Abstract
Background
The long term prognosis in terms of risk or predictors of developing HCC among patients with sustained virological response (SVR) remains unclear.
Methods
We conducted a retrospective cohort study using data from the VA HCV Clinical Case Registry in patients with positive HCV RNA between 10/1999 and 8/2009 and follow up through 1/2010. HCV treatment (interferon with or without ribavirin) and SVR (RNA test negative at least 12 weeks after the end of treatment) were determined. We used Cox proportional hazards models to calculate hazard ratios (HR) for potential predictors (demographic, virological and clinical) associated with HCC development following SVR.
Results
We identified 33,005 HCV-infected individuals who received treatment, of whom 10,817 patients achieved SVR. Among these patients, 100 developed new HCC during a total follow of 30,562 person years for an overall incidence rate 0.33% per year. The annual risk of HCC remained considerably high among patients with cirrhosis (1.39%) and those cured after age 64 (0.95%). Patients with diabetes (adjusted HR =1.88, 1.21 – 2.91) or genotype 3 infection (adjusted HR =1.62, 0.96 – 2.734) were significantly more likely to develop HCC.
Conclusions
The risk of HCC after HCV cure, while considerably reduced, remains relatively high at 0.33% per year. Older age and/or presence of cirrhosis at the time of SVR are associated with a high enough risk to warrant surveillance. Diabetes is also a risk factor for post SVR HCC.
Keywords: epidemiology, outcomes, Veterans Administration, automated data, sustained viral response, cirrhosis, cure
INTRODUCTION
Most persons who acquire hepatitis C virus (HCV) develop chronic hepatitis C (CHC) infection. CHC is estimated to affect 2.7 to 3.9 million individuals1 in the United States, where it remains the major cause of cirrhosis, hepatocellular carcinoma (HCC) and liver transplantation. CHC is also associated with increased liver disease and overall mortality.2 However, estimates of natural history and clinical course of HCV come from studies of mostly untreated or uncured cohorts with CHC.3
The recent advent of interferon-free directly acting antivirals (DAA) has changed the short-term landscape of CHC, with high cure rates (>90%) of HCV for most patient groups as well as high acceptability by patients and providers.4 The rapid rate of dissemination and use of DAAs, despite their high price, is likely to result in curing most people with known CHC within the next few years.5 However, the intermediate and long-term prognosis of patients cured of HCV is unclear. Previous studies, mostly from single centers in Japan, have indicated that cured CHC patients have a significant reduction in their risk of developing HCC compared to untreated or treated but not cured controls.6 However, given the low cure rate coupled with low treatment rate with interferon based therapies, few studies could assemble a large enough number of cured patients; present long term follow up to allow meaningful conclusions about relatively rare outcomes such as HCC, or examine the determinants of these outcomes.7–13
The required clinical follow up for patients who achieve SVR are unclear. For example, HCC surveillance is recommended in most clinical practice guidelines in high risk groups that are defined based on >1.0% annual risk of developing HCC.14 However, the available information does not allow for precise estimation of HCC risk or the temporal change of that risk.15 Furthermore, apart from presence of cirrhosis at or before SVR, there have been no consistently identified risk factors for HCC post SVR. This information has become important for clinical practice as well as for setting clinical research agenda in an era of increasingly cured HCV.
We therefore conducted a retrospective cohort study among 10,738 CHC patients with SVR during 1999–2009 to estimate the risk of HCC and examine potential determinants of this risk.
METHODS
Data Sources
This study was approved by Baylor College of Medicine’s Institutional Review Board, and all procedures conformed to the ethical guidelines of the 1975 Declaration of Helsinki. We used data from the VHA HCV Clinical Case Registry (CCR), which contains health information for all known HCV-infected patients from 128 VHA facilities nationwide. Data elements in the CCR include date of birth, laboratory test results, outpatient and inpatient VHA pharmacy data and inpatient and outpatient diagnoses and procedure codes.16 VA Vital Status file, which captures death and corresponding date with up to 97.6% agreement with the National Death Index, was used to ascertain date of death.17
Study Population
The study cohort included patients with CHC, defined as a positive test for HCV RNA in plasma by qualitative or quantitative assays or genotype test between October 1, 1999, and December 31, 2009. We included patients who received interferon or pegylated interferon therapy (with and without ribavirin) and achieved an SVR. We defined SVR as last HCV RNA test being negative at least 12 weeks after treatment completion, as previously described.2;18 We defined the date of earliest indication of SVR (first negative RNA test date of the terminal sequence of negative RNA tests) as the index date for this analysis. We also evaluated HCC incidence in a group of patients who received treatment but had no SVR (last HCV RNA test after treatment end date was positive) using the last date of treatment as their index date. The non SVR group was not included in any other analysis.
