Abstract
Inflammation-related changes in the concentrations of inflammatory mediators such as c-reactive protein (CRP), interleukin 1 (IL-1), and IL-6 as well as kynurenine metabolites are associated with major depressive disorder (MDD) and affect depressive behavior, cognition, and hippocampal plasticity in animal models. We previously reported that the ratios of kynurenic acid (KynA) to the neurotoxic metabolites, 3-hydroxykynurenine (3HK) and quinolinic acid (QA), were positively correlated with hippocampal volume in depression. The hippocampus is critical for autobiographical memory (AM) recall which is impaired in MDD. Here we tested whether the ratios, KynA/3HK and KynA/QA were associated with AM recall performance as well as hippocampal activity during AM recall. Thirty-five unmedicated depressed participants and 25 healthy controls (HCs) underwent fMRI scanning while recalling emotionally-valenced AMs and provided serum samples for the quantification of kynurenine metabolites, CRP, and cytokines (IL-1 receptor antagonist - IL-1RA; IL-6, tumor necrosis factor alpha - TNF, interferon gamma -IFN- , IL-10). KynA/3HK and KynA/QA were lower in the MDD group relative to the HCs. The concentrations of the CRP and the cytokines did not differ significantly between the HCs and the MDD group. Depressed individuals recalled fewer specific AMs and displayed increased left hippocampal activity during the recall of positive and negative memories. KynA/3HK was inversely associated with left hippocampal activity during specific AM recall in the MDD group. Further, KynA/QA was positively correlated with percent negative specific memories recalled in the MDD group and showed a non-significant trend toward a positive correlation with percent positive specific memories recalled in HCs. In contrast, neither CRP nor the cytokines were significantly associated with AM recall or activity of the hippocampus during AM recall. Conceivably, an imbalance in levels of KynA versus QA-pathway metabolites may adversely impact the function of the hippocampus and AM recall, raising the possibility that kynurenine pathway may affect emotion-dependent memory within the context of depression.
INTRODUCTION
Major depressive disorder (MDD) is the most disabling disorder worldwide as measured by years lived with disability (Whiteford et al., 2013), yet we still do not have a good understanding of its pathophysiology. A negative view of the self is a central feature of depression. Thus not surprisingly a deficit in autobiographical memory (AM) consistently is reported in MDD. Depressed individuals not only tend to recall relatively more negative than positive memories, but also display AM overgenerality (i.e. difficulty recalling specific memories that occurred at identified times and places, coupled with the tendency to recall general memories without episodic details) (Williams et al., 2007). Dysfunction of the AM system potentially links deficits in social problem solving, rumination, social avoidance, and feelings of hopelessness (Arie et al., 2008, Hermans et al., 2005). Moreover, a recent meta-analysis found that AM was significantly less specific and more general in depressed patients with a history of suicide attempt relative to those without such a history (Richard-Devantoy et al., 2014).
We have identified a consistent and robust neurophysiological marker of AM impairment in depression, characterized by an increase in the BOLD response of a hippocampus-centric network during the cued recall of AMs, an effect we interpret to reflect more effortful AM recall in depression (Young et al., 2013b, 2014b, Young et al., 2012). Nevertheless, the biological factors associated with these neurophysiological changes during AM recall remain unclear.
Inflammation constitutes one potential mechanism underpinning cognitive dysfunction in MDD. Meta-analyses have found that on average, the circulating concentrations of pro-inflammatory cytokines such as IL-1, IL-6, and TNF are elevated in people with depression (Black and Miller, 2015, Dowlati et al., 2010, Howren et al., 2009), while several studies have reported depression-associated increases in pro-inflammatory cytokine protein and/or mRNA levels in the cerebrospinal fluid in vivo (CSF) (Levine et al., 1999, Lindqvist et al., 2009) or brain tissues postmortem (Pandey et al., 2012, Rao et al., 2010).
Pro-inflammatory cytokines not only regulate synaptic plasticity and neurogenesis in the hippocampus at low concentrations, but at elevated concentrations compromise neuronal survival and synaptic transmission, causing learning and memory impairments (Yirmiya and Goshen, 2011). IL-1 in particular, plays an important role in learning, memory, and long term potentiation (Yirmiya and Goshen, 2011), and has been associated with hippocampal-dependent memory impairment following infection or intracerebroventricular injection in experimental studies (Barrientos et al., 2009, Gibertini et al., 1995, Oitzl et al., 1993, Palin et al., 2004). Extant evidence indicates that this effect may be mediated in part by altered NMDA receptor signaling and glutamatergic neurotransmission (Yirmiya and Goshen, 2011), such that inflammatory stimuli activate the IL-1 receptor leading to NMDA receptor 2-B phosphorylation and enhanced CA2+ influx (Balosso et al., 2008, Viviani et al., 2003).
Kynurenic acid (KynA) and quinolinic acid (QA) act as endogenous antagonists and agonists of the NMDA receptor, respectively (Schwarcz et al., 2012). A recent study found that IL-1-induced activation of the kynurenine pathway in human hippocampal progenitor cells in vitro led to a 12–30 fold upregulation of the enzymes that produce 3HK and QA (indoleamine 2,3 dioxygenase (IDO), kynurenine 3-monooxygenase (KMO), and kynureninase), and further that treatment with a KMO inhibitor reversed the suppressive effects of IL-1 (Zunszain et al., 2012). Consistent with these data, in mouse models of Alzheimer’s Disease (AD) and Huntington’s Disease (HD) peripheral administration of an inhibitor of KMO was shown to elevate brain levels of KynA, decrease extracellular glutamate, synaptic loss, and spatial memory deficits (Zwilling et al., 2011). Further, (Heisler and O'Connor, 2015) demonstrated that mice deficient in IDO or KMO were protected from endotoxin-induced impairments in novel object recognition, a task that is dependent on normal function of the hippocampus and parahippocampal region (Andre et al., 2008). Interestingly, in healthy humans, low-dose endotoxin has been shown to impair short-term recall (Reichenberg et al., 2001) and inflammatory challenge with the typhoid vaccine was shown to compromise spatial memory, an effect mediated by decreased glucose metabolism in the parahippocampal region (Harrison et al., 2014). Nevertheless, the relationship between inflammation and memory, especially specific forms of memory such as AM, is not yet clear in the context of MDD.
