Abstract
Liver fibrosis is a common outcome of chronic liver disease and leads to liver cirrhosis and hepatocellular carcinoma. No FDA-approved targeted anti-fibrotic therapy exists. Activated hepatic stellate cells (aHSCs) are the major cell types responsible for liver fibrosis; therefore, eradication of aHSCs, while preserving quiescent HSCs and other normal cells, is a logical strategy to stop and/or reverse liver fibrogenesis/fibrosis. However, there are no effective approaches to specifically deplete aHSCs during fibrosis without systemic toxicity. aHSCs are associated with elevated expression of death receptors (DRs) and become sensitive to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced cell death. Treatment with recombinant TRAIL could be a potential strategy to ameliorate liver fibrosis; however, the therapeutic application of recombinant TRAIL is halted due to its very short half-life. To overcome this problem, we previously generated PEGylated TRAIL (TRAILPEG) that has a much longer half-life in rodents than native-type TRAIL. Here, we demonstrate that intravenous TRAILPEG has a markedly extended half-life over native-type TRAIL in non-human primates and has no toxicity in primary human hepatocytes. Intravenous injection of TRAILPEG directly induces apoptosis of aHSCs in vivo and ameliorates carbon tetrachloride-induced fibrosis/cirrhosis in rats by simultaneously down-regulating multiple key fibrotic markers that are associated with aHSCs. In conclusion, TRAIL-based therapies could serve as new therapeutics for liver fibrosis/cirrhosis and possibly other fibrotic diseases.
Keywords: apoptosis, cirrhosis, death receptors, fibrosis, hepatic stellate cells, TRAIL
INTRODUCTION
The mechanisms that underlie the pathogenesis of liver fibrosis have been extensively studied, yet no medications have emerged as effective antifibrotic agents. Chronic liver injury from liver diseases, such as viral hepatitis, alcoholic hepatitis, nonalcoholic steatohepatitis, biliary diseases, metabolic disorders, or autoimmune conditions, stimulates the accumulation of excessive extracellular matrix (ECM) that results in liver fibrosis. Progressive liver fibrosis leads to cirrhosis and remodeling of the hepatic vascular architecture that can result in liver failure, cancer and premature death.1–4 Hepatic stellate cells (HSCs) are the major cell type that produce excessive ECM, leading to liver fibrosis. Quiescent HSCs (qHSCs) are initially activated by several factors such as damaged hepatocytes, apoptotic bodies, cytokines and chemokines produced by resident hepatic macrophages (Kupffer cells) and infiltrating inflammatory cells during liver injury. Activated stellate cells (aHSCs) express platelet-derived growth factor (PDGF) and PDGF receptors (PDGF-Rs). PDGF induces HSC proliferation, resulting in increased production of pro-fibrogenic cytokines such as transforming growth factor-β (TGF-β), which further activate HSCs to up-regulate alpha smooth muscle actin (α-SMA) expression and stimulate ECM secretion. aHSCs and Kupffer cells also express tissue inhibitors of metalloproteinases (TIMPs) which inhibit matrix-degrading metalloproteinase activity and promote HSC survival, altering the balance between ECM secretion and degradation. Based on their role in the fibrotic cascade, aHSCs are a major target for antifibrotic therapy.5, 6 Selectively eradicating aHSCs but not qHSCs or other liver cells is anticipated to induce strong antifibrotic effects, because the originator cells of fibrogenesis are depleted and key fibrogenic components are simultaneously inhibited. However, there is a lack of robust methods by which aHSCs may be inactivated or eradicated. Moreover, aHSCs are known to be resistant to apoptotic stimuli including Fas ligand (FasL), tumor necrosis factor alpha (TNF-α), or DNA intercalating agents such as anticancer drugs and oxidative stress mediators.7
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a type II transmembrane protein in the TNF-α superfamily due to sequence homology with TNF and FasL.8, 9 TRAIL can be proteolytically cleaved from the cell surface and released in soluble form. Soluble TRAIL is intrinsically a homotrimer and subsequently trimerizes TRAIL-receptors after binding. Five TRAIL receptors have been identified in humans, but only two TRAIL-R1/DR4 and TRAIL-R2/DR5 receptors initiate apoptosis similar to Fas/FasL and TNF-R/TNF signaling pathways. TRAIL receptor binding stimulates formation of death-inducing signaling complex (DISC) with the recruited adaptor protein, Fas-associated protein with death domain (FADD). FADD recruits procaspases 8 and 10, and DISC allows auto-activation of these caspases. Downstream of this signaling is the proteolytic cleavage and activation of caspases 3/6/7, resulting in apoptosis. Another pathway of apoptosis is the induction of mitochondrial dysfunction and membrane permeabilization causing release of cytochrome c that activates caspase 9 and finally cleavage of caspase 3/7 resulting in apoptosis. TRAIL can also bind to its decoy receptors, TRAIL-R3/DcR1 and TRAIL-R4/DcR2, but these receptors lack a functional death domain and are unable to induce apoptosis. Dulanermin, recombinant human TRAIL, has been investigated as an anti-cancer therapy; but, its clinical efficacy was disappointing,10, 11 probably due to TRAIL-resistance in primary cancer cells and the short half-life of the protein (less than 5 min in rodents and 30 min in humans).12, 13
The aHSC cell line, LX2, up-regulates DR4 and DR5 and becomes sensitive to TRAIL-induced cell death.14 Despite promising in vitro studies, the role of TRAIL signaling in liver fibrogenesis has not been fully investigated. Furthermore, an effective molecule that can selectively induce apoptosis in aHSCs with limited hepatotoxicity has not been developed, so the translation of antifibrotic therapies from “bench to bedside” has been limited. Herein, we determine if such a strategy has therapeutic potential in liver fibrosis and cirrhosis. We verified if TRAIL is a suitable target for anti-fibrotic therapy by comparing TRAIL receptor expression levels in activated primary human HSCs and in liver tissue samples from healthy patients and in patients with liver fibrosis/cirrhosis. To address the poor clinical potency of recombinant TRAIL in oncologic clinical studies, we utilized a long-acting TRAIL consisting of a PEGylated human trimeric isoleucine-zipper fused TRAIL (TRAILPEG). The antifibrotic potency of long-acting TRAIL was investigated in carbon tetrachloride (CCl4)-induced fibrosis rat models at various stages of injury. We explore the role of systemic TRAILPEG in liver fibrogenesis in vivo and mechanisms of TRAIL sensitization in primary human HSCs. The results warrant further investigation into stable TRAIL-based materials as antifibrotic therapeutic strategies.
MATERIALS AND METHODS
Human liver samples
The Liver Tissue Procurement Distribution System (LTPDS, the Division of Pediatric Gastroenterology and Nutrition, University of Minnesota, Minneapolis, MN) provided frozen alcoholic cirrhotic liver samples and paraffin embedded liver samples from patients with HBV, HCV, ALD, or ALD/HCV with end-stage cirrhosis who received liver transplantation. Liver diseases were diagnosed by LTPDS and based on a history of alcohol drinking, infected viral markers and liver histology. Liver pathology of these specimens showed bridging fibrosis and cirrhosis. LTPDS also provided paraffin embedded normal healthy liver specimens obtained from human donor livers not used for transplantation. The LTPDS was funded by NIH Contract #N01-DK-9-2310. The protocol for using these liver samples has been approved by the LTPDS of the University of Minnesota and the National Institutes of Health. Frozen normal human liver tissues were purchased from TRL (Triangle Research Labs LLC, Durham, NC) for analysis of protein expression by western blot as controls.
Human primary hepatocyte culture and TRAILPEG treatment
Cryopreserved human primary hepatocytes, human hepatocyte plating medium, and thawing medium were obtained from TRL (Triangle Research Labs, LLC, Durham, NC). According to the manufacturer’s instructions, cryopreserved hepatocytes were thawed in thawing medium and cultured in human hepatocyte plating medium in a 6-well plate of collagen type I Biocoat (BD Biosciences, San Jose, CA). Cells were cultured overnight and then treated with TRAILPEG or recombinant human His-iLZ-TRAIL for 3 h. After cells were harvested, the expression of TRAIL receptors (DR4/DR5) and apoptosis markers were determined by Western blot analysis, and cell viability was analyzed by MTT assays.
