Abstract
Background
Gaps in the literature exist regarding health-related quality of life (HRQOL) early after left ventricular assist device (LVAD) surgery. The purposes of our study were to describe HRQOL over time, by age and gender, and identify risk factors for poor HRQOL early after LVAD implant.
Methods
Patients (n=7,353) from INTERMACS received a continuous flow LVAD as a primary implant at 133 U.S. hospitals. Of these, 5,640 patients had pre LVAD HRQOL data, and 3,353 patients had 6 month post LVAD HRQOL data. There were 2,748 patients with data at both time periods. HRQOL was measured using the EQ-5D-3L instrument. Data were collected pre-implant and 3 and 6 months post-operatively. Statistical analyses included chi square, t-tests, Pearson correlation coefficients, and multiple regression.
Results
Overall HRQOL and dimensions of HRQOL improved from before to 6 months after device implant when examined by age and gender. However, younger patients and women reported significantly more problems regarding all dimensions before implant and significantly more problems regarding pain/discomfort and anxiety/depression at both 3 and 6 months after implant. An increase in overall HRQOL from before to 6 months after implant was related to pre implant INTERMACS level 1. Factors related to a decrease in HRQOL from before to 6 months after implant were listed for heart transplant before surgery, co-morbidities, better preoperative HRQOL, adverse events within 6 months after implant, and bridge to transplant moderately likely and unlikely, and NYHA class 4 at 6 months post LVAD (R2=41%).
Conclusions
Overall HRQOL and dimensions of HRQOL improve in subgroups of patients from before to 6 months after surgery, although differences in improvement exist. Adverse events are risk factors for decreased HRQOL across time and support the ongoing need to improve device technology with the aim of reducing adverse events.
Left ventricular assist device (LVAD) implantation alters the disease trajectory of advanced heart failure and contributes to improved survival and health-related quality of life (HRQOL) for as long as 2+ years after implant.1–6 Understanding of HRQOL in LVAD patients has increased, yet gaps persist in understanding HRQOL by subgroups (e.g., age and gender) and risk factors for poor HRQOL outcomes. Adamson et al.7 compared outcomes in a small sample of older versus younger patients implanted with continuous flow LVADs for bridge to transplant or destination therapy and reported improved overall HRQOL in both groups from before to 6 months after surgery. We reported improvement in HRQOL in older and younger continuous flow LVAD patients implanted for destination therapy from before to 12 months after surgery, noting that HRQOL was better at both time periods in the oldest cohort.8 In a study of gender-based outcomes, Bogaev et al.9 reported similarly improved overall HRQOL in both men and women 6 months after bridge to transplant continuous flow LVAD implant. Dimensions of HRQOL were not examined in two of the above studies.7,9
Risk factors for decreased survival after adult primary continuous flow LVAD implantation include age, gender, INTERMACS levels 1 and 2, right heart dysfunction, kidney dysfunction, and history of cardiac surgery or stroke.1 Risk factors for decreased HRQOL after implant are largely unknown, but may include factors similar to those which contribute to decreased survival. We recently reported that re-hospitalization was related to a decrement in HRQOL from before to 1 year after implant for destination therapy.8 Re-hospitalization was, most likely, a proxy for adverse events.
Understanding pre and post implant risk factors for poor post implant outcomes may inform selection criteria for device implantation and reinforces the ongoing need to improve device technology, in order to reduce rates of adverse events. Furthermore, when patients consider the option of LVAD implantation, it is important to inform them about risks for poor post-operative outcomes, including HRQOL.10 Understanding differences in HRQOL by subgroups may assist clinicians to target monitoring of HRQOL in higher risk patients and tailor HRQOL-specific care.
The purposes of this study were to (1) identify differences in overall HRQOL and dimensions of HRQOL across time by age and gender, and (2) identify pre and post implant factors related to change in overall HRQOL from before to 6 months after LVAD implantation. We defined HRQOL as “the functional effect of an illness and its consequent therapy upon a patient, as perceived by the patient”.11 Overall HRQOL and dimensions of HRQOL (mobility, self-care, usual activities, anxiety/depression, and pain/discomfort) were measured.12, 13 We focused on the early post implant time period (i.e., < 6 months post implant) because it is a time period within which patients are adjusting to “life on a device” physically, mentally, and socially, while potentially dealing with early post implant adverse events.14
METHODS
Theoretical Framework
The theoretical framework which guided these analyses is modified from the framework of Spilker and Revicki11 which demonstrates the effect of disease and treatment on HRQOL (figure 1). As per the framework, MCS is directed toward relieving heart failure and is associated with benefits and adverse events and also directly influences overall HRQOL. Demographic characteristics, heart failure and co-morbidities, and MCS-related clinical benefits and adverse events also directly influence HRQOL. Variables in INTERMACS were identified within each group of factors thought to influence HRQOL, including demographics, pre implant heart failure and co-morbidities, MCS (including implant strategy and type of support), post implant clinical benefits, and adverse events. We did not analyze relationships among these factors, but rather focused only on how these factors are related to overall HRQOL.
Figure 1.
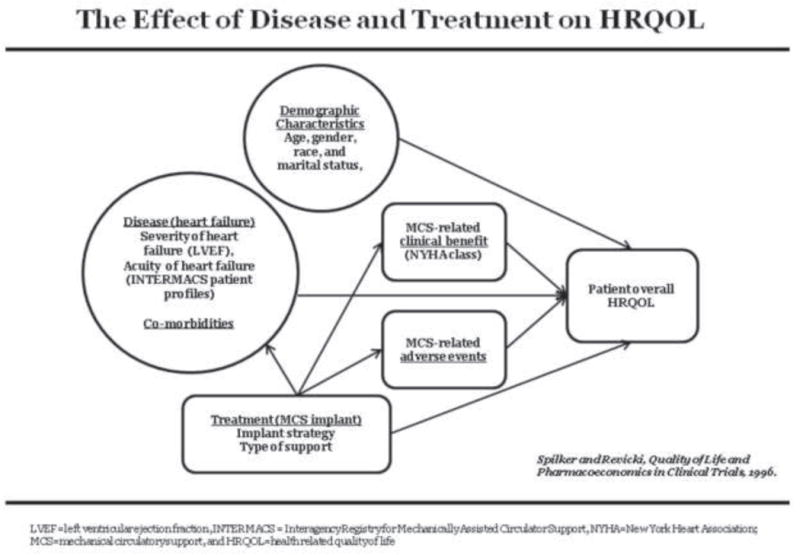
Theoretical Framework of the Effect of Disease and Treatment on HRQOL, modified
Sample
Subjects were enrolled in the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS).15 Adult patients (>19 years) in this INTERMACS cohort received an FDA-approved continuous flow LVAD as a primary implant. LVAD patients were from a total pool of 7,353 patients implanted at 133 U.S. hospitals between June, 2006 and March, 2013 with follow-up through September, 2013. Of these, 5,640 patients had pre LVAD HRQOL data, and 3,353 patients had 6 month post LVAD HRQOL data. There were 2,748 patients with data at both time periods. Our age cut-off for some analyses is derived from the gerontology literature, which defines old age by subgroup as follows: 60–69=young-old, 70–79=middle-old, and 80+=old-old. We combined the subgroups (young-old, middle-old and old-old) and thus define old as 60+.16
Data collection
HRQOL was measured using the EQ-5D-3L, a generic, self-report instrument which includes a brief health profile and single measure of health status.12, 13 Five dimensions of HRQOL (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) are assessed. The response format has three levels: no problems, some or moderate problems, and extreme problems. Patients also rate their overall HRQOL (i.e., health status) on a graduated (0–100), 20 cm vertical visual analog scale (VAS), with 0 = worst imaginable health state and 100 = best imaginable health state. Psychometric support for this instrument has been previously reported, including in patients with cardiovascular disease.17, 18
Pre and post implant medical records data were collected including demographic and behavioral data, medical and surgical history, co-morbidities, implant strategy, INTERMACS profile, hospital length of stay, re-hospitalization, survival, tests (laboratory and procedures), and device-related adverse events.
Procedures
The INTERMACS registry was approved by the Institutional Review Boards of participating institutions. Patients were consented prior to device implant or as soon as possible post-implant. For this report, EQ-5D and medical records data were collected pre-implant and at 3 and 6 months post-operatively until device removal, transplant, or death. Data were also collected regarding reasons for EQ-5D non-completion. Data were entered electronically into the INTERMACS database and analyzed at the INTERMACS data coordinating center, University of Alabama, Birmingham, AL.