In morphometric MRI studies we previously found reductions in serum KynA/QA in MDD along with positive correlations between KynA/QA and gray matter volumes of the hippocampus in MDD (Savitz et al., 2015b). Further, we reported a positive correlation between KynA/3HK and hippocampal volume in depressed patients with bipolar disorder (Savitz et al., 2015a). The fact that we consistently have found the ratio of KynA to QA-pathway metabolites to be the most reproducible abnormality in mood disorders and the strongest correlate with gray matter volumes, may reflect the competing actions of KynA and 3HK/QA. Specifically, 3HK and QA are produced in the brain by microglia and infiltrating macrophages. While 3HK is a free radical generator, QA acts as an NMDA receptor agonist and additionally plays a role in lipid peroxidation, disruption of the blood-brain barrier, and the initiation of inflammatory responses (Schwarcz et al., 2012, Stone et al., 2013). In contrast, KynA is a pleiotropic molecule that acts inter alia as an NMDA receptor antagonist and an enhancer of nerve growth factor expression (Stone et al., 2013). These data raise the possibility that 3HK and QA may compromise memory function while KynA may exert protective effects on memory in the context of depression. It may therefore be more informative to consider the balance between activation of the KynA and QA branches of the kynurenine pathway rather than the absolute levels of these metabolites.
In sum: 1) Pro-inflammatory cytokines (e.g. IL-1 and IL-6), and neurotoxic kynurenine metabolites have been shown to be associated with hippocampal atrophy and/or memory deficits in animal studies. 2) We previously reported that greater KynA to QA-pathway metabolites in the periphery are associated with larger hippocampal volumes in mood disorders. 3) We have previously shown that AM performance is impaired in patients with MDD and that this deficit is associated with an elevated hemodynamic response in the hippocampus during AM recall. 4) In preclinical work, KMO-dependent neurotoxic kynurenine metabolism has been reported to at least partly mediate endotoxin-induced recognition memory impairment.
Therefore we hypothesized that depressed individuals with higher levels of pro-inflammatory cytokines such as TNF, IL-6, and IL-1RA (a surrogate marker of IL-1) and lower levels of KynA to QA-pathway metabolites would show deficits in AM generation (i.e. decreased percentage of specific memories recalled) along with an increased BOLD response in the hippocampus during cued AM recall.
MATERIALS AND METHODS
Participants
Participants provided written informed consent after receiving a full explanation of the study procedures and risks as approved by the local IRB.
All MDD participants (n=35) and healthy controls (HCs, n=25) were interviewed with the Structured Clinical Interview for the DSM-IV-TR (First et al., 2002), and the Hamilton Depression Rating Scale (HDRS-21 item) (Hamilton, 1960). In addition, unstructured psychiatric interviews with board-certified psychiatrists were conducted with all MDD participants.
MDD participants were male or female outpatients between ages 18 and 55 years who met DSM-IV-TR criteria for primary MDD in a current major depressive episode ((APA), 2000) and had not received any psychotropic medication for at least 3 weeks (8 for fluoxetine) before blood draw and MRI scanning (mean length of time off medication: 67.2 ± 68.5 months, 19 participants were treatment naïve). Exclusion criteria included serious suicidal ideation or behavior; bipolar disorder; medical conditions or concomitant medications likely to influence CNS or immunological function including cardiovascular, respiratory, endocrine, and neurological diseases; a history of drug or alcohol abuse within 6 months or of drug or alcohol dependence within 1 year (DSM-IV-TR criteria), and general MRI exclusions such as paramagnetic implants or claustrophobia. Details of non-psychiatric medications and comorbid medical disorders of the participants are provided in tables S2 and S3, respectively.
Healthy controls (HC) met the same criteria as MDD participants except they had no personal or family history (in first degree relatives) of psychiatric illness according to the Family Interview for Genetic Studies (Maxwell, 1992).
fMRI
Details of the fMRI data acquisition parameters and autobiographical memory task were reported previously (Young et al., 2013a). Briefly, blood-oxygen-level dependent fMRI was performed on a 3T GE Discovery MR750 scanner and eight-channel head coil. Participants were presented with an emotionally valenced or neutral cue word (20 positive, 20 negative, 20 neutral). During fMRI, participants were presented with a cue word for 12s and instructed to recall a past experience. Following the cue, participants rated the retrieved memory on the specificity (specific, categorical, extended, semantic, repeat, no memory) and the valence (negative, somewhat negative, neutral, somewhat positive, positive, no memory). Participants had 10s to assign each rating. Participants were instructed as to the definitions of the standard memory categorizations of specific, categorical, extended, and semantic (Williams et al., 2007), and were able to provide and classify examples of each type before fMRI. A specific memory was defined as a memory for an event that occurred at an identified place and lasted up to one day (e.g. I failed an exam last Tuesday). A categorical memory referred to a category of events containing a number of episodes without reference to one specific event (e.g., all exams failed without reference to one particular exam). An extended memory referred to an extended period of time without reference to a specific event within the time frame (e.g. a week-long vacation). A semantic memory was defined as a statement of fact without an associated event (e.g., ‘I’ve never been dancing’). While participants were told they should try to recall a specific memory, no instruction was given regarding the valence of the recalled memory; therefore the valence of the cue and the valence of the memory did not necessarily match. For analysis, the reported memory valence was used.
AM recall was compared to a semantic example generation condition in order to control for abstract or general knowledge retrieval. Specifically, participants were presented with an emotionally valenced or neutral cue word and instructed to think of seven examples from the category, then rate the ease with which they generated examples and the number generated. Following the presentation of each cue and each set of ratings, participants engaged in a riser detection task as a control for visual input/attention. All example generation/memory cue words were scrambled into lowercase non-word letter strings and participants were instructed to count the number of risers in the string, defined as a letter with a part rising above the tops of the other letters (e.g., “gulmnh” has the risers l and h). The presentation of each letter string was jittered with an average presentation time of 6s. For a randomly selected one-half of these strings, a 2s period followed where participants selected whether the number of risers in the previous string was even or odd.
The order of memory and example cue word presentations was pseudo-randomized with restrictions on order presentation to prevent sequential presentations of a particular valence. Within each of ten runs, participants were presented with six memory cue words, three example generation cue words, and 18 riser letter strings in the order: cue word–riser (1/2 followed by odd/even question) – ratings – riser (1/2 followed by odd/even question).