Study Exposure and Outcomes
The primary outcome of the study was new (incident) cases of HCC (ICD-9 code 155.0) that was first recorded after the index date. Patients with HCC recorded any time prior to index date (prevalent cases) were excluded to ensure that HCC was newly diagnosed after treatment. The ICD-9 code-based definition for HCC was validated in a previous study against detailed medical-record reviews and shown to have a high positive predictive value.19 The study follow-up ended at the time of HCC, patient’s death, last visit in the VHA, or January 1. 2010.
Potential Risk Factors for HCC
We ascertained several baseline risk factors that may be associated with an accelerated or decreased progression to HCC in patients with SVR: age (<45, 45–54, 55–64, 65+), race (African American, non-Hispanic White, Hispanic, other, or unknown), gender, HCV genotype, cirrhosis, diabetes, alcohol use, HIV infection, hepatitis B virus (HBV) infection, and BMI. We identified HIV, diabetes, and alcohol use by the presence of outpatient or inpatient ICD-9 diagnosis codes recorded any time prior to the SVR index date. We defined cirrhosis as the presence of cirrhosis ICD-9 codes (571.2, 571.5, 571.6)20; we also examined alternate definitions using an AST, platelets ratio index (APRI)>2 any time prior to the SVR index date and analyzed three definitions of cirrhosis: codes only, APRI> 1.77 and APRI> 2.0. We defined patients with HBV coinfection as subjects with a positive HBV surface antigen test during the study period. BMI was defined using the height and weight closest to (and prior to) the SVR index date. Diabetes was examined as a time dependent exposure variable by continually updating it throughout the study period until event or censor time.
Data Analysis
We calculated the annualized incidence rates of HCC by dividing the number of incident cases by a denominator of patient years (PY) follow-up, and compared these rates among patients who received antiviral treatment based on SVR and no SVR status. We first examined the incidence rate of HCC overall in patients who were treated with and without SVR.
The remainder of the analyses was limited to the SVR cohort. We estimated the annualized HCC incidence in several subgroups defined by demographic and clinical characteristics in patients with SVR. We generated cumulative hazard function curves, using Fine-Gray adaptation of the Kaplan-Meier estimation method21 to illustrate and compare the cumulative incidence of HCC (with death as a competing risk) overall and stratified by several variables of interest (age, cirrhosis, and diabetes) starting at SVR date till the end of the follow-up period. We used the log rank test to evaluate the differences among these rates by risk factor (cirrhosis, age at time of SVR). We then constructed Cox proportional hazards models to examine the association between risk factors and time to development of HCC. Variables with a univariate p-value <0.20 were considered candidate variables for the model and all variables with a p-value <0.10 were retained in the final model.
We conducted secondary analyses excluding HCC cases that developed during the first year of follow-up; these cases may have been prevalent cases and or have a different biology and risk factors than those that develop later on. We also conducted an analysis restricted to patients without significant risk factors to potentially identify patients at very low HCC risk who can be excluded from HCC surveillance programs.
The results of these regressions were expressed as hazard ratios (HRs) and corresponding 95% confidence intervals (CIs). No significant departures from proportional hazards were found for the included predictors.
RESULTS
Study Cohort
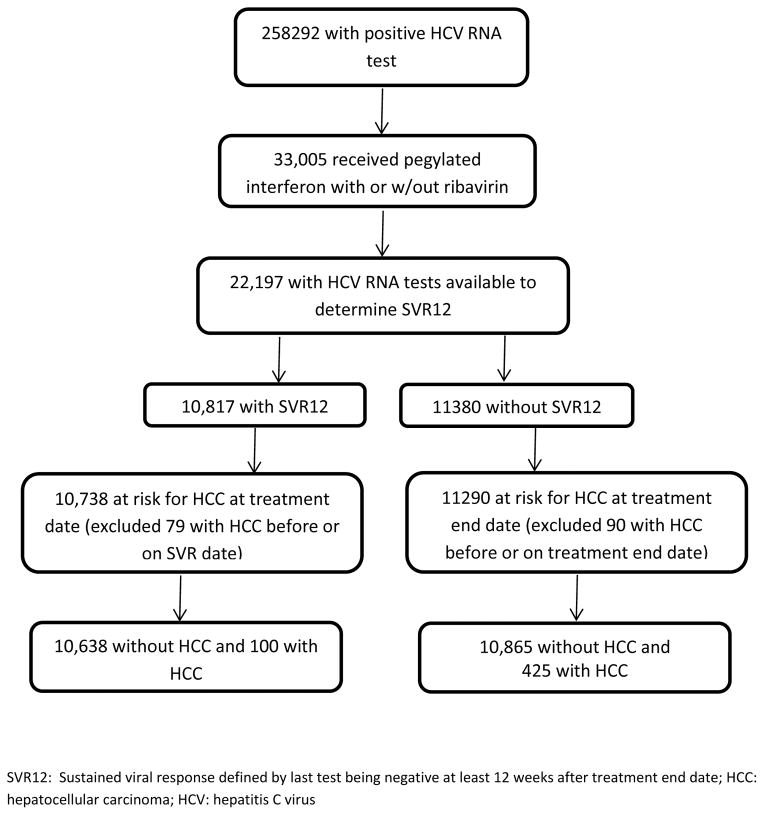
There were 258,292 patients with a positive HCV RNA test identified from the HCV CCR; of these, 33,005 received interferon/pegylated interferon with or without ribavirin. SVR could be determined in 22,197 patients, of whom 10,817 had a documented negative RNA test more than 12 weeks after treatment end date, and 11,380 in whom SVR was not achieved (Figure 1). We subsequently excluded 79 patients in whom HCC was diagnosed on or before the SVR date, resulting in 10,738 patients in the SVR cohort, and excluded 90 patients in whom HCC was diagnosed on or before the treatment end date, resulting in 11,290 in the no SVR cohort (Figure 1).
