Abstract
Background
Heart failure (HF) guidelines recommend brain natriuretic peptide (BNP) and multivariable risk-scores such as the Seattle HF Model (SHFM) to predict risk in HF with reduced ejection fraction (HFrEF). A practical way to integrate information from these two prognostic tools is lacking. We sought to establish a SHFM+BNP risk-stratification algorithm.
Methods
The retrospective derivation cohort included consecutive patients with HFrEF at Mayo. One-year outcome (death, transplantation or ventricular assist device) was assessed. The SHFM+BNP algorithm was derived by stratifying patients within SHFM-predicted risk categories (≤2.5%, 2.6–≤10%, >10%) according to BNP above or below 700 pg/mL and comparing SHFM-predicted and observed event rates within each SHFM+BNP category. The algorithm was validated in a prospective, multicenter HFrEF registry (Penn HF Study).
Results
Derivation (n=441; one-year event rate 17%) and validation (n=1513; one-year event rate 12%) cohorts differed with the former being older and more likely ischemic with worse symptoms, lower EF, worse renal function, higher BNP and SHFM scores. In both cohorts, across the three SHFM-predicted risk strata, a BNP>700 pg/ml consistently identified patients with approximately three-fold the risk that the SHFM would have otherwise estimated regardless stage of HF, intensity and duration of HF-therapy, and comorbidities. Conversely, the SHFM was appropriately calibrated in patients with a BNP<700 pg/ml.
Conclusion
The simple SHFM+BNP algorithm displays stable performance across diverse HFrEF cohorts and may enhance risk stratification to enable appropriate decisions regarding HF therapeutic or palliative strategies.
Introduction
Accurate risk stratification in heart failure (HF) patients is needed to facilitate informed decisions regarding medications, defibrillators, transplantation, ventricular assist devices (LVAD), experimental therapies and palliative or end-of-life care1. Clinical characteristics, biomarkers, exercise performance and imaging parameters have all been utilized to assess risk2. Recent HF guidelines recommend use of natriuretic peptides assays and multivariable clinical risk-scores to quantify risk1.
The Seattle HF Model (SHFM)3 is a risk-score that integrates clinical, pharmacological, device and laboratory characteristics, offering a comprehensive profile of the HF patient. It has been reported to predict outcomes in clinical trial and registry cohorts4–14. However, it does not capture pertinent prognostic factors such as medication doses, delivered ICD therapies and HF hospitalizations. Elevated B-type natriuretic peptide (BNP) levels predict HF events and mortality in a wide variety of HF- cohorts15 and may detect such facets of the HF clinical profile. However, despite decreasing costs of the BNP assay, its increased availability and widespread use16, for an individual patient, the predictive implications of the BNP level is difficult to ascertain15. BNP varies with age, sex, body size and renal function and declines with appropriate therapy. Hence, while elevated BNP values connote increased risk, interpretation of BNP levels must integrate information regarding patient characteristics, intensity of therapy and HF-stage. Notably, while guidelines recommend the use of BNP for risk stratification, they do not stipulate a specific BNP level above which its calibration is robust enough to identify high-risk patients that may benefit the most from particular management strategies.
Integrating BNP levels with the highly patient-specific characterization provided by the SHFM has great complementary potential. When added to the SHFM, BNP confers statistically significant improvement in discrimination (c-index) or reclassification as quantitated by net (NRI) or integrated (IDI) reclassification indices17–19. However, methods to translate these statistical indices to quantification of risk provided by the SHFM combined with BNP levels in clinical practice are lacking.
We sought to derive and validate a simple, clinically useful risk-assessment algorithm that incorporates both the SHFM and a specific BNP cutoff in patients with HF and reduced ejection fraction (HFrEF) seen across a spectrum of care environments and providers, at different stages in the natural history of HF, with variable intensity and duration of HF-therapy and with varying comorbidity burden.
Methods
Study Population
Derivation Cohort
We identified a retrospectively compiled cohort of consecutive HF patients seen at Mayo Outpatient Clinics and associated hospitals in Rochester, MN from July 1st, 2007 through December 31st, 2007, a time-frame intentionally chosen to pre-date the era of widespread LVAD referrals. Using a modification of a previously-described natural language processing program20, all electronic clinical notes were searched for non-negated terms (Supplemental Table 1) consistent with HF. Patients with ejection fraction (EF) ≤ 35% documented within two years were included in the study and underwent detailed medical record review. The date of the most recent echocardiogram was considered the fiducial point for assessment of risk-scores, comorbidities and outcomes. New York Heart Association functional class (NYHA) was verified by record review. Comorbidities, medications, electrocardiogram, echocardiogram and laboratory findings were extracted from the electronic medical record. Only patients with BNP levels on or within 30 days of the date of the echocardiogram were included in our cohort. The Triage® BNP assay, as previously reported21, 22, was utilized. The ascertainment of death (query date 3/1/2009) was determined from the Mayo registration database and included several procedures, as previously described23. Accurint®, an institutionally approved Web-based resource and location service that includes data from the Social Security Death Index was queried. Also, in addition to the deaths noted during clinical care, all death certificates for Olmsted County residents are obtained every year from the county office. Further, the Mayo Clinic registration office records the obituaries and notices of deaths in the local newspapers. Finally, data on all Minnesota deaths are obtained from the State of Minnesota every year. Heart transplantation or LVAD implantation was assessed by chart review and by cross-match with the surgical transplant and LVAD database of all heart transplantation or LVAD implantations at Mayo. One-year survival free from death, transplantation or LVAD implantation for all patients was ascertained. Only records of patients with consent for medical record use for research purposes were included. This study was approved by the Mayo institutional review board (IRB).
