Abstract
Purpose
This case series highlights the novel use of intravitreal melphalan for non-vitreous retinoblastoma. It assesses the efficacy and toxicity of intravitreal melphalan for non-vitreous retinoblastoma.
Methods
This observational small case series, investigates three patients treated with intravitreal melphalan for non-vitreous retinoblastoma that was refractory to multiple-course ophthalmic artery chemosurgery (OAC). Patients’ demographics, response to treatment, toxicity of treatment as clinically evaluated and measured by electroretinogram (ERG).
Patients
Three eyes of three patients received a median of 7 weekly intravitreal melphalan injections (30μg/0.07cc) for persistent retinal or subretinal tumors refractory to treatment with multiple-course OAC.
Results
Eyes remain tumor-free at a median of 14 months follow-up. One eye was enucleated due to a vitreous hemorrhage that obscured fundus details. One eye had extinguished ERG recordings prior to injections and two eyes had a decrease in ERG responses over the intravitreal treatment course. The eye with subretinal seeding demonstrated marked retinopathy by ophthalmoscopy and fluorescein angiography and one eye was enucleated due to the development of a vitreous hemorrhage.
Conclusion
This small case series highlights that non-vitreous disease that is refractory or persistent despite prior OAC can regress with intravitreal melphalan. However this treatment may result in retinal toxicity.
Keywords: retinoblastoma, melphalan, intravitreal injection, chemotherapy, retina
Introduction
In 2014, there were at least eight papers dedicated to the subject of intravitreal melphalan for vitreous disease1-8. With the use of intravitreal melphalan, eyes that were once enucleated for refractory vitreous seeds now demonstrate historically high ocular survival rates1,9,10. While there has not been a formal head-to-head study, intravitreal melphalan is considered superior to ophthalmic artery chemosurgery for controlling vitreous seeds11.
This present study reviews our initial experience with a novel indication for intravitreal melphalan: refractory retinal or subretinal disease. We describe three cases of retinal or subretinal disease that progressed despite, or was refractory to, multiple-course ophthalmic artery chemosurgery (OAC) and was subsequently treated with intravitreal melphalan injections in an attempt to avoid enucleation.
Methods and Materials
This was an observational study for which Institutional Review Board approval was acquired from Memorial Sloan Kettering and informed consent was obtained for each patient. This study included patients who received and completed intravitreal melphalan injections for the unlabeled treatment of non-vitreous disease that was refractory to multiple-course ophthalmic artery chemosurgery (with or without focal therapy). Follow-up period is from the initial intravitreal melphalan injection.
The intravitreal melphalan injection was prepared and administered in a manner that has been previously been described5. All weekly intravitreal injections were composed of 30μg/0.07mL melphalan, injected approximately 3 to 3.5mm posterior to the limbus and treated with cryotherapy at the injection site during needle withdrawal. The needle was positioned in the eye according to the type of disease and proximity of tumor: the needle shaft was inserted with the shaft internalized approximately 10% for the case of subretinal disease; and inserted fully into the center of the eye for retinal disease. In the former instance, the intent was to inject as close to the retinal surface as possible to increase the concentration of the dose at the retinal surface to target the subretinal disease (which, due to the absence of subretinal fluid, was in close proximity to the outer retinal surface). Electroretinogram (ERG) recordings were obtained during regularly scheduled examination under anesthesia as previously described5. Pre-treatment measurements from the day of the first injection are compared with those taken one month following the last injection, and measurements at the most recent follow-up.
Case Reports
Patient, tumor, treatment and ERG outcomes are summarized in Table 1. Patient 1, a 67-month-old male was diagnosed with bilateral disease at 1 month of age. He received 3 cycles of intravenous chemotherapy, transpupillary thermotherapy (TTT) to both eyes and 3 infusions of OAC (melphalan and topotecan) to the left eye. Over the next four years, he developed 3 episodes of juxtapapillary retinal recurrence and was treated with 3 additional courses of OAC (5 infusions) and TTT. However, the retinal tumor recurred and he was treated with 8 injections of 30μg melphalan with complete resolution of the tumor (figure 1). He remains tumor free at 14 months follow-up.
Table 1.