Analysis of fMRI data was performed using AFNI (http://afni.nimh.nih.gov/afni), and consisted of slice timing correction, within-subject realignment, co-registration between anatomical and functional images, spatial normalization to a common stereotaxic array (Talairach and Tournoux, 1988), and spatial smoothing (Gaussian kernel, 4mm full-width at half-maximum). General linear model analysis was performed using AFNI’s 3dDeconvolve program. For each participant, the hemodynamic response to each event type was modeled as a boxcar function convolved with a canonical hemodynamic response function. The main effects-of-interest were cue word presentations that prompted specific memory recall of any valence, positive-specific memory recall, negative-specific memory recall, example generation in response to cues of any valence, example generation in response to positive cues, and example generation in response to negative cues. In addition to regressors modeling main effects and subject motion, each design matrix included regressors modeling rating selection and cue presentation where other types of memories were recalled (categorical, extended, semantic). The non-word letter strings used as stimuli for the riser detection task were modeled as the baseline, and used to determine whether the group difference in the contrasts of interest (memory recall versus example generation) was being driven by differences during memory recall or during example generation.
We defined regions-of-interest for the left and right hippocampus using Talairach masks within AFNI. For each participant, the 3dDeconvolve output was resampled and the mask applied (AFNI’s 3dROIstats) to calculate the percent signal change within each region-of-interest for each of the following contrasts: Specific Memories versus Example Generation, Specific Positive Memories versus Positive Example Generation, and Specific Negative Memories versus Negative Example Generation. The resulting percent signal changes for each comparator contrast were used for the second-level analyses.
Kynurenine Pathway Metabolites
A blood sample was obtained from each subject within one week prior to completing the MRI scan. Subjects fasted overnight and the blood sample was drawn between 8am and 11am. Serum samples were collected with BD Vacutainer serum tubes, processed according to the standard BD Vacutainer protocol, and stored at −80°C.
All assays were performed blind to diagnosis. Concentrations of tryptophan (TRP), kynurenine (KYN), KynA, 3HK, and QA were measured by Brains Online, LLC (www.brainsonline.org). The serum metabolite concentrations were determined by high-performance liquid chromatography (HPLC) with tandem mass spectrometry (MS/MS) detection using their standard protocols. IL-6, TNF, IL-10, and IFN- were measured with the Mesoscale Discovery (MSD) platform. High-sensitivity C-reactive protein (CRP) was measured in a clinical laboratory using the Kamiya Biomedical K-Assay while IL-1RA was measured with plate-bound ELISA (R&D Systems). The lower limit of quantification and the coefficients of variation for the analytes are provided in table S4, and inter-correlations of the immunological markers in the combined sample are provided in table S1.
Statistical Analysis
Statistical tests were performed using SYSTAT 13 (Systat Software Inc., USA). All statistical tests were two-tailed and non-normally distributed variables were log normalized prior to parametric analyses.
Analysis of Variance with sex, age, and BMI as covariates was used to test for mean differences between the MDD and HC groups in memory performance, hippocampal activity, and serum concentration of immune markers. The criterion for significance for KynA/3HK, KynA/QA, memory performance, and hippocampal activity was set at p<0.05 because we previously had shown group differences for these variables, and here were reproducing these differences in the current sample. For group differences in the individual kynurenine metabolites, cytokines, and CRP we used a Bonferroni correction for multiple comparisons.
To measure the relationship between left and right hippocampal activity and cytokines, CRP, and the kynurenine metabolites, a linear regression analysis was performed with the covariates age, sex, and BMI. The criterion for significance was set at a Bonferroni corrected p-value of <0.002 (26 comparisons). Similarly, to measure the relationship between memory performance (percent of specific, specific positive, and specific negative memories recalled) and the cytokines, CRP, and the kynurenine metabolites, a linear regression analysis was performed with the covariates age, sex, and BMI. The criterion for significance was set at a Bonferroni corrected p-value of <0.004 (13 comparisons). Furthermore, correlations found to be significant in one group were compared between groups via Fisher’s r-to-z transformation to evaluate the significance of the difference between the correlation coefficients.
RESULTS
Table 1 provides the means, standard deviations, and ANOVA results for each group. Regarding the immunological data, KynA/3HK and KynA/QA were reduced in the MDD group relative to the HCs, and this difference remained significant after regressing out sex, age, and BMI. There was no significant difference between groups in the individual kynurenine pathway metabolites (TRP, KYN, KynA, 3HK, QA), cytokines (IL-1RA, IL-6, TNF, IFN- , and IL-10), and CRP. As expected, activation of the kynurenine pathway as indexed by Kyn/Trp was positively correlated with CRP, IL-6, TNF, IL-10, and IFN- . Further, KynA/3HK was negatively correlated with CRP, IL-1RA, IL-6, and TNF while KynA/QA was negatively correlated with TNF (table S1).
Table 1.
Mean demographic, immunological, and autobiographical memory data for the MDD and HC groups (numbers in parenthesis indicate one standard deviation of the mean).