Validation Cohort
The Penn HF Study is a National Heart, Lung and Blood Institute sponsored, prospective, multicenter registry of outpatients with chronic HF recruited from the University of Pennsylvania (Philadelphia, PA), Case Western University (Cleveland, OH), and the University of Wisconsin (Madison, WI)18, 24, 25. The primary inclusion criterion was a clinical diagnosis of HF as determined by a HF-specialist. Participants were excluded if they have a non-cardiac condition resulting in an expected mortality of less than six months as judged by the treating physician, or if they were unable to provide consent. At time of study entry, clinical data were obtained using standardized questionnaires administered to the patient and physician, with verification through medical records. Blood samples were obtained at enrollment and BNP was measured using standard ARCHITECT immunoassays (Abbott Laboratories, Abbott Park, IL) as previously described26. Echocardiography was performed within 30 days of blood sampling. Follow-up events including all-cause mortality and cardiac transplantation or LVAD implantation were prospectively ascertained every six months through patient contact and verified through death certificates, medical records, or contact with patients families by research personnel. All participants provided written informed consent, and the study protocol was approved by IRBs.
SHFM
The SHFM is a multivariable risk-prediction score based on clinical, pharmacological, device and laboratory characteristics (Supplemental table 2). It has been validated in multiple HF populations as a predictor of mortality, cardiac transplantation or LVAD placement3. The version of the score used in this study was the SHFM-D, abbreviated as SHFM. The derivation and validation of the SHFM has been previously described4. Missing variables were quantified (Supplemental Table 2). For calculation of the SHFM, imputation of the mean value was performed.
Derivation of the SHFM+BNP Algorithm
We derived a SHFM+BNP algorithm in the derivation cohort by stratifying patients within pre-specified categories of clinically relevant one-year risk [low (≤2.5%), intermediate (2.6–≤10%), and high (>10%)] per SHFM-predicted probabilities, calculated using the published SHFM score for one-year survival free of cardiac transplantation or LVAD placement. Next we stratified patients within each risk category according to BNP levels above or below the optimal partition-value based on ROC-analysis in the entire derivation cohort.
Statistical Analysis
Baseline characteristics were summarized using standard descriptive statistics. Due to the skewed distribution of BNP, analyses were performed after log transformation. ROC curves were used to measure the ability of the SHFM and BNP (log-transformed, continuous variable) to discriminate those patients who died or required cardiac transplantation or LVAD implantation within one year. Using the derivation cohort ROC-analysis, we identified the optimal BNP value that maximized sensitivity and specificity and used it as the partition-value for the SHFM+BNP algorithm. We then estimated its corresponding sensitivity and specificity within the derivation and validation cohorts. Next, we compared the observed to the mean predicted risk within BNP subgroups of the SHFM-predicted risk categories within the derivation and validation cohorts.
We determined whether the prognostic potential associated with BNP varied across values of the SHFM by examining the statistical significance of the interaction term (SHFM by BNP partition value) in Cox-regression models. A statistically significant interaction term would suggest that the prognostic potential of BNP depended on the SHFM. For these analyses, we used a composite time-to-event outcome of time to death, cardiac transplantation or LVAD implantation. Patients who did not experience an outcome prior to one year were censored at one year. Sensitivity analyses examined the SHFM+BNP algorithm for the endpoint of one-year mortality alone, the use of optimal partition-value based on ROC analysis in the validation cohort, and use of N -terminal pro-B-type natriuretic peptide (NT-proBNP), which was available in a subset of the validation cohort.
In secondary analyses, we assessed whether BNP (as a log-transformed, continuous variable) provided incremental predictive information to the SHFM by comparing the c-index for scores without and with BNP. To assess discrimination, we derived Harrell’s concordance index [c-index (95% confidence interval), ROC curve equivalent for right-censored data27] and compared as previously described28. Risk reclassification (net reclassification improvement; NRI) and discrimination (integrated discrimination improvement; IDI) analyses29, 30 were performed as per recent recommendations31.
A p-value < 0.05 was considered statistically significant. Statistical analyses were performed using JMP version-7.0.1 (SAS Institute), MedCalc version-12.3.0 and R 3.0.1 (R Development Core Team, Vienna, Austria).