Statistics
Data were analyzed using SAS software, version 9.1 (Carey, NC). Overall HRQOL was examined for the entire cohort; overall HRQOL and dimensions of HRQOL were examined by age (<60 years vs > 60 years) and gender, using mean + standard deviation for the VAS and frequencies for the five dimensions. Statistical analyses included chi square, t-tests, Pearson correlation coefficients, and forward stepwise multiple linear regression. For all analyses, level of significance was set at p<0.05. All available data were analyzed for each time point. Subsequently, paired t-tests and chi square were used to test the robustness of our analyses for patients with complete data pre and 6 months post implant. Change in the VAS score was also examined over time and a priori, a change of > 10 units was considered to be clinically important. This decision was based on the cancer literature, which estimates a change of 8–12 in VAS scores as a “minimally important difference (MID)” for self-rated health status among cancer patients.19 We did not find MIDs for VAS scores in other disease states in the literature.
A VAS rating of 0 was assigned to patients too sick to respond, which increased the size of the patient group with complete data. This rating was assigned based on the spread of scores for patients who responded in a previous report on HRQOL by INTERMACS profile, wherein patients with profile 1 (i.e., critical cardiogenic shock) had pre implant VAS scores of <10.6 Sensitivity analyses, not including patients assigned a VAS rating of 0, were conducted regarding the regression analyses.
A response level of “Extreme problems” was assigned to patients too sick to respond for the dimensions of mobility, self-care, and usual activities to reduce the potential for overestimation of HRQOL in patients who were most severely ill. Notably, 95–99% of pre implant patients with INTERMACS profile 1 reported having problems with mobility, self-care and usual activities and approximately 90%–95% of these patients reported “extreme problems” in a previous INTERMACS report.6 No assignment of responses was made for too sick patients for the pain/discomfort and anxiety/depression dimensions, as being too sick does not necessarily indicate extreme problems regarding pain or negative emotions.
The dependent variable for the HRQOL multiple regression analysis was change in the mean VAS score from before to 6 months post LVAD implantation (n=2,748). Independent variables included the pre implant VAS, demographic characteristics (age [continuous variable], gender, race, and marital status), NYHA class, behavioral variables (current smoker and alcohol abuse), pre implant clinical variables (INTERMACS patient profile, implant strategy, co-morbidities [previous cancer, pre chronic obstructive pulmonary disease, diabetes, stroke, ascites, coronary artery disease, congenital heart disease, and history of coronary artery bypass grafting and valve surgery], interventions before implant [inotropes, dialysis, ventilator support, and implantable cardioverter defibrillator]), left ventricular ejection fraction, laboratory variables (creatinine, INR, cholesterol, and blood type), and post implant variables (adverse events [, bleeding, infection, neurological dysfunction, device malfunction, hemolysis, hepatic dysfunction, hypertension, psychiatric episode, cardiac arrhythmias, pericardial drainage, myocardial infarction, renal dysfunction, respiratory failure, right heart failure, arterial non-CNS thromboembolism, venous thromboembolism, and would dehiscence], 6 month NYHA class, and implant strategy, and resource variables [intensive care length of stay and rehospitalization]).
RESULTS
Descriptive analyses
Demographic and clinical characteristics
Characteristics of patients are described in table 1. INTERMACS patients who completed the pre-implant EQ-5D (n=5,640) were typically middle-aged, white males. Most patients were INTERMACS profile20 2 (i.e., progressive decline on inotropic support) or 3 (i.e., stable but inotrope dependent) at implant and listed as a bridge to transplant, likely to be listed, or destination therapy. The vast majority of patients who completed an EQ-5D pre implant were NYHA class IV. As compared to pre implant patients who completed the EQ-5D (n=5,640), non-completers (n=1,713) were significantly more likely to be non-white, less educated, and less acutely ill (e.g., lower frequency of inotrope use, ventilator support, and other VAD support) (Table 1). Regarding resource use and adverse events at 6 months after implant, patients who completed the EQ-5D survey (n=4,174) had higher rates of neurological dysfunction than non-completers (n=1,757) (Table 1).
Table 1.
Characteristics of Continuous Flow LVAD Recipients at Pre and 6 months Post Implant
| Pre-Implant Characteristics | Pre-implant LVAD recipients with EQ-5D data (n=5640) | Pre-implant LVAD recipients without EQ-5D data (n=1713) | p-value |
|---|---|---|---|
| Demographic and behavioral characteristics | |||
| Age at implant (mean years + SD) | 56.7 (± 12.86) | 57.1 (± 13.01) | 0.22 |
| Male (%) | 78.6 | 80.8 | 0.05 |
| Race (% white) | 70.5 | 67.7 | 0.02 |
| Married at time of implant (%) | 66.9 | 65.3 | 0.22 |
| > High school education (%) | 53.4 | 48.6 | 0.004 |
| Current smoker (%) | 8.6 | 6.0 | 0.0006 |
| Current alcohol abuse (%) | 11.0 | 8.4 | 0.003 |
| Current drug abuse (%) | 1.6 | 1.1 | 0.10 |
| Pre implant clinical characteristics | |||
| Primary cardiac diagnosis (%) | |||
| Ischemic cardiomyopathy | 40.3 | 40.8 | 0.69 |
| Dilated cardiomyopathy | 47.8 | 50.0 | 0.21 |
| Other | 11.9 | 9.6 | 0.01 |
| NYHA class = IV (%) | 80.6 | 73.7 | <0.0001 |
| Inotrope therapy (%) | 82.5 | 74.6 | <0.0001 |
| Intra aortic balloon pump (%) | 28.5 | 27.0 | 0.23 |
| Ventilator (%) | 6.8 | 4.1 | <0.0001 |
| Other ventricular assist device (%) | 1.9 | 0.7 | 0.0008 |
| Implantable cardioverter defibrillator (%) | 81.5 | 83.9 | 0.03 |
| RVEF (%) | 19.3 | 19.4 | 0.95 |
| INTERMACS profile at implant (%) | |||
| 1 | 15.3 | 10.8 | < 0.0001 |
| 2 | 40.8 | 35.8 | 0.0002 |
| 3 | 27.2 | 26.4 | 0.55 |
| 4 | 12.4 | 18.9 | < 0.0001 |
| 5 | 2.3 | 4.8 | < 0.0001 |
| 6 | 1.3 | 2.0 | 0.04 |
| 7 | 1.2 | 0.7 | 0.04 |
| Device strategy (%) | |||
| Bridge to transplant – listed | 27.9 | 24.7 | 0.01 |
| Bridge to transplant – likely to be eligible | 22.8 | 27.1 | 0.0002 |
| Bridge to transplant – moderately likely to be eligible | 10.2 | 9.4 | 0.34 |
| Bridge to transplant-unlikely to become eligible | 3.2 | 3.6 | 0.50 |
| Destination Therapy | 34.8 | 36.0 | 0.88 |
| Bridge to Recovery | 0.6 | 0.6 | 0.81 |
| 6 Month Post Implant Characteristics | 6 month post implant LVAD recipients with EQ-5D data (n=4174) | 6 month post implant LVAD recipients without EQ-5D data (n=1757) | p-value |
| 6 month post implant clinical characteristics | |||
| Implant hospital length of stay (days + SD) | 25.4 ± 23.1 | 24.4 ± 24.8 | 0.14 |
| Discharge to home (%) | 77.1 | 75.5 | 0.21 |
| Re-hospitalization within 6 months of discharge (%) | 55.5 | 56.2 | 0.51 |
| Most common adverse events through 6 months post implant (%) | |||
| Bleeding | 32.9 | 31.2 | 0.21 |
| Infection | 33.3 | 34.9 | 0.24 |
| Cardiac Arrhythmia | 23.4 | 22.7 | 0.59 |
| Renal Dysfunction | 6.1 | 5.5 | 0.37 |
| Neurological Dysfunction | 10.0 | 7.8 | 0.007 |
| Respiratory Failure | 12.3 | 11.2 | 0.23 |
NYHA=New York Heart Association; RVEF=right ventricular ejection fraction; INTERMACS=Interagency Registry for Mechanically Assisted Circulatory Support
EQ-5D-3L questionnaire completion rates
Of the 7,353patients who received continuous flow LVADs, completion rates for the EQ-5D were as follows: pre implant=77%, 3 months post implant=56%, and 6 months post implant=57% (Table 2). Reasons for EQ-5D non-completion across time are listed in table 2.