| HC | MDD | |||
|---|---|---|---|---|
| Demographics | 2 | p value | ||
| N (% F) | 25 (60.0) | 35 (62.9) | 0.12 F(1,58) |
0.72 p value |
| Age | 35.1 (8.66) | 37.3 (8.60) | 0.12 | 0.73 |
| HDRS | 0.63 (1.35) | 19.3 (6.38) | 16.8 | <0.001 |
| BMI | 26.5 (6.12) | 27.14 (5.21) | 0.18 | 0.67 |
| Immune Data | F(1,41) | p value | ||
| CRP (ng/ml) | 1.16 (1.94) | 1.03 (1.55) | 0.47 | 0.63 |
| IL-1RA (pg/ml) | 223 (274) | 354 (487) | 1.41 | 0.24 |
| IL-6 (pg/ml) | 0.32 (0.24) | 0.44 (0.35) | 2.18 | 0.15 |
| IL-10 (pg/ml) | 0.16 (0.11) | 0.25 (0.55) | 0.51 | 0.48 |
| TNF (pg/ml) | 0.94 (0.49) | 1.14 (0.76) | 1.34 | 0.25 |
| IFN- (pg/ml) | 1.94 (1.95) | 2.10 (2.35) | 0.97 | 0.33 |
| TRP (µM) | 57.5 (6.39) | 54.3 (12.2) | 1.09 | 0.37 |
| KYN (nM) | 1.89 (0.32) | 1.89 (0.53) | 0.83 | 0.51 |
| KynA (nM) | 45.7 (15.4) | 38.1 (9.87) | 2.01 | 0.11 |
| 3HK (nM) | 31.9 (9.88) | 34.6 (13.0) | 1.06 | 0.39 |
| QA (nM) | 336 (117) | 448 (205) | 1.72 | 0.16 |
| KynA/3HK | 1.48 (0.42) | 1.13 (0.33) | 9.72 | <0.001* |
| KynA/QA | 0.14 (0.05) | 0.09 (0.03) | 6.86 | <0.001* |
| Memory Data | ||||
| Specific Memories (%) | 63.0 (9.87) | 40.0 (14.1) | 15.4 | <0.001* |
| Positive | 60.3 (12.0) | 49.8 (8.52) | 4.31 | 0.01* |
| Negative | 24.0 (7.51) | 32.7 (10.8) | 2.07 | 0.12 |
| Neutral | 15.7 (7.23) | 17.5 (12.2) | 0.57 | 0.73 |
|
Percentage BOLD Signal Change During Memory Recall versus Example Generation | ||||
| L Hippocampus | ||||
| Specific | 0.07 (0.01) | 0.21 (0.03) | 2.67 | 0.04* |
| Specific Positive | 0.07 (0.02) | 0.14 (0.02) | 4.86 | 0.003* |
| Specific Negative | 0.07 (0.01) | 0.18 (0.03) | 3.69 | 0.01* |
| R Hippocampus | ||||
| Specific | 0.18 (0.03) | 0.16 (0.02) | 1.03 | 0.41 |
| Specific Positive | 0.13 (0.02) | 0.09 (0.01) | 1.65 | 0.18 |
| Specific Negative | 0.15 (0.02) | 0.12 (0.03) | 1.22 | 0.32 |
Abbreviations: HC = healthy control; MDD = major depressive disorder; HDRS = 21 item Hamilton Rating Scale for Depression. BMI = body mass index; CRP = C-reactive protein; IL-1RA = interleukin 1 receptor antagonist; IL-6 = interleukin 6; IL-10 = interleukin 10; TNF= tumor necrosis factor alpha; IFN- = interferon gamma; TRP = tryptophan; KYN = kynurenine; KynA = kynurenic Acid; 3HK = 3-hydroxykynurenine; QA = quinolinic acid.
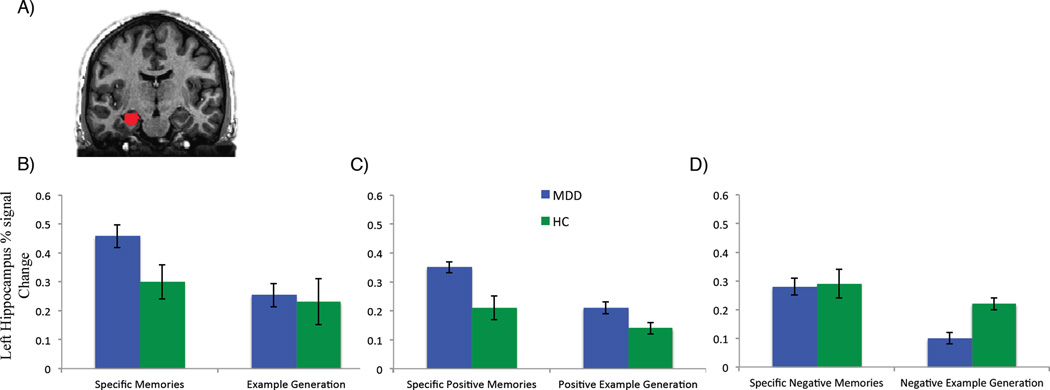
The MDD group recalled fewer specific autobiographical memories overall, as well as fewer specific memories rated as positive in valence (table 1). There was no significant group difference in the proportion of negative or neutral specific memories recalled. With regard to hippocampal activity, the MDD group had increased left hippocampal activity while recalling specific memories regardless of valence, as well as increased left hippocampal activity during the recall of positively and negatively-valenced specific memories (Figure 1). There was no statistically significant group difference in right hippocampal activity during AM recall.
Figure 1.
Left hippocampal activity during autobiographical memory recall relative to visual baseline and example generation relative to visual baseline for each participant group. (A) Coronal anatomical MRI slices showing the left hippocampal mask overlay. (B–D) show the percent signal change in the left hippocampus during (B) Specific autobiographical memory recall versus visual baseline and example generation versus visual baseline (all valences combined). (C) Specific autobiographical memory recall of memories rated positive in valence versus visual baseline and example generation in response to positive cue words versus visual baseline, and (D) Specific autobiographical memory recall of memories rated negative in valence versus visual baseline and example generation in response to negative cue words versus visual baseline.
Error bars indicate +/− one standard error of the mean. HC = healthy control, MDD = major depressive disorder
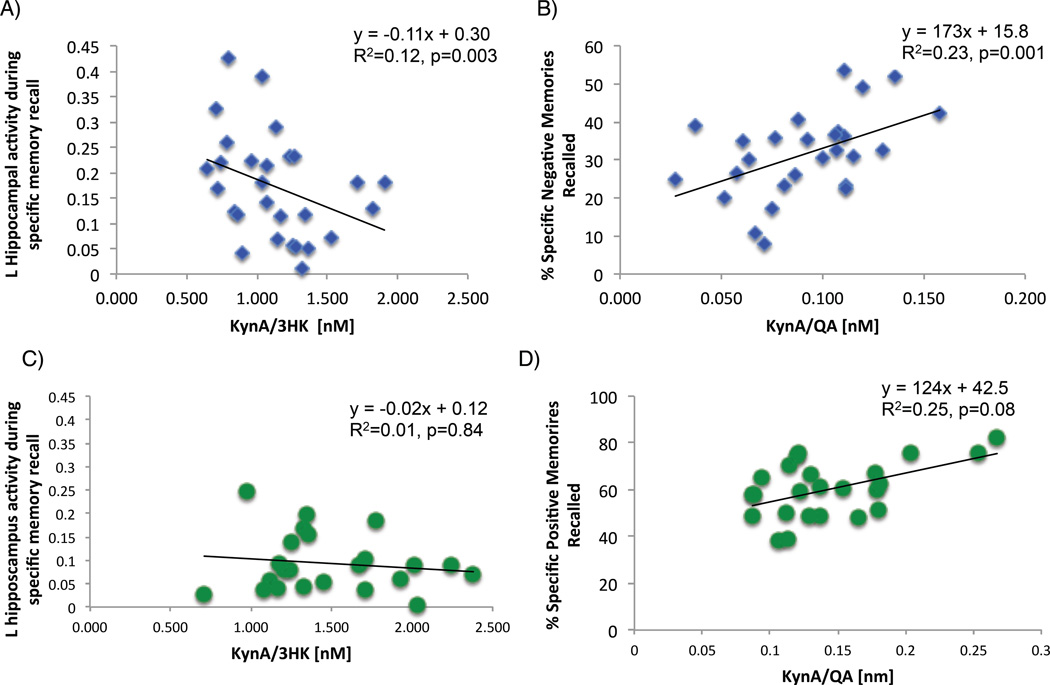
As hypothesized, in the combined MDD and HC sample, KynA/3HK was negatively associated with left hippocampal activity during specific memory recall regardless of valence (R2adjusted=0.09, t=2.86, p=0.002). Crucially this correlation was significant in the MDD group alone (R2adjusted=0.12, t=2.83, p=0.003, Figure 2A) but not the HCs (Figure 2C), and the difference in correlation coefficients between MDD and HC (R2=0.01) participants approached significance (p=0.07). Additionally, in the MDD group there was a significant positive association between KynA/QA and the percent of negative memories recalled (R2adjusted=0.23, t=2.99, p=0.001; Figure 2B), which significantly differed from the correlation between KynA/QA and the percent of negative memories recalled in the HC group (p=0.001). Further, there was a trend in the HCs towards a positive correlation between KynA/QA and the percent of positive specific memories recalled (R2adjusted=0.25, t=1.81, p=0.084; Figure 2D), which was significantly different from the correlation observed in the MDD group (R2=0.10; difference p=0.02). No other correlations approached significance in the HC group (R2adjusted <0.05, ts<0.72, ps>0.48). Further, there were no significant correlations between AM recall performance or hippocampal activity during AM recall and IL-1RA, IL-6, IL-10, TNF, INFγ, or CRP concentrations in either the MDD or HC groups (R2adjusted <0.02, ts<1.67, ps>0.11), nor was there a significant correlation between any immune marker and hippocampal activity during example generation relative to visual baseline (R2adjusted <0.07, ts<1.98, ps>0.06).