Results
Baseline Characteristics and outcomes
In the derivation cohort, of the 441 consecutive patients identified; 268 (61%) were community patients (residing within 100 miles of center) and 308 (70%), 101 (23%), and 32 (7%) were seen in cardiovascular, non-cardiovascular subspecialty and primary care settings, respectively. In the validation cohort, 1513 patients were included. Baseline characteristics from each cohort are shown (Table 1). Missing data was rare with one patient having no creatinine level, 12 having no NYHA classification and four having no sodium level available. The derivation cohort was older, more often male, had a higher prevalence of ischemic cardiomyopathy, lower BMI, lower EF, higher NYHA and more severe renal dysfunction. The derivation cohort patients were less likely to be on ACE-inhibitors, angiotensin-receptor blockers, aldosterone antagonists and beta-blockers. On average, the derivation cohort had higher BNP levels and SHFM scores, consistent with higher risk.
Table 1.
Baseline Characteristics of Derivation and Validation Cohorts
| PATIENT CHARACTERISTICS | Derivation Cohort n=441 |
Validation Cohort n=1513 |
|---|---|---|
| Age (years), mean ± SD | 69 ± 14 | 56 ± 15 |
| Male Sex, n (%) | 317 (72) | 1000 (66) |
| Race, n (%) | ||
| White | 410 (93) | 1114 (74) |
| African American | 9 (2) | 330 (22) |
| Other | 22 (5) | 69 (4) |
| Body Mass Index (Kg/m2), mean ± SD | 29 ± 6 | 30 ± 7 |
| Systolic BP (mmHg), Mean ± SD | 115 ± 20 | 114 ± 20 |
| Ejection Fraction (%), mean ± SD | 26 ± 6 | 34 ± 17 |
| Ischemic Cardiomyopathy, n (%) | 250 (57) | 455 (30) |
| NYHA Functional Class III/IV, n (%) | 294 (67) | 558 (37) |
| Implantable Defibrillator, n (%) | 181 (44) | 636 (42) |
| Estimated GFR, mL/min/1.73m2 | 58 ± 25 | 84 ± 32 |
| SHFM Score, mean ± SD | 0.80 ± 0.9 | −0.07 ± 1.0 |
| LABORATORIES | ||
| BNP (pg/mL), median (IQR) | 547 (276,1146) | 171 (47,576) |
| Creatinine (mg/dL), median (IQR) | 1.3 (1.0, 1.6) | 0.9 (0.8,1.3) |
| Sodium (mEq/L), mean ± SD | 139 ± 3.7 | 139 ± 3.4 |
| Uric Acid (mg/dL), median (IQR) | 7.8 (5.9,9.8) | 7.0 (5.7,8.8) |
| MEDICATIONS (%) | ||
| ACE inhibitors or ARBs | 71 | 87 |
| Aldosterone Antagonists | 25 | 34 |
| Beta-Blockers | 81 | 88 |
| Digoxin | 41 | 39 |
| Diuretics | 76 | 78 |
| Statin | 53 | 50 |
Abbreviations; BNP, brain natriuretic peptide; BP, blood pressure; GFR, glomerular filtration rate; NYHA, New York Heart Association, SHFM, Seattle Heart Failure Model.
Follow-up was available for all patients in the derivation cohort at one year, at which time 61 (14%) had died, 8 (2%) had received cardiac transplantation and 6 (1%) had received a LVAD. The validation cohort was followed for at least one year, at which time 89 (12%) patients had died, 70 (5%) had received cardiac transplantation and 18 (1%) had received a LVAD.
Predictive Characteristics of SHFM and BNP
Derivation Cohort
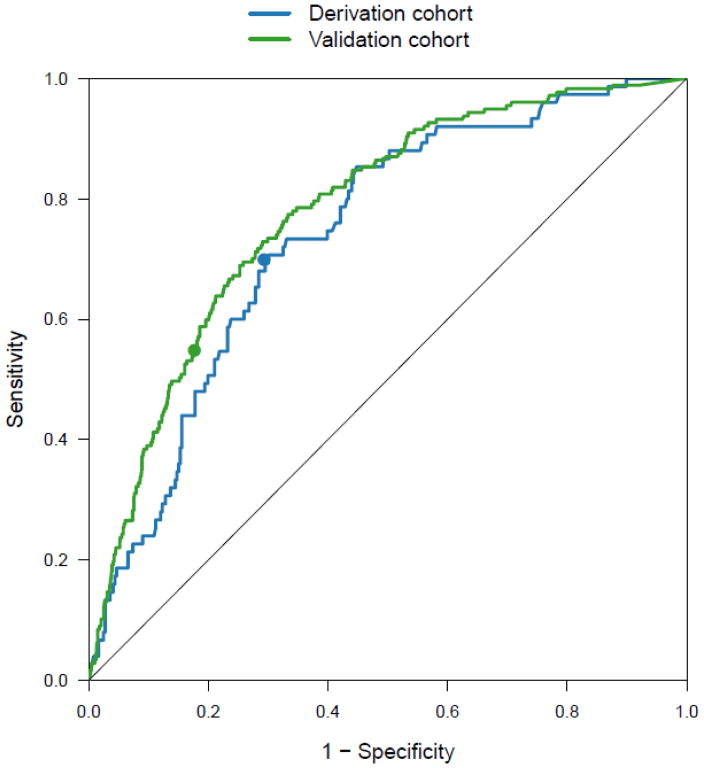
C-indices of the SHFM [0.75 (0.71,0.79)] and BNP [0.74 (0.70,0.78)] were significantly greater than 0.50 (Figure 1a). SHFM and BNP were each associated with one-year survival free of cardiac transplantation, or LVAD and offered similar (p=0.75) risk discrimination. There was only a moderate correlation between SHFM and BNP (R=0.43; p<0.001), suggesting that these variables assess different prognostic factors. BNP as a log-transformed, continuous variable enhanced the performance of the SHFM in predicting the composite one-year endpoint with a significant improvement in discrimination and reclassification criteria (Δ c-index of 0.03, NRI 10%, IDI 2%, p ≤ 0.04 for each).