Liver fibrosis and cirrhosis induced by CCl4 in rats
Animal studies were undertaken according to an approved protocol reviewed by the Johns Hopkins Animal Care and Use Committee. Sprague Dawley (SD) male rats at the age of 5–6 weeks (BW 120–150 g) were purchased from Charles River (Germantown, MD). Rats were divided into 4 groups: 1) olive oil and phosphate buffered saline (PBS) treated groups, 2) olive oil and TRAILPEG, 3) CCl4 and PBS and 4) CCl4 and TRAILPEG. For fibrotic rats, rats were administered with 2 mL/kg of CCl4 (Sigma-Aldrich, 20% CCl4 in olive oil) three times per week through intraperitoneal (i.p.) injection or olive oil as control groups for a total of 4 weeks. At day 29, rats were then treated with 4 mg/kg of TRAILPEG via intravenous (i.v.) injection every day for ten days or treated with the same amount of PBS for control groups. Rats were anesthetized at day 40, and blood and liver tissues were collected for analysis. To induce liver cirrhosis, rats were divided into four groups just as the fibrosis groups and administered with CCl4 (20% CCl4 in olive oil, 2 mL/kg) three times per week via i.p. injection or olive oil as control groups for a total of 7 weeks. Starting on day 50, rats were treated with 4 mg/kg of TRAILPEG via i.v. injection every day for fourteen days or treated with the same amount of PBS for control groups. Rats were anesthetized at day 65, and blood and liver tissues were collected for analysis.
Statistical Analysis
All data were analyzed using GraphPad Prism 6 software. Differences between two means were assessed by a paired or unpaired t-test. Differences among multiple means were assessed, as indicated, by one-way ANOVA followed by Tukey’s post-hoc test or by the Student’s t-test as appropriate. Error bars represent s.d. or s.e.m., as indicated. Probabilities of P < 0.05 or as indicated were considered statistically significant.
Other methods
Additional methods are described in the Supporting Information.
RESULTS
TRAILPEG has an extended half-life in vivo without toxicity in primary human hepatocytes
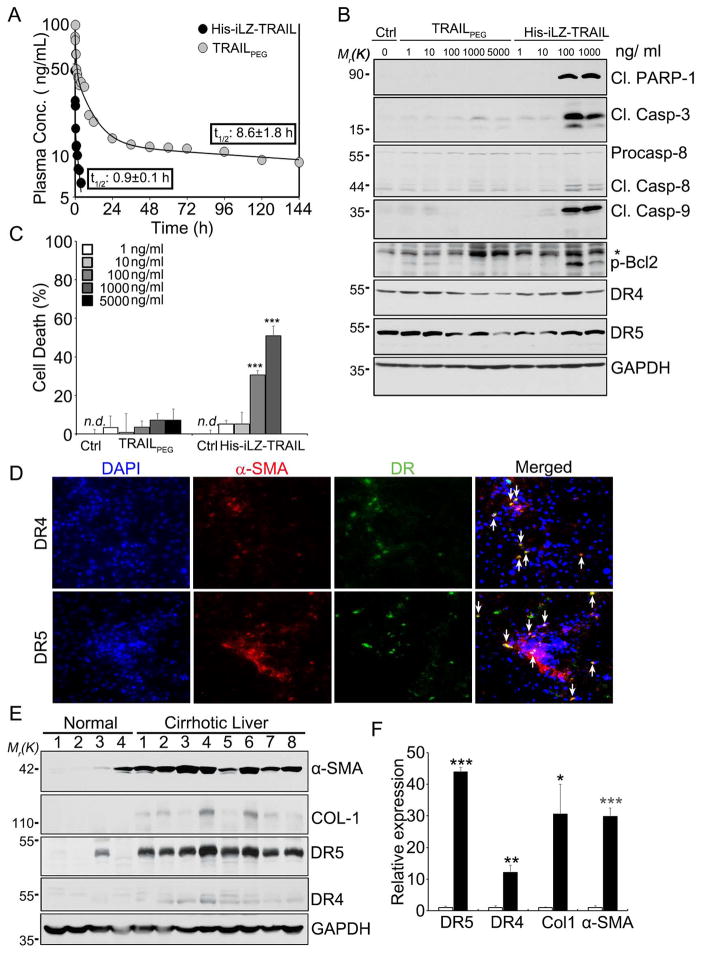
The extremely short half-life and low in vivo potency of recombinant human TRAIL makes it difficult to provide continuous and potent TRAIL-induced cell apoptosis. In addition, many TRAIL-based therapies have been unstable in solution and can aggregate at high concentrations, leading to toxicity and dose limitations in clinical studies. To overcome these disadvantages, we developed TRAILPEG by stabilizing a potent homotrimer TRAIL comprised of isoleucine-zipper amino acid motifs at the N-terminus that favor trimer formation (His-iLZ-TRAIL) with a 5 kDa poly(ethylene glycol) (PEG).15, 16 PEGylation is a highly efficient commercial strategy to extend half-life of protein drugs as well as reduce protein aggregation.17,18 We demonstrated that TRAILPEG has an improved pharmacodynamic (PD) profile in a tumor xenograft model and pharmacokinetic (PK) profile in rats compared to recombinant TRAIL.15 TRAILPEG demonstrated a substantially extended half-life (8.6 h) in cynomolgus monkeys after i.v. injection compared to His-iLZ-TRAIL (0.9 h) (Fig. 1A and Table S1). Native-type TRAIL is non-toxic to primary hepatocytes, because hepatocytes, like many non-transformed cells, are resistant to TRAIL-induced apoptosis despite expressing TRAIL receptors.19 However, some TRAIL variants, particularly His-tagged or Flag-tagged TRAIL, are prone to uncontrolled aggregation and induce pronounced apoptosis in hepatocytes.20, 21 To investigate potential liver toxicity, primary human hepatocytes were treated in vitro with varying concentrations of TRAILPEG, and apoptotic signaling and cell death was compared against its non-PEGylated analog. His-iLZ-TRAIL induced apoptosis in human hepatocytes in vitro at concentrations higher than 100 ng/mL as evidenced by increased cleaved PARP-1 and cleaved caspases (Fig. 1B) as well as by quantified cell death (Fig. 1C). In contrast, TRAILPEG did not show toxicity at concentrations up to 5,000 ng/mL. Steatotic hepatocytes, which are known to be more sensitive to TRAIL-mediated cytotoxicity,22 undergo apoptosis when treated with high concentrations of His-iLZ-TRAIL but demonstrate no cell death with TRAILPEG at the same TRAIL concentration (Fig. S1). Despite having a lower toxicity profile, TRAILPEG maintained equal cancer killing efficiency when compared to His-iLZ- TRAIL with significantly improved solubility (reduced aggregation) at high concentrations in neutral pH, as we demonstrated previously.15
Figure 1. Intravenously injected TRAILPEG shows extended half-life in non-human primates and no toxicity in primary human hepatocytes. TRAIL receptors, DR5 and DR4, are upregulated in human cirrhotic livers and co-localized in aHSCs.
(A) Pharmacokinetic profiles of His-iLZ-TRAIL and TRAILPEG (12.5 μg/kg) after intravenous injection in cynomolgus monkeys (n = 2 per group) labeled with quantified half-lives. (B, C) Safety of TRAILPEG in primary human hepatocytes. Control (Ctrl) group is untreated. (B) Western blot analysis of hepatocytes treated with various concentrations of TRAILPEG or His-iLZ-TRAIL for 3 h. (C) Quantified cell death analyzed by the MTT assay after treatment of primary hepatocytes with TRAILPEG or His-iLZ-TRAIL. Data expressed as mean ± s.d. *** P < 0.001 vs. non-treated groups (Ctrl). n.d., non-detectable. (D) Double immunofluorescence micrographs of cirrhotic liver sections stained for nuclei (DAPI, blue) with DR4 or DR5 (green) and α-SMA (red). Arrows indicate co-localized DR4 or DR5 with α-SMA-positive cells, aHSCs. Original magnification 200x. (E) Western blot analyses of human normal (4 human samples, lanes labeled 1–4) and alcoholic cirrhotic liver tissues (8 patient samples, lanes labeled 1–8) (ALC). (F) Densitometry analysis of western blots from (E) shown as relative protein expression normalized to healthy liver tissue. Data expressed as mean ± s.e.m *P < 0.05, **P < 0.01, ***P < 0.001 vs. non-treated groups (Ctrl).