Figure 1.
The study flow diagram with criteria used to identify the study cohort.
HCC Incidence in Treated Cohorts With and Without SVR
There were 100 incident cases of HCC diagnosed during 30,562 person-years (PY) of follow-up after SVR [mean duration of follow up after SVR was 2.8 years (s.d. 2.0)]; this yielded an annual HCC incidence rate of 3.27/1000 PY (or 0.327%). This rate was considerably and significantly lower than the 1.32 per 100 PY incidence rate in the group of patients with no SVR (425 HCC cases during 45,509.9 PY follow up in 11,290 patients treated with no SVR); unadjusted HR of 0.49 (95% CI (%, 0.46 – 0.53) for SVR vs. non-SVR.
We hand reviewed a convenience sample of 22 cases of the 100 HCCs among patients with SVR and confirmed HCC diagnosis in 21 of these cases (i.e., positive predictive value [PPV] for ICD-9 codes of 95%). Of these cases, 12 had both cirrhosis diagnosis and high APRI, 6 had high APRI but no cirrhosis diagnosis, and 3 had no cirrhosis diagnosis and no high APRI.
HCC Incidence and Risk Factors in Patients with SVR
Table 1 presents the demographic and clinical characteristics of the SVR cohort as well as the annual HCC incidence rate and unadjusted HR for subgroups of patients with each characteristic. The mean age of the SVR cohort was 53.1 (sd=6.3) year, and consisted of mostly male patients (95.3%) of white race (64.4%). Most were infected with HCV genotype 1 (53.6%) before achieving SVR.
Table 1.
Demographic and clinical characteristics of 10,738 Veterans with HCV who achieved an SVR12 of whom 100 developed HCC during follow up
| Variable | Total N=10,738 N (%) | HCC N=100 | Total PY | Annual Incidence Rate (%) (95% CI) | Unadjusted HR (95% CI) |
|---|---|---|---|---|---|
| Age | |||||
| < 45 | 791 (7.4) | 2 | 2593.7 | 0.077 (0.074 – 0.080) | 0.36 (0.087 – 1.499) |
| 45 – 54 | 5471 (50.9) | 36 | 16916.6 | 0.213 (0.210 – 0.216) | 1.0 (ref) |
| 55 – 64 | 4154 (38.7) | 54 | 10213.1 | 0.529 (0.518 – 0.539) | 2.46 (1.614 – 3.763) |
| 65+ | 322 (3.0) | 8 | 839.4 | 0.953 (0.889 – 1.018) | 4.43 (2.060 – 9.539) |
| Gender | |||||
| Female | 506 (4.7) | 1 | 1422.8 | 0.070 (0.067 – 0.074) | 0.20 (0.029 – 1.466) |
| Male | 10232 (95.3) | 99 | 29139.9 | 0.340 (0.336 – 0.344) | 1.0 (ref) |
| Race | |||||
| White | 6919 (64.4) | 63 | 20159.8 | 0.313 (0.308 – 0.317) | 1.0 (ref) |
| Black | 1387 (12.9) | 13 | 3695.6 | 0.352 (0.340 – 0.363) | 1.13 (0.619 – 2.044) |
| Hispanic | 368 (3.4) | 8 | 1259.0 | 0.635 (0.600 – 0.671) | 2.03 (0.973 – 4.252) |
| Asian | 34 (0.3) | 1 | 89.3 | 1.120 (0.888 – 1.352) | 3.62 (0.503 – 26.143) |
| Other | 85 (0.8) | 1 | 217.5 | 0.460 (0.399 – 0.521) | 1.47 (0.204 – 10.614) |