Figure 1.
Receiver operating characteristic (ROC) curves demonstrating the prediction accuracy of BNP for the composite outcome of death, cardiac transplantation or ventricular assist device implantation at one year in the derivation and validation cohorts. Circles indicate (1–specificity, sensitivity) for the BNP cut-point of 700 pg/mL.
Validation Cohort
C-indices of the SHFM [0.76 (0.72, 0.80)] and BNP [0.78 (0.75, 0.81)] were significantly greater than 0.50. SHFM and BNP were each associated with one-year survival free of cardiac transplantation, or LVAD and offered similar (p=0.32) risk discrimination. There was only a moderate correlation between SHFM and BNP (R=0.54; p<0.001).
SHFM+BNP Algorithm
In the derivation cohort, a BNP value of 700 pg/mL maximized sensitivity (0.71) and specificity (0.70) for predicting one-year events (Figure 1). In the validation cohort, this value corresponded to a sensitivity of 0.55 and specificity of 0.82 and hence was used as the partition-value for the SHFM+BNP algorithm.
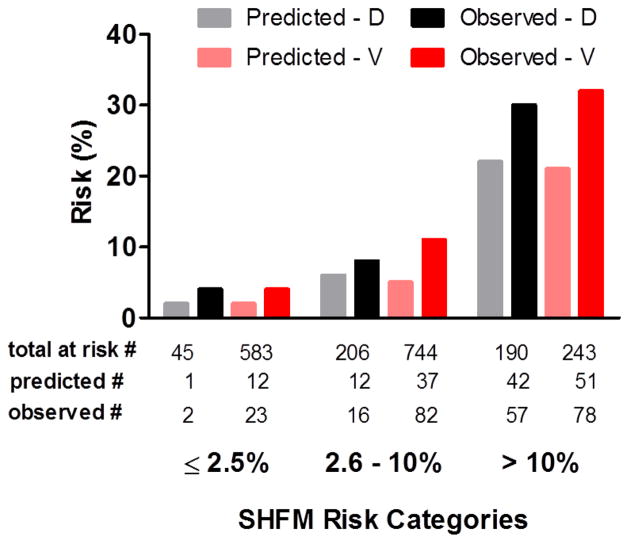
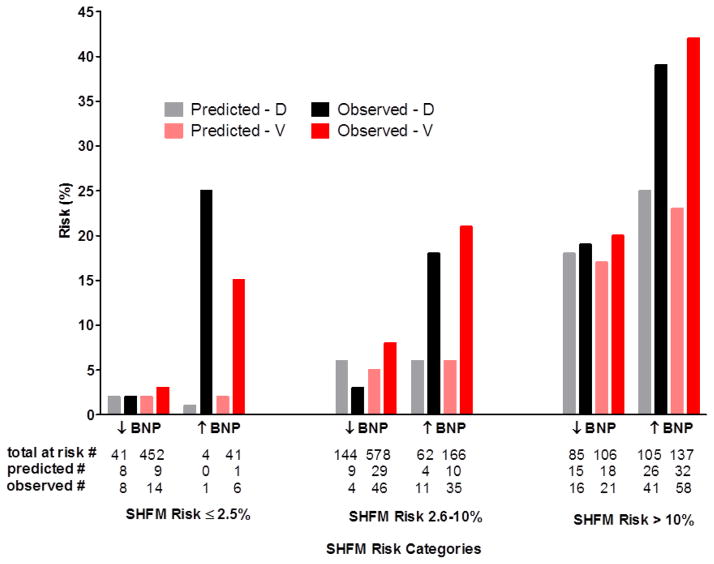
The ratio of observed-to-predicted risk was similar across the three categories of SHFM-predicted risk, indicating similar calibration of the SHFM within each cohort (Figure 2). The SHFM consistently underestimated risk in both cohorts. Across each SHFM-risk category, those with elevated BNP (≥700 pg/mL) had higher observed events than predicted by the SHFM. Differences in observed versus predicted risk within the BNP subgroups were similar in the derivation and validation cohort across the three SHFM-risk categories (Figure 3).
Figure 2.
Calibration histograms in each of derivation (D) and validation (V) cohorts. Patients are stratified within the pre-specified categories of clinically relevant one-year risk [low (≤2.5%), intermediate (2.6–≤10%), and high risk (>10%)] according to SHFM -predicted probabilities. One-year predicted SHFM mortality risk in each of the derivation cohort (grey bar) and validation cohort (pink bar). Observed composite one-year outcome of mortality, cardiac transplantation or ventricular assist device implantation in each of the derivation cohort (black bar) and validation cohort (red bar).