TRAIL receptor expression levels are upregulated in HSCs in human cirrhotic livers
To validate the clinical relevance of our strategy, we measured TRAIL receptor expression levels in aHSCs/myofibroblasts from liver sections of patients with liver cirrhosis. Immunohistochemical analysis and immunofluorescence double staining revealed that DR4 and DR5 expression was elevated in human cirrhotic liver tissues associated with hepatitis B virus (HBV), hepatitis C virus (HCV) infection, or chronic alcohol consumption (Fig. S2), and co-localized with α-SMA+ aHSCs (Fig. 1D). Western blot analyses of liver tissues from patients with alcoholic cirrhosis showed strong up-regulation of DR5 and moderate upregulation of DR4 compared to healthy livers (Fig. 1E, 1F). These data validate DRs as a clinical target for aHSCs and imply that TRAIL-based molecules can target aHSCs. By verifying DR expression on aHSCs and developing a stable and safe TRAIL receptor agonist with a prolonged PK in non-human primates, we were motivated to explore TRAILPEG and its ability to eradicate aHSC in preclinical models of liver fibrosis and cirrhosis.
Intravenously injected TRAILPEG ameliorates CCl4-induced liver fibrosis in rats
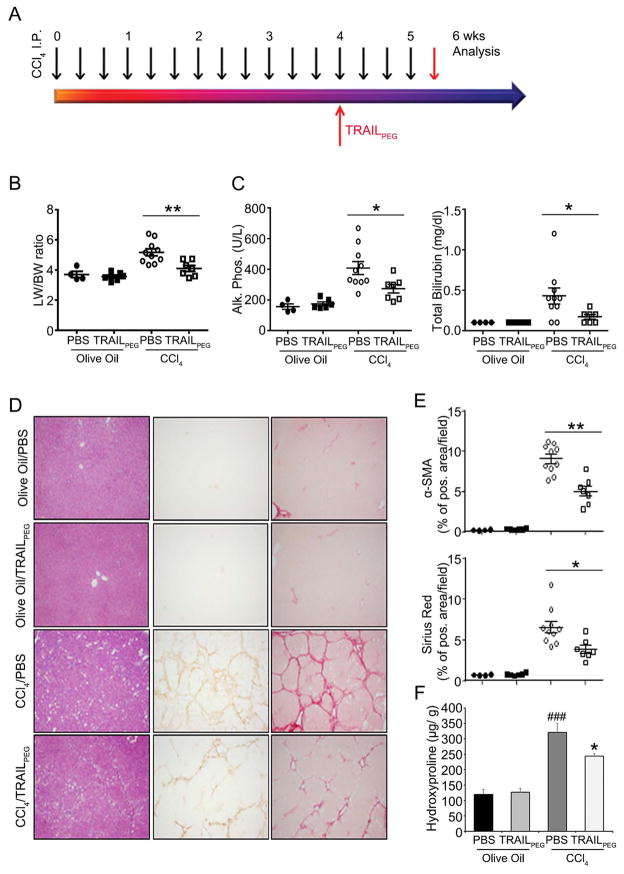
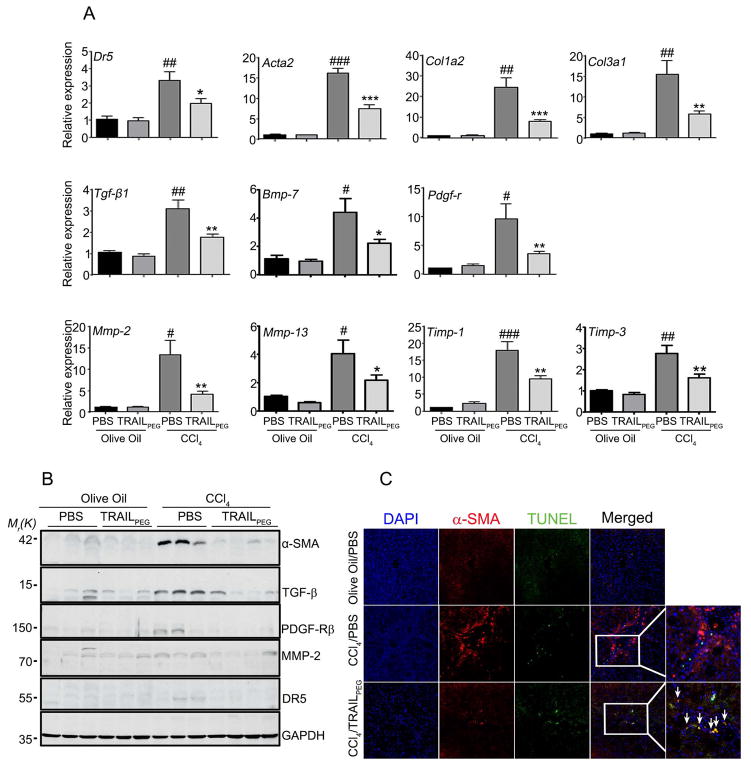
To investigate the effect of TRAILPEG on fibrosis in vivo, liver fibrosis was induced by thrice weekly treatment of CCl4 in rats,23 as shown in the treatment schedule in Fig. 2A. Two control groups received olive oil alone. After four weeks of CCl4 exposure, fibrotic rats received TRAILPEG (4 mg/kg, protein-based, at day 29) or PBS daily for a total of ten days while continuing to receive CCl4. Olive oil served as the vehicle control for CCl4 exposure in rats, and PBS served as the vehicle control for TRAILPEG treatment. In the rats with CCl4-induced liver fibrosis, liver weight-body weight ratio was significantly lower in TRAILPEG-treated rats compared with the PBS-treated group (Fig. 2B). Alkaline phosphatase (ALP) and total bilirubin (Fig. 2B) were significantly lower in sera from TRAILPEG treated fibrotic rats than the PBS-treated fibrotic rats. Alanine transaminase and aspartate transaminase (ALT and AST, respectively) were not significantly different between TRAILPEG and PBS treated groups, likely due to continuous CCl4-induced liver damage during the study (Fig. S3). Immunohistochemistry and computerized image analyses clearly showed markedly reduced positive areas of fibrosis detected by α-SMA and collagen deposition staining (Sirius Red) in liver specimens from TRAILPEG-treated rats compared with the PBS-treated group (Fig. 3D, 3E). In addition, hydroxyproline levels, a quantification of collagen deposition in liver tissue, were lower in the TRAILPEG-treated disease model over the untreated (PBS) study group (Fig. 3F). Fibrotic and TRAIL signaling markers in liver tissues during CCl4 and TRAILPEG treatments were analyzed at mRNA and protein levels. Quantitative real time PCR (qPCR) of mRNA obtained from TRAILPEG-treated liver tissues revealed an obvious reduction of multiple, highly up-regulated genes associated with the transition of qHSCs to the aHSC/myofibroblast phenotype, including Dr5 (TRAIL-R), Acta-2 (α-SMA), Col1a2 (collagen I), Col3a1 (collagen III), Tgf-β1 (transforming growth factor-beta 1), Bmp-7 (bone morphogenetic protein-7), Pdgf-r (platelet-derived growth factor receptors, PDGF-R), Mmp-2, Mmp-13 (matrix metalloproteinases-2 and -13), Timp-1, -3 (tissue inhibitor of metalloproteinase-1 and -3) and (Fig. 3A). Western blot analyses confirmed a significant decrease in expression levels of these proteins in the TRAILPEG-treated group (Fig. 3B, S4). All the tested markers were statistically reduced by at least 50% at mRNA and protein levels. To verify selective TRAIL-induced apoptosis in aHSCs, we double-stained the liver sections from control (olive oil) or CCl4-induced fibrotic rats treated with PBS or TRAILPEG for α-SMA+ aHSCs and apoptosis using TUNEL (Terminal deoxynucleotidyl transferase dUTP nick end labeling). In control (olive oil-treated) healthy livers, no strong α-SMA and TUNEL staining was observed. In TRAILPEG-treated fibrotic livers, TUNEL staining co-localized with α-SMA, validating that apoptosis occurred in α-SMA+ aHSCs (Fig. 3C, S5). Importantly, continuous systemic administration of TRAILPEG in oil alone to normal rats did not induce any noticeable toxicity, particularly in the liver. Animal groups treated with olive oil/PBS and olive oil/TRAILPEG demonstrated the same levels of ALT and AST (Fig. S3).