| unreported | 1945 (18.1) | 14 | 5141.6 | 0.272 (0.265 – 0.280) | 0.87 (0.488 – 1.556) |
| HCV Genotype | |||||
| 1 | 5757 (53.6) | 53 | 15876.2 | 0.334 (0.329 – 0.339) | 1.0 (ref) |
| 2 | 2679 (24.9) | 15 | 7812.4 | 0.192 (0.188 – 0.196) | 0.58 (0.325 – 1.022) |
| 3 | 1454 (13.5) | 22 | 4117.7 | 0.534 (0.518 – 0.551) | 1.60 (0.974 – 2.632) |
| 4 | 73 (0.7) | 0 | 222.0 | 0.000 (0.000 – 0.000) | --- |
| 5/6 | 3 (0.0) | 0 | 10.9 | 0.000 (0.000 – 0.000) | --- |
| M | 772 (7.2) | 10 | 2523.6 | 0.396 (0.381 – 0.412) | 1.17 (0.591 – 2.303) |
| HBV coinfection | |||||
| No | 10365 (96.5) | 97 | 29466.3 | 0.329 (0.325 – 0.333) | 1.0 (ref) |
| Yes | 373 (3.5) | 3 | 1096.5 | 0.274 (0.257 – 0.290) | 0.84 (0.267 – 2.659) |
| HIV coinfection | |||||
| No | 10428 (97.1) | 96 | 29700.0 | 0.323 (0.320 – 0.327) | 1.0 (ref) |
| Yes | 310 (2.9) | 4 | 853.8 | 0.468 (0.437 – 0.500) | 1.45 (0.533 – 3.939) |
| Alcohol | |||||
| No | 5944 (55.3) | 46 | 17633.8 | 0.261 (0.257 – 0.265) | 1.0 (ref) |
| Yes | 4794 (44.7) | 54 | 12928.9 | 0.418 (0.410 – 0.425) | 1.62 (1.089 – 2.424) |
| Cirrhosis | |||||
| No | 9190 (85.6) | 42 | 26398.7 | 0.159 (0.157 – 0.161) | 1.0 (ref) |
| Yes | 1548 (14.4) | 58 | 4164.1 | 1.393 (1.351 – 1.435) | 8.97 (5.986 – 13.440) |
| APRI>2 | |||||
| 0 | 7416 (69.1) | 20 | 21592.7 | 0.093 (0.091 – 0.094) | 1.0 (ref) |
| 1 | 3322 (30.9) | 80 | 8970.02 | 0.892 (0.873 – 0.910) | 9.19 (5.620 – 15.035) |
| Diabetes | |||||
| No | 8712 (81.3) | 69 | 25210.5 | 0.274 (0.270 – 0.277) | 1.0 (ref) |
| Yes | 2026 (18.7) | 31 | 5352.28 | 0.579 (0.564 – 0.595) | 2.11 (1.368 – 3.240) |
| BMI | |||||
| < 18.5 | 101 (0.9) | 1 | 284.8 | 0.351 (0.310 – 0.392) | 1.16 (0.157 – 8.589) |
| 18.5 – 25 | 2824 (26.3) | 24 | 8012.3 | 0.300 (0.293 – 0.306) | 1.0 (ref) |
| 25 – 30 | 4268 (39.7) | 38 | 12364.3 | 0.307 (0.302 – 0.313) | 1.03 (0.617 – 1.715) |
| 30+ | 3350 (31.2) | 35 | 9398.4 | 0.372 (0.365 – 0.380) | 1.24 (0.740 – 2.092) |
| no BMI | 195 (1.8) | 2 | 502.9 | 0.398 (0.363 – 0.432) | 1.17 (0.276 – 4.964) |
BMI: Body Mass Index; APRI: AST, Platelet Ratio Index;, HCV: hepatitis C virus; HBV: hepatitis B virus
Among patients with SVR who developed HCC, the median age at time of HCC diagnosis was 58.0 with IQR 55.0–61.5. The time from SVR to HCC ranged from 0.04 to 7.33 years, with a median of 1.66 years (IQR: 0.61 – 3.19). Patients with cirrhosis diagnostic codes had the highest annual incidence of HCC incidence (1.39%). Patients with APRI >2 had an annual HCC incidence of 0.89% (95% CI, 0.87 – 0.91). A total of 3906 (36.4%) had either cirrhosis codes or APRI>2 before SVR date and in these patients the annual incidence of HCC was 0.83% (95% CI, 0.82–0.85).