Figure 3.
Calibration histograms in each of derivation (D) and validation (V) cohorts are shown with one-year predicted SHFM mortality risk in each of the derivation cohort (grey bar) and validation cohort (pink bar). Observed composite one-year outcome of mortality, cardiac transplantation or ventricular assist device implantation in each of the derivation cohort (black bar) and validation cohort (red bar). Patients are stratified within the pre-specified categories of clinically relevant one-year risk [low (≤2.5%), intermediate (2.5–≤10%), and high risk (>10%)] according to SHFM-predicted probabilities and subsequently according to BNP levels above (↑) or below (↓) the optimal partition-value (700 pg/ml) based on ROC-analysis in the derivation cohort.
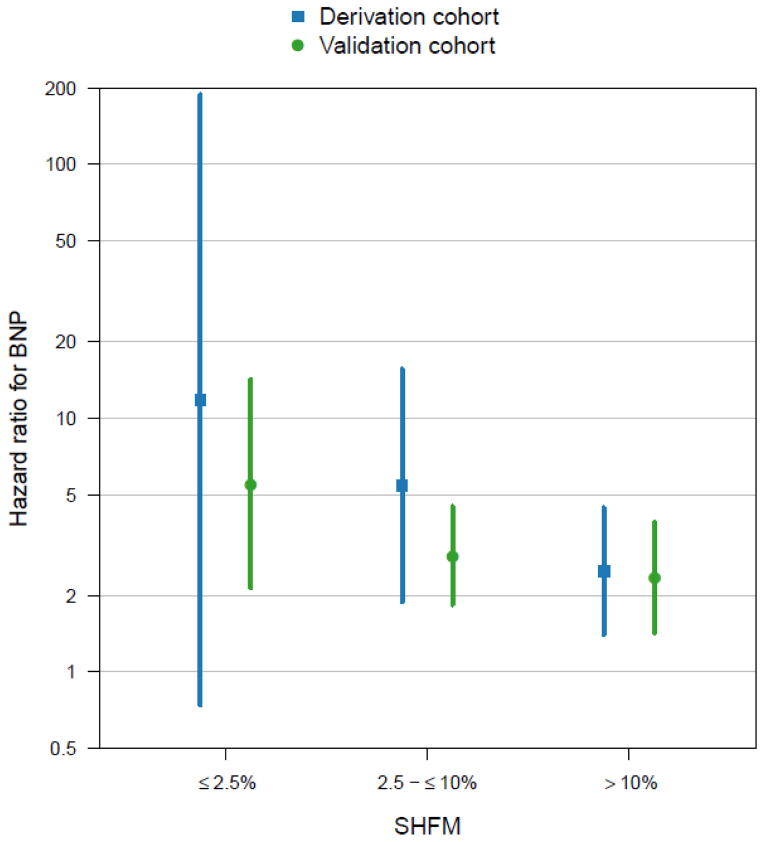
Figure 4 displays hazard ratios (HR) comparing the hazard of the composite outcome of death, cardiac transplantation or LVAD implantation between BNP ≥700 pg/mL and BNP <700 pg/mL, stratified by categories of clinically relevant one-year risk (≤2.5%, 2.5–≤10%, and >10%) per the SHFM. Although the estimated HR decreased with increasing SHFM, there was no evidence to suggest that the HR differed by SHFM category in the derivation (P=0.29) or validation (P=0.33) cohorts. Confidence intervals (CI) were broad for the derivation cohort due to limited sample size, particularly among those with SHFM risk ≤2.5% (n=45).
Figure 4.
Hazard ratios for the composite outcome of death, cardiac transplantation or ventricular assist device implantation between BNP ≥ 700 pg/mL and BNP < 700 pg/mL, stratified by categories of clinically relevant one-year risk (≤2.5%, 2.5–≤10%, and >10%) according to the SHFM. Sample sizes in each group are provided in Figure 3.
We then fit Cox-regression models for the composite outcome that included SHFM as a continuous variable, BNP cut-point (≥700 versus <700 pg/mL) and their interaction to determine whether the prognostic potential of BNP was consistent across all levels of the SHFM. There was no evidence to suggest that the prognostic potential of BNP depended on the SHFM in either the derivation (P=0.11) or validation (P=0.11) cohorts.
In the absence of a statistically significant interaction, we estimated the prognostic potential of BNP in addition to the SHFM from Cox-regression models for the combined outcome that included SHFM as a continuous variable and BNP cut-point (≥700 versus <700 pg/mL). In the derivation cohort, the HR associated with a BNP ≥700 versus <700 pg/mL was 2.6, 95% CI: (1.5, 4.4). In the validation cohort, the HR was 3.4, 95% CI: (2.5, 4.7).