Figure 2. Intravenously injected TRAILPEG ameliorates CCl4-induced liver fibrosis in rats.
(A) TRAILPEG (4 mg/kg) was intravenously treated daily in control and in fibrotic livers induced by CCl4 (three times per week) in rats. TRAILPEG or PBS-treatment was initiated at day 29 in control (olive oil-treated) and CCl4-treated rats. Livers and blood samples were obtained at day 40. (B) Liver weight (LW)/body weight (BW) analyses. (C) Serum levels of alkaline phosphatase (Alk. Phos.) and total bilirubin. (D) Representative photomicrographs of liver sections from control (olive oil-treated) or chronic CCl4-treated rats with or without TRAILPEG stained with hematoxylin and eosin (H&E) and immunohistochemistry of activated HSC marker (α-SMA) and collagen deposition (Sirius Red). Original magnification 40x. (E) Digital image quantification of α-SMA and Sirius Red (collagen) staining. (F) Quantification of collagen by hydroxyproline analysis in total livers. Data expressed as mean ± s.e.m. *P < 0.05, **P < 0.01 vs. CCl4 + PBS groups. ###P < 0.001 vs. olive oil + PBS groups.
Figure 3. Intravenously injected TRAILPEG effectively targets aHSCs and simultaneously downregulates multiple fibrogenic components during liver fibrogenesis.
(A) Downregulated gene expression profiles of TRAIL receptor (DR5), HSC activation and fibrogenic markers from TRAILPEG treated fibrotic livers (n = 4–10). (B) Western blot analyses of rodent TRAIL receptor (DR5) and α-SMA expression, representative of HSC activation, along with other fibrogenic markers. (C) Immunofluorescence micrographs of liver sections from control (olive oil-treated) or chronic CCl4 treatment with or without TRAILPEG stained for nuclei (DAPI, blue), aHSCs (α-SMA, red), apoptosis (TUNEL, green), and merged image. Original magnification 100x. Arrows indicate TRAILPEG-induced apoptosis in aHSCs. Arrows indicate overlap of TUNEL and α-SMA staining. Additional markers in the sera of rats are shown in Supplementary Figure 3. Data expressed as mean ± s.e.m. #P < 0.05, ##P < 0.01, ###P < 0.001 vs. olive oil + PBS groups. *P < 0.05, **P < 0.01, ***P < 0.001 vs. CCl4 + PBS groups.
Intravenously injected TRAILPEG ameliorates CCl4-induced liver cirrhosis in rats
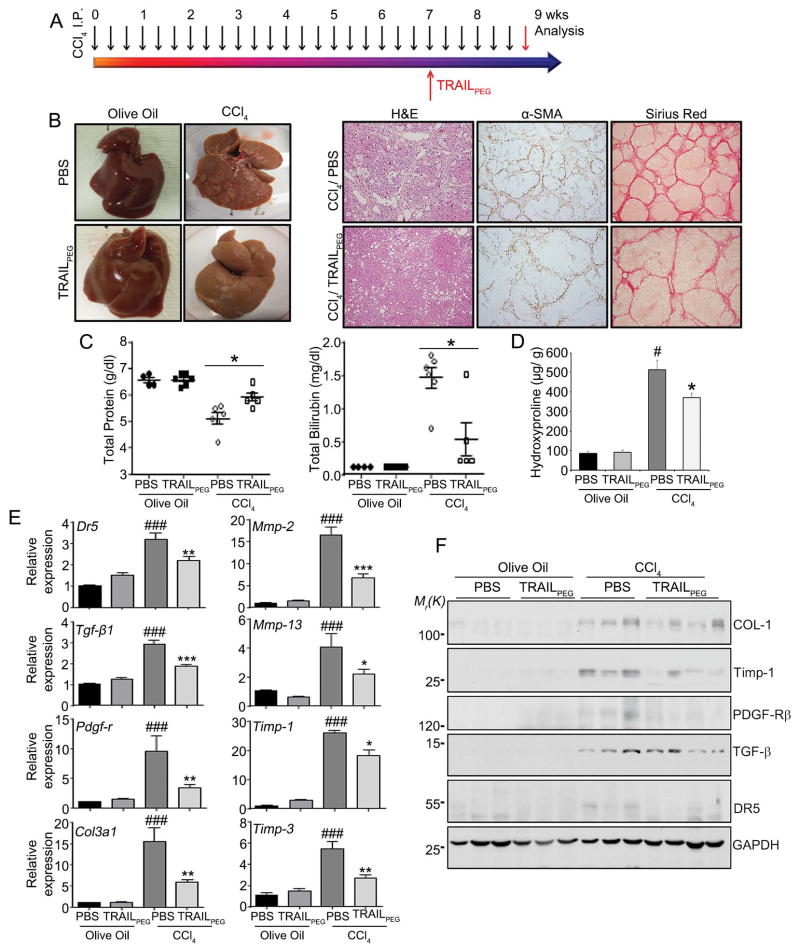
After demonstrating clear antifibrotic activity of TRAILPEG in liver fibrosis, we hypothesized that it can also reverse the fibrotic process in cirrhotic livers. In cirrhotic rats after long-term CCl4-treatment,23 TRAILPEG (4 mg/kg, protein-based, at day 50) was injected daily for fourteen days along with continued CCl4-treatment, and samples were collected and examined at day 65 of CCl4 treatment (Fig. 4A). Just as in the fibrosis study, olive oil served as the vehicle control for CCl4 exposure in rats, and PBS served as the vehicle control for TRAILPEG treatment. As illustrated in Fig. 4B, PBS-treated cirrhotic livers revealed advanced development of fibrosis such as nodule formation, demonstrated by intensely stained α-SMA positive areas and collagen depositions. On the other hand, cirrhotic rats exposed to systemic TRAILPEG showed a clear difference in macroscopic morphological appearance along with a marked reduction of α-SMA positive areas and collagen deposition as evidenced by immunohistochemistry analysis (Fig. 4B, S6A). TRAILPEG treated cirrhotic animal models had increased serum levels of total protein and albumin with significantly lower total bilirubin, direct bilirubin and hydroxyproline levels (Fig. 4C, 4D, S6B) than untreated cirrhotic animals. At the mRNA and protein levels, TRAILPEG treatment resulted in substantially down-regulated molecules associated with fibrogenesis (Fig. 4E, 4F, S6C). In TRAILPEG-treated cirrhotic rats, the relative fold decrease of multiple genes compared to control, including Dr5, Tgf-β1, Timp-1, Timp-3, Col3a1, Pdgf-r, Mmp-2, Mmp-13, and Bmp-7, was more pronounced than in TRAILPEG-treated fibrotic livers. Taken together, these data suggest that systemically delivered TRAILPEG, at a modest dose, histologically and functionally reverses CCl4-induced liver cirrhosis to early-stage fibrosis.
Figure 4. Intravenously injected TRAILPEG ameliorates CCl4-induced liver cirrhosis in rats.