Table 2.
EQ-5D Completion Rates and Reasons for Non-completion
| Status | Pre-implant (n=7353) | 3 months (n=6599*) | 6 months (n=5931*) |
|---|---|---|---|
| EQ-5D Completed | 4005 (54%) | 3258 (49%) | 3088 (52%) |
| Too Sick** | 1635 (22%) | 431 (7%) | 265 (5%) |
|
| |||
| Total ‘Completed’ | 5640 (77%) | 3689 (56%) | 3353 (57%) |
| Incomplete reasons | |||
| Coord did not contact/Admin | 752 | 471 | 333 |
| Unable to obtain F/U | — | 348 | 410 |
| Patient unavailable | 36 | 22 | 10 |
| Pt unwilling or unable | 191 | — | — |
| Pt not seen in clinic/hospital | 315 | 205 | 199 |
| Pt seen in clinic/hospital but EQ5D not done (due) | — | 1277 | 1188 |
| Other | 77 | 155 | 34 |
| Unknown | 342 | 532 | 404 |
|
| |||
| Total | 1713 (23%) | 2910(44%) | 2578 (43%) |
number of patients with the opportunity for follow-up where the patient has not died, been transplanted or recovered.
completed forms includes patients who filled out the EQ-5D and also those patients captured as ‘too sick’ to complete the EQ-5D. ‘Too sick’ patients were assigned a value of 0 for the VAS and ‘extreme problems’ for the 3 physical dimensions of Mobility, Self Care and Usual Activities
HRQOL from before to after LVAD implantation
Overall HRQOL (i.e., mean VAS score) improved significantly for the entire cohort, using all available data, from before to 6 months after LVAD implantation (Figure 2a). Pre LVAD, the majority of patients (76%) had VAS scores <50 while at 6 months post LVAD, the majority of patients (78%) had VAS scores >50 (Table 3). The majority of patients (69%) with pre and 6 mo post LVAD data (n=2,748) increased their VAS scores by >10 points, while 10% increased their VAS scores by < 10%, and 21% of patients had no change or decreased their VAS scores (Table 3). We considered a change in VAS score of >10 points as the minimal clinically important difference.19
Figure 2.
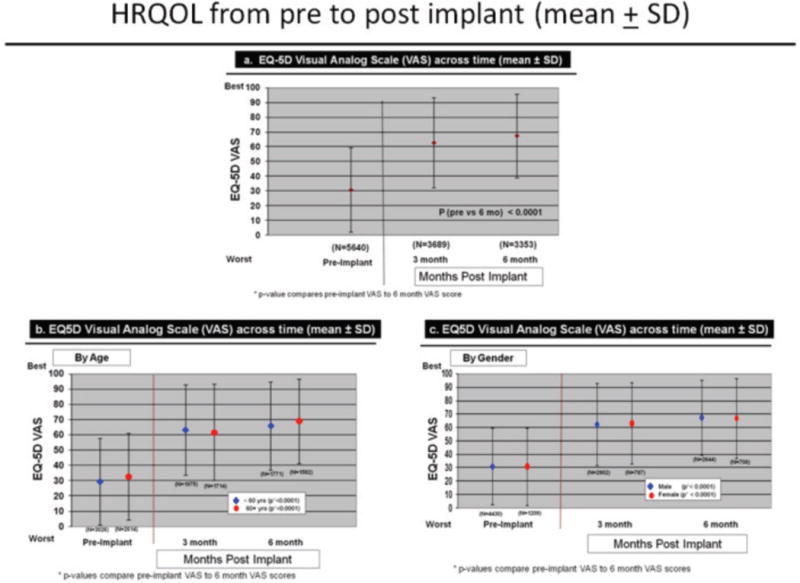
Change in HRQOL Dimensions Across Time by Age
Table 3.
VAS Scores pre and post implant & Change in VAS Scores Over Time
| VAS | Pre-implant (n=5640**) | 6 months (n=3353**) | |
|---|---|---|---|
| 0 – 25 | 2714 (48.1%) | 404 (12.1%) | |
| 26 – 50 | 1589 (28.2%) | 347 (10.3%) | |
| 51 – 75 | 908 (16.1%) | 974 (29.0%) | |
| 76 – 100 | 429 (7.6%) | 1628 (48.6%) | |
|
| |||
| Total | 5640 (100%) | 3353 (100%) | |
| Change in VAS(n=2748*) | n | % | |
|
| |||
| Increase | |||
| >20 | 1637 | 59.6% | |
| 11 – 20 | 254 | 9.2% | |
| 1 – 10 | 274 | 10.0% | |
| Decrease (or no change) | |||
| 0 – 10 | 396 | 14.4% | |
| 11 – 20 | 62 | 2.3% | |
| > 20 | 125 | 4.6% | |
Only includes paired data (patients with both pre and post 6 months completed EQ-5D)
completed forms includes patients who filled out the EQ-5D and also those patients captured as ‘too sick’ to complete the EQ-5D. ‘Too sick’ patients were assigned a value of 0 for the VAS and ‘extreme problems’ for the 3 physical dimensions of Mobility, Self Care and Usual Activities
Comparisons of HRQOL by age and gender
Change over time in overall HRQOL and for dimensions was similar by demographic characteristics, using all available data. Overall HRQOL improved significantly (pre to 6 months post implant) for older and younger patients (Figure 2b). Older and younger patients reported significantly fewer problems from before to 6 months after LVAD implantation for all dimensions (Figures 3–7). Similarly, men and women reported significantly improved overall HRQOL (pre to 6 months post implant) (Figure 2c) and significantly fewer problems from before to 6 months after LVAD implant for all dimensions (Figures 8–12).
Figure 3.
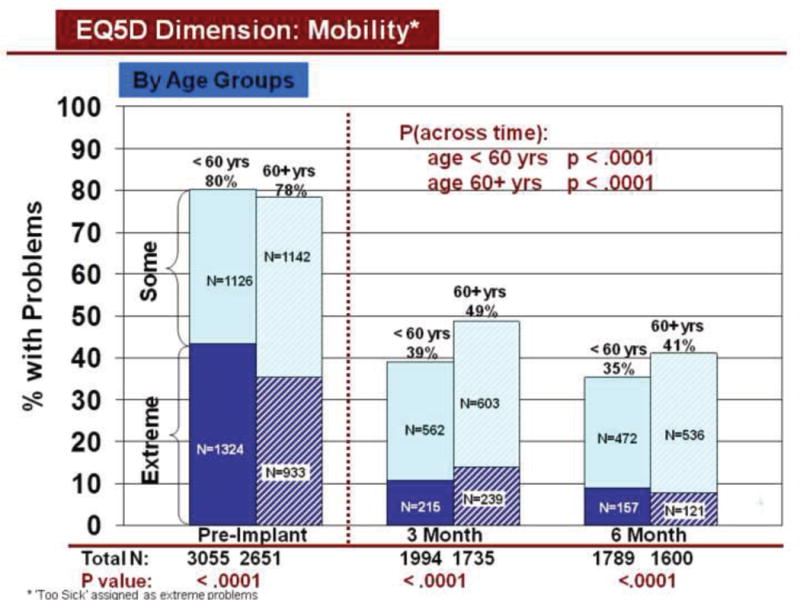
Change in Mobility Across Time by Age
Figure 7.
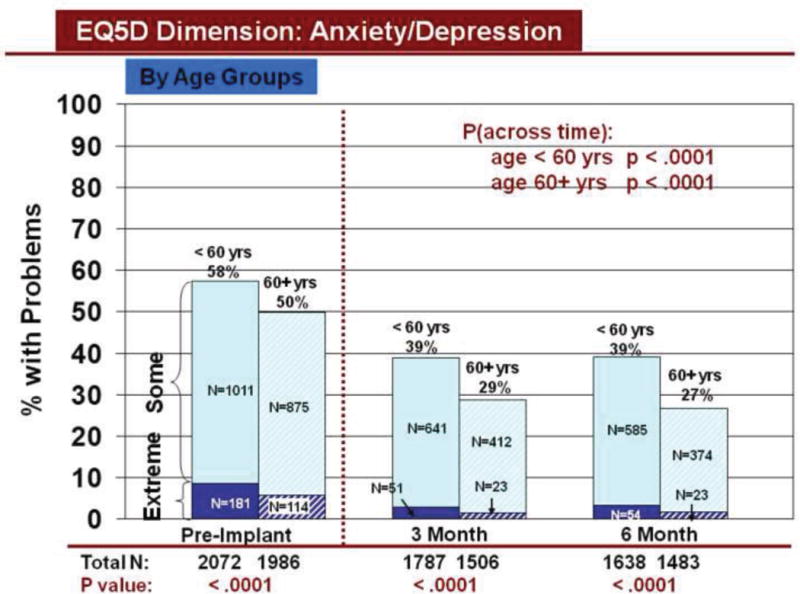
Change in Anxiety/Depression Across Time by Age
Figure 8.