Characteristics of patients, tumors, treatment and ERG outcomes
| Patient | Age (mos) | RE | IC | Laterality | Disease type | Prior treatment | Prior OAC (infusions) | No. of injections | Follow-up (mos) | ERG measurements |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | IIA | B | Bilateral | Retinal | IVC, L, Cr | 8 | 8 | 14.2 | Very good to good |
| 2 | 27 | IIIA | D | Bilateral | Retinal | IVC, L | 3 | 7 | 11.0 | Stable at extinguished |
| 3 | 13 | VA | D | Bilateral | Subretinal | IVC, L | 3 | 2 | 15.6 | Fair to extinguished |
Age = age at first injection, RE = Reese-Ellsworth, IC = International Classification, IVC = intravenous chemotherapy, L = laser, Cr = cryotherapy, OAC = ophthalmic artery chemosurgery, No. = number, ERG = electroretinogram.
Figure 1.
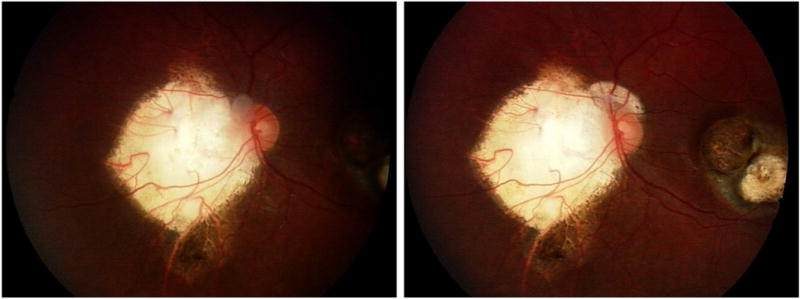
Patient 1 showing response of retinal disease to intravitreal melphalan. Left: Left eye of patient 1 showing pre/peripapillary tumor recurrence following a treatment course of systemic chemotherapy, laser and four courses of ophthalmic artery chemosurgery (OAC) (8 infusions). Right: Left eye of patient 1 with tumor regression following 8 injections of intravitreal melphalan.
Patient 2, a 3-month-old female was diagnosed with bilateral retinoblastoma and was treated with systemic chemotherapy at an outside institution. She was referred to our center and had 3 OAC infusions to the left eye (3-4mg melphalan and 0.3-0.4mg topotecan). She responded well and a type 2 regressed extension jutting from her main calcified tumor was observed. Over the next 18 months, this proboscis-like extension continued to vascularize, enlarge in size and form impending seed-like buds. Vascularization of this suspicious area was confirmed with fluorescein angiography. Following 7 injections of 30μg melphalan, this proboscis-like area regressed into a calcified fragment. Eleven months following the injections, the eye developed vitreous hemorrhage, which obscured fundus details and limited the ability to clinically follow for active disease. The eye was enucleated and no viable tumor was found on pathology.
Patient 3, a 5-month-old female was diagnosed with bilateral retinoblastoma and due to anomalous carotid arteries, OAC was not possible at presentation. She was treated with 5 cycles of systemic chemotherapy. The main macula tumor responded, but persistent subretinal seeds spanned nine clock-hours of the peripheral retina. At 11 months of age, after the carotid artery had time to further develop, OAC was re-attempted. Three infusions of OAC were successfully given via the traditional ophthalmic artery route but subretinal disease persisted, and treatment options were limited. The family agreed to intravitreal melphalan, and following 2 injections of 30μg melphalan, the patient developed a severe retinopathy (figure 2). One month following this, the subretinal seeds, hemorrhage, edema and vasculitis resolved and the patient remains tumor free 16 months later.
Figure 2.