(A) TRAILPEG (4 mg/kg) was intravenously treated daily for two weeks in control and rat models of CCl4-treated (three times a week) liver cirrhosis. TRAILPEG or PBS-treatment was initiated at day 50 in control (olive oil-treated) and CCl4-treated rats. Livers and blood samples were obtained at day 65. (B) Representative photos of normal and cirrhotic livers from rats (left) and representative photomicrographs of liver sections stained with H&E and immunohistochemistry of activated HSC marker (α-SMA) and collagen deposition (Sirius Red) treated with or without TRAILPEG, according to timeline in (A), at day 65. Original magnification 40x. (C) Serum levels of total protein and bilirubin. (D) Quantification of collagen by hydroxyproline analysis in total livers (n = 4–6). (E) Gene expression profiles of TRAIL receptor (DR5), HSC activation markers and fibrogenic markers from TRAILPEG- or PBS-treated cirrhotic livers as well as TRAILPEG- or PBS-treated normal livers (n = 4–6). (F) Western blot analyses of TRAIL receptor (DR5) and fibrogenic markers. Control (Ctrl) is PBS-treated normal (olive oil-treated) liver samples. Additional markers in the sera of rats and gene expression profiles are shown in Supplementary Figure 6B. Data expressed as mean ± s.e.m. #P < 0.05, ##P < 0.01, ###P < 0.001 vs. olive oil + PBS groups. *P < 0.05, **P < 0.01, ***P < 0.001 vs. CCl4 + PBS groups.
TRAILPEG-induced apoptosis in primary human hepatic stellate cells
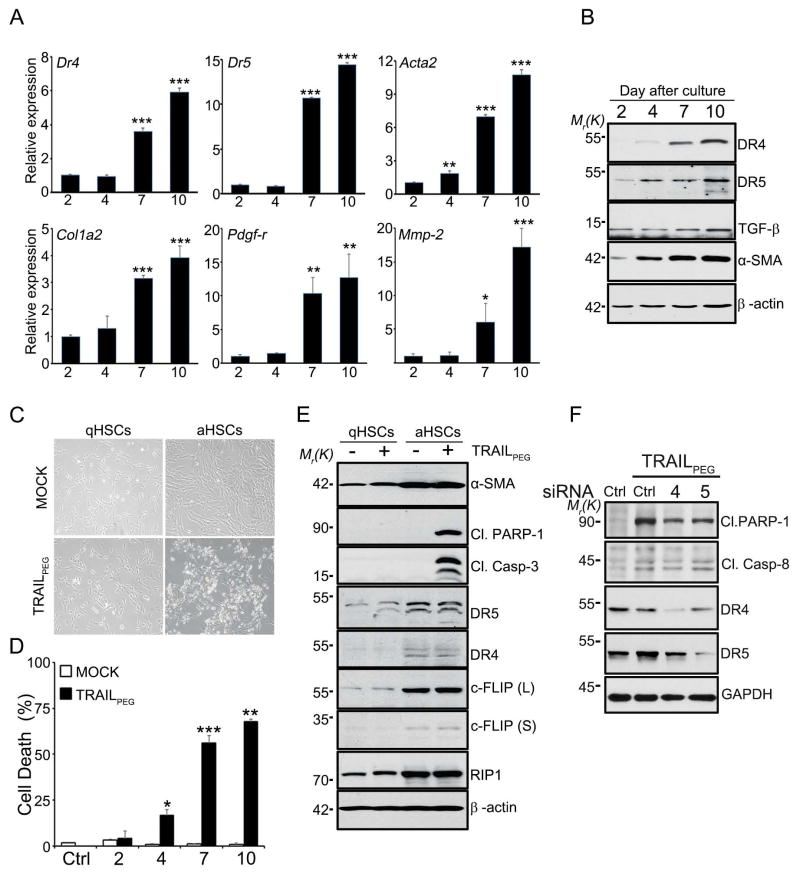
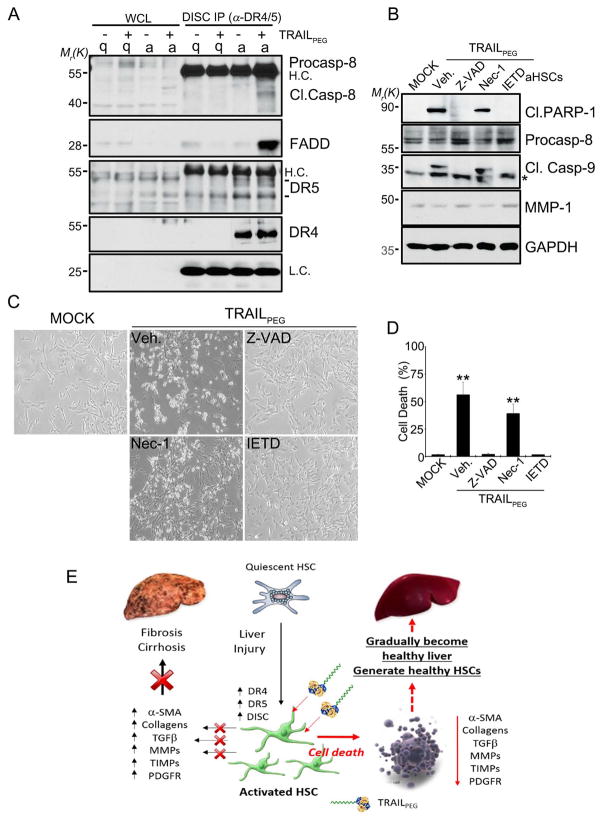
To investigate the mechanism of TRAIL sensitization in HSCs, changes in TRAIL signaling and TRAIL resistance-related components were studied by analyzing apoptotic and anti-apoptotic proteins in vitro. After we confirmed up-regulation of DRs in immortalized LX2 human HSC cell lines (Fig. S7) consistent with previous reports,14 we set out to test TRAIL-induced apoptosis in primary human HSCs. Human HSCs were cultured for 2 days (quiescent) and 7 or 10 days (activated). Culture-activated HSCs (Day 7 and 10) showed morphological changes and distinct induction of fibrogenic markers, DRs and decoy receptors (Dcr1 and Dcr2), compared to qHSCs (Day 2) at the mRNA and protein levels (Fig. 5A, 5B, S8A). These changes correlated with cell surface staining for functional DR5 and DR4 in aHSCs (Fig. S8B). During activation, HSCs deplete the anti-apoptotic protein, XIAP, and augment the pro-apoptotic protein, BAK (Fig. S9A), which could contribute to the increased sensitivity of aHSCs to TRAIL-induced cell death (Figs. 5C, 5D). Interestingly, however, activated HSCs also highly up-regulate a series of anti-apoptotic proteins such as BCL-2, BCL-XL, MCL-1, c-IAP1 (Figs. S9A, S9B) as well as c-FLIP, all of which are well known for inhibiting TRAIL-induced apoptosis in various cancer cells (Fig. 5E). In addition, receptor-activating protein 1 (RIP1), a kinase involved in necroptosis (programmed necrosis),24 was up-regulated in aHSCs (Fig. 5E). Despite increased expression of anti-apoptotic proteins, TRAILPEG (1 μg/mL) treatment rapidly induced apoptosis in aHSCs in 3 hrs as indicated by highly expressed apoptotic modulator levels, including DRs, cleaved PARP-1, and caspase-3.25 Bright-field microscopy images of HSCs and results from cell death assays indicate that HSCs became highly sensitive to TRAILPEG during activation (Fig. 5C, 5D, S9C). Based on gene knockdown studies, we demonstrate that TRAILPEG-induced apoptosis in aHSCs is mediated by either DR4 or DR5 (Fig. 5F). We also validated that activated primary HSCs are resistant to apoptosis when incubated with conventional toxic agents such as doxorubicin, cisplatin, H2O2, as well as in serum deprivation for 24 hr, but not against TRAILPEG (Fig. S4E). We subsequently assessed DISC immunoprecipitation (IP) with DR4 and DR5 antibodies in HSCs and confirmed that TRAILPEG-induced DISC formation comprised of caspase 8 and FADD in aHSCs only (Fig. 6A). Downstream, these complexes activate caspase-8 and thereby trigger extrinsic apoptosis. Caspase-8, FLIP, DR4 and DR5 were detected in DISC after exposure of aHSCs to FLAG-TRAIL, verifying TRAIL-induced aHSC apoptosis is a results of caspase-8 activation in DISC initiated by triggered DR4/DR5 (Fig. S10). To determine if TRAILPEG induces apoptosis via a caspase-dependent pathway or necroptosis pathway, aHSCs were incubated with the caspase-8 inhibitor, z-IETD-fmk, and the pan-caspase inhibitor, Z-VAD-fmk and treated with TRAILPEG. Western blot analysis data, microscopic images, and MTT assays corroborate that the treatment of caspase-8 and pan-caspase inhibitors blocked TRAIL induced apoptosis (Fig. 6B–D).
