [PubMed] [Google Scholar]
- 47.Lee-Baggley D, DeLongis A, Voorhoeave P, Greenglass E. Coping with the threat of severe acute respiratory syndrome: Role of threat appraisals and coping responses in health behaviors. Asian Journal of Social Psychology. 2004;7:9–23. doi: 10.1111/j.1467-839X.2004.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of Neuroscience. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Francoeur RB. Using an innovative multiple regression procedure in a cancer population (Part 1): Detecting and probing relationships of common interacting symptoms (pain, fatigue/weakness, sleep problems) as a strategy to discover influential symptom pairs and clusters . OncoTargets and therapy. 2015;8:45–56. doi: 10.2147/OTT.S66465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Appleton AA, Buka SL, Loucks EB, Gilman SE, Kubzansky LD. Divergent associations of adaptive and maladaptive emotion regulation strategies with inflammation. Health Psychology. 2013;32:748–756. doi: 10.1037/a0030068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lam S, Dickerson SS, Zoccola PM, Zaldivar F. Emotion regulation and cortisol reactivity to a social-evaluative speech task. Psychoneuroendocrinology. 2009;34:1355–1362. doi: 10.1016/j.psyneuen.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Miller G, Chen E, Cole SW. Health psychology: Developing biologically plausible models linking the social world and physical health. Annual Review of Psychology. 2009;60:501–524. doi: 10.1146/annurev.psych.60.110707.163551. [DOI] [PubMed] [Google Scholar]
- 53.Gross JJ, Levenson RW. Hiding feelings: The acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology. 1997;106:95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- 54.Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: The process and practice of mindful change. 2 ed. Guilford Press; 2012. [Google Scholar]
- 55.Farchione TJ, Fairholme CP, Ellard KK, Boisseau CL, Thompson-Hollands J, Carl JR, Barlow DH. Unified protocol for transdiagnostic treatment of emotional disorders: a randomized controlled trial. Behavior therapy. 2012;43:666–678. doi: 10.1016/j.beth.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson DS, Hayes SC, Biglan A, Embry DD. Evolving the future: Toward a science of intentional change. Behavioral and Brain Sciences. 2014;37:395–416. doi: 10.1017/S0140525X13001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sauer-Zavala S, Boswell JF, Gallagher MW, Bentley KH, Ametaj A, Barlow DH. The role of negative affectivity and negative reactivity to emotions in predicting outcomes in the unified protocol for the transdiagnostic treatment of emotional disorders . Behaviour research and therapy. 2012;50:551–557. doi: 10.1016/j.brat.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davis M, Morina N, Powers M, Smits J, Emmelkamp P. A meta-analysis of the efficacy of acceptance and commitment therapy for clinically relevant mental and physical health problems. Psychotherapy and Psychosomatics. 2015;84:30–36. doi: 10.1159/000365764. [DOI] [PubMed] [Google Scholar]
- 59.Leng SX, McElhaney JE, Walston JD, Xie D, Fedarko NS, Kuchel GA. ELISA and multiplex technologies for cytokine measurement in inflammation and aging research. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2008;63:879–884. doi: 10.1093/gerona/63.8.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheung Y, Ng T, Shwe M, Ho H, Foo K, Cham M, Yong W. Association of pro-inflammatory cytokines and chemotherapy-associated cognitive impairment in breast cancer patients: A multi-centered, prospective, cohort study. Annals of Oncology. 2015 doi: 10.1093/annonc/mdv206. Advance online publication:doi:10.1093/annonc/mdv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamidullah, Changkija B, Konwar R. Role of interleukin-10 in breast cancer. Breast Cancer Research and Treatment. 2012;133:11–21. doi: 10.1007/s10549-011-1855-x. [DOI] [PubMed] [Google Scholar]
- 62.Stanton AL, Low CA. Expressing emotions in stressful contexts: Benefits, moderators, and mechanisms. Current directions in psychological science. 2012;21:124–128. [Google Scholar]
- 63.Kvillemo P, Bränström R. Coping with breast cancer: A meta-analysis. PLoS One. 2014;9:e112733. doi: 10.1371/journal.pone.0112733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chapman BP, Fiscella K, Kawachi I, Duberstein P, Muennig P. Emotion suppression and mortality risk over a 12-year follow-up. Journal of Psychosomatic Research. 2013;75:381–385. doi: 10.1016/j.jpsychores.2013.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Classen C, Koopman C, Angell K, Spiegel D. Coping styles associated with psychological adjustment to advanced breast cancer. Health Psychology. 1996;15:434–437. doi: 10.1037//0278-6133.15.6.434. [DOI] [PubMed] [Google Scholar]
- 66.Schlatter MC, Cameron LD. Emotional suppression tendencies as predictors of symptoms, mood, and coping appraisals during AC chemotherapy for breast cancer treatment. Annals of Behavioral Medicine. 2010;40:15–29. doi: 10.1007/s12160-010-9204-6. [DOI] [PubMed] [Google Scholar]
- 67.Gross J. Emotional expression in cancer onset and progression. Social Science & Medicine. 1989;28:1239–1248. doi: 10.1016/0277-9536(89)90342-0. [DOI] [PubMed] [Google Scholar]
- 68.Tamagawa R, Giese-Davis J, Speca M, Doll R, Stephen J, Carlson LE. Trait mindfulness, repression, suppression, and self-reported mood and stress symptoms among women with breast cancer. Journal of Clinical Psychology. 2013;69:264–277. doi: 10.1002/jclp.21939. [DOI] [PubMed] [Google Scholar]
- 69.Giese-Davis J, Conrad A, Nouriani B, Spiegel D. Exploring emotion-regulation and autonomic physiology in metastatic breast cancer patients: Repression, suppression, and restraint of hostility. Personality and Individual Differences. 2008;44:226–237. doi: 10.1016/j.paid.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fitzpatrick F, Wheeler R. The immunopharmacology of paclitaxel (Taxol®), docetaxel (Taxotere®), and related agents. International immunopharmacology. 2003;3:1699–1714. doi: 10.1016/j.intimp.2003.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.