HCC incidence was also higher among older (≥65 years old) (0.95%) compared with younger patients. There was a trend towards a higher HCC incidence rate in patients with genotype 3, HIV coinfection, alcohol use, diabetes, and elevated BMI; however the HRs were not statistically significant. The annual incidence of HCC occurred remained fairly stable through the first 5 years after SVR, whereas there very few HCC cases recorded in years 6–8 to allow for precise estimation of risk (Table 2). The 5 year cumulative risk of HCC after SVR was 0.33%.
Table 2.
The annual risk of hepatocellular carcinoma with HCV who achieved an SVR.
| Year after SVR | At risk patients | PY follow up | Incident HCC Cases | Incidence Rates per 100 (95% CI) |
|---|---|---|---|---|
| 1 | 10735 | 9381.3 | 35 | 0.373 (0.366 – 0.381) |
| 2 | 8312 | 7370.0 | 26 | 0.353 (0.345 – 0.361) |
| 3 | 6493 | 5680.0 | 13 | 0.229 (0.223 – 0.235) |
| 4 | 4842 | 4038.2 | 16 | 0.396 (0.384 – 0.408) |
| 5 | 3284 | 2561.2 | 6 | 0.234 (0.225 – 0.243) |
| 6 | 1856 | 1223.4 | 1 | 0.082 (0.077 – 0.086) |
| 7 | 661 | 331.9 | 2 | 0.603 (0.538 – 0.667) |
| 8 | 85 | 29.1 | 1 | 3.444 (2.191 – 4.696) |
PY: patient-years, CI: confidence intervals; SVR: sustained virological response
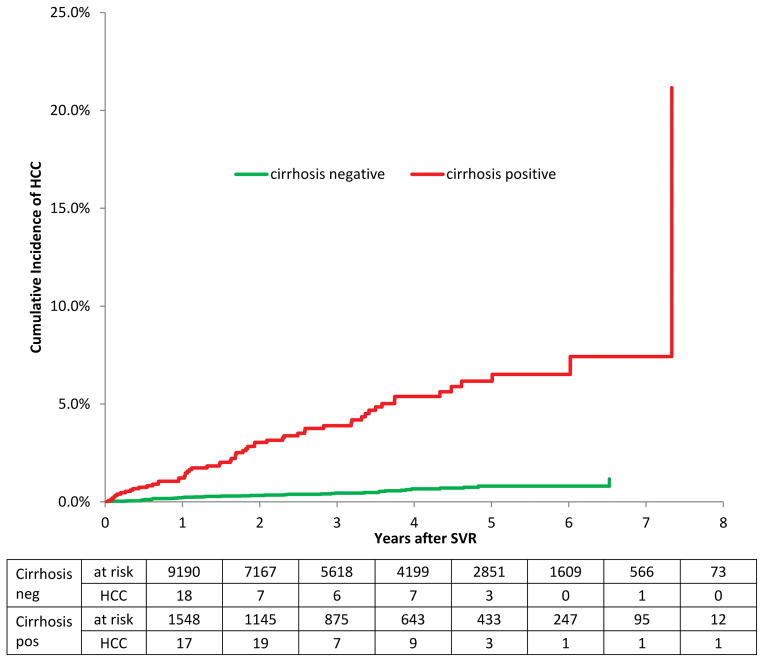
The cumulative incidence steadily increased after SVR through year 8. Similar patterns were seen for subgroups of patients of different ages and with and without cirrhosis. The older age group had a higher cumulative incidence than younger patients (log-rank p-value <0.0001). There cumulative incidence of HCC was both higher with longer follow up and with older age groups (Gray’s log-rank p < .0001) (Supplemental Figure 1). Patients with cirrhosis had a significantly higher cumulative incidence of HCC than patients without it (Figure 2; log-rank test p-value <0.0001).
Figure 2.
The cumulative incidence of hepatocellular carcinoma among patients with HCV who achieved SVR, stratified by the presence or absence of cirrhosis at the time of SVR. Gray’s log-rank test p <.0001
Predictors of HCC Risk After SVR
In the multivariable Cox PH model, several risk factors were significant predictors of developing HCC after SVR (Table 3). The highest risk of developing HCC was associated with presence of cirrhosis (adjusted HR=6.69; 95% CI, 4.32 – 10.35). Patients with diabetes were almost 2 times more likely to develop HCC after SVR than patients without diabetes (adjusted HR=1.88; 95%CI: 1.21 – 2.91). In addition, patients with genotype 3 (adjusted HR=1.62; 95% CI, 0.96 – 2.73) were more likely to develop HCC. Finally, patients who were older (age 55–64: 2.04; 1.29 – 3.23 and age 65+: 4.51; 1.96 – 10.40 vs. 45 – 54 years old) and Hispanic ethnicity (adjusted HR: 2.27; 95 CI%, 1.07 – 4.80) were more likely to develop HCC after having an SVR than younger and white patients. We further examined the effect of APRI score with or without cirrhosis diagnosis. High APRI (>2.0) in the presence of cirrhosis diagnosis was associated with the highest HCC risk (2% per year) compared to no cirrhosis and low APRI group (0.06% per year). The HCC risk was intermediate and equivalent with those who had a diagnosis of cirrhosis with low APRI (0.53% per year) or had had APRI but no cirrhosis diagnosis (0.48% per year) (supplemental Table 1).