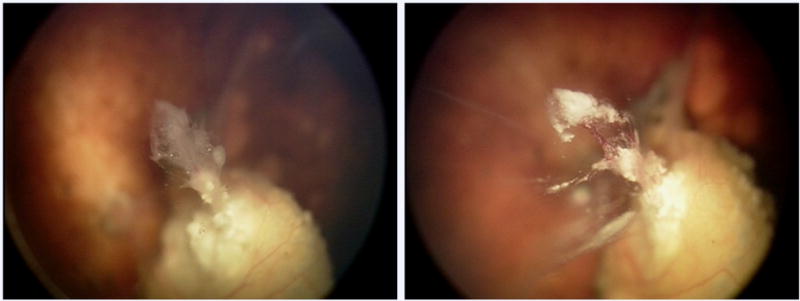
Patient 2 showing response of retinal disease to intravitreal melphalan. Left: Following three courses of ophthalmic artery chemosurgery (OAC), the right eye of patient 2 shows active tumor extending from the main calcified tumor. Right: Right eye of patient 2 with calcific tumor regression following 7 injections of intravitreal melphalan.
Discussion
This report describes three cases of non-vitreous disease that persisted or recurred despite treatment with OAC, and was successfully treated with intravitreal melphalan. Cases include pre-/peri-papillary tumor, a proboscis of tumor extending from a calcified tumor and peripheral subretinal seeding all refractory to, or persistent despite laser or OAC.
As is demonstrated in this series, intravitreal melphalan injections may result in degraded ERG responses5. For example, the patient with clinical retinitis/vasculitis (patient 3) experienced extinguished ERG responses following two injections. This toxicity was in the face of imminent enucleation and is presumably related to the proximal delivery of the melphalan to the retinal surface, intentionally targeting the subretinal disease. One eye developed a vitreous hemorrhage that may have been related to treatment, and even though it came to enucleation, there was no viable tumor found on pathology.
This series suggests that in certain instances, the indication for intravitreal melphalan may be extended to include retinal and subretinal refractory disease, although this may be at the expense of retinal toxicity. We look forward to confirmation of these preliminary observations by a larger series of cases and long-term follow up.
Supplementary Material
Figure 3.
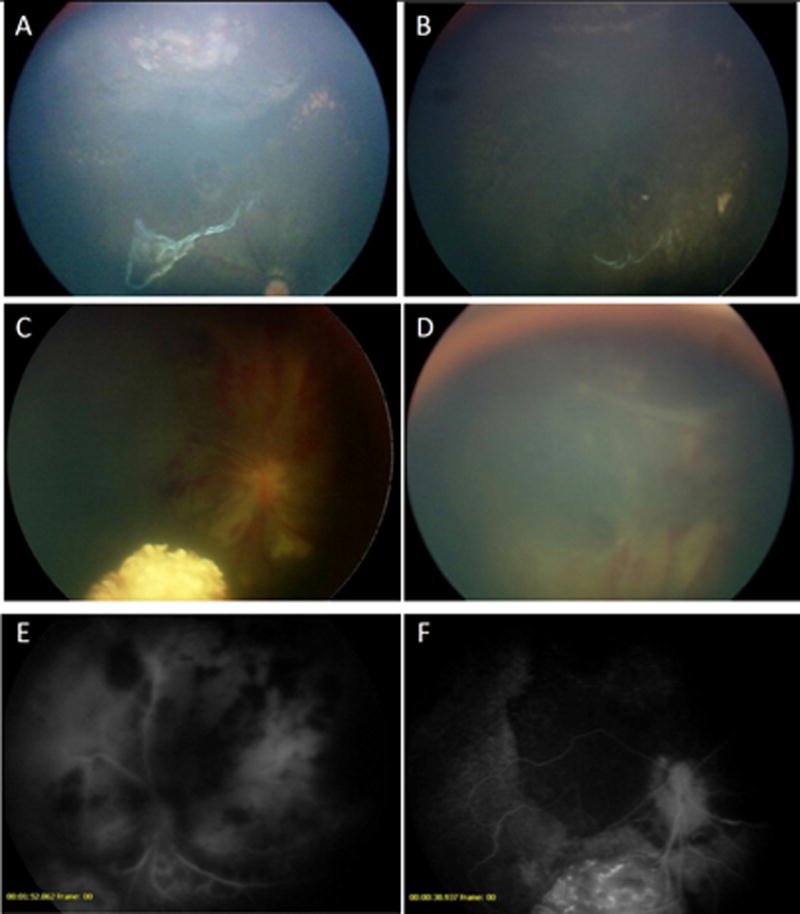
Patient 3 showing response of subretinal disease to intravitreal melphalan. A. Persistent subretinal disease following systemic chemotherapy and 3 infusions of OAC. B. Nine months following 2 intravitreal injections of melphalan, the eye remains tumor free. C & D. One week following the second intravitreal melphalan injection, four quadrants of intraretinal hemorrhage with edema and vasculitis were observed. E. Fluorescein angiogram at that time demonstrates blockage from the hemorrhage, and leaky retinal and choroidal blood vessels. F. The fluorescein angiogram 3 months later demonstrates patchy hypoperfusion of the choroid and retina, particularly superiorly, where the injection was given.