Figure 2.
Scatterplots showing the correlation between (A) KynA/3HK and activity of the hippocampus during specific autobiographical memory recall in the MDD group, (B) KynA/QA and the percent of specific autobiographical memories rated as negative in valence in the MDD group, (C) Kyn/3HK and activity of the hippocampus during specific autobiographical memory recall in the healthy control group, and (D) KynA/QA and the percent of specific autobiographical memories rated as positive in valence in the HC group.
DISCUSSION
The main finding of this study is that within the MDD group, a higher value of the putatively neuroprotective KynA/3HK ratio was associated with less left hippocampal activity during specific AM recall (regardless of the valence of the recalled memory). This finding appears consistent with the hypothesis that greater activation during AM recall is required to compensate for less efficient hippocampal function in order to maintain function near the normative level. This type of compensatory effect has previously been described in the literature. For instance, adults at risk for Alzheimer’s disease (APOE 4 carriers) show increased cerebral blood flow in the medial temporal lobes compared to non 4 carriers, a result that has been interpreted to reflect a compensatory response to an increased demand for glucose and oxygen to support neuronal activity in preclinical disease (Bangen et al., 2012, Fleisher et al., 2009). Additionally, the KynA/QA index, which also is putatively neuroprotective, was associated with significantly greater memory recall for negative AMs in the MDD group, as well as a nonsignificant trend toward greater recall of positive AMs in the HC group. These findings remained significant after correction for multiple comparisons (applying this correction was conservative, since the hypothesis concerning the relationship between AM and KynA/3HK or KynA/QA was made a priori). The results extend our previously reported structural MRI findings in the hippocampus and raise the possibility that the relative balance between activation of the KynA pathway versus the QA pathway also may influence hippocampal function (i.e., the ability to retrieve emotionally-valenced AMs and the neurophysiological activity concurrent with this process).
Increased hippocampal activity previously has been reported in depressed and remitted depressed groups relative to HCs during specific AM recall (Young et al., 2014a) and is hypothesized to reflect the increased effort that MDD individuals require to overcome the volumetric reductions in order to successful retrieve a specific memory (Videbech and Ravnkilde, 2004). The negative association between KynA/3HK and BOLD response in the left hippocampus thus appears consistent with the hypothesis that higher levels of neurotoxic, QA-pathway metabolites may lead to, or be associated with, a dendritic atrophy process in the hippocampus that impairs AM recall.
The observation that these findings were limited to the left hippocampus is intriguing given the results of preclinical studies showing that NMDA receptors containing NR2B subunits are more densely expressed in left than in right hippocampus (Kawakami et al., 2003, Shinohara et al., 2008), and that NMDA-mediated excitotoxicity is dependent on NMDA receptors containing NR2B subunits (Liu et al., 2007). Conceivably, the left hippocampus may be more susceptible to glutamate-induced neuroplasticity. Moreover, studies using PET to assess cerebral glucose metabolism, which predominantly reflects glutamatergic transmission, found that limbic-thalamo-cortical activity is elevated particularly in the left hemisphere in depressed subjects with MDD (Price and Drevets, 2010).
The positive association between KynA/QA and the percent of specific negative memories recalled in the MDD group also is consistent with our hypothesis that QA-pathway metabolites may influence AM recall. As MDD participants show a bias towards recalling negative information (Mathews and MacLeod, 2005), it appears the KynA/QA ratio facilitates the learned memory bias, such that greater KynA/QA is associated with improved hippocampal function and AM recall, irrespective of the emotional valence of the memory. For instance, in the HCs, a non-significant trend was evident towards a positive correlation between the KynA/QA ratio and the percent of specific positive memories recalled. The lack of significant correlation between KynA/QA and memory recall in the HC group is not surprising as the variance in both the kynurenine metabolite concentrations and the percentage of specific memories recalled was greatly reduced relative to the MDD group.
It is noteworthy that the effect of KynA and QA-pathway metabolites on AM recall may be context dependent, and that the relationships reported here may be limited to MDD because depressed patients display reductions in KynA and elevations in glutamate transmission in limbic-thalamo-cortical circuits (Dantzer et al., 2008, Sanacora et al., 2012). For instance, in non-depressed rats, intra-cerebroventricular infusion of 10µl KynA reduced the extracellular glutamate levels in hippocampus, leading to an impairment in spatial memory (Pocivavsek et al., 2011), while deletion of kynurenine aminotransferase II (KAT II), a major biosynthetic enzyme of brain KYNA, led to reduced hippocampal KynA, increased extracellular glutamate, and enhanced cognitive function (Potter et al., 2010). These contrasting results raise the possibility that an imbalance in kynurenine pathway metabolism in either direction may adversely affect memory performance. However, it also is important to emphasize that the association between kynurenine metabolites and memory performance observed here, is limited to emotion-dependent memory rather than “cold” (emotion-independent) cognition since differences in hippocampal activity were driven by the emotional memory component and not by the cognitive component of the AM task (general knowledge retrieval).
