Abstract
We recently reported that immune stimulation can be compromised if animals are simultaneously subjected to stressful conditions. To test the generalizability of these findings, and to elucidate neuroendocrine mediating mechanisms, we herein employed CpG-C, a novel TLR-9 immune-stimulating agent. Animals were subjected to ongoing stress (20-hrs of wet cage exposure) during CpG-C treatment, and antagonists to glucocorticoids, β-adrenoceptor, COX2, or opioids were employed (RU486, nadolol, etodolac, naltrexone). In F344 rats, marginating-pulmonary NK cell numbers and cytotoxicity were studied, and the NK-sensitive MADB106 experimental metastasis model was used. In Balb/C mice, experimental hepatic metastases of the CT-26 colon tumor were studied; and in C57BL/6J mice, survival rates following excision of B16 melanoma was assessed – both mouse tumor models involved surgical stress. The findings indicated that simultaneous blockade of glucocorticoid and β-adrenergic receptors improved CpG-C efficacy against MADB106 metastasis. In mice bearing B16 melanoma, long-term survival rate was improved by CpG-C only when employed simultaneously with blockers of glucocorticoids, catecholamines, and prostaglandins. Prolonged stress impaired CpG-C efficacy in potentiating NK activity, and in resisting MADB106 metastasis in both sexes, as also supported by in vitro studies. This latter effect was not blocked by any of the antagonists or by adrenalectomy. In the CT26 model, prolonged stress only partially reduced the efficacy of CpG-C. Overall, our findings indicate that ongoing behavioral stress and surgery can jeopardize immune-stimulatory interventions and abolish their beneficial metastasis-reducing impacts. These findings have implications for the clinical setting, which often involve psychological and physiological stress responses during immune-stimulation.
Keywords: immune stimulation, CpG-C, NK, ongoing stress, glucocorticoids, catecholamines, prostaglandins
1. Introduction
Immune stimulation by biological response modifiers (BRMs), is becoming an effective therapeutic modality in various medical settings and pathologies [1, 2]. Unfortunately, anti-cancer immunostimulatory interventions have been markedly more successful in animal studies than in clinical trials [3, 4]. Our previous studies provided evidence suggesting that exposure to stress simultaneously with immunostimulatory treatment can impair the efficacy of immune stimulation [5, 6]. Unlike in common animal models, patients are often subjected to physiological and psychological stressors while treated with immunostimulatory agents, which may partially account for the lower success rate of immunostimulatory treatment in the clinical setting.
CpG-C is a synthetic oligodeoxynucleotide (ODN), containing unmethylated cytosine deoxynucleotide followed by guanine deoxynucleotide motifs, which was shown to increase antigen presentation and to enhance anti-tumor responses [7, 8]. Through ligand binding of TLR-9, CpG-C induces secretion of type 1 interferons and expression of co-stimulatory molecules by plasmacytoid dendritic cells (pDCs), which consequently activate cell-mediated immune responses, carried through natural killer (NK) cells, cytotoxic T cells (CTLs), and T-helper-1 (TH1) cells [9, 10]. We have previously reported that CpG-C increased NK cell cytotoxicity in F344 rats, protected NK cells from suppression by β-adrenoceptor activation, and markedly improved post-operative immune status, as reflected by in vivo NK-dependent resistance to experimental metastases, and by increased levels of plasma IL-12 and pDC maturation markers [11-13].
Stress hormones, including glucocorticoids (GCs) and catecholamines (CAs), were shown to exert immunosuppressive effects through impacting the TH1/TH2 cytokine balance. In our previous studies, we have shown that CAs, GCs, and prostaglandins can suppress several aspects of cell-mediated immunity in vivo, including levels of NK activity and NK-dependent resistance to experimental metastasis [14-19], and plasma levels of IL-12 [20]. Opioids were also implicated in immune modulation and were shown to inhibit cellular and humoral immunity, including cytokine production and NK cell activity [21]. It is worthy to note that various types of immune stimulation (BRMs) were reported to induce stress responses, including activation of the SNS and HPA axis [22], which could constitute a negative feedback loop.
These actions of stress hormones may counteract the beneficial effects of CpG-C described above, as both CpG-C and stress hormones seem to exert their effects via altering the TH1/TH2 cytokine balance. Indeed, in our previous studies in mice, stress during treatment with CpG-C decreased the elevation in IL-12 plasma levels, the number of circulating pDCs (and other DC subtypes), and the expression of co-stimulatory molecules (i.e. CD80 and CD86) on these cells [12]. In rats, we have demonstrated that various stress paradigms during treatment with IL-12 significantly disrupted the ability of IL-12 to generate immune stimulation, and in some cases completely abolished it [23]. Interestingly, it remains unclear whether the disruption of immune-stimulation by stress is the mere summation of two opposing influences on the same outcome - the beneficial effects of a BRM versus the deleterious effects of stress - or rather a result of a unique mechanism through which stress-induced responses actively inhibit the capacity of immunocytes to be stimulated by BRMs.
In the current study we focused on in vivo approaches, aiming to assess the efficacy of CpG-C immune stimulation in different animal models of metastatic progression. We aimed to (i) test whether subjecting animals to ongoing stress concurrently with CpG-C immune stimulation could dampen the treatment's efficacy, and (ii) explored the involvement of specific immune mechanisms and different stress hormones, in vitro and in vivo, in modulating the efficacy of CpG-C in the context of prolonged stress exposure (or stress hormones).
2. Materials and Methods
2.1 Animals and counterbalancing
Male and female Fischer 344 (F344) rats, and Balb/C, and C57BL/6J mice (Harlan Laboratories, Jerusalem, Israel), 12-13 weeks old, were housed 4 per cage with free access to food and water on a 12:12 light:dark cycle at 22-24°C. Animals were acclimated to the vivarium for at least 3 weeks prior to experimentation and rats were handled daily during the last week prior to experimentation to reduce potential procedural stress. Order of drug administration and tumor injection was counterbalanced across groups in each experiment. Housing conditions are regularly monitored by the Institutional Animal Care and Use Committee of Tel Aviv University, which also approved all studies described herein.
2.2 The induction of acute stress through surgery or β-adrenoceptor stimulation
In addition to prolonged stress exposure concurrently with CpG-C treatment, the final outcome of CpG-C immune stimulation was often tested in the context of acute stressors, such as surgery or exposure to a β-adrenergic challenge. These acute stressors were used for two reasons - they often characterize the clinical setting where immune-stimulatory approaches are used or could be beneficial (e.g., having cancer or undergoing surgical removal of a primary tumor), and because they often better indicate the beneficial effects of immune stimulation [11, 23].