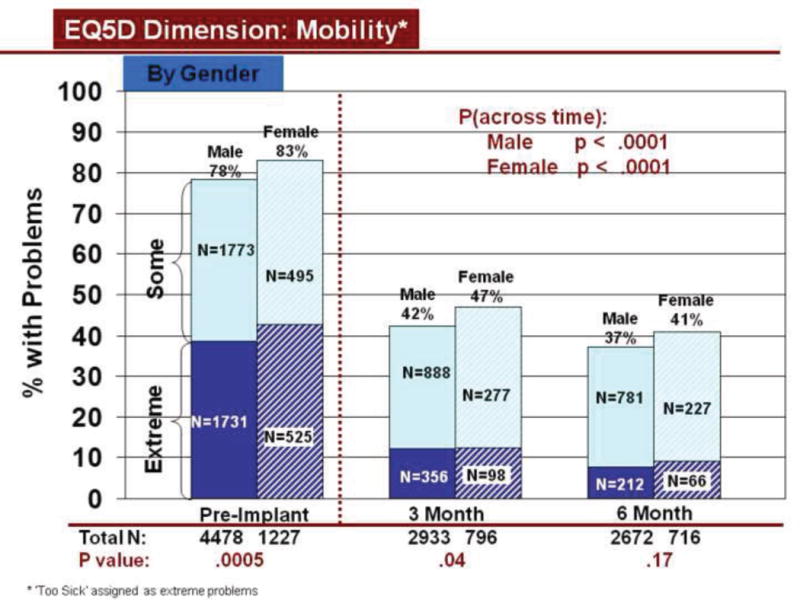
Change in Mobility Across Time by Gender
Figure 12.
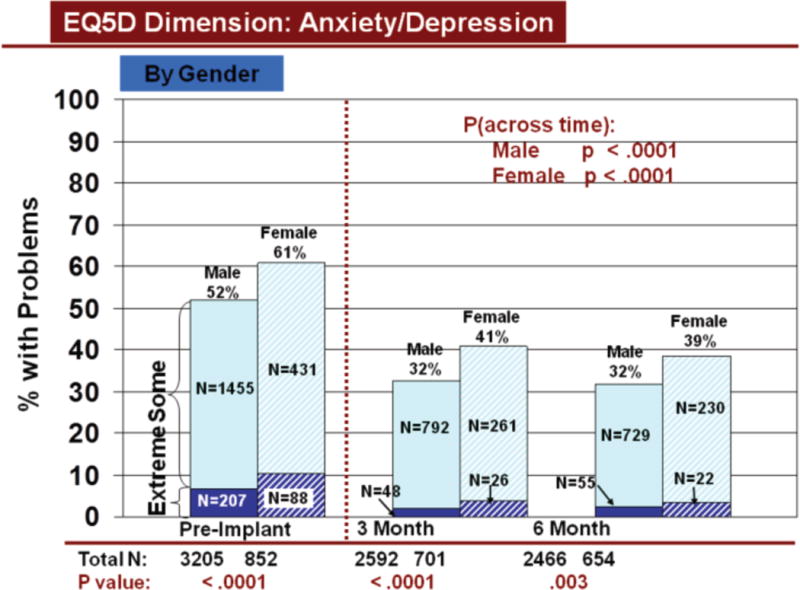
Change in Anxiety/Depression Across Time by Gender
Rates of problems for HRQOL dimensions were also compared cross-sectionally for age and gender within each time period, using all available data. Before LVAD implant, younger patients reported significantly more problems than older patients for all dimensions (Figures 3–7). At both 3 and 6 months after device implant, older patients reported significantly more problems than younger patients for mobility, but fewer problems regarding pain/discomfort and anxiety/depression. Prior to LVAD implantation, women reported significantly more problems for all dimensions, than men (figures 8–12). Women also reported significantly more problems than men at both 3 and 6 months after implant for usual activities, pain/discomfort, and anxiety/depression.
Sensitivity analyses
Sensitivity analyses for overall HRQOL were conducted using paired t-tests (n=1981 [excluding patients assigned a VAS score of 0]). Overall HRQOL, including by age and gender, significantly improved between pre and 6 months post implant (data not shown). Using Chi square, and paired data, (excluding patients assigned “extreme problems” if too sick to respond) significant improvement also occurred for all dimensions by both age and gender from pre to 6 months after implant (data not shown). These analyses support our findings using all available data.
Multivariable analyses
Multiple regression analyses were conducted to identify factors related to change in mean VAS score from before to 6 months after LVAD implant for patients with complete data at both time periods (including patients assigned a VAS score of 0, n=2,748) (Table 4). Pre implant INTERMACS level 1 (i.e., critical cardiogenic shock) was associated with an increase in VAS score from before to 6 months after LVAD implant. Variables associated with a decrease in VAS score from before to after device implant were: listed for heart transplant at implant, chronic obstructive pulmonary disease, ascites, alcohol abuse, higher VAS score at implant, adverse events within 6 months of implant (renal dysfunction, respiratory failure, neurological dysfunction, and infection), and bridge to heart transplant moderately likely and unlikely and NYHA class 4 at 6 months post LVAD (R2=41%, p<0.0001).
Table 4.
Factors Associated with Change in HRQOL Pre-implant – 6 months post implant
| Risk Factors | Estimates (SE) | p value |
|---|---|---|
| Pre-implant conditions | ||
| INTERMACS Level 1 | 5.1 (1.6) | 0.002 |
| BTT: Listed | −3.7 (1.2) | 0.002 |
| Pre COPD | −10.4 (2.67) | 0.0001 |
| Ascites | −3.7 (2.3) | 0.10 |
| Alcohol abuse | −4.4 (1.7) | 0.01 |
| Pre-implant VAS Score | −0.78 (0.02) | < 0.0001 |
| COPD * Pre-implant VAS score interaction | 0.17 (0.06) | 0.008 |
| Clinical Course | ||
| BTT: Unlikely at 6 months | −9.9 (2.9) | 0.0006 |
| BTT: Mod likely at 6 months | −4.7 (1.9) | 0.01 |
| NYHA 4 at 6 months | −15.3 (2.9) | < 0.0001 |
| Events within first 6 months | ||
| Renal Dysfunction | −5.3 (2.5) | 0.03 |
| Respiratory Failure | −4.8 (1.8) | 0.008 |
| Neurological Dysfunction | −5.6 (1.9) | 0.004 |
| Infection | −2.8 (1.1) | 0.01 |
Intercept = 65.0, R2 = 41% n=2748, p < 0.0001
HRQOL=health-related quality of life; INTERMACS=interagency Registry for Mechanically Assisted Circulatory Support; BTT=bridge to transplant; COPD=chronic obstructive pulmonary disease; VAS=visual analog scale
Negative coefficients indicate the decrement in change
The Intercept indicates the amount of change (improvement) for a patient with no ‘risk factors’
We plotted the effect of adverse events on change in VAS scores over 6 months by INTERMACS profiles. As per figures 13–16, greater improvement in VAS scores occurred in patients with lower INTERMACS profiles (i.e., higher severity of illness) and fewer post-operative adverse events. Patients were at approximately the same level of VAS score at 6 months after implant.
Figure 13.
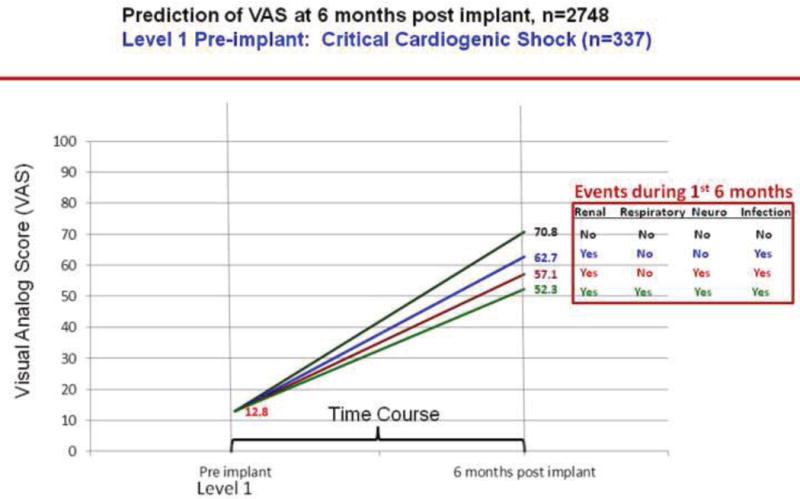
Predictions of post implant VAS score by pre implant INTERMACS profile 1
Figure 16.