The absence of group differences in CRP and cytokines is surprising given the well established relationship between inflammation and depression. Meta-analyses of CRP and cytokine concentrations in MDD generally are indicative of small (d=0.15–0.25) (Howren et al., 2009) to moderate effect sizes (d=0.3–0.4) (Goldsmith et al., 2016, Hiles et al., 2012) although there is substantial variation from one cytokine to another, and there also is evidence of publication bias in favor of positive studies (Hiles et al., 2012). Thus is it possible that our study was underpowered to detect group differences in CRP and cytokine concentrations (or associations between these inflammatory mediators and hippocampal function). Secondly, it is conceivable that our relatively strict exclusion criteria resulted in the recruitment of MDD participants who did not manifest symptom complexes or co-morbidities that have been associated with chronic inflammation in the literature. For example, the MDD and HC were well matched for BMI, a major contributor to peripheral inflammation.
Several additional limitations of this study merit comment. First, a cross-sectional design was used to investigate the relationship between cytokines, kynurenine pathway metabolites and AM. The data reported thus are correlative in nature and do not provide information concerning causality. Additionally, the cross-sectional design introduced the potential confound that unmeasured differences in the experience of recall between groups and even between subjects may have influenced the results. Longitudinal designs following individuals as they transition from prodromal or high-risk, asymptomatic phases into a depressive episode are warranted to confirm our findings. Secondly, it would have been advantageous to include additional measures of cognition because our results did not clarify whether memory function per se, as opposed to memory for emotionally valenced autobiographical information, was enhanced in depressed individuals with increased KynA/3HK and/or KynA/QA. However, the condition to which autobiographical memory recall was compared during fMRI was a task in which participants were instructed to generate examples of categories, and the group differences in hippocampal function observed during specific memory recall were driven by differences in autobiographical memory recall, as activity during example generation did not differ between groups (Figure 1B). This observation thus provides assurance that our results are specific to the autobiographical memory system and not simply to general memory retrieval. Thirdly, we did not require a threshold HDRS score in order to meet inclusion criteria. Therefore, we have substantial variability in the severity of depression across participants. While this allowed us to have more variance in the measures of interest, which is important for detecting significant correlations and may be one reason there were no significant correlations in the HC group, not mandating a severity threshold may have accounted for the failure to obtain significant between group differences in the blood-based biomarkers. Furthermore, the large variance observed in the MDD group in the concentrations of inflammatory biomarkers and kynurenine metabolites also may have reduced our power to detect significant differences between groups on these measures. Nonetheless, the larger variance in the concentrations of inflammatory biomarkers is consistent with the literature indicating that only a subset of people with MDD have an immune-related etiology (Raison and Miller, 2013). Fourthly, for ethical reasons we could not include participants demonstrating suicidal behavior or severe suicidal ideation, a phenotype that has been strongly linked to increases in QA (Bay-Richter et al., 2015, Erhardt et al., 2013). Fifthly, for a minority of subjects, there was up to a one week delay between blood sampling and scanning. Our expectation is that the concentration of kynurenine metabolites will be relatively consistent over time, such that the ratios of KynA to QA-pathway metabolites obtained one week before testing are likely to be similar to that obtained at the time of testing. For instance, we previously reported that the ratio of KynA to QA was decreased in both current depressed and remitted MDD patients (Savitz et al., 2015c). Further, Brundin and colleagues found reductions in KynA, and increases in QA in the CSF of suicide attempters that were maintained for at least 18 months post suicide attempt (Bay-Richter et al., 2015). The stability of CRP and cytokine measurements over time has been studied. Over the course of 6 weeks, intraclass correlations (ICC) of 0.92 (TNF), 0.62 (CRP), and 0.48 (IL-6) were obtained in healthy controls (Navarro et al., 2012). Thus with possible exception of IL-6, we do not think that the time interval between blood sampling and imaging would have significantly affected the results obtained herein, although data are not available for IFN , IL-1RA, and IL-10.
Finally, we measured cytokines and kynurenine metabolites in serum rather than in brain, and our data thus may not reflect the central kynurenine metabolite concentrations. Nonetheless, multiple sources of evidence suggest that peripheral and central measures of kynurenine metabolites are significantly correlated. Firstly, KYN and 3HK are able to cross the blood–brain barrier (Fukui et al., 1991), potentially explaining why increased production of KynA from kynurenine in the periphery through exercise training appears to be neuroprotective (i.e. in the context of inflammation, less kynurenine can enter into the brain to be transformed into QA) (Agudelo et al., 2014). Secondly, the reductions in Kyn/3HK and Kyn/QA in the MDD group relative to the HC group are consistent with the results of (Bay-Richter et al., 2015), who found persistent decreases in KynA and increases in QA in the CSF of predominantly depressed subjects up to 2 years after a suicide attempt. Thirdly, preclinical studies demonstrated a major role for peripheral kynurenine metabolites in neurotoxicity and depressive behavior. For instance, (Zwilling et al., 2011) reported that in mouse models of AD and HD the peripheral administration of an experimental drug that inhibits KMO resulted in elevated brain KynA levels together with decreased glutamate release and excitotoxicity in the brain parenchyma, despite the fact that neither the prodrug nor its metabolite appeared to cross the blood brain barrier.
In conclusion, our results raise the possibility that dysregulation of the kynurenine pathway contributes to the functional neurophysiological abnormalities and memory biases observed in some patients with MDD.
Supplementary Material
Highlights.
MDD patients recalled fewer specific autobiographical memories than healthy controls
MDD subjects displayed increased left hippocampal activity during memory recall
KynA/3HK was inversely correlated with hippocampal activity during memory recall in MDD
KynA/QA was correlated with % negative specific memories recalled in the MDD group
Acknowledgments
This study was funded by a grant from the National Institute of Mental Health to JS (K01MH096077). This research was also supported by the Laureate Institute for Brain Research and The William K. Warren Foundation. The funders had no influence on the design or conduct of the study, collection, management, analyses, or interpretation of the data, or in the preparation, review or approval of the manuscript.