2.3 The wet cage stress paradigm
Animals were placed in cages filled with room-temperature water to the height of 2 cm (for rats) or 1 cm (for mice), with free access to food and water, for a total period of 20 hrs, with a 2 hrs recess period (in the animals' home-cages) that began 9 hrs after initiation of the protocol. This stress protocol was initiated 4-6 hrs after the onset of the light period.
2.4 Assessment of plasma corticosterone levels
Blood for assessment of plasma corticosterone (CORT) levels was drawn from the tail vein into heparinized test tubes. Plasma CORT levels were measured by radioimmunoassay (RIA) (ImmuChem double antibody corticosterone 125I RIA kit, MP Biomedicals, Orangeburg, NY), per manufacturer's instructions.
2.5 Drugs and their administration
For additional information regarding the pharmacokinetic profile of each drug and their respected half-life, please refer to the Supplementary data.
2.5.1 CpG-C
(Sigma, Israel), CpG-C (ODN 2395: 5’-TCGTCGTTTTCGGCGCGCGCCG-3’) with a phosphorothioate backbone. Purity examination by the limulus amebocyte lysate assay resulted with undetectable levels of endotoxin. CpG-C was dissolved in PBS (phosphate-buffered saline) and was administered intraperitoneally (i.p.) at a dose of 165 μg/kg to rats and 50μg per animal to mice [11].
2.5.2 Metaproterenol
(Sigma, Israel), a non-selective β-adrenergic agonist with a higher affinity to β2 than to β1 receptors [24]. Metaproterenol was dissolved in PBS and administered subcutaneously (s.c.) at a dose of 3 mg/kg, This dose was based on our previous studies, mimicking the effects caused by other stressors such as surgery or swim stress [16, 25].
2.5.3 Epinephrine
(Sigma, Israel), adrenoceptor agonist. The drug was dissolved in a slow release vehicle (see below) and administered s.c. at a dose of 1.2 mg/kg [26].
2.5.4 RU486
(Sigma, Israel), progesterone and glucocorticoid receptor antagonist. The drug was dissolved in corn oil and was administered s.c. at a dose of 25 mg/kg [19].
2.5.5 Nadolol
(Sigma, Israel), a non-selective β-adrenergic blocker. The drug was dissolved in PBS and administrated s.c. at a dose of 0.4 mg/kg [27].
2.5.6 Propranolol
(Sigma, Israel) a non-selective β-adrenergic blocker. The drug was dissolved in a slow release vehicle (see below) and administered s.c. at a dose of 5 mg/kg [15].
2.5.7 Naltrexone
(Sigma, Israel), a wide-range opioid receptor antagonist. The drug was dissolved in PBS and administrated i.p. at a dose of 2 mg/kg [28].
2.5.8 Etodolac
a semi-selective COX2 inhibitor, kindly donated by Taro, Israel. The drug was dissolved in corn oil and was administered s.c. at a dose of 50 mg/kg [15].
2.5.9 Slow release vehicle
The slow release vehicle (SRV) is an emulsion used to extend absorption time of drugs, and is based on 4 parts PBS, 3 parts mineral oil (Sigma, Israel), and 1 part mannide-monooleate (a non-specific surface active emulsifier, Sigma, Israel). Unpublished data from our laboratory indicated that a β-adrenergic antagonist administered in the slow release vehicle exerted its effects 9 and 12 hrs following injection, whereas the same dose of drug administered in saline had no effects at these time points.
2.6 Experimental laparotomy
The procedure has been described in details ‘elsewhere [29]. Briefly, rats were anesthetized and maintained with 2.5% isoflurane and a 4 cm midline abdominal incision was performed. The small intestine was externalized, rubbed with a PBS-soaked gauze pad and left hydrated with a PBS-soaked gauze pad for 30 mins. Finally, the intestine was internalized and the abdomen sutured.
2.7 Adrenalectomy and sham operation
Rats were anesthetized and maintained with 2.5% isoflurane and a 4 cm midline abdominal incision was performed. Both adrenal glands were exposed and removed completely using standard surgical techniques. Sham-operated rats underwent the exact same procedure without removal of the adrenal glands. In order to expedite recovery from surgery, adrenalectomized rats were subsequently injected s.c. three times, 3 hrs apart, with 1 mg/kg corticosterone per injection. Following surgery, and to ensure complete recovery, rats were kept in their home cages undisturbed for 4–6 weeks before entering experiments. To overcome the lack of mineralocorticoids, and to approximate normal levels of corticosterone, adrenocorticotropic hormone (ACTH), and their circadian rhythms, the regular drinking water of adrenalectomized rats was replaced with 0.9% NaCl (saline) containing 15 mg corticosterone/liter. Previous studies showed that this method approximates normal circadian corticosterone levels (~20–100 ng/L, [30] and unpublished data from our laboratory), as rats drink mostly during their first activity hours [31]. Two hours before experimentation, and to ensure low physiological corticosterone levels, adrenalectomized rats were given only saline without corticosterone as their drinking water.
2.8 Surgical procedure for CT26 tumor cell injection through the spleen
Mice were anesthetized and maintained with 2.5% isoflurane and a 0.5 cm abdominal incision was performed adjacent to the spleen (a left flank incision approximately 2 cm left of the abdominal midline). 2×104 CT26 tumor cells in 100μl PBS were injected into the spleen (and naturally drained into the splenic and portal veins, and deposited in the liver), using a 31G needle, which was maintained in the spleen tissue for two minutes following injection. A 4/0 blue polypropylene monofilament non-absorbable suture was placed across the hilum of the spleen to prevent bleeding, and a splenectomy was then performed. After the excision of the contaminated spleen, the peritoneum and skin were sutured. The animals were allowed to recover in their home cages.
Assessment of metastatic development
Animals were monitored daily for general well-being after tumor injection, and euthanized with an overdose of isoflurane on the 20th day. Livers were then harvested and weighed, and surface-hepatic metastases were counted by an experimenter blind to the experimental group of each animal. Metastases were identified as being greater than 1 mm in diameter, forming a spherical solid and distinct formation.
2.9 B16 melanoma tumor cell inoculation, and tumor development and excision
Mice were orthotopically injected with 105 B16 melanoma cells into the right footpad subcutaneous space (in a volume of 20 μl). Developing tumors were visually inspected daily thereafter. Once a tumor reached a 100μl volume (tumor length × width × height), the mouse underwent tumor excision by paw amputation. For the amputation, mice were anesthetized and maintained with 3% isoflurane, and the tumor-bearing hind-paw was amputated with surgical scissors 2mm above the ankle joint. The wound was treated with an antiseptic paste (10% povidone iodine). Mice were subsequently monitored for morbidity signs on a daily basis for an 80-day period (more than 3 weeks following the last morbidity incidence). Mice showing sickness behavior or manifesting cancer recurrence above the amputation site were overdosed with isoflurane and autopsied to determine malignant foci. Sickness behavior was defined by slow body movements, non-responsiveness to environmental stimuli, tremor, or loss of more than 10% body weight.