We obtained similar results in a sensitivity analysis in which the outcome was limited to mortality alone (Supplemental Figure 1). In the derivation cohort, the HR associated with a BNP ≥700 versus <700 pg/mL was 2.0, 95% CI: (1.1, 3.5). In the validation cohort, the HR was 4.2, 95% CI: (2.7, 6.6).
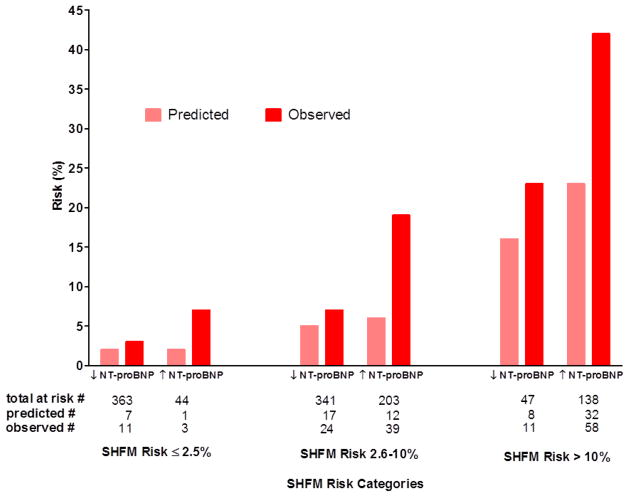
Sensitivity analysis of a SHFM+NT-proBNP algorithm was performed in the validation cohort (NT-proBNP levels were not available in derivation cohort). Consistently, across each SHFM risk category, using an optimal NT-proBNP cut-point of 1110 pg/mL, those patients with elevated NT-proBNP had higher observed events than those with lower NT-proBNP (Figure 5). The HR associated with a NT-proBNP ≥1110 versus <1110 pg/ml was 3.2, 95% CI: (2.1, 4.6).
Figure 5.
NT-proBNP sensitivity analysis within validation cohort. Calibration histograms shown: one-year observed mortality, cardiac transplantation or ventricular assist device implantation (red bar) and SHFM-predicted mortality risk (pink bar). Patients are stratified within the pre-specified categories of clinically relevant one-year risk [low (≤2.5%), intermediate (2.5–≤10%), and high risk (>10%)] according to SHFM -predicted probabilities and subsequently according to NT-proBNP levels above (↑) or below (↓) the optimal partition-value (NT-proBNP > 1100) based on ROC-analysis in the validation cohort.
Discussion
In a single-center, retrospectively defined, derivation cohort of patients with HFrEF seen across a spectrum of care environments and providers, application of a simple SHFM+BNP risk-stratification algorithm revealed that regardless of patients SHFM predicted category of risk, a BNP≥700 pg/mL identifies patients with ≥ two-fold risk of mortality than the SHFM would suggest while a BNP<700 pg/ml identifies patients in whom the SHFM risk is well calibrated. In an external, prospective, validation cohort that was younger, less symptomatic, more commonly with non-ischemic etiology, higher EF, better renal function and lower BNP and SHFM scores, the SHFM+BNP algorithm performed similarly. This algorithm provides a method to integrate information from two well validated risk-prediction tools and provides a partition-value (BNP ≥ 700 pg/mL) that has reliable performance when interpreted in the context of the SHFM-predicted risk.
As compared to the average annual SHFM estimated mortality risk of approximately 20% in the high-risk category of our cohort, patients presented to the advanced HF therapeutics committee at a large HF center and listed as UNOS status II had an average annual SHFM estimated mortality risk of 16%5. This highlights the external validity and clinical relevance of the findings in our cohort that indeed is representative of patients being considered for advanced HF therapies and for whom better risk categorization is of benefit.
Consistent with previous reports17–19, our data shows that the combination of SHFM and BNP provides incremental, statistically significant risk prediction information. In a cohort of nonclinical trial, hospital-based patients with HF, BNP was found to add predictive information to the SHFM for predicting one-year survival free from death, transplantation or LVAD implantation (Δ c-index of 0.05)17. Likewise, Ky et al. showed that adding NT-proBNP to the SHFM improved prediction of transplant-free survival at one year in a population of ambulatory patients with chronic HF (Δ c-index of 0.02)18.
While this and other studies demonstrate that BNP assays provide statistically significant increments in the prognostic information provided by the SHFM, a practical way to integrate the information from these risk-assessment tools has been lacking. Ideally, the most rigorous method of complementing prognostic information from the SHFM with BNP would be an incorporation of BNP into the SHFM model whereby beta-estimates are used to generate an enhanced model. This would require a large population of patients with values for each of the 16 SHFM variables and simultaneous BNP levels. This is not feasible; hence, our proposal. To our knowledge, this is the first risk-assessment algorithm incorporating both the SHFM and BNP for clinical use in HF.
The information provided by the SHFM allows one to interpret the BNP level in the context of HF-stage and extent of HF-therapy. A limitation of BNP assays is that any given value of BNP may have different connotations depending on the duration or HF-stage and the intensity of medical therapy. A high level in a decompensated patient with new-onset HF on little or no medical therapy does not necessarily carry the same risk as an identical level in a patient on maximal medical therapy and high-dose diuretics.