Figure 5. HSCs upregulate fibrogenic components and death receptors during activation and become sensitive to TRAILPEG-induced apoptosis.
Human primary HSCs were culture-activated for various time points (2–10 days) and treated with TRAILPEG or vehicle followed by quantitative real time PCR (qPCR) or western blot analyses. (A) qPCR of aHSC markers and TRAIL receptors during transition from the quiescent (day 2) to the culture-activated phenotype (days 4, 7 and 10). (B) Western blot analyses of anti-apoptotic and pro-apoptotic markers in primary human HSCs during transition from the quiescent (day 2 in culture) to an activated phenotype (days 4, 7 and 10). α-SMA was used as a activation bio-marker for HSCs. (C) Representative photos of qHSCs (day 2) and aHSCs (day 7) treated with TRAILPEG (1 μg/mL) for 3 h. (D) Quantified TRAILPEG-induced cell death during HSC activation (day 2–10) analyzed by MTT assay. (E) Western blot analysis of apoptotic markers and TRAIL receptors from qHSCs (day 2) and aHSCs (day 7) treated with vehicle or TRAILPEG (1 μg/mL) for 3 h. (F) Western blot analysis of apoptotic markers from LX-2 cells transfected with control siRNA (Ctrl), DR4 siRNA, or DR5 siRNA and treated with TRAILPEG (1 μg/mL) for 8 h. Data expressed as mean ± s.d. *P < 0.05, **P < 0.01, ***P < 0.001 vs. qHSC (day 2) (A) or MOCK (D).
Figure 6. TRAILPEG induces apoptosis in aHSCs, but not quiescent HSCs (qHSCs), through death-inducing signaling complex (DISC) and caspase-dependent pathway.
(A) DISC immunoprecipitation (IP) studies. Western blot analysis of qHSCs (q, 2 days) and aHSCs (a, 7 days) treated with TRAILPEG (2 μg/mL, 60 min) and then immunoprecipitated with anti-DR4 and anti-DR5 antibodies. Whole cell lysates (WCL) are indicated (left). H.C., heavy chain, L.C. light chain. (B) Western blot analysis of apoptotic markers from aHSCs co-treated with TRAILPEG (1 μg/mL) and various inhibitors of pan-caspase (Z-VAD, 20 μM), caspase-8 (IETD, 20 μM) and necroptosis (Nec-1, 50 μM) for 3 h. (C) Representative photos of aHSCs co-treated with TRAILPEG (1 μg/mL) and various inhibitors of pan-caspase (Z-VAD, 20 μM), caspase-8 (IETD, 20 μM) and necroptosis (Nec-1, 50 μM) for 3 h. (D) Quantified cell death analyses by MTT assay. Data expressed as mean ± s.d. *P < 0.05, **P < 0.01 vs. MOCK (D). (E) Summarized effect of TRAILPEG on HSC activation and liver fibrogenesis.
DISCUSSION
By addressing the known limitations of TRAIL agonists in previous clinical studies and validating TRAIL receptors as a target in fibrosis, we demonstrate that TRAIL receptor agonists can have a significant therapeutic role in liver fibrosis and cirrhosis. Here, we target and eradicate the major originator of liver fibrosis, aHSCs, using an engineered TRAIL agonist as an antifibrotic agent (Fig. 6E). Liver fibrosis is the excess accumulation of ECM as a result of chronic inflammation induced by multiple causes including alcoholic steatohepatitis, viral hepatitis, non-alcoholic steatohepatitis (NASH), metabolic disorders, and autoimmune diseases. Even after elimination of the causative trigger, persistent chronic damage may result in extensive scarring in the tissue and a steep decline of potential reversibility. This can lead to cirrhosis and vasculature distortion, which causes liver failure, portal hypertension, hepatocellular carcinoma (HCC) and premature death. Because liver fibrosis patients may be asymptomatic for decades and typically seek treatment only at late stages of fibrosis or cirrhosis,26 preventative strategies during early stages of fibrosis may not always be clinically relevant. Therefore, antifibrotic drugs that prevent liver fibrosis progression toward cirrhosis or induce regression of advanced fibrosis and cirrhosis are urgently needed.
By nature, aHSCs are a major target for antifibrotic therapy, because they are the primary ECM-producing cell in the liver and orchestrate liver fibrogenesis. Thus inhibition of HSC activation or removal of aHSCs is a rational strategy for liver fibrosis therapy. A few studies that alter HSC activation by targeting intracellular signaling molecules modulating HSC activation27 or inhibiting extracellular fibrogenic components have been reported.6, 28 As an alternative, our strategy selectively removes aHSCs and hence down-regulates downstream fibrogenic signaling by facilitating targeted aHSC death while leaving normal hepatic cells and regenerated quiescent HSCs unharmed. By targeting the TRAIL-induced cell death pathway in aHSCs in vivo, we demonstrate a promising antifibrotic strategy that may have broader implications towards drug development in diverse fibrotic diseases.
Although aHSCs do not undergo spontaneous apoptosis and are resistant to various proapoptotic stimuli,7 including conventional cytotoxic agents (Fig. S9D), we confirm that aHSCs are highly sensitive to TRAIL-induced apoptosis (Fig. 5C–E). Notably, unlike recombinant TRAIL11 and certain TRAIL receptor agonists29 investigated for cancer, our engineered TRAIL did not induce apoptosis in off-target cells, including qHSCs or other normal cells such as hepatocytes (Fig. 1B, 1C, S1). The potential of utilizing recombinant TRAIL as an antifibrotic agent has been suggested by Gores et al., when they firstly demonstrated TRAIL-induced cell death in aHSCs in LX2 cell lines.14 However, limited studies demonstrate TRAIL as a potential strategy for aHSC eradication in vitro and only speculate at in vivo therapeutic activity. We recently showed that systemic administration of hyaluronic acid-conjugated TRAIL can prevent fibrosis in a mild model of fibrosis in rats,30 but TRAIL-induced HSC cell death was not established. To date, there are no studies that demonstrate antifibrotic efficacy of TRAIL in established fibrosis and cirrhosis animal models.
Recombinant TRAIL has an extremely short half-life, is unstable and aggregates at high concentrations; thus, unmodified TRAIL has limited uses for in vivo drug development. More than ten PEGylated biologics are approved to date31 and over twenty PEGylated therapies are being investigated in the clinic,32 demonstrating that PEGylation is an effective and, to the best of our knowledge, safe method to improve protein drug delivery. In addition, PEGylated proteins are considered less immunogenic than their respective non-PEGylated counterparts.17 Some clinical concerns have been raised for high molecular weight PEGylated protein drugs, but clinical doses are drastically lower than those demonstrated for PEG toxicity.33 TRAILPEG overcomes the inherent limitations of TRAIL by improving its stability, extending its circulating half-life and reducing its aggregation while retaining its intrinsic biological activity (Fig. 1A–C).15 In this study, we also defined up-regulation of DRs on aHSC/myofibroblasts in the parenchyma, α-SMA positive myofibroblasts, and inflammatory cells in fibrotic septa of human livers with alcoholic or viral cirrhosis (Fig. S2). The effects of site-specific PEGylation further enabled the in vivo investigation of TRAIL. We assessed the antifibrotic effects of TRAILPEG in animal models of liver fibrosis and cirrhosis induced by CCl4 administration (Fig. 2–4). Our results demonstrate that systemic administration of TRAILPEG significantly ameliorates liver fibrosis and cirrhosis in animal models, as confirmed by reduced α-SMA and collagen deposition levels along with down-regulated expression of multiple fibrotic components in liver tissues (Fig. 2–4).