2.10 Tumor cell lines and their maintenance
2.10.1 MADB106
MADB106 is a selected variant cell line obtained from a pulmonary metastasis of a chemically induced mammary adenocarcinoma (MADB100) in the F344 rat [32]. MADB106 tumor cells metastasize only to the lungs, a process that is dependent upon NK cells [32]. The lung tumor retention (LTR) of MADB106 cells is highly indicative of the number of metastases that would have developed weeks later [16, 32-34]. Additionally, because the metastatic process of MADB106 is sensitive to NK activity predominantly in the first 24 hours following inoculation [32, 33], LTR is more reflective of in vivo NK activity levels than the number of actual metastases [16]. The MADB106 cell line was maintained in monolayer cultures in complete media (CM) (RPMI-1640 media supplemented with 10% heat-inactivated fetal calf serum (FCS), 50μg/mL of gentamicin, 2mM of L-glutamine, 0.1mM of non-essential amino-acids, and 1mM of sodium pyruvate, Biological Industries, Kibbutz Biet Haemek, Israel) in 100% humidity, 5% CO2 at 37°C. Cells were removed from the culture flask with trypsin solution (0.25% in PBS), and were washed with CM. This cell line was used for both in vivo assessment of lung tumor retention and ex-vivo examination of NK cytotoxicity.
Radiolabeling of MADB106 tumor cells for the assessment of lung tumor retention
Tumor cell DNA radiolabeling for assessment of LTR was accomplished by adding 0.5 μCi/ml of 125Iododeoxyuridine (125IDUR, Eisenberg bros., Israel) to the cell culture for 24 hrs.
Radiolabeling of MADB106 tumor cells for the assessment of MP-NK cytotoxicity
Radiolabeling of the target cells was conducted by incubating them for 1 hour with 100 μCi 51Cr (Rotem Taassiot, Dimona, Israel) in 100μl saline, 100μl FCS, and 50μl CM. Following this incubation, target cells were washed 3 times (300×g for 10 min) in CM and their concentration adjusted to 5×104/ml.
2.10.2 CT26
The CT26 murine colon carcinoma cell line is a chemically-induced undifferentiated carcinoma, syngeneic to the Balb/C strain [35]. Cells were grown in monolayer cultures in CM, at 37°C, 100% humidity, and 5% CO2.
Cells were removed from the culture flask with a 0.25% trypsin solution in PBS, washed once in PBS containing 0.1mg/ml BSA (335×g for 10 min), and adjusted to a final concentration of 2×105/ml in PBS-BSA for spleen injection at a volume of 100μl per animal. Cells were kept on ice during the entire injection procedure in each of the experiments. No effects of duration of this pre-inoculation, in vitro tumor cell maintenance, on the number of developing liver metastases, were evident.
2.10.3 B16F10.9
B16F10.9 (B16) melanoma cells, syngeneic to the C57BL/6J mouse strain, were kindly provided by Prof. Amiram Raz (Department of Biochemistry, Faculty of Life Sciences, Tel-Aviv University, obtained from Drs. M.L. Kripke and I.J. Fidler from the University of Texas -M.D. Anderson Hospital, Houston, TX). Cells were grown in cultures in 5% CO2, 100% humidity, 37°C, in CM.
2.11 Inoculation with MADB106 tumor cells and assessment of lung tumor retention
Rats were lightly anesthetized with isoflurane, and 4×105/kg MADB106 tumor cells in 2ml/kg PBS containing 0.1% bovine serum albumin (BSA) were injected into their tail vein. For assessment of LTR, animals were sacrificed with CO2 24 hrs after inoculation with 125IDUR-labeled tumor cells, their lungs were removed and placed in a -counter to assess percent radioactivity retained in this organ. LTR was calculated using the following formula: (radioactivity count of lung – background radioactivity) × 100/(radioactivity count of the total injected cell suspension – background radioactivity).
2.12 In vitro assessment of NK cytotoxicity
Harvesting and preparing marginating-pulmonary leukocytes for assessment of NK cytotoxicity
Rats were sacrificed with an overdose of isoflurane and the peritoneal and chest cavities opened. Marginating-pulmonary leukocytes were harvested by perfusing the heart with 30U/ml of heparinized PBS. PBS was injected into the right ventricle and perfusate was collected from the left ventricle. The first 3ml of blood and 3ml of perfusate, which were contaminated with blood, were discarded, and the following 25ml were collected and concentrated to 1ml. This was achieved by centrifuging the perfusate (400×g for 10 min), discarding the supernatant, and suspending the pellet in 3ml of CM, centrifuging the perfusate again (400×g for 10 min) and concentrating the perfusate into 1ml.
Assessment of NK cytotoxicity
A 4-hrs 51Cr release assay was used. This procedure assesses anti-tumor NK cell cytotoxicity per ml of effector cells without prior purification of marginating-pulmonary leukocytes. Earlier studies have indicated that cytotoxicity measured using this procedure is attributable to NK cells, rather than other cell types or soluble factors, as the selective depletion of NK cells abolishes all target-cell killing [36, 37]. The advantages of this procedure include shorter duration, less interference with the effector cells, and better representation of the original in vivo milieu of cell composition. Six different effector to target (E:T) ratios were formed by serially diluting 150 μl aliquots of the effector-cell preparation in a microtiter plate. Then, 5,000 radiolabeled target cells (MADB106) in 100μl CM were added to each well on top of the effector-cell preparation. Spontaneous and maximal releases of radioactivity were determined by substituting effector cells with CM or Triton-X100 (Sigma, Rehovot, Israel), respectively. Prior to and following a 4 h incubation period (100% humidity, 5% CO2 at 37°C) plates were centrifuged (400g for 10 min, at 25°C and 4°C, respectively). Finally, 100μl samples of supernatant, which reflect specific target cell lysis, were recovered for the assessment of radioactivity in a γ-counter.