While the SHFM offers adequate risk discrimination in advanced HF, Kalogeropoulos et al.6 showed that it systematically overestimated survival and underestimated risk, especially in blacks and in patients with devices. This finding was more prominent when including transplantation and LVAD implantation as an end point. Here, in a cohort that included up to 42% of patients with devices and 22% blacks, we show that the information provided by the BNP level overcomes this tendency of the SHFM to underestimate risk. This is likely explained by the ability of BNP to provide additional information regarding the heart structure and function, wall stress32 and level of humoral activation and inflammatory/oxidative stress33.
The implications of our approach are three-fold: First, it identifies that patients with a BNP>700 have approximately three-fold the risk that the SHFM would estimate regardless of what HF-stage a patient is evaluated in, and irrespective of the intensity and duration of HF-therapy and comorbidity burden. Second, it allows a simple approach for risk-assessment relative to decisions for aggressive optimization of medical therapy, appropriate transition to advanced HF therapies and palliative or end-of-life care. Third, the stable performance of our algorithm across varied HF-cohorts suggests that this approach affords a means of indexing HF-populations for risk, something that may prove useful in clinical trials and daily practice.
Limitations
Our study should be interpreted in the context of inherent methodological limitations of retrospective studies. NYHA, comorbidities and medications were obtained by individualized chart review and were limited by care providers documentation. Missing values were imputed for calculation of composite scores. Other studies validating composite risk-scores have been retrospective and had similar rates of missing data that had to be imputed5. The similar performance in a prospectively enrolled registry of patients suggests that the retrospectively derived data were valid. NT-proBNP levels were not available in the derivation cohort. Many facilities now use NT-proBNP rather than BNP. The sensitivity analysis in the validation cohort provides a level for NT-proBNP to use in a SHFM+NT-proBNP algorithm, but this requires replication. Cause of death was not determined and we are unable to determine whether the tool specifically identifies death to progressive pump failure, arrhythmia or non-HF related causes. Finally, we determined whether the prognostic potential of BNP was consistent across levels of the SHFM based on a statistical test for the interaction between the SHFM and BNP cut-point. It is possible that we were simply underpowered to detect an interaction.
Conclusions
Establishing a specific BNP cutoff that identifies a higher-risk subset of patients, our proposed algorithm offers a simple approach to integrate prognostic information provided by SHFM and BNP. Its stable characteristics and performance across these two diverse cohorts support its utility today in diverse clinical settings as physicians counsel HF patients regarding medications, defibrillators, advanced HF therapies, experimental therapies and palliative or end-of-life care.
Supplementary Material
Acknowledgments
Funding
Dr. Omar AbouEzzeddine is funded by The NIH Training Grant (T-91160) and support for mentoring Dr. AbouEzzeddine is provided by the HFCRN Skills Development Core (HL 84907). Statistical Support was provided by the CTSA (5UL1RR024150-05). Dr. Thomas Cappola was provided additional funding by NIH R01HL088577. Dr. Nancy Sweitzer received funding to support this study from the University of Wisconsin Department of Medicine. Dr. Fang received funding to support this study from the Harrington Heart and Vascular Institute, Cleveland, OH.
Footnotes
Disclosures
Dr. Cappola reports receiving support for running biomarker assays from Abbott Diagnostics. Dr. Jaffe has or presently consults for most of the major diagnostic companies including those whose assays are used in this analysis. Drs. French, Fang, Sweitzer, AbouEzzeddine and Redfield have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Abouezzeddine OF, Redfield MM. Who has advanced heart failure?: Definition and epidemiology. Congest Heart Fail. 2011;17:160–8. doi: 10.1111/j.1751-7133.2011.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: Prediction of survival in heart failure. Circulation. 2006;113:1424–33. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 4.Levy WC, Lee KL, Hellkamp AS, et al. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation. 2009;120:835–842. doi: 10.1161/CIRCULATIONAHA.108.816884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorodeski EZ, Chu EC, Chow CH, Levy WC, Hsich E, Starling RC. Application of the Seattle Heart Failure Model in ambulatory patients presented to an advanced heart failure therapeutics committee. Circ Heart Fail. 2010;3:706–14. doi: 10.1161/CIRCHEARTFAILURE.110.944280. [DOI] [PubMed] [Google Scholar]
- 6.Kalogeropoulos AP, Georgiopoulou VV, Giamouzis G, Smith AL, Agha SA, Waheed S, et al. Utility of the Seattle Heart Failure Model in patients with advanced heart failure. J Am Coll Cardiol. 2009;53:334–42. doi: 10.1016/j.jacc.2008.10.023. [DOI] [PubMed] [Google Scholar]
- 7.Pitt B, Poole-Wilson PA, Segal R, et al. Effect of losartan compared with captopril on mortality in patients with symptomatic heart failure: Randomised trial--the Losartan Heart Failure Survival Study ELITE II. Lancet. 2000;355:1582–7. doi: 10.1016/s0140-6736(00)02213-3. [DOI] [PubMed] [Google Scholar]