Table 3.
Predictors of HCC in Veterans with HCV who achieved an SVR. Results from Cox proportional hazards model while adjusting for the competing risk of death.
| variable | level | Hazard Ratio (95% CI) | p-value |
|---|---|---|---|
| Diabetes (time-varying) | no | 1.0 (ref) | |
| yes | 1.876 (1.211 – 2.906) | 0.0048 | |
| Race | White | 1.0 (ref) | |
| Black | 1.398 (0.730 – 2.679) | 0.3121 | |
| Hispanic | 2.269 (1.073 – 4.798) | 0.0319 | |
| Asian | 3.016 (0.411 – 22.138) | 0.2777 | |
| Other | 1.684 (0.231 – 12.248) | 0.6069 | |
| Missing | 1.301 (0.716 – 2.362) | 0.3875 | |
| Cirrhosis at SVR | no | 1.0 (ref) | |
| yes | 6.686 (4.319 – 10.350) | <.0001 | |
| Alcohol | no | 1.0 (ref) | |
| yes | 1.676 (1.082 – 2.595) | 0.0207 | |
| Age at SVR | < 45 | 0.559 (0.133 – 2.345) | 0.4269 |
| 45 – 54 | 1.0 (ref) | ||
| 55 – 64 | 2.043 (1.292 – 3.231) | 0.0023 | |
| 65+ | 4.509 (1.955 – 10.400) | 0.0004 | |
| HCV genotype | 1 | 1.0 (ref) | |
| 2 | 0.591 (0.324 – 1.077) | 0.0859 | |
| 3 | 1.620 (0.960 – 2.734) | 0.0709 |
CI: confidence intervals; SVR: sustained virological response
Secondary analyses
We repeated the analysis excluding 35 HCC cases that developed during the first year of follow up. Similar to the original analysis, the significant risk factors for HCC in this analysis remained cirrhosis, diabetes, Hispanic ethnicity, older age and genotype 3 (supplemental Table 2), with cirrhosis as the strongest risk factor. We also estimated HCC incidence among patients with and without cirrhosis but restricted to patients with prior genotype 1 and 2, no baseline diabetes, and younger than 65 years. HCC was diagnosed in 31 cases during 2552 PY follow up in 981 patients with cirrhosis, for a relatively high incidence rate (1.215% per year, 95% 1.167 – 1.262), however it was low in those without cirrhosis where a total of 25 HCC cases developed during 19147 PY follow-up (in 6673 patients with SVR and no cirrhosis pre SVR for an incidence rate of 0.131 (95% CI 0.129 – 0.132).
Discussion
This is the first large scale US cohort study to assess the risk of HCC following achievement of SVR among patients with CHC. As expected, successful treatment led to a considerable reduction in HCC risk. However, the annual risk of HCC among patients who cleared HCV was not negligible and ranged between 0.1% and 1.55% (overall 0.33%) in various subgroups. The highest residual HCC risk was observed among patients with cirrhosis at the time of treatment (1.39% annual risk), followed by patients who achieved SVR after age 65 (0.95% annual risk) irrespective of cirrhosis. The presence or development of diabetes constituted a further significant risk factor for HCC, whereas having HCV genotype 3 before SVR was the only virological factor associated with increased HCC risk after SVR. The risk of HCC appeared to be constant for several years after SVR. These findings have important implications for surveillance in the many patients who will achieve SVR with the new DAAs.
Our current findings argue strongly in favor of early treatment before the development of cirrhosis and in implementing or continuing HCC surveillance among patients with cirrhosis even after achieving SVR. The best possible time to achieve SVR is before the development of advanced fibrosis or cirrhosis. On the other hand, achieving SVR after the development of cirrhosis was still associated with a significantly elevated HCC risk; that risk (1.39%) exceeded the 1% per year threshold for which some clinical practice guidelines advocate continue HCC surveillance. Having an APRI greater than 2.0 was also predictive of increased HCC risk especially in those with documented cirrhosis (close to 2% per year), whereas absence of cirrhosis combined with low APRI was associated with the lowest risk of HCC in this cohort (0.06% per year); these two factors can be easily applied in clinical practice. While older age at time of SVR was a major risk factor for HCC, the annual incidence of HCC post SVR also seems to be constant over the first 5 years (Table 2) which argues against existing cancers before SVR which would have been detected early on in follow-up, or solely aging related cancer which would been seen mostly in late years of follow-up.