2.13 Flow Cytometry
Standard procedures were used to prepare cells for flow cytometric analysis [38]. MP-NK cells from the lung perfusate were identified by the FITC-conjugated anti-NKR-P1 mAb (PharMingen, San Diego) as being NKR-P1bright (CD161bright) cells. This criterion has been shown to exclusively identify more than 95% of cells that exhibit NK activity[39]. T cells were identified using a PE-conjugated anti-CD5 mAb (eBioscience, San Diego) and NKT cells were identified as NKR-P1+CD5+ lymphocytes. Granulocytes and lymphocytes were identified based on forward and side scatters. Flow cytometry analysis was conducted using a FACScan (Becton Dickinson). To assess the total number of cells per μl of sample (or a specific cell subtype), 300 polystyrene microbeads (20μm, Duke Scientific, Palo Alto) per μl sample were added to each sample, and the following formula was used: (# of cells in sample/ # of microbeads in sample) × 300.
2.14 Statistical Analysis
One, two, or three-way factorial analyses of variance (ANOVA) with a pre-determined significance level of 0.05 were conducted. Provided significant group differences were found, Fisher's protected least significant differences (Fisher's PLSD) contrasts were performed to compare specific pairs of groups, based on a priori hypotheses. Repeated measures ANOVA was conducted for NK cytotoxicity (E:T ratio being the repeated measure). In cases where the ANOVA assumption of homogeneity of variances was violated (e.g., Exp. 2), pair-wise comparisons were conducted using unpaired t-test, based on a priori hypotheses.
3. Results
3.1 Exp. 1: Prolonged stress during CpG-C treatment attenuated its beneficial effects on MADB106 LTR in the context of a surgical procedure
Male F344 rats (n=56) were subjected to the wet cage stress paradigm (see methods) or remained in their home cages as controls (HCC). Two hours after the onset of stress, rats received either immune stimulation with a single CpG-C injection (165 μg/Kg, i.p.) or a single PBS injection. Twenty hours following CpG-C injection (2 hrs after the end of the prolonged stress paradigm), all animals underwent laparotomy, followed by the LTR assessment procedure (see methods).
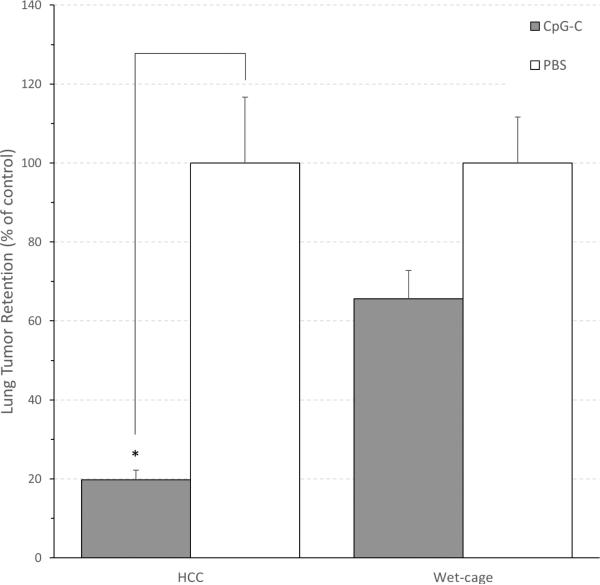
To better reflect the efficacy of CpG-C, data is presented as % of the respective control for each condition (wet cage or HCC). The absolute %LTR levels of PBS injected animals that were subjected to wet cage or that served as HCC were 3.429 and 2.703, respectively. A two-way ANOVA revealed significant main effects for wet cage (F (1,52) = 5.187, p < 0.05 ), and for CpG-C (F (1,52) = 32.393, p < 0.05), and a significant interaction (F (1,52) = 5.188, p < 0.05), indicating that the decrease in LTR levels by CpG-C in HCC animals was greater than in the wet cage condition (Fig. 1).
Fig. 1. Wet-cage stress exposure concurrently with CpG-C treatment attenuated its beneficial effects on MADB106 LTR.
The improvement (decrease) in LTR levels by CpG-C in the wet cage condition was significantly smaller than in the home cage control (HCC) condition.* indicates a significant difference from PBS at p < 0.05. Data is shown as mean ± SEM and is presented as percent of the respective control (PBS) LTR level for the HCC or wet cage condition (see Results for absolute LTR levels).
3.2 Exp. 2: Prolonged stress during CpG-C treatment abolished its beneficial effects on MADB106 LTR, both in the context of adrenergic challenge and without it
Female F344 rats (n=80) were subjected to the wet cage stress paradigm or served as HCC. Two hours after the onset of stress, rats were injected with CpG-C (165 μg/Kg, i.p.) or with PBS. Twenty hours following CpG-C injection (2 hrs after the end of the prolonged stress paradigm), animals were administered with either the β-adrenergic agonist metaproterenol (3 mg/kg, s.c.), or with PBS, simultaneously with the inoculation of radiolabeled MADB106 tumor cells for the assessment of LTR.
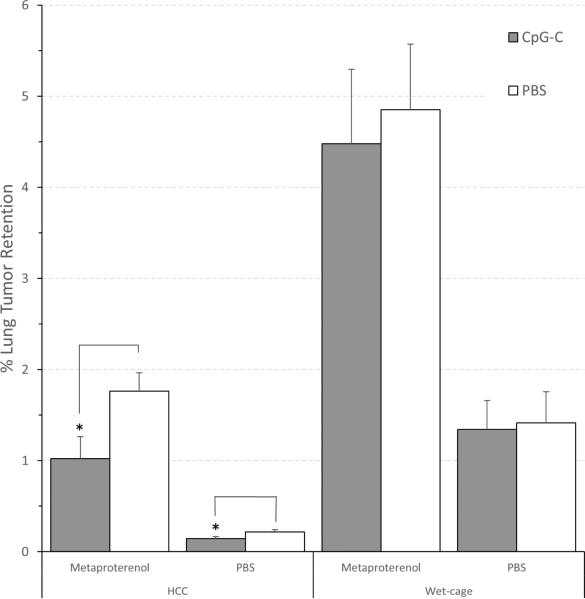
A three-way ANOVA revealed significant main effects for wet cage (F (1,72) = 53.130, p < 0.05), for metaproterenol (F (1,72) = 53.852, p < 0.05), and a significant interaction between wet cage and metaproterenol (F (1,72) = 11.450, p < 0.05). The following pair-wise comparisons were conducted using unpaired t-test, as the ANOVA assumption of homogeneity of variances was violated. In HCC animals, a decrease in LTR levels by CpG-C was evident both in animals subjected to adrenergic challenge (t (18) = 2.362, p < 0.05) and in its absence (t (18) = 2.142, p < 0.05), whereas in animals subjected to wet cage, no effects of CpG-C were evident (with or without adrenergic challenge) (Fig. 2).
Fig. 2. Wet-cage stress exposure concurrently with CpG-C treatment abolished its beneficial effects on MADB106 LTR, both in the context of adrenergic challenge (metaproterenol) and without it.