The authors also thank all the research participants and wish to acknowledge the contributions of Brenda Davis, Debbie Neal, Chibing Tan, and Ashlee Taylor from the laboratory of TKT at the University of Oklahoma Integrative Immunology Center towards the transport, processing and handling of all blood samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
WCD is currently an employee of Janssen Pharmaceuticals, LLC, of Johnson & Johnson, Inc. The other authors have no financial conflicts of interest or disclosures to declare.
REFERENCES
- (APA), A. P. A. Diagnostic and Statistical Manual of Mental Disorders. Fourth. Washington, DC: American Psychiatric Association; 2000. Text Revision. [Google Scholar]
- Agudelo LZ, Femenia T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, Correia JC, Izadi M, Bhat M, Schuppe-Koistinen I, Pettersson AT, Ferreira DM, Krook A, Barres R, Zierath JR, Erhardt S, Lindskog M, Ruas JL. Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014;159:33–45. doi: 10.1016/j.cell.2014.07.051. [DOI] [PubMed] [Google Scholar]
- Andre C, O'Connor JC, Kelley KW, Lestage J, Dantzer R, Castanon N. Spatio-temporal differences in the profile of murine brain expression of proinflammatory cytokines and indoleamine 2,3-dioxygenase in response to peripheral lipopolysaccharide administration. J Neuroimmunol. 2008;200:90–99. doi: 10.1016/j.jneuroim.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arie M, Apter A, Orbach I, Yefet Y, Zalsman G. Autobiographical memory, interpersonal problem solving, and suicidal behavior in adolescent inpatients. Compr Psychiatry. 2008;49:22–29. doi: 10.1016/j.comppsych.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Balosso S, Maroso M, Sanchez-Alavez M, Ravizza T, Frasca A, Bartfai T, Vezzani A. A novel non-transcriptional pathway mediates the proconvulsive effects of interleukin-1beta. Brain. 2008;131:3256–3265. doi: 10.1093/brain/awn271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangen KJ, Restom K, Liu TT, Wierenga CE, Jak AJ, Salmon DP, Bondi MW. Assessment of Alzheimer's disease risk with functional magnetic resonance imaging: an arterial spin labeling study. J Alzheimers Dis. 2012;31(Suppl 3):S59–S74. doi: 10.3233/JAD-2012-120292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Hein AM, Higgins EA, Watkins LR, Rudy JW, Maier SF. Time course of hippocampal IL-1 beta and memory consolidation impairments in aging rats following peripheral infection. Brain, behavior, and immunity. 2009;23:46–54. doi: 10.1016/j.bbi.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bay-Richter C, Linderholm KR, Lim CK, Samuelsson M, Traskman-Bendz L, Guillemin GJ, Erhardt S, Brundin L. A role for inflammatory metabolites as modulators of the glutamate N-methyl-d-aspartate receptor in depression and suicidality. Brain Behav Immun. 2015;43:110–117. doi: 10.1016/j.bbi.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Black C, Miller BJ. Meta-Analysis of Cytokines and Chemokines in Suicidality: Distinguishing Suicidal Versus Nonsuicidal Patients. Biol Psychiatry. 2015;78:28–37. doi: 10.1016/j.biopsych.2014.10.014. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctot KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Lim CK, Linderholm KR, Janelidze S, Lindqvist D, Samuelsson M, Lundberg K, Postolache TT, Traskman-Bendz L, Guillemin GJ, Brundin L. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology. 2013;38:743–752. doi: 10.1038/npp.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. Patient Edition (SCID-I/P) New York, NY: New York State Psychiatric Institute, Biometrics Research; 2002. [Google Scholar]
- Fleisher AS, Podraza KM, Bangen KJ, Taylor C, Sherzai A, Sidhar K, Liu TT, Dale AM, Buxton RB. Cerebral perfusion and oxygenation differences in Alzheimer's disease risk. Neurobiol Aging. 2009;30:1737–1748. doi: 10.1016/j.neurobiolaging.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- Gibertini M, Newton C, Friedman H, Klein TW. Spatial learning impairment in mice infected with Legionella pneumophila or administered exogenous interleukin-1-beta. Brain Behav Immun. 1995;9:113–128. doi: 10.1006/brbi.1995.1012. [DOI] [PubMed] [Google Scholar]
- Goldsmith DR, Rapaport MH, Miller BJ. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. 2016 doi: 10.1038/mp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Doeller CF, Voon V, Burgess N, Critchley HD. Peripheral inflammation acutely impairs human spatial memory via actions on medial temporal lobe glucose metabolism. Biol Psychiatry. 2014;76:585–593. doi: 10.1016/j.biopsych.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler JM, O'Connor JC. Indoleamine 2,3-dioxygenase-dependent neurotoxic kynurenine metabolism mediates inflammation-induced deficit in recognition memory. Brain Behav Immun. 2015 doi: 10.1016/j.bbi.2015.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D, Defranc A, Raes F, Williams JM, Eelen P. Reduced autobiographical memory specificity as an avoidant coping style. Br J Clin Psychol. 2005;44:583–589. doi: 10.1348/014466505X53461. [DOI] [PubMed] [Google Scholar]
- Hiles SA, Baker AL, de Malmanche T, Attia J. A meta-analysis of differences in IL-6 and IL-10 between people with and without depression: exploring the causes of heterogeneity. Brain, behavior, and immunity. 2012;26:1180–1188. doi: 10.1016/j.bbi.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- Kawakami R, Shinohara Y, Kato Y, Sugiyama H, Shigemoto R, Ito I. Asymmetrical allocation of NMDA receptor epsilon2 subunits in hippocampal circuitry. Science. 2003;300:990–994. doi: 10.1126/science.1082609. [DOI] [PubMed] [Google Scholar]
- Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40:171–176. doi: 10.1159/000026615. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, Hansson O, Bjorkqvist M, Traskman-Bendz L, Brundin L. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66:287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wong TP, Aarts M, Rooyakkers A, Liu L, Lai TW, Wu DC, Lu J, Tymianski M, Craig AM, Wang YT. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J Neurosci. 2007;27:2846–2857. doi: 10.1523/JNEUROSCI.0116-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Maxwell E. Family Interview for Genetic Studies (FIGS): A Manual for FIGS. Clinical Neurogenetics Branch. Bethesda, MD: Intramural Research Program, National Institute of Mental Health; 1992. [Google Scholar]