The concurrent down-regulation of fibrotic markers is a resounding characteristic of the TRAILPEG-treated fibrotic and cirrhotic animals, and we verify that it is predominantly a result of TRAIL-induced aHSC apoptosis in vivo. The levels of profibrogenic markers, TGF-β and PDGF-R, were significantly reduced in the TRAILPEG-treated group in both fibrotic and cirrhotic animal models (Fig. 3A, 3B, 4F, 4G). TGF-β and PDGF-R are typically produced by platelets and Kupffer cells/macrophages in early stages of liver damage; but after activation of HSCs, aHSCs become the major source of these cytokines, which act in an autocrine manner to promote further HSC activation, cell migration and ECM formation.28, 34 In addition to diminished levels of TGF-β and PDGF-R in the TRAILPEG-treated group, there was decreased expression of collagens, MMPs and TIMPs in fibrotic and cirrhotic livers (Fig. 3 and 4). Therapeutic antibodies or small molecule inhibitors targeting individual fibrogenic molecules have been investigated as antifibrotic agents.6 Instead of independently targeting one of many fibrogenic components such as TGF-β, PDGF-R, MMPs or TIMPS, the TRAILPEG treatment strategy introduced here simultaneously inhibits all of their activities. Using this single agent, we demonstrate that depleting the significant originator cell of fibrosis, aHSCs, is a viable and powerful therapeutic strategy for reversing liver fibrosis and cirrhosis. In addition, based on blood chemistry and histology, TRAILPEG did not induce any noticeable toxicity in vivo, including in the liver, after repetitive injections.
Immortalized LX2 cells and activated primary human HSCs demonstrate a significant up-regulation of DRs and undergo TRAIL-induced apoptosis (Fig. 5, S7–S10). To investigate the mechanisms of TRAIL sensitization in HSCs, changes in TRAIL signaling and TRAIL resistance were studied by analyzing apoptotic and anti-apoptotic proteins in both primary human HSCs and LX2 cells. Interestingly, we discovered that aHSCs up-regulate not only DRs but also multiple anti-apoptotic proteins that are well known to strongly inhibit TRAIL-induced apoptosis in various cancer cells such as BCL-2, BCL-XL, MCL-1 and FLIP (Fig. 5E, S9). In the field of oncology, extensive studies demonstrate that TRAIL-resistance in cancer cells can be overcome by using inhibitors of anti-apoptotic proteins to potentiate apoptosis.35, 36 However, we did not observe TRAIL-resistance in aHSCs. Based on our molecular analysis, apoptosis is the main cell death pathway in aHSCs treated with TRAILPEG. Yet, we also show evidence that necroptosis may be involved in TRAILPEG-induced cell death, based on the slight reduction in cell death over control when HSCs were treated with TRAILPEG and necroptosis inhibitor, Nec-1 (Fig. 6D) as well as the increase in RIP1 expression during HSC activation (Fig. 5E). Since TRAIL-induced apoptosis has been predominantly investigated in cancer cells and not in primary human HSCs, we are currently exploring mechanisms of TRAIL resistance and sensitivity by comparing TRAIL death pathways between HSCs and cancer cells.37 TRAILPEG could also target cirrhosis-associated HCC when combined with an appropriate TRAIL sensitizer. Further investigation is needed to explore the synergistic effect of TRAILPEG to eradicate liver tumor cells while simultaneously reversing cirrhosis. Furthermore, the effect of TRAILPEG on other types of cells that play a key role in liver fibrosis must be elucidated. During liver fibrosis, hepatic resident cells including liver sinusoidal endothelial cells, Kupffer cells and injured hepatocytes contribute to HSC activation by producing distinct cytokines and growth factors. It is conceivable that such cells can be targeted by systemically administered TRAILPEG and may be partly responsible for the antifibrotic effect of TRAIL-based therapy. In vivo resistance or sensitivity of hepatic resident cells against systemically administered TRAIL during liver injuries must be clearly elucidated. Lastly, to fully understand the role of antifibrotic efficacies of TRAILPEG in a preclinical setting, the drug should be evaluated in mechanistically distinct animal models of human hepatic fibrosis. Overall, further studies into the role of TRAIL signaling in aHSCs, the effect of TRAILPEG on hepatic resident cells during liver fibrosis in vivo, and antifibrotic activity in additional fibrotic animal models is required. Our studies in severe CCl4-induced fibrosis and cirrhosis rat models warrant the further development of TRAILPEG for human liver fibrosis.
We introduce a new strategy to ameliorate liver fibrosis and cirrhosis in vivo by targeting DR-expressing aHSCs through systemically administered, long-acting TRAIL agonist, TRAILPEG. Our studies identify up-regulated TRAIL receptors in aHSCs as an in vivo clinical target to treat liver fibrosis and cirrhosis and validate an intravenously administered recombinant TRAIL agonist as a promising antifibrotic agent. Combined with the high unmet clinical need for effective antifibrotic therapies, our study warrants clinical translation of TRAIL-based therapies for liver fibrosis and potentially other fibrotic disorders.
Supplementary Material
Acknowledgments
Financial Support
This study was supported by the US National Institutes of Health (NIH) grants EB013450 (NIBIB, to S.L.), AA000369 (NIAAA, to B.G.) and the US Department of Defense grant, CA130460 (to Y.O. and S.L.) and the National Research Foundation of Korea grants NRF-2013R1A1A2062043 (to K.C.L.), NRF-2013R1A1A2064165 (to S.M.L), NRF-2013K1A1A2A02050115 (to J.S.P. and K.K.).
We thank Theraly Pharmaceuticals for providing TRAILPEG.
Abbreviations
- CCl4
carbon tetrachloride
- qHSC
quiescent hepatic stellate cells
- aHSC
activated hepatic stellate cells
- TRAIL
Tumor necrosis factor-related apoptosis-inducing ligand
- TRAILPEG
PEGylated TRAIL
- PEG
poly(ethylene glycol)
- DR
death receptors
- DcR
decoy receptor
- ECM
extracellular matrix
- α-SMA
alpha smooth muscle actin
- MMP
matrix metalloproteinase
- TIMP
tissue inhibitor of metalloproteinase
- TGF-β
transforming growth factor beta
- PDGF
platelet-derived growth factor
- DISC
death inducing signaling complex
- FADD
fas-associated death domain
- Bcl-2
B cell lymphoma 2
- Bcl-xL
B cell lymphoma-extra large
- MCL-1
myeloid cell leukemia 1
- PARP-1
poly (ADP-ribose) polymerase 1
- c-FLIP
cellular (FADD-like IL-1β-converting enzyme)-inhibitory protein
Contributor Information
Yumin Oh, Email: yoh11@jhmi.edu.
Ogyi Park, Email: opark5@jhmi.edu.
Magdalena Swierczewska, Email: maggies@jhu.edu.
James P. Hamilton, Email: jpahamilton@jhmi.edu.
Jong-Sung Park, Email: jpark222@jhmi.edu.
Tae Hyung Kim, Email: tigerk@jhu.edu.
Sung-Mook Lim, Email: smlim@skku.edu.
Hana Eom, Email: sm11-jo@nate.com.
Dong Gyu Jo, Email: jodg@skku.edu.
Choong-Eun Lee, Email: celee@skku.edu.
Raouf Kechrid, Email: rkechd@mail.nih.gov.
Panagiotis Mastorakos, Email: pmastor1@jhmi.edu.
Clark Zhang, Email: czhang30@jhmi.edu.
Sei Kwang Hahn, Email: skhanb@postech.ac.kr.
Ok-Cheol Jeon, Email: ocjeon@gmail.com.
Youngro Byun, Email: yrbyun@snu.ac.kr.
Kwangmeyung Kim, Email: kim@kist.re.kr.
Justin Hanes, Email: hanes@jhmi.edu.
Kang Choon Lee, Email: kclee@skku.edu.
Martin G. Pomper, Email: mpomper@jhmi.edu.
Bin Gao, Email: bgao@mail.nih.gov.
Seulki Lee, Email: slee343@jhmi.edu.