- 8.Cohn JN, Tognoni G Valsartan Heart Failure Trial Investigators. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–75. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 9.Mann DL, McMurray JJ, Packer M, et al. Targeted anticytokine therapy in patients with chronic heart failure: results of the Randomized Etanercept Worldwide Evaluation (RENEWAL) Circulation. 2004;109:1594–1602. doi: 10.1161/01.CIR.0000124490.27666.B2. [DOI] [PubMed] [Google Scholar]
- 10.Maggioni AP, Opasich C, Anand I, et al. Anemia in patients with heart failure: Prevalence and prognostic role in a controlled trial and in clinical practice. J Card Fail. 2005;11:91–8. doi: 10.1016/j.cardfail.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 11.Giamouzis G, Kalogeropoulos AP, Georgiopoulou VV, et al. Incremental value of renal function in risk prediction with the Seattle Heart Failure Model. Am Heart J. 2009;157:299–305. doi: 10.1016/j.ahj.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan MD, Levy WC, Crane BA, Russo JE, Spertus JA. Usefulness of depression to predict time to combined end point of transplant or death for outpatients with advanced heart failure. Am J Cardiol. 2004;94:1577–80. doi: 10.1016/j.amjcard.2004.08.046. [DOI] [PubMed] [Google Scholar]
- 13.Ketchum ES, Moorman AJ, Fishbein DP, et al. Predictive value of the Seattle Heart Failure Model in patients undergoing left ventricular assist device placement. J Heart Lung Transplant. 2010;29:1021–5. doi: 10.1016/j.healun.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Levy WC, Mozaffarian D, Linker DT, Farrar DJ, Miller LW. Can the Seattle Heart Failure Model be used to risk-stratify heart failure patients for potential left ventricular assist device therapy? J Heart Lung Transplant. 2009;28:231–6. doi: 10.1016/j.healun.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 15.Maisel AS, Daniels LB. Breathing not properly 10 years later: what we have learned and what we still need to learn. J Am Coll Cardiol. 2012;60:277–82. doi: 10.1016/j.jacc.2012.03.057. [DOI] [PubMed] [Google Scholar]
- 16.Braunwald E. Heart failure. JACC Heart Fail. 2013;1:1–20. doi: 10.1016/j.jchf.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 17.May HT, Horne BD, Levy WC, et al. Validation of the Seattle Heart Failure Model in a community-based heart failure population and enhancement by adding b-type natriuretic peptide. Am J Cardiol. 2007;100:697–700. doi: 10.1016/j.amjcard.2007.03.083. [DOI] [PubMed] [Google Scholar]
- 18.Ky B, French B, Levy WC, et al. Multiple biomarkers for risk prediction in chronic heart failure. Circ Heart Fail. 2012;5:183–90. doi: 10.1161/CIRCHEARTFAILURE.111.965020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cabassi A, de Champlain J, Maggiore U, et al. Prealbumin improves death risk prediction of bnp-added seattle heart failure model: Results from a pilot study in elderly chronic heart failure patients. Int J cardiol. 2013;168:3334–9. doi: 10.1016/j.ijcard.2013.04.039. [DOI] [PubMed] [Google Scholar]
- 20.Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA. 2006;296:2209–16. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 21.Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of b-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–7. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 22.Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC., Jr Plasma brain natriuretic peptide concentration: impact of age and gender. J Am Coll Cardiol. 2002;40:976–82. doi: 10.1016/s0735-1097(02)02059-4. [DOI] [PubMed] [Google Scholar]
- 23.Roger VL, Weston SA, Redfield MM, et al. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–50. doi: 10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 24.Cappola TP, Matkovich SJ, Wang W, et al. Loss-of-function DNA sequence variant in the clcnka chloride channel implicates the cardio-renal axis in interindividual heart failure risk variation. Proc Natl Acad Sci U S A. 2011;108:2456–61. doi: 10.1073/pnas.1017494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ky B, Kimmel SE, Safa RN, et al. Neuregulin-1 beta is associated with disease severity and adverse outcomes in chronic heart failure. Circulation. 2009;120:310–17. doi: 10.1161/CIRCULATIONAHA.109.856310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Apple FS, Panteghini M, Ravkilde J, et al. Quality specifications for b-type natriuretic peptide assays. Clin Chem. 2005;51:486–93. doi: 10.1373/clinchem.2004.044594. [DOI] [PubMed] [Google Scholar]
- 27.Harrell FE. Regression modeling strategies : With applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001. [Google Scholar]
- 28.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 29.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the roc curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 30.Pencina MJ, D’Agostino RB, Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: A scientific statement from the American Heart Association. Circulation. 2009;119:2408–16. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iwanaga Y, Nishi I, Furuichi S, et al. B-type natriuretic peptide strongly reflects diastolic wall stress in patients with chronic heart failure: comparison between systolic and diastolic heart failure. J Am Coll Cardiol. 2006;47:742–8. doi: 10.1016/j.jacc.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 33.Talha S, Charloux A, Enache I, Piquard F, Geny B. Mechanisms involved in increased plasma brain natriuretic peptide after heart transplantation. Cardiovasc Res. 2011;89:273–81. doi: 10.1093/cvr/cvq331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.