- Navarro SL, Brasky TM, Schwarz Y, Song X, Wang CY, Kristal AR, Kratz M, White E, Lampe JW. Reliability of serum biomarkers of inflammation from repeated measures in healthy individuals. Cancer Epidemiol Biomarkers Prev. 2012;21:1167–1170. doi: 10.1158/1055-9965.EPI-12-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl MS, van Oers H, Schobitz B, de Kloet ER. Interleukin-1 beta, but not interleukin-6, impairs spatial navigation learning. Brain Res. 1993;613:160–163. doi: 10.1016/0006-8993(93)90468-3. [DOI] [PubMed] [Google Scholar]
- Palin K, Bluthe RM, Verrier D, Tridon V, Dantzer R, Lestage J. Interleukin-1beta mediates the memory impairment associated with a delayed type hypersensitivity response to bacillus Calmette-Guerin in the rat hippocampus. Brain Behav Immun. 2004;18:223–230. doi: 10.1016/j.bbi.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Rizavi HS, Ren X, Fareed J, Hoppensteadt DA, Roberts RC, Conley RR, Dwivedi Y. Proinflammatory cytokines in the prefrontal cortex of teenage suicide victims. J Psychiatr Res. 2012;46:57–63. doi: 10.1016/j.jpsychires.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocivavsek A, Wu HQ, Potter MC, Elmer GI, Pellicciari R, Schwarcz R. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology. 2011;36:2357–2367. doi: 10.1038/npp.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter MC, Elmer GI, Bergeron R, Albuquerque EX, Guidetti P, Wu HQ, Schwarcz R. Reduction of endogenous kynurenic acid formation enhances extracellular glutamate, hippocampal plasticity, and cognitive behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:1734–1742. doi: 10.1038/npp.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. Do cytokines really sing the blues? Cerebrum : the Dana forum on brain science. 2013;2013:10. [PMC free article] [PubMed] [Google Scholar]
- Rao JS, Harry GJ, Rapoport SI, Kim HW. Increased excitotoxicity and neuroinflammatory markers in postmortem frontal cortex from bipolar disorder patients. Mol Psychiatry. 2010;15:384–392. doi: 10.1038/mp.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Richard-Devantoy S, Berlim MT, Jollant F. Suicidal behaviour and memory: A systematic review and meta-analysis. World J Biol Psychiatry. 2014:1–23. doi: 10.3109/15622975.2014.925584. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Dantzer R, Wurfel BE, Victor TA, Ford BN, Bodurka J, Bellgowan PS, Teague TK, Drevets WC. Neuroprotective kynurenine metabolite indices are abnormally reduced and positively associated with hippocampal and amygdalar volume in bipolar disorder. Psychoneuroendocrinology. 2015a;52:200–211. doi: 10.1016/j.psyneuen.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowan PS, Bodurka J, Teague TK, Dantzer R. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology. 2015b;40:463–471. doi: 10.1038/npp.2014.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Wurfel BE, Ford BN, Bellgowan PS, Victor TA, Bodurka J, Teague TK, Dantzer R. Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain Behav Immun. 2015c;46:55–59. doi: 10.1016/j.bbi.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nature reviews. Neuroscience. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara Y, Hirase H, Watanabe M, Itakura M, Takahashi M, Shigemoto R. Left-right asymmetry of the hippocampal synapses with differential subunit allocation of glutamate receptors. Proc Natl Acad Sci U S A. 2008;105:19498–19503. doi: 10.1073/pnas.0807461105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone TW, Stoy N, Darlington LG. An expanding range of targets for kynurenine metabolites of tryptophan. Trends in pharmacological sciences. 2013;34:136–143. doi: 10.1016/j.tips.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System - an Approach to Cerebral Imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. The American journal of psychiatry. 2004;161:1957–1966. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Viviani B, Bartesaghi S, Gardoni F, Vezzani A, Behrens MM, Bartfai T, Binaglia M, Corsini E, Di Luca M, Galli CL, Marinovich M. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J Neurosci. 2003;23:8692–8700. doi: 10.1523/JNEUROSCI.23-25-08692.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet. 2013;382:1575–1586. doi: 10.1016/S0140-6736(13)61611-6. [DOI] [PubMed] [Google Scholar]
- Williams JM, Barnhofer T, Crane C, Herman D, Raes F, Watkins E, Dalgleish T. Autobiographical memory specificity and emotional disorder. Psychological bulletin. 2007;133:122–148. doi: 10.1037/0033-2909.133.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, behavior, and immunity. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Young K, Bellgowan P, Bodurka J, Drevets W. Neurophysiological correlates of autobiographical memory deficits in currently and formerly depressed subjects Psychological medicine. 2014a;44:2951–2963. doi: 10.1017/S0033291714000464. [DOI] [PubMed] [Google Scholar]
- Young K, Bellgowan P, Bodurka J, Drevets WC. Behavioral and Neurophysiological Correlates of Autobiographical Memory Deficits in Patients With Depression and Individuals at High Risk for Depression. Journal of the American Medical Association psychiatry. 2013a;70:698–708. doi: 10.1001/jamapsychiatry.2013.1189. [DOI] [PubMed] [Google Scholar]
- Young KD, Bellgowan PS, Bodurka J, Drevets WC. Behavioral and neurophysiological correlates of autobiographical memory deficits in patients with depression and individuals at high risk for depression. JAMA Psychiatry. 2013b;70:698–708. doi: 10.1001/jamapsychiatry.2013.1189. [DOI] [PubMed] [Google Scholar]
- Young KD, Bellgowan PS, Bodurka J, Drevets WC. Neurophysiological correlates of autobiographical memory deficits in currently and formerly depressed subjects. Psychol Med. 2014b;44:2951–2963. doi: 10.1017/S0033291714000464. [DOI] [PubMed] [Google Scholar]
- Young KD, Erickson K, Nugent AC, Fromm SJ, Mallinger AG, Furey ML, Drevets WC. Functional anatomy of autobiographical memory recall deficits in depression. Psychol Med. 2012;42:345–357. doi: 10.1017/S0033291711001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM, Thuret S, Price J, Pariante CM. Interleukin-1beta: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37:939–949. doi: 10.1038/npp.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwilling D, Huang SY, Sathyasaikumar KV, Notarangelo FM, Guidetti P, Wu HQ, Lee J, Truong J, Andrews-Zwilling Y, Hsieh EW, Louie JY, Wu T, Scearce-Levie K, Patrick C, Adame A, Giorgini F, Moussaoui S, Laue G, Rassoulpour A, Flik G, Huang Y, Muchowski JM, Masliah E, Schwarcz R, Muchowski PJ. Kynurenine 3-monooxygenase inhibition in blood ameliorates neurodegeneration. Cell. 2011;145:863–874. doi: 10.1016/j.cell.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.