References
- 1.Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med. 2013;5:167sr1. doi: 10.1126/scitranslmed.3004700. [DOI] [PubMed] [Google Scholar]
- 2.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altamirano J, Bataller R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat Rev Gastroenterol Hepatol. 2011;8:491–501. doi: 10.1038/nrgastro.2011.134. [DOI] [PubMed] [Google Scholar]
- 4.Friedman SL. Evolving challenges in hepatic fibrosis. Nat Rev Gastroenterol Hepatol. 2010;7:425–436. doi: 10.1038/nrgastro.2010.97. [DOI] [PubMed] [Google Scholar]
- 5.Friedman SL. Hepatic stellate cells. Prog Liver Dis. 1996;14:101–130. [PubMed] [Google Scholar]
- 6.Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest. 2013;123: 1887–1901. doi: 10.1172/JCI66028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Novo E, Marra F, Zamara E, Valfre di Bonzo L, Monitillo L, Cannito S, Petrai I, Mazzocca A, Bonacchi A, De Franco RS, Colombatto S, Autelli R, Pinzani M, Parola M. Overexpression of Bcl-2 by activated human hepatic stellate cells: resistance to apoptosis as a mechanism of progressive hepatic fibrogenesis in humans. Gut. 2006;55:1174–1182. doi: 10.1136/gut.2005.082701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, Smith C, Smolak P, Goodwin RG, Rauch CT, Schuh JC, Lynch DH. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 9.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 10.Soria JC, Mark Z, Zatloukal P, Szima B, Albert I, Juhasz E, Pujol JL, Kozielski J, Baker N, Smethurst D, Hei YJ, Ashkenazi A, Stern H, Amler L, Pan Y, Blackhall F. Randomized phase II study of dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell lung cancer. J Clin Oncol. 2011;29:4442–4451. doi: 10.1200/JCO.2011.37.2623. [DOI] [PubMed] [Google Scholar]
- 11.Lemke J, von Karstedt S, Zinngrebe J, Walczak H. Getting TRAIL back on track for cancer therapy. Cell Death Differ. 2014;21:1350–1364. doi: 10.1038/cdd.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelley SK, Harris LA, Xie D, Deforge L, Totpal K, Bussiere J, Fox JA. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther. 2001;299:31–38. [PubMed] [Google Scholar]
- 13.Ashkenazi A, Holland P, Eckhardt SG. Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/Tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL) J Clin Oncol. 2008;26:3621–3630. doi: 10.1200/JCO.2007.15.7198. [DOI] [PubMed] [Google Scholar]
- 14.Taimr P, Higuchi H, Kocova E, Rippe RA, Friedman S, Gores GJ. Activated stellate cells express the TRAIL receptor-2/death receptor-5 and undergo TRAIL-mediated apoptosis. Hepatology. 2003;37:87–95. doi: 10.1053/jhep.2003.50002. [DOI] [PubMed] [Google Scholar]
- 15.Chae SY, Kim TH, Park K, Jin CH, Son S, Lee S, Youn YS, Kim K, Jo DG, Kwon IC, Chen X, Lee KC. Improved antitumor activity and tumor targeting of NH(2)-terminal-specific PEGylated tumor necrosis factor-related apoptosis-inducing ligand. Mol Cancer Ther. 2010;9:1719–1729. doi: 10.1158/1535-7163.MCT-09-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim TH, Youn YS, Jiang HH, Lee S, Chen X, Lee KC. PEGylated TNF-related apoptosis-inducing ligand (TRAIL) analogues: pharmacokinetics and antitumor effects. Bioconjug Chem. 2011;22:1631–1637. doi: 10.1021/bc200187k. [DOI] [PubMed] [Google Scholar]
- 17.Harris JM, Chess RB. Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 18.Kang JS, Deluca PP, Lee KC. Emerging PEGylated drugs. Expert Opin Emerg Drugs. 2009;14:363–380. doi: 10.1517/14728210902907847. [DOI] [PubMed] [Google Scholar]
- 19.Lawrence D, Shahrokh Z, Marsters S, Achilles K, Shih D, Mounho B, Hillan K, Totpal K, DeForge L, Schow P, Hooley J, Sherwood S, Pai R, Leung S, Khan L, Gliniak B, Bussiere J, Smith CA, Strom SS, Kelley S, Fox JA, Thomas D, Ashkenazi A. Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med. 2001;7:383–385. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- 20.Ganten TM, Koschny R, Sykora J, Schulze-Bergkamen H, Buchler P, Haas TL, Schader MB, Untergasser A, Stremmel W, Walczak H. Preclinical differentiation between apparently safe and potentially hepatotoxic applications of TRAIL either alone or in combination with chemotherapeutic drugs. Clin Cancer Res. 2006;12:2640–2646. doi: 10.1158/1078-0432.CCR-05-2635. [DOI] [PubMed] [Google Scholar]
- 21.Nair PM, Flores H, Gogineni A, Marsters S, Lawrence DA, Kelley RF, Ngu H, Sagolla M, Komuves L, Bourgon R, Settleman J, Ashkenazi A. Enhancing the antitumor efficacy of a cell-surface death ligand by covalent membrane display. Proc Natl Acad Sci U S A. 2015;112:5679–5684. doi: 10.1073/pnas.1418962112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malhi H, Barreyro FJ, Isomoto H, Bronk SF, Gores GJ. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56:1124–1131. doi: 10.1136/gut.2006.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Constandinou C, Henderson N, Iredale JP. Modeling liver fibrosis in rodents. Methods Mol Med. 2005;117:237–250. doi: 10.1385/1-59259-940-0:237. [DOI] [PubMed] [Google Scholar]
- 24.Vandenabeele P, Galluzzi L, Vanden Berghe T, Kroemer G. Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol. 2010;11:700–714. doi: 10.1038/nrm2970. [DOI] [PubMed] [Google Scholar]
- 25.Jin ZY, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4:139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- 26.Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38:S38–53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 27.Bartneck M, Warzecha KT, Tacke F. Therapeutic targeting of liver inflammation and fibrosis by nanomedicine. Hepatobiliary Surg Nutr. 2014;3:364–376. doi: 10.3978/j.issn.2304-3881.2014.11.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seki E, Schwabe RF. Hepatic inflammation and fibrosis: functional links and key pathways. Hepatology. 2015;61:1066–1079. doi: 10.1002/hep.27332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papadopoulos KP, Issacs R, Bilic S, Kentsch K, Huet HA, Hofmann M, Rasco D, Kundamal N, Tang Z, Cooksey J, Mahipal A. Unexpected hepatotoxicity in a phase I study of TAS266, a novel tetravalent agonistic Nanobody® targeting the DR5 receptor. Cancer Chemother Pharmacol. 2015;75:887–895. doi: 10.1007/s00280-015-2712-0. [DOI] [PubMed] [Google Scholar]
- 30.Yang JA, Kong WH, Sung DK, Kim H, Kim TH, Lee KC, Hahn SK. Hyaluronic acid-tumor necrosis factor-related apoptosis-inducing ligand conjugate for targeted treatment of liver fibrosis. Acta Biomater. 2015;12:174–182. doi: 10.1016/j.actbio.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Alconcel SNS, Baas AS, Maynard HD. FDA-approved poly(ethylene glycol)-protein conjugate drugs. Polym Chem. 2011;2:1442–1448. [Google Scholar]
- 32.Swierczewska M, Lee KC, Lee S. What is the future of PEGylated therapies? Expert Opin Emerg Drugs. doi: 10.1517/14728214.2015.1113254. Article in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Webster R, Elliott V, Park BK. PEG and PEG conjugates toxicity: towards an understanding of the toxicity of PEG and its relevance to PEGylated biologicals. In: Veronese FM, editor. PEGylated Protein Drugs: Basic Science and Clinical Applications. Switzerland: Birkhauser Verlag; 2009. pp. 127–146. [Google Scholar]
- 34.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hellwig CT, Rehm M. TRAIL signaling and synergy mechanisms used in TRAIL-based combination therapies. Mol Cancer Ther. 2012;11:3–13. doi: 10.1158/1535-7163.MCT-11-0434. [DOI] [PubMed] [Google Scholar]
- 36.Voelkel-Johnson C. TRAIL-mediated signaling in prostate, bladder and renal cancer. Nat Rev Urol. 2011;8:417–427. doi: 10.1038/nrurol.2011.81. [DOI] [PubMed] [Google Scholar]
- 37.Yin C, Evason KJ, Asahina K, Stainier DY. Hepatic stellate cells in liver development, regeneration, and cancer. J Clin Invest. 2013;123:1902–1910. doi: 10.1172/JCI66369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.