Most previous studies that evaluated risk HCC factors following SVR were conducted in Japan, and included predominantly patients without cirrhosis.8–13 In these studies, the reported HCC incidence ranged from 1.5 to 5% in the first 5 years following SVR.15 In our study the 5 year rate was 0.33%%. Ours is the largest study to date of patients with SVR. The large number of patient years follow up (30562.8 PY) and a sufficient number of outcome events (i.e., HCC) allowed precise calculation of HCC incidence rates as well as relative risk estimates of potentially important demographic, clinical, and virological HCC risk factors. Our study also had a major advantage of limiting the comparison to those who received treatment -- thus reducing the bias that could result from incomplete adjustment of contraindications for treatment (e.g., cirrhosis, alcohol use, HIV) which are also major confounders for HCC risk as well as overall survival (e.g., comorbid medical and psychiatric conditions).
Diabetes both that was present at time of SVR or developed during follow up was a significant risk factor for HCC. The presence of diabetes among those who progressed to HCC may be a reflection of the severity of underlying liver disease and/or development of cirrhosis, but may also exert carcinogenic effects indirectly via promoting hepatic steatosis or directly via disrupted insulin-IGF pathway and or inflammation.22 HCV genotype 3 was associated with higher HCC risk than other genotypes. Some of this observed association may be related to the known higher risk of cirrhosis with HCV genotype 323, and while we adjusted for cirrhosis, residual confounding by environment-gene joint effects24, as well as misclassification (particularly of early stages of cirrhosis) were likely present.
The retrospective study design, while allowing for an efficient well powered study, was limited by diagnostic bias related to nonstandard use of testing or surveillance for HCC. However, given the relatively long follow up we believe that most HCCs in this cohort would have declared themselves within the study period. Similarly, misclassification was possible in potential risk factors of HCC (e.g., diabetes, obesity). We did not have information on duration of obesity, glycemic control in diabetes, or dose or duration of alcohol drinking. However, it was highly unlikely that patients with ongoing or severe alcohol use were treated with interferon and therefore we do not believe that alcohol abuse was an important factor in this study. While we had information on cirrhosis, we did not have more granular information on the severity of hepatic fibrosis and no information on hepatic steatosis. HCC diagnosis may have been misclassified in some cases; however, of the 22 cases of the 100 HCCs that were chart reviewed 21 were verified as true HCC (i.e., PPV 95%). While the study showed a beneficial effect of treatment in reducing HCC, selection bias related to who received the treatment was likely to be present. However, this was not the main purpose of the study; we rather focused on treated patients and compared those with SVR vs no SVR; this analysis eliminates the selection bias related to decision to treat. Lastly the generalizability of the findings to non-veterans and women is unknown and will need to be examined in future studies. However, all patients who achieved SVR in this study did so with the use of interferon with or without ribavirin. It is not clear whether the same HCC risk reduction will be seen with SVR related to DAAs. It is possible that interferon related anti proliferative properties would result in a greater HCC risk reduction than interferon-free DAAs.
An increasing number of patients are getting treated and cured of their CHC. Understanding the prognosis of these patients is timely as well as important. HCC risk was considerably and significantly lowered among those who achieved SVR than those who were treated but with no SVR. The high efficacy and safety of interferon free DAA in patients with cirrhosis is likely to result in a number of patients with HCV cured and cirrhosis that is much larger than we have seen before.5;7
We found in a large national VA cohort of patients who achieved SVR with interferon-based therapy have a considerably reduced risk compared to those treated with no SVR. However, the risk of HCC remains elevated for several years after SVR especially in subgroups of patients with cirrhosis, diabetes and the elderly. These findings support early HCV treatment before the development of cirrhosis and continued HCC surveillance even after SVR among those who already developed cirrhosis.