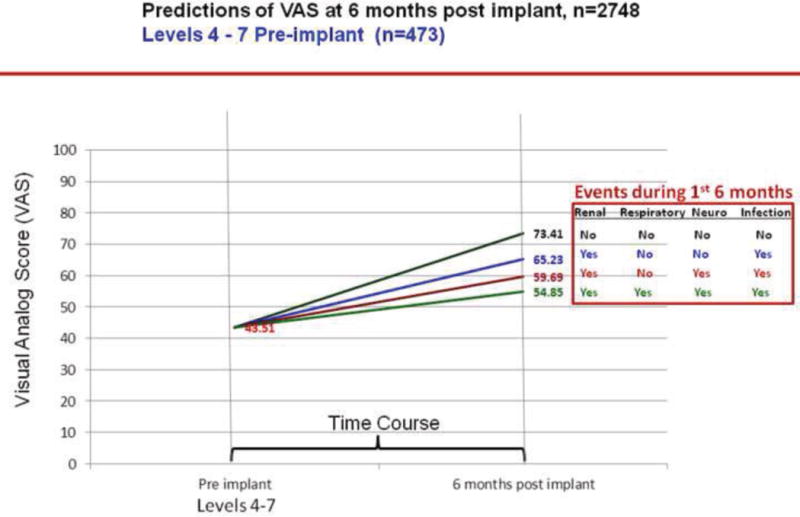
Predictions of post implant VAS score by pre implant INTERMACS profiles 4–7
Sensitivity analyses
Sensitivity analyses (for patients with complete data at both time periods, excluding patients assigned a VAS score of 0, n=1981) were conducted regarding change in VAS score over time, using the same multiple regression approach, as the original analyses (Table 5). INTERMACS level 1 remained significantly associated with an increase in VAS score over time, and INTERMACS level 2 (progressive decline on inotropic support) also became significant, as did age and white race (not significant in the original regression analyses). A higher pre implant VAS score and bridge to transplant moderately likely at 6 months remained significantly associated with a decrease in VAS score from before to after implant; however, co-morbidities, bridge to heart transplant unlikely and NYHA class 4 at 6 months post LVAD were no longer significant. Regarding post implant adverse events, respiratory failure and neurological dysfunction were similarly significant; however, renal dysfunction and infection were no longer risk factors, while right heart failure, which was not significant in the original regression analyses, became significant in the sensitivity analyses. While the sensitivity analyses had fewer significant risk factors and some of them were different, the sensitivity analyses, none-the-less support our original regression analyses, as severity of illness, pre implant VAS score, clinical course, and post implant adverse events were significant variables in both analyses.
Table 5.
Factors Associated with Change in HRQOL Pre-implant – 6 months post implant (Sensitivity Analyses)
| Risk Factors | Estimates (SE) | p value |
|---|---|---|
| Demographic | ||
| Age (log) | 6.2 (1.7) | 0.0002 |
| White race | 3.6 (1.1) | 0.0009 |
| Pre-implant conditions | ||
| INTERMACS Level 1 | 10.9 (2.1) | < 0.0001 |
| INTERMACS Level 2 | 4.5 (1.2) | 0.0002 |
| Pre-implant VAS Score | −0.82 (0.02) | < 0.0001 |
| Clinical Course | ||
| BTT: Mod likely at 6 months | −3.3 (1.6) | 0.04 |
| Events within first 6 months | ||
| Respiratory Failure | −5.1 (1.6) | 0.002 |
| Neurological Dysfunction | −4.2 (1.7) | 0.01 |
| Right Heart Failure | −2.9 (1.4) | 0.04 |
Intercept = 38.6, R2 = 53.1%, n=1981, p < .0001
HRQOL=health-related quality of life; INTERMACS=interagency Registry for Mechanically Assisted Circulatory Support; BTT=bridge to transplant; VAS=visual analog scale
Negative coefficients indicate the decrement in change
The Intercept indicates the amount of change (improvement) for a patient with no ‘risk factors’
DISCUSSION
Advanced heart failure patients experienced improvement in overall HRQOL and dimensions of HRQOL from before to after LVAD implantation which was sustained through 6 months postoperatively, with no significant differences by age and gender. Before implant, younger patients and women reported significantly more problems in HRQOL dimensions than older patients and men. After implant, frequencies of problems differed for some dimensions by age and gender. As described within our theoretical framework, per Spilker and Reviciki,11 change in HRQOL from before to 6 months after implant was significantly related to clinical variables, including pre implant INTERMACS level and co-morbidities, and post operative adverse events.
Our finding of improved HRQOL across time, by age and gender, from before to after implant, is similar to other reports in the VAD literature.7–9 Our cross-sectional findings by subgroup deserve additional comment. Older patients reported more problems with mobility, as compared to younger patients, early after implant. This finding is similar to findings regarding physical function and activities of daily living after heart transplantation21, 22 and in other chronic illness populations who undergo cardiac surgery23 or procedures.24, 25 We previously reported more disability in older heart transplant patients compared to younger patients regarding ambulation and work at 1 year21 and ambulation and body care/movement at 5–10 years22 after heart transplantation. Older dialysis patients also have worse physical function than younger dialysis patients.24, 25 Older age was related to decline in physical function > 6 months after cardiac surgery.23 Our current report of increased anxiety/depression in younger versus older patients before and within 6 months after implant is supported by our previous findings of more depression in younger versus older patients after heart transplantation,26 and more anxiety and depression in younger patients in the cardiac surgical27 and heart failure28 literature.
We also found that women reported more problems with usual activities, pain/discomfort, and anxiety/depression than men before and at both time periods after LVAD implant. We similarly reported that women reported worse physical functional disability overall and regarding ambulation, mobility, self-care and home management, as compared to men, at 1 year after heart transplantation.21 Long-term after heart transplantation, we also found that female gender was associated with more overall physical functional disability, and disability related to ambulation, mobility, and body care/movement than male gender.22 Reports of differences in physical function, favoring men, have also been reported in the general cardiac surgical literature.29, 30 Differences in anxiety/depression by gender are also supported by the literature, early and later after heart transplantation,26, 31, 32 after cardiac surgery,33, 34 and in patients with heart failure.35 Our findings suggest an opportunity to focus attention on subgroups of patients to maximize HRQOL before and after device implantation.
We addressed an important gap in the VAD literature by identifying factors related to change in HRQOL from before to early after implant. In support of these findings, we previously reported that adverse events after heart transplantation are related to HRQOL both early36 and later37 after heart transplantation. Similarly, post-operative complications were related to HRQOL early38, 39 and later40 after myocardial revascularization. Lastly, the relationship between the pre implant VAS score and change in the VAS score after implant has also been demonstrated in the cardiac surgical literature. Patients with worse preoperative HRQOL had higher gains in HRQOL than patients with better preoperative HRQOL after myocardial revascularization.41, 42
Our report on HRQOL from INTERMACS has limitations. Survivorship bias may have contributed to overly optimistic findings. Also, lack of questionnaire completion before and after LVAD implantation limits interpretation and generalizability of our findings. However, our post hoc assignment of scores, in patients too sick to respond, enhanced completion rates and may have reduced overestimation of HRQOL across time. Additionally, we used a generic health profile rather than a heart failure specific HRQOL instrument which may not have captured disease-specific concerns. Anxiety and depression were also combined as one question in the EQ-5D, which may be better understood as separate questions. Finally, there are variables, not collected in INTERMACS, which may have been related to MCS HRQOL, such as symptoms and specific coping mechanisms.