In the absence of prolonged stress (HCC), CpG-C decreased LTR levels both in animals administered with metaproterenol (simultaneously with tumor challenge) or with placebo (PBS) (*), whereas in animals subjected to the Wet cage stress condition, no effects of CpG-C were evident in either condition. * indicates a significant difference from PBS at p < 0.05. Data is shown as mean ± SEM.
3.3 Exp. 3, 4, and 5: Prolonged stress during CpG-C treatment abolished its beneficial effects on MADB106 LTR; Blockade of (i) the GR by RU486, (ii) the β-adrenergic and GR by nadolol and RU486, or (iii) the opioid receptors by naltrexone, did not prevent the deleterious effects of prolonged stress
Three experiments with the same 2×2×2 experimental design and time schedule were conducted – differing only in the specific antagonists used. In the first experiment, male F344 rats (n=61) were subjected to the wet cage paradigm or served as HCC, and received two injections of the GR antagonist RU486 (25 mg/kg, s.c.) or oil, 30 min before exposure to stress, and again 30 min before the second half of the paradigm. In the second experiment, male F344 rats (n=61) were subjected to the wet cage paradigm or served as HCC, and received two injections of RU486 (25 mg/kg, s.c.) together with the β-blocker nadolol (0.4 mg/kg, s.c.) (“blockers”) or oil. In the third experiment, male F344 rats (n=34) were subjected to the wet cage paradigm or served as HCC, and received two injections of the opioid receptor antagonist naltrexone (2 mg/kg, s.c.) or PBS.
In all three experiments, the above four groups were further subdivided to receive either a CpG-C injection (165 μg/Kg, i.p.), or a PBS injection, two hours after the onset of stress. Twenty hrs following CpG-C injection (2 hrs after the end of the prolonged stress paradigm), all animals were administered with epinephrine (1.2 mg/kg, s.c., dissolved in slow release vehicle), and 1.5 hrs after, rats were inoculated with radiolabeled MADB106 tumor cells for the assessment of LTR.
Exp. 3 - RU486
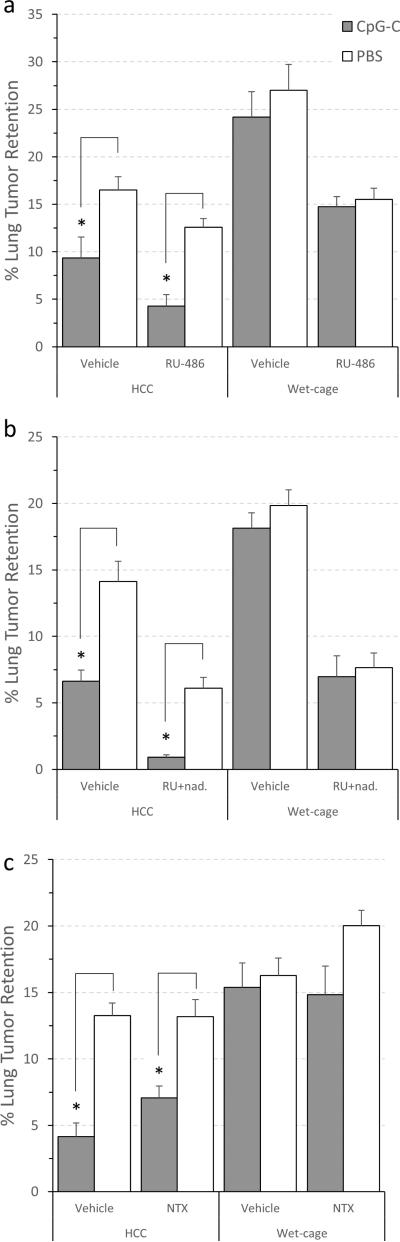
A three-way ANOVA revealed significant main effects for wet cage (F (1,53) = 59.015, p < 0.05 ), for CpG-C (F (1,53) = 14.289, p < 0.05), and for RU486 (F (1,53) = 35.091, p < 0.05). A significant interaction between wet cage and CpG-C was evident (F (1,53) = 5.558, p < 0.05), indicating the deleterious effects of stress on CpG-C efficacy. A three-way interaction was not significant suggesting that RU486 did not prevent the deleterious effects of prolonged stress on CpG-C efficacy. Specifically, in HCC animals, a decrease in LTR levels by CpG-C was evident (both in animals administered with RU486 and in its absence), whereas in animals subjected to wet cage, no effects of CpG-C were evident with or without the RU486 administration (Fig. 3A).
Fig. 3. The blockade of (i) GRs by RU486, (ii) β-adrenoceptors and GRs by nadolol and RU, and (iii) opioid receptors by naltrexone did not prevent the deleterious effects of stress on the efficacy of CpG-C treatment.
CpG-C decreased MADB106 LTR levels in the absence of stress (HCC) (*), and Wet cage stress exposure prevented this beneficial effect. The use of the GR blocker RU486 alone (a), the GR blocker RU with the β-adrenoceptor antagonist nadolol (b), or the opioid receptor antagonist naltrexone (c), did not prevent the effects of stress. * indicates a significant difference from PBS at p < 0.05. Data is shown as mean ± SEM.
Exp. 4 - nadolol and RU486
The exact same pattern of results evident in Exp. 3 was evident in this experiment: For wet cage (F (1,53) = 59.668, p < 0.05 ), for CpG-C (F (1,53) = 22.072, p < 0.05), for blockers (F (1,53) = 133.371, p < 0.05), and for the two-way interaction (F (1,53) = 10.292, p < 0.05)) (Fig. 3B). Thus the combined blockade of GR and β-adrenoceptor did not prevent the deleterious effects of prolonged stress on the efficacy of CpG-C.
Exp. 5 - naltrexone
A three-way ANOVA revealed significant main effects for wet cage (F (1,55) = 53.49, p < 0.05 ), and for CpG-C (F (1,55) = 29.09, p < 0.05). However, the interaction between these two factors did not reach statistical significance, nor did the three-way interaction. No effects of naltrexone were evident (Fig. 3C). Fisher's PLSD contrasts indicated significant effects of CpG-C in the HCC groups (p<0.05), but not in the prolonged stress (wet-cage) groups. Thus, no evidence for the involvement of opioids in the deleterious effects of prolonged stress is apparent.