Supplementary Material
Reference List
- 1.Mohd HK, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57:1333–1342. doi: 10.1002/hep.26141. [DOI] [PubMed] [Google Scholar]
- 2.Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509–516. doi: 10.1016/j.cgh.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Goodgame B, Shaheen NJ, Galanko J, El-Serag HB. The risk of end stage liver disease and hepatocellular carcinoma among persons infected with hepatitis C virus: publication bias? Am J Gastroenterol. 2003;98:2535–2542. doi: 10.1111/j.1572-0241.2003.07678.x. [DOI] [PubMed] [Google Scholar]
- 4.Muir AJ, Naggie S. Hepatitis C Virus Treatment: Is It Possible To Cure All Hepatitis C Virus Patients? Clin Gastroenterol Hepatol. 2015;13:2166–2172. doi: 10.1016/j.cgh.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sussman NL, Remien CH, Kanwal F. The end of hepatitis C. Clin Gastroenterol Hepatol. 2014;12:533–536. doi: 10.1016/j.cgh.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013;158:329–337. doi: 10.7326/0003-4819-158-5-201303050-00005. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt WN, Nelson DR, Pawlotsky JM, Sherman KE, Thomas DL, Chung RT. Direct-acting antiviral agents and the path to interferon independence. Clin Gastroenterol Hepatol. 2014;12:728–737. doi: 10.1016/j.cgh.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda M, Fujiyama S, Tanaka M, et al. Risk factors for development of hepatocellular carcinoma in patients with chronic hepatitis C after sustained response to interferon. J Gastroenterol. 2005;40:148–156. doi: 10.1007/s00535-004-1519-2. [DOI] [PubMed] [Google Scholar]
- 9.Chang KC, Hung CH, Lu SN, et al. A novel predictive score for hepatocellular carcinoma development in patients with chronic hepatitis C after sustained response to pegylated interferon and ribavirin combination therapy. J Antimicrob Chemother. 2012;67:2766–2772. doi: 10.1093/jac/dks269. [DOI] [PubMed] [Google Scholar]
- 10.Arase Y, Kobayashi M, Suzuki F, et al. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology. 2013;57:964–973. doi: 10.1002/hep.26087. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita N, Ohho A, Yamasaki A, Kurokawa M, Kotoh K, Kajiwara E. Hepatocarcinogenesis in chronic hepatitis C patients achieving a sustained virological response to interferon: significance of lifelong periodic cancer screening for improving outcomes. J Gastroenterol. 2014;49:1504–1513. doi: 10.1007/s00535-013-0921-z. [DOI] [PubMed] [Google Scholar]
- 12.Huang CF, Yeh ML, Tsai PC, et al. Baseline gamma-glutamyl transferase levels strongly correlate with hepatocellular carcinoma development in non-cirrhotic patients with successful hepatitis C virus eradication. J Hepatol. 2014;61:67–74. doi: 10.1016/j.jhep.2014.02.022. [DOI] [PubMed] [Google Scholar]
- 13.Chang KC, Tseng PL, Wu YY, et al. A polymorphism in interferon L3 is an independent risk factor for development of hepatocellular carcinoma after treatment of hepatitis C virus infection. Clin Gastroenterol Hepatol. 2015;13:1017–1024. doi: 10.1016/j.cgh.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 14.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li DK, Chung RT. Impact of hepatitis C virus eradication on hepatocellular carcinogenesis. Cancer. 2015;121:2874–2882. doi: 10.1002/cncr.29528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Backus LI, Gavrilov S, Loomis TP, et al. Clinical Case Registries: simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc. 2009;16:775–783. doi: 10.1197/jamia.M3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer JR, Kanwal F, Richardson P, Mei M, El-Serag HB. Gaps in the achievement of effectiveness of HCV treatment in national VA practice. J Hepatol. 2012;56:320–325. doi: 10.1016/j.jhep.2011.05.032. [DOI] [PubMed] [Google Scholar]
- 19.Davila JA, Weston A, Smalley W, El-Serag HB. Utilization of screening for hepatocellular carcinoma in the United States. J Clin Gastroenterol. 2007;41:777–782. doi: 10.1097/MCG.0b013e3180381560. [DOI] [PubMed] [Google Scholar]
- 20.Kramer JR, Davila JA, Miller ED, Richardson P, Giordano TP, El-Serag HB. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27:274–282. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94:496–509. [Google Scholar]
- 22.El-Serag HB, Kanwal F. Obesity and hepatocellular carcinoma: hype and reality. Hepatology. 2014;60:779–781. doi: 10.1002/hep.27172. [DOI] [PubMed] [Google Scholar]
- 23.Kanwal F, Kramer JR, Ilyas J, Duan Z, El-Serag HB. HCV genotype 3 is associated with an increased risk of cirrhosis and hepatocellular cancer in a national sample of U.S. Veterans with HCV. Hepatology. 2014;60:98–105. doi: 10.1002/hep.27095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanwal F, White DL, Jiao L, et al. Genetic Variants in Interleukin-28B Are Associated with Diabetes and Diabetes-Related Complications in Patients with Chronic Hepatitis C Virus Infection. Dig Dis Sci. 2015;60:2030–2037. doi: 10.1007/s10620-015-3545-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.