CONCLUSION
Overall HRQOL and dimensions of HRQOL improve in older and younger patients, as well as in men and women, from before to 6 months after LVAD implantation. Importantly, while dimensions of HRQOL improve from before to early after implant, approximately 1/3 to 1/2 of all patients report some problems after implant, which must be addressed while patients adjust to living with mechanical circulatory support. Patients who are ‘sickest’ have the greatest opportunity for major improvement in HRQOL after implant. Patients with co-morbidities that prevent listing for heart transplantation may have limited HRQOL improvement, although notably, we have previously reported that HRQOL also improves from before to after implant for destination therapy.8 Adverse events have a major detrimental effect on HRQOL at 6 months. Reducing adverse events should be a central focus in efforts to further improve HRQOL. These findings support the ongoing need to evaluate co-morbid risks before implant and improve device technology to enhance post LVAD HRQOL.
Figure 4.
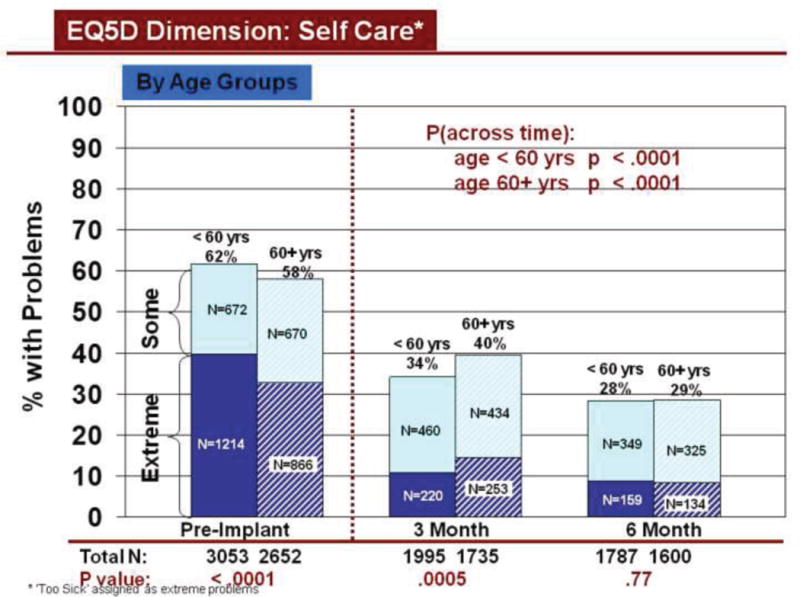
Change in Self Care Across Time by Age
Figure 5.
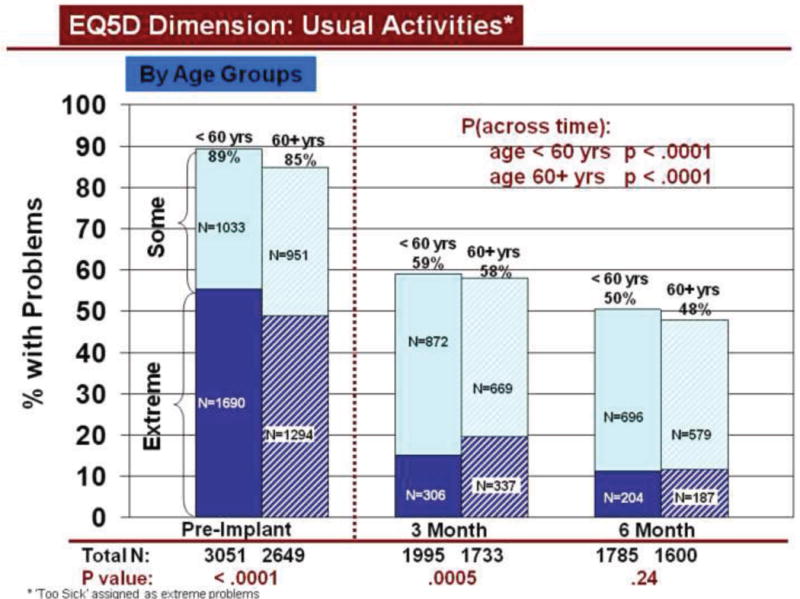
Change in Usual Activities Across Time by Age
Figure 6.
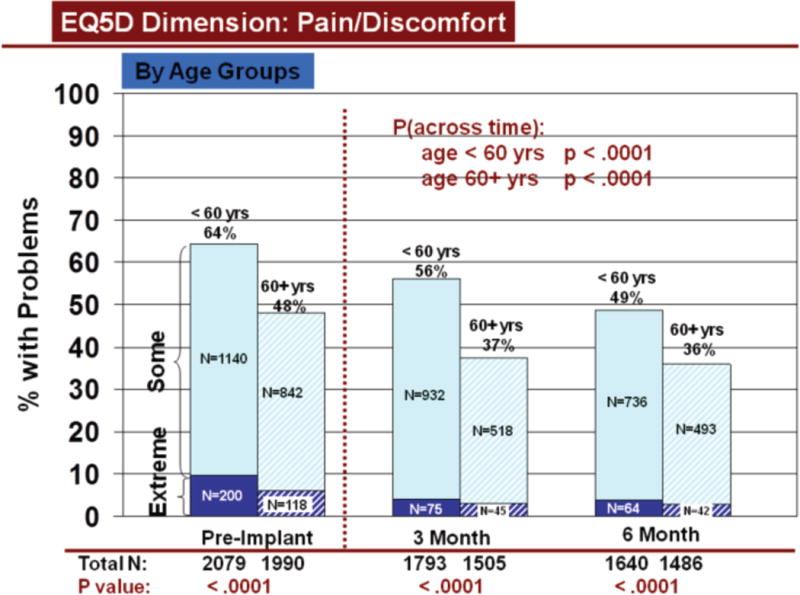
Change in Pain/Discomfort Across Time by Age
Figure 9.
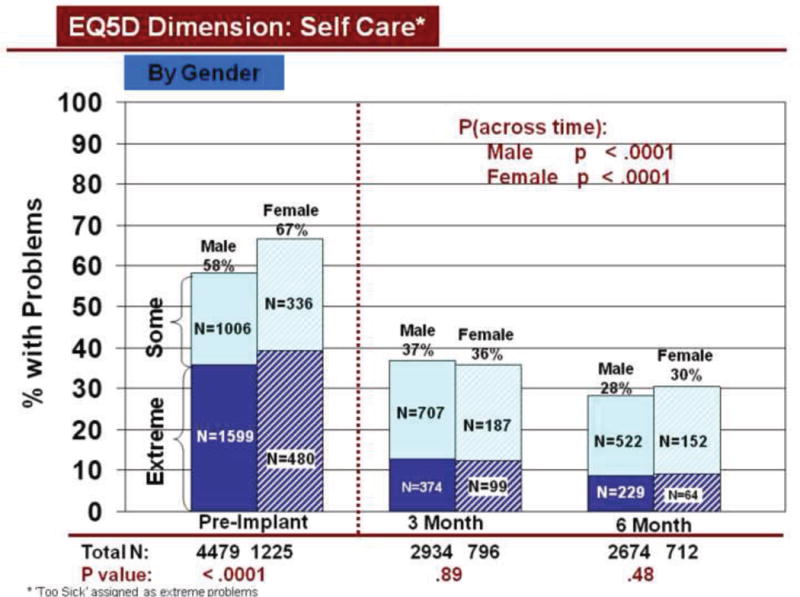
Change in Self Care Across Time by Gender
Figure 10.
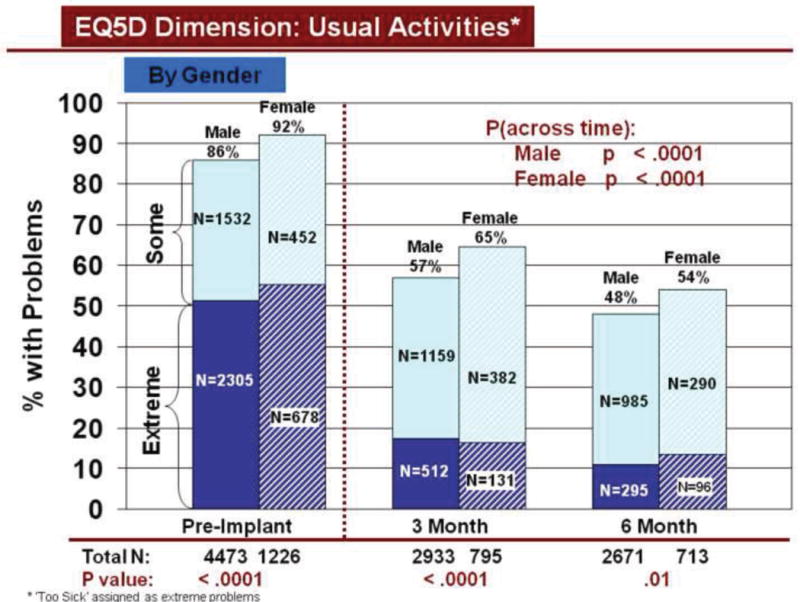
Change in Usual Activities Across Time by Gender
Figure 11.