3.4 Exp. 6: Adrenalectomy did not prevent the deleterious effects of prolonged stress on the efficacy of CpG-C treatment
The experiment was conducted in two replicates. F344 male rats (n=73 in each replicate) underwent adrenalectomy or sham operation and recovered for three weeks.. After the recovery period, animals were subjected to the wet cage paradigm or served as HCC. Two hours after the onset of stress, rats received either immune stimulation with a single CpG-C injection (165 μg/Kg, i.p.), or injection of PBS. Twenty hours following CpG-C injection (2 hrs after the end of the prolonged stress paradigm), all animals were administered with epinephrine (1.2 mg/kg, s.c., dissolved in slow release vehicle), and 1.5 hrs later, rats were injected with radiolabeled MADB106 tumor cells for the assessment of LTR.
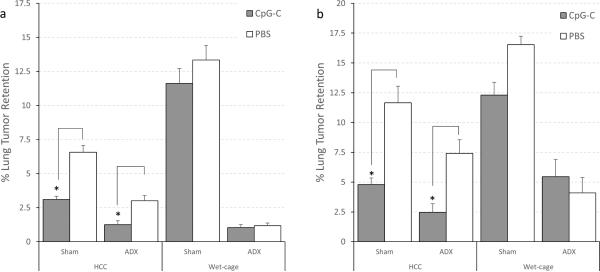
In both replicates, a three-way ANOVA revealed significant main effects for wet cage (F (1,65) = 14.21, p < 0.05 and F (1,65) = 64.129, p < 0.05), CpG-C (F (1,65) = 20.969, p < 0.05 and F (1,65) = 18.444, p < 0.05), and adrenalectomy (F (1,65) = 64.915, p < 0.05 and F (1,65) = 289.812, p < 0.05). A two-way interaction between wet cage and CpG-C was significant (F (1,65) = 7.730, p < 0.05 and F (1,65) = 4.112, p < 0.05) indicating that wet cage prevented the beneficial effects of CpG-C. The three-way interaction was not significant, and thus, and as clearly seen in Fig. 4a and 4b, adrenalectomy did not prevent the deleterious effects of prolonged stress.
Fig. 4. Wet-cage stress concurrently with CpG-C treatment attenuated its beneficial effects on LTR, and adrenalectomy did not prevent the deleterious effects of stress.
(a) and (b) are two replicates of the same study, showing the exact same pattern of results. In home cage control (HCC) animals, a decrease in LTR levels by CpG-C was evident both in adrenalectomized (ADX) and sham-operated (Sham) animals (*), whereas in animals subjected to the wet cage condition, the effects of CpG-C were attenuated in both ADX and Sham animals. * indicates a significant difference from PBS at p < 0.05. Data is shown as mean ± SEM.
3.5 Exp. 7: The in vitro effects of CpG-C and of corticosterone, catecholamines, and prostaglandins on blood production of IL-12 and TNF-α
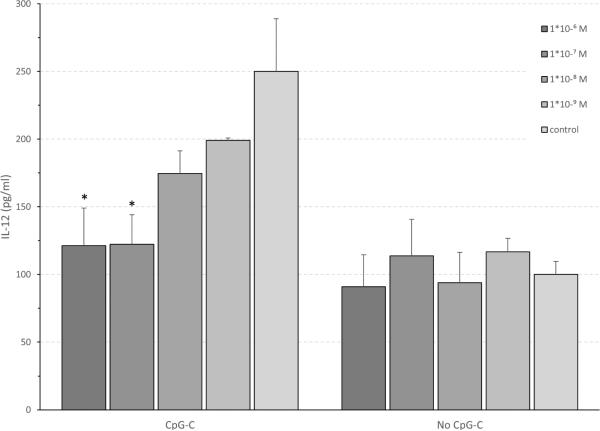
To test the direct in vitro effects of specific stress hormones on leukocyte production of IL-12 and TNF-α, the following experiment was conducted.Blood was withdrawn from 6 F344 male rats into EDTA-coated syringes (1.8mg/mL blood), pooled, and washed 3 times to discard endogenous IL-12 and TNF-α. Aliquots of 500 μl washed blood were mixed with 500 μl of CM, incubated with one stress factor, and 2 hrs later with/without CpG-C (5 μg/ml). The stress factors used were CORT, epinephrine, MP, and PGE2. For each factor, four consecutive concentrations were used (1×10−5M, 1×10−6M, 1×10−7M, 1×10−8M, 1×10−9M; differing between each factor), ranging between high concentrations commonly used in similar studies, and physiological concentrations, aiming to simulate in vivo plasma levels observed at baseline and following stress (see Methods). The study was conducted in triplicates, but in several conditions (mostly vehicle), more aliquots were used. Plates were incubated for 24 hrs following CpG-C addition, and supernatants were collected for assessment of IL-12 and TNF-α levels employing ELISA (Peprotech-Asia, Rehovot, Israel).
Corticosterone and IL-12
Two-way ANOVA indicated a significant main effect for CpG-C (F(1,61) = 12.228 , p < 0.001), a marginal effect for corticosterone (p = 0.077), and a marginal interaction between these factors (p = 0.066). Specifically, CpG-C increased IL-12 production, and corticosterone dose-dependently decreased this effect of CpG-C without impacting IL-12 production in blood not exposed to CpG-C. Within the CpG-C conditions the two higher concentrations of CORT significantly suppressed IL-12 production (PLSD, p < 0.01) (Fig. 5).
Fig. 5. CpG-C increases in vitro IL-12 levels, and CORT dose-dependently attenuated this increase.
Physiological CORT levels dose-dependently reduced the efficacy of CpG-C in elevating IL-12 levels. This effect of CORT was observed beginning at low physiological levels (10−9M) that characterized baseline (no stress) conditions. Marginal effect for CORT (p = 0.077). Within the CpG-C conditions the two higher concentrations of CORT significantly suppressed IL-12 production. * indicates a significant difference from relevant PBS at p < 0.05. Data is shown as mean ± SEM.
Corticosterone and TNF-α
CpG-C increased TNF-α production (F(1,59) = 15.660, p < 0.001) and corticosterone at the doses tested had no impact on this outcome.
All other factors
PGE2, epinephrine, or MP had no impact on the levels of IL-12 and TNF-α in any of the conditions tested.