Summary Statement.
This series reports on 3 patients with non-vitreous disease that failed OAC including retinal and subretinal retinoblastoma which responded to intravitreal melphalan injections but at the expense of retinal toxicity.
Acknowledgments
This work was supported by the Fund for Ophthalmic Knowledge.
Footnotes
None of the authors have any financial disclosures or conflicts.
References
- 1.Munier FL. Classification and management of seeds in retinoblastoma. Ellsworth Lecture Ghent August 24th 2013. Ophthalmic Genet. 2014;35(4):193–207. doi: 10.3109/13816810.2014.973045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shah NV, Pham DG, Murray TG, et al. Intravitreal and subconjunctival melphalan for retinoblastoma in transgenic mice. Journal of Ophthalmology. 2014;2014:829879. doi: 10.1155/2014/829879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith SJ, Smith BD, Mohney BG. Ocular side effects following intravitreal injection therapy for retinoblastoma: a systematic review. Br J Ophthalmol. 2014;98(3):292–297. doi: 10.1136/bjophthalmol-2013-303885. [DOI] [PubMed] [Google Scholar]
- 4.Ghassemi F, Shields CL, Ghadimi H, Khodabandeh A, Roohipoor R. Combined intravitreal melphalan and topotecan for refractory or recurrent vitreous seeding from retinoblastoma. JAMA Ophthalmol. 2014;132(8):936–941. doi: 10.1001/jamaophthalmol.2014.414. [DOI] [PubMed] [Google Scholar]
- 5.Local and Systemic Toxicity of Intravitreal Melphalan for Vitreous Seeding in Retinoblastoma: A Preclinical and Clinical Study.
- 6.buitrago E, Lagomarsino E, Mato G, Schaiquevich P. Stability of Melphalan Solution for Intravitreal Injection for Retinoblastoma. JAMA Ophthalmol. 2014 doi: 10.1001/jamaophthalmol.2014.2324. [DOI] [PubMed] [Google Scholar]
- 7.Francis JH, Marr BP, Brodie SE, Gobin YP, Abramson DH. Tethered vitreous seeds following intravitreal melphalan for retinoblastoma. JAMA Ophthalmol. 2014;132(8):1024–1025. doi: 10.1001/jamaophthalmol.2014.436. [DOI] [PubMed] [Google Scholar]
- 8.Shields CL, Manjandavida FP, Arepalli S, Kaliki S, Lally SE, Shields JA. Intravitreal melphalan for persistent or recurrent retinoblastoma vitreous seeds: preliminary results. JAMA Ophthalmol. 2014;132(3):319–325. doi: 10.1001/jamaophthalmol.2013.7666. [DOI] [PubMed] [Google Scholar]
- 9.Ghassemi F, Shields CL. Intravitreal melphalan for refractory or recurrent vitreous seeding from retinoblastoma. Arch Ophthalmol. 2012;130(10):1268–1271. doi: 10.1001/archophthalmol.2012.1983. [DOI] [PubMed] [Google Scholar]
- 10.Munier FL, Gaillard M-C, Balmer A, Beck-Popovic M. Intravitreal chemotherapy for vitreous seeding in retinoblastoma: Recent advances and perspectives. Saudi J Ophthalmol. 2013;27(3):147–150. doi: 10.1016/j.sjopt.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abramson DH, Marr BP, Dunkel IJ, et al. Intra-arterial chemotherapy for retinoblastoma in eyes with vitreous and/or subretinal seeding: 2-year results. Br J Ophthalmol. 2012;96(4):499–502. doi: 10.1136/bjophthalmol-2011-300498. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