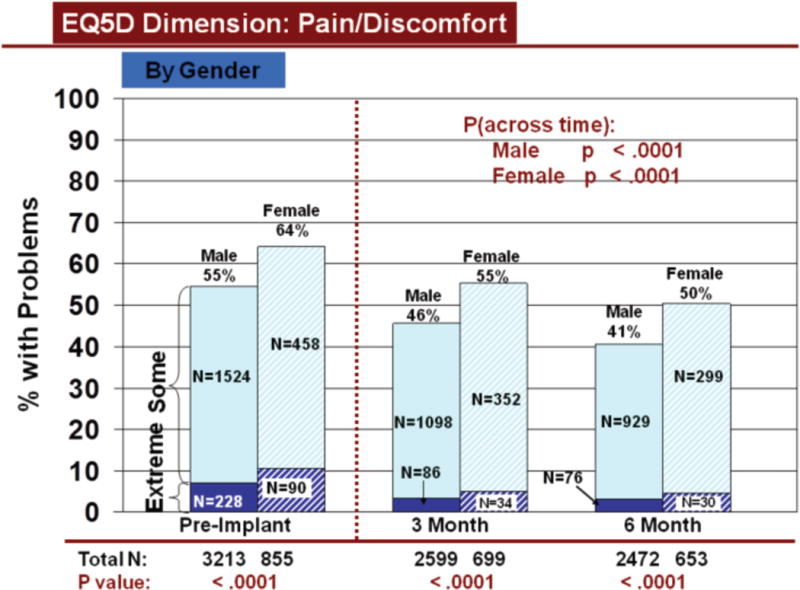
Change in Pain/Discomfort Across Time by Gender
Figure 14.
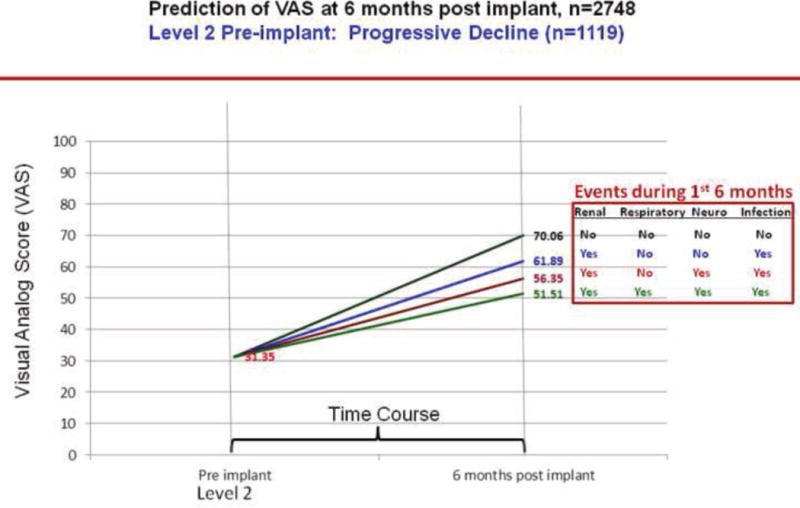
Predictions of post implant VAS score by pre implant INTERMACS profile 2
Figure 15.
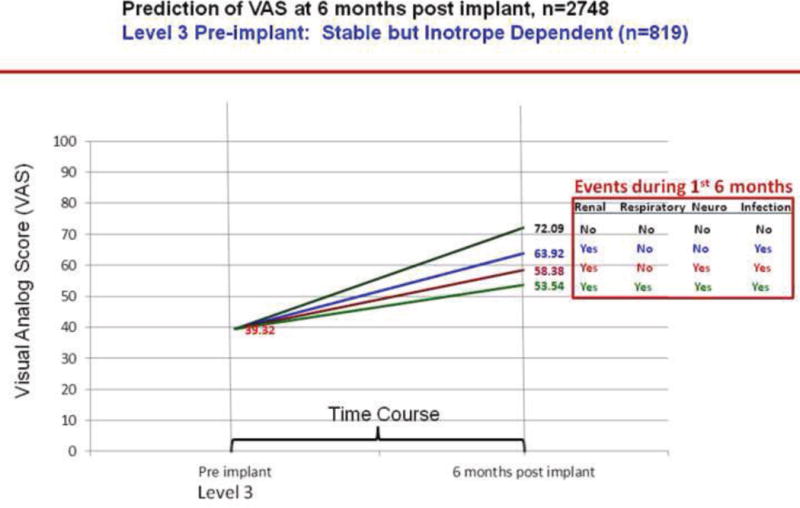
Predictions of post implant VAS score by pre implant INTERMACS profile 3
Acknowledgments
Disclosure statement
This work was sponsored by the National Institutes of Health, National Heart, Lung and Blood Institute (NHLBI), Registry of Mechanical Circulatory Support Devices for End-Stage Heart Failure (INTERMACS). Contract No. HHSN268200548198C.
Kathleen L. Grady, PhD, RN, MS - NHLBI and NIA funding
Sherri Wissman, RN- no disclosures
David C. Naftel, PhD- consultant HeartWare and Thoratec Corporations
Susan Myers, BA - no disclosures
Annetine Gelijins, PhD- no disclosures
Alan Moskowitz, MD- no disclosures
Francis D. Pagani, PhD, MD- no disclosures
James B. Young, MD- no disclosures
John A. Spertus, MD - Grant support from ACCF, NIH, PCORI, Amorcyte, Lilly, Genentech and Gilead.
Owns the copyright to the KCCQ. Serves as a consultant to the Scientific Advisory Board of United Healthcare, Amgen and Jannsen.
James K. Kirklin, MD – no disclosures
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kathleen L. Grady, Northwestern University, Chicago, IL.
Sherri Wissman, Ohio State University, Medical Center, Columbus, OH.
David C. Naftel, University of Alabama, Birmingham, Birmingham, AL.
Susan Myers, University of Alabama, Birmingham, Birmingham, AL.
Annetine Gelijins, Mount Sinai Medical Center, New York, NY.
Alan Moskowitz, Mount Sinai Medical Center New York, NY.
Francis D. Pagani, University of Michigan, Ann Arbor, MI.
James B. Young, The Cleveland Clinic, Cleveland, OH.
John A. Spertus, St. Luke’s Mid America Heart Institute and UMKC, Kansas City, MO.
James K. Kirklin, University of Alabama, Birmingham, Birmingham, AL.