3.6 Exp. 8: Potential in vivo involvement of corticosterone and catecholamine levels in modulating the efficacy of CpG-C in non-stressed animals
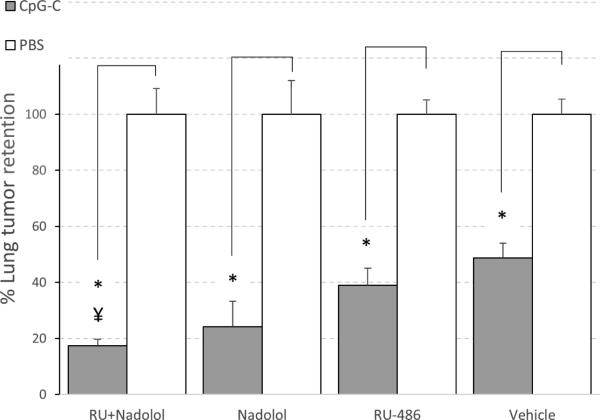
In Exp. 3 and 4, the use of RU486 alone, or the combination of nadolol and RU486 seemed to improve the efficacy of CpG-C in animals not subjected to prolonged stress (HCC), specifically when considering the ratio between the CpG-C group and the PBS group. As these 4 groups were also characterized by lower variance (than the groups that also underwent prolonged stress), we conducted a separate analysis in these groups. Data was transformed to percent of respective PBS treated group, and a 2×2 ANOVA was conducted and yielded a marginally significant interaction between the effects of the blockers (e.g., RU486 and nadolol in Exp. 4) and the effects of CpG-C (F (1,27) = 3.315, p = 0.079), toward improving CpG-C efficacy. Therefore, to further investigate potential beneficial effects of blocking CORT or β-adrenergic stimulation (separately or combined) on CpG-C efficacy in the home-cage condition, we conducted the following experiment, combined its results with the equivalent data collected in Exp. 3 and 4, and analyzed the beneficial effects of CpG-C as percent from its respective PBS group.
Male F344 rats (n=53) were administered with either RU486 (25 mg/kg, s.c.), nadolol (0.4 mg/kg, s.c.), both blockers, or oil, simultaneously with CpG-C (165 μg/Kg, i.p.) or PBS injection. Twenty hours following CpG-C injection, all animals were administered with epinephrine (1.2 mg/kg, s.c., dissolved in slow release vehicle), and 1.5 hrs later, rats were inoculated with radiolabeled MADB106 tumor cells for the assessment of LTR.
Data was calculated as percent of respective PBS injected group to better reflect the magnitude of the effect of CpG-C within each treatment (blocker) condition. A two-way ANOVA revealed a significant main effect for CpG-C (F (1,107) = 185.4, p < 0.01), reducing LTR levels; a significant effect for the different blockers (F (3,107) = 2.69, p < 0.05), alone and together improving the efficacy of CpG-C; and a significant interaction between blockers and the effects of CpG-C (F (3,107) = 2.72, p < 0.05). Specifically, in animals treated with the combination of nadolol and RU486, CpG-C reduced LTR levels to 17% of PBS (no-CpG-C) levels, whereas in animals not treated with blockers (vehicle - oil) CpG-C reduced LTR levels only to 49% of PBS (no-CpG-C) levels, a difference that was also statistically significant (Fisher's PLSD, p < 0.05) (Fig. 6). It is worthy to note that the same pattern of effects was evident when separately analyzed in each of the three studies.
Fig. 6. The combined blockade of GC and β-adrenoceptors improved the efficacy of CpG-C.
Animals in this study were not subjected to prolonged stress. In Vehicle treated animals, CpG-C reduced LTR levels to 48% of PBS treatment animals (no CpG-C), while in animals treated with RU486 and Nadolol (RU+nad), CpG-C reduced LTR significantly more, reaching 17% of PBS (no CpG-C) treatment levels. * indicates a significant difference from PBS at p < 0.05. Data presented as percent of respective control (PBS) levels, and is shown as mean ± SEM.
3.7 Exp. 9: Prolonged stress during CpG-C treatment abolished the beneficial effects of CpG-C on ex-vivo NK cytotoxicity
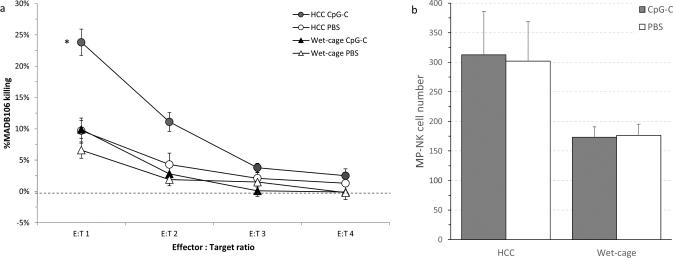
A 2×2 design was used. F344 male rats (n=50) were subjected to the wet cage paradigm or served as HCC. Two hours after the onset of stress, rats received either immune stimulation with a single CpG-C injection (165 μg/Kg, i.p.), or PBS injection. Twenty hrs following CpG-C injection (2 hrs after the end of the prolonged stress paradigm), all animals were sacrificed and marginating-pulmonary leukocytes were harvested for the ex-vivo assessment of NK-cell numbers and activity levels against the syngeneic MADB106 target line.
NK cytotoxicity
A two-way ANOVA revealed significant main effects for wet cage (F (1,46) = 24.512, p < 0.05) and CpG-C (F (1,46) = 13.518, p < 0.05), and a significant interaction between wet cage and CpG-C (F (1,46) = 8.235, p < 0.05) indicating that only HCC animals exhibited marked increase in MADB106 killing levels following CpG-C (Fig. 7a).
Fig. 7. Wet-cage stress concurrently with CpG-C treatment prevented the increase in marginating-pulmonary (MP)-NK ex-vivo cytotoxicity against MADB106 tumor cells.
(a) MP-NK activity: CpG-C markedly elevated MADB106 killing levels in home cage control (HCC) animals, but not in animals subjected to the wet cage condition. (b) MP-NK cell number: No significant effects of CpG-C were evident, suggesting that the increase in NK activity levels evident in (a) are per cell. Data is shown as mean ± SEM.
NK-cell numbers
A two-way ANOVA revealed a significant main effect for wet cage (F (1,46) = 7.617, p < 0.05). No significant effects for CpG-C and no interaction were evident (Fig. 7b), suggesting that changes in NK activity caused by CpG-C (Fig 7a) did not depend on changes in numbers of NK cells, but rather reflected changes in cytotoxicity per cell.