References
- 1.Slaughter MS, Pagani FD, McGee EC, et al. HeartWare ventricular assist system for bridge to transplant: combined results of the bridge to transplant and continued access protocol trial. J Heart Lung Transplant. 2013 Jul;32(7):675–83. doi: 10.1016/j.healun.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009 Dec 3;361(23):2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 3.Pagani FD, Miller LW, Russell SD, Aaronson KD, John R, Boyle AJ, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54(4):312–21. doi: 10.1016/j.jacc.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 4.Rogers J, Aaronson KD, Boyle AJ, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55:1826–34. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 5.Kirklin JK, Naftel DC, Pagani FD, et al. Sixth INTERMACS annual report: A 10,000-patient database. J Heart Lung Transplant. 2014;33:555–564. doi: 10.1016/j.healun.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 6.Grady KL, Naftel DC, Stevenson L, et al. Overall quality of life improves to similar levels after mechanical circulatory support regardless of severity of heart failure before implantation. J Heart Lung Transplant. 2014;33:412–421. doi: 10.1016/j.healun.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adamson RM, Stahovich M, Chillcott S, et al. Clinical strategies and outcomes in advanced heart failure patients older than 70 years of age receiving the HeartMate II left ventricular assist device. J Am Coll Cardiol. 2011;57:2487–95. doi: 10.1016/j.jacc.2011.01.043. [DOI] [PubMed] [Google Scholar]
- 8.Grady KL, Naftel DC, Myers S, et al. Change in health-related quality of life from before to after destination therapy mechanical circulatory support is similar for older and younger patients: Analyses from INTERMACS. J Heart Lung Transplant. 2015;34:213–221. doi: 10.1016/j.healun.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogaev R, Pamboukian S, Moore S, et al. Comparison of outcomes in women versus men using a continuous flow left ventricular assist device as a bridge to transplantation. J Heart Lung Transplant. 2011;30:515–22. doi: 10.1016/j.healun.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Allen L, Stevenson L, Grady K, Goldstein N, Matlock D, Arnold R, Cook N, Felker GM, Francis GS, Hauptman P, Havranek E, Krumholz H, Mancini D, Riegel B, Spertus J. Decision making in advanced heart failure: A scientific statement from the American Heart Association. Circulation. 2012 Apr 17;125(15):1928–1952. doi: 10.1161/CIR.0b013e31824f2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spilker B. Quality of life and pharmacoeconomics in clinical trials. 2nd. New York: Lippincott Williams & Wilkins; 1996. [Google Scholar]
- 12.EuroQol group. EuroQol a new facility for the measurement of health related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 13.Rabin R, de Charro F. EQ-5D: A measure of health status from the EuroQol Group. Ann Med. 2001;33:337–43. doi: 10.3109/07853890109002087. [DOI] [PubMed] [Google Scholar]
- 14.Grady K, Magasi S, Hahn EA, McGee E, Jr, Yancy C. A patient-centric conceptual framework for health-related quality of life in mechanical circulatory support. J Heart Lung Transplant. 2015 May 4; doi: 10.1016/j.healun.2015.04.003. pii: S1053-2498(15)01206-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Kirklin JK, Naftel DC, Stevenson L, et al. INTERMACS database for durable devices for circulatory support: First annual report. J Heart Lung Transplant. 2008;27:1065–72. doi: 10.1016/j.healun.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Forman DE, Berman AD, McCabe3 CH, Baim DS, Wei JY. PTCA in the elderly: the “young-old” versus the “old-old. J Am Geriatr Soc. 1992;40(1):19–22. doi: 10.1111/j.1532-5415.1992.tb01823.x. [DOI] [PubMed] [Google Scholar]
- 17.Van Agt H, Essink-Bot ML, Krabbe P, Bonsel G. Test-retest reliability of health state valuations collected with the EuroQoL questionnaire. Soc Sci Med. 1994;39:1537–44. doi: 10.1016/0277-9536(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 18.Dyer M, Goldsmith K, Sharples L, Buxton M. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health Qual Life Outcomes. 2010;8:13. doi: 10.1186/1477-7525-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pickard AS, Neary MP, Cella D. Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health and Quality of Life Outcomes. 2007;5:70. doi: 10.1186/1477-7525-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevenson LW, Pagani FD, Young JB, et al. INTERMACS profiles of advanced heart failure: the current picture. J Heart Lung Transplant. 2009;28:535–41. doi: 10.1016/j.healun.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 21.Jalowiec A, Grady KL, White-Williams C. Gender and age differences in symptom distress and functional disability at 1 year after heart transplant surgery. Heart & Lung. 2011;40(1):21–30. doi: 10.1016/j.hrtlng.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grady KL, Naftel DC, Young JB, et al. Patterns and predictors of physical functional disability at 5–10 years after heart transplantation. The Journal of Heart and Lung Transplantation. 2007;26:1182–1191. doi: 10.1016/j.healun.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grady KL, Lee R, Subacius H, et al. Improvements in Health-related Quality of Life Before and After Isolated Cardiac Operations. Ann Thorac Surg. 2011;91:777–783. doi: 10.1016/j.athoracsur.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 24.Taveras Ae, Bekui AM, Gorban-Brennan N, Raducu R, Finkelstein FO. Peritoneal dialysis in patients 75 years of age and older – a 22-year experience. Adv Perit Dial. 2012;28:84–8. [PubMed] [Google Scholar]
- 25.Lamping DL, Constantinovici N, Roderick P, et al. Clinical outcomes, quality of life, and costs in the North Thames Dialysis study of elderly people on dialysis: a prospective cohort study. The Lancet. 2000;356:1543–50. doi: 10.1016/S0140-6736(00)03123-8. [DOI] [PubMed] [Google Scholar]
- 26.Rybarczyk B, Grady K, Naftel D, et al. Emotional adjustment five years after heart transplant: A multi-site study. Rehabilitation Psychology. 2007;52:206–214. [Google Scholar]
- 27.Krannich JH, Weyers P, Lueger S, et al. Presence of depression and anxiety before and after coronary artery bypass graft surgery and their relationship to age. BMC Psychiatry. 2007;7(47):1–6. doi: 10.1186/1471-244X-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moser D, Heo S, Lee K, et al. ‘It could be worse … lot’s worse!’ Why health-related quality of life is better in older compared with younger individuals with heart failure. Age and Ageing. 2013;42:626–32. doi: 10.1093/ageing/aft078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindquist R, Dupuis G, Terrin M, et al. Comparison of health-related quality of life outcomes of men and women after coronary artery bypass surgery through 1 year: Findings from the POST CABG Biobehavioral Study. Am Heart J. 2003;146:1038–44. doi: 10.1016/S0002-8703(03)00451-4. [DOI] [PubMed] [Google Scholar]
- 30.Herlitz J, Wiklund I, Sjoland H, et al. Relief of symptoms and improvement of health-related quality of life five years after coronary artery bypass graft in women and men. Clin Cardiol. 2001;24(5):385–92. doi: 10.1002/clc.4960240508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dew MA, DiMartini AF, DeVito Dabbs AJ, et al. Onset and risk factors for anxiety and depression during the first 2 years after lung transplantation. Gen Hosp Psy. 2012;34:127–38. doi: 10.1016/j.genhosppsych.2011.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dew MA, Kormos RL, DiMartini AE, et al. Prevalence and risk of depression and anxiety-related disorders during the first three years after heart transplantation. Psychosomatics. 2001;42(4):300–13. doi: 10.1176/appi.psy.42.4.300. [DOI] [PubMed] [Google Scholar]
- 33.Modica M, Ferratini M, Spezzaferri R, et al. Gender differences in illness behavior after cardiac surgery. J Cardiopulm Rehab Prev. 2014;34:123–29. doi: 10.1097/HCR.0000000000000043. [DOI] [PubMed] [Google Scholar]
- 34.Nemati M, Astaneh B. The impact of coronary artery bypass graft surgery on depression and anxiety. J Cardiovasc Med. 2011;12:401–04. doi: 10.2459/JCM.0b013e32834358e9. [DOI] [PubMed] [Google Scholar]
- 35.Eastwood J, Moser D, Riegel B, et al. Commonalities and differences in correlates of depressive symptoms in men and women with heart failure. Euro J Cardiovasc Nurs. 2012;11(3):356–65. doi: 10.1177/1474515112438010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grady K, Jalowiec A, White-Williams C. Predictors of Quality of Life in Patients at 1 Year After Heart Transplantation. The Journal of Heart and Lung Transplantation. 1999;18(3):202–210. doi: 10.1016/s1053-2498(98)00048-5. [DOI] [PubMed] [Google Scholar]
- 37.Grady K, Naftel D, Kobashigawa J, et al. Patterns and predictors of quality of life at 5 – 10 years after heart transplantation. The Journal of Heart and Lung Transplantation. 2007;26:535–43. doi: 10.1016/j.healun.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peric V, Borzanovic M, Stolic R, et al. Predictors of worsening of paitents’ quality of life six months after coronary artery bypass surgery. J Card Surg. 2008;23:648–54. doi: 10.1111/j.1540-8191.2008.00667.x. [DOI] [PubMed] [Google Scholar]
- 39.Lie I, Arnesen H, Sandvik L, et al. Predictors for physical and mental health 6 months after coronary artery bypass grafting: A cohort study. Euro J Cardiovasc Nurs. 2010;9:238–243. doi: 10.1016/j.ejcnurse.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Herlitz J, Brandrup-Wognsen G, Caidahl K, et al. Determinants for an impaired quality of life 10 years after coronary artery bypass surgery. Int J Cardiol. 2005;98:447–52. doi: 10.1016/j.ijcard.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 41.Noyez L, Markou A, van Breugel F. Quality of life one year after myocardial revascularization. Is preoperative quality of life important? Inter Cardiovasc Thorac Surg. 2006;5:115–20. doi: 10.1510/icvts.2005.120113. [DOI] [PubMed] [Google Scholar]
- 42.Rumsfeld JS, Magid DJ, O’Brien M, et al. Changes in health-related quality of life following coronary artery bypass graft surgery. Ann Thorac Surg. 2001 Dec;72(6):2026–2032. doi: 10.1016/s0003-4975(01)03213-1. [DOI] [PubMed] [Google Scholar]