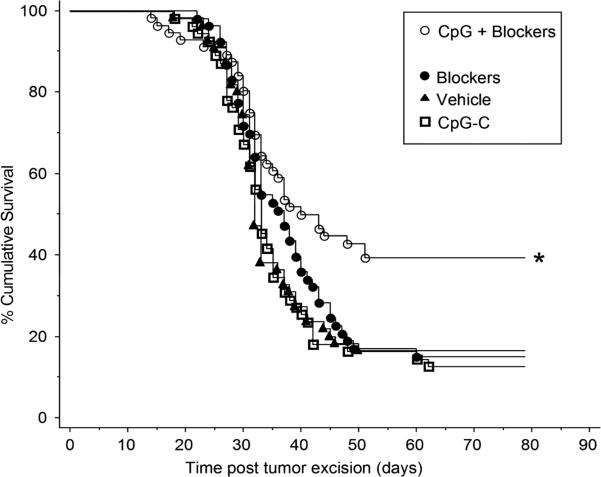
3.8 Exp. 10: Only the combination of CpG-C and blockers for surgical stress responses increased long-term survival rates following excision of a primary metastasizing tumor in C57BL/6J mice
Male and female C57BL/6J mice (n=219) were injected with B16F10.9 melanoma cells intrafootpad. Once a tumor reached 100μl in volume, mice were assigned to 1 of 4 groups: (1) a cocktail of three blockers for surgical stress responses, (2) CpG-C (50 μg, i.p.), (3) the cocktail plus CpG-C, or (4) vehicle injections. The cocktail was comprised of the β-adrenergic antagonist, propranolol (5 mg/kg in slow release vehicle, s.c.), the glucocorticoid receptor antagonist RU486 (25 mg/kg, s.c.), and the COX2 synthesis inhibitor etodolac (50 mg/kg, s.c.). Twenty-four hours later, mice received the same treatment and the tumor was excised by paw amputation. Mice were monitored for morbidity signs/mortality for 80 days, which was more than 3 weeks following last morbidity/mortality. Morbid mice were sacrificed and cancer recurrence was verified.
Only mice that received CpG-C together with the three blockers exhibited significantly higher survival rates compared to the other groups, as indicated by Tarone-Ware test for pairwise group comparisons in the Kaplan-Meier model for survival rates (p < 0.05) (Fig. 8). No sex differences were observed in any of the outcomes.
Fig. 8. Only the combination of CpG-C and blockers improved survival rates in C57BL/6J mice following tumor excision.
Whereas CpG-C alone did not improve long-term survival rates in C57BL/6J mice undergoing B16 melanoma tumor excision, the simultaneous inhibition of COX2, and GR and β-adrenoceptor blockade (Blockers), increased survival rates (*). The blockers alone had no effect. * indicates a significant difference at p < 0.05.
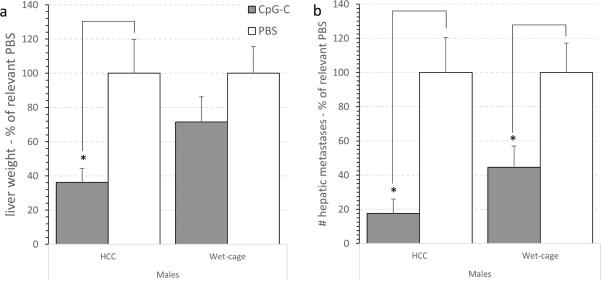
3.9 Exp. 11: Prolonged stress attenuated the beneficial effects of CpG-C treatment on number of liver metastases and liver weight in the CT-26 experimental hepatic metastasis model in male Balb/c mice
The study was conducted in 4 replicates, each containing all 4 groups specified below. A total of 70 male Balb/c mice were subjected to the wet cage paradigm or served as HCC. Two hours after the onset of stress, mice received either immune stimulation with a single CpG-C injection (20 μg/mouse, i.p.) or PBS injection. Twenty hrs following CpG-C injection (2 hrs after the end of stress), all animals underwent laparotomy for the intra-splenic CT-26 injection procedure. Twenty days after tumor injection, animals were sacrificed and livers were removed, weighed, and surface hepatic metastases were counted.
Data was calculated as percent of relevant PBS group, indicating effect size of CpG-C within each condition (baseline levels were similar in the two PBS groups).
Liver weight
A two-way ANOVA revealed a significant main effect for CpG-C (F (1,66) = 9.782, p < 0.05) , but no significant main effect for wet cage stress or for interaction. However, CpG-C significantly reduced liver weight in HCC, but not in the wet cage condition, as is also indicated by pair wise Fisher's PLSD contrasts (p < 0.05) (Fig. 9a).
Fig. 9. Wet-cage stress exposure concurrently with CpG-C treatment attenuated its beneficial effects on liver weight and number of CT26 metastases in males.
(a) CpG-C significantly reduced liver weight in HCC (*), but not in animals subjected to the wet cage condition. Weight is shown as % of relevant PBS group. (b) Assessing the number of metastases yielded a significant effect for CpG-C in reducing metastases number under HCC (*), which was partially attenuated under the wet cage condition (*). Number of metastases is shown as % of relevant PBS group. * indicates a significant difference from PBS at p < 0.05. In both indices (a) and (b). Data is shown as mean ± SEM.
Metastases number
A similar pattern of results were evident, but the pattern of statistical significance differed. A two-way ANOVA revealed a significant main effect for CpG-C (F (1,64) = 23.006, p < 0.05). No significant interaction was evident. CpG-C improved tumor resistance by 80% under HCC condition, but only by 50% under stress conditions, and both were found significant by pair wish Fisher's PLSD contrasts (p < 0.05) (Fig. 9b).
4.0 Discussion
In this study we explored the potential impact of ongoing stress and stress hormones on the efficacy of immune stimulation with CpG-C by quantifying NK cytotoxicity and by using different tumor models of experimental and spontaneous metastasis. Additionally, we attempted to elucidate neuroendocrine mechanisms modulating the efficacy of CpG-C in (i) the absence of environmental stressor, and (ii) when CpG-C is used concurrently with exposure to stress or to surgery. As is elaborated below, prolonged stress attenuated or abolished the immune-stimulatory and metastasis-inhibitory effects of CpG-C, as was manifested through the indices of NK activity and resistance to metastasis of several cancer types. Additionally, host neuroendocrine responses to CpG-C and its concomitant effect, specifically the activation of SNS and HPA, dampen the efficacy of CpG-C. These outcomes are likely to have biological and clinical ramifications regarding the use of immune-stimulatory interventions in the clinical context of cancer treatment, which most likely involves stress responses of various origins.
We employed three tumor models of metastasis to assess and generalize the biological significance of the impact of stress on immune stimulation. The MADB106 experimental metastasis model was used in rats. This model is sensitive to in vivo activity levels of marginating-pulmonary (MP)-NK cells [27, 32, 40-42], and the in vivo index of MADB106 LTR used herein was shown to be significantly improved by CpG-C through activating NK cells [11, 13]. Therefore, MP-NK numbers and activity were also studied herein (ex-vivo against MADB106 target cells), and the in vivo index of MADB106 LTR was used to assess the impact of stress and specific stress hormones on the efficacy of CpG-C.
The results directly indicated that exposure to ongoing stress during CpG-C treatment markedly impaired its immune-stimulatory effect. Whereas in control animals CpG-C markedly elevated ex-vivo MP-NK cell cytotoxicity of MADB106 cells, CpG-C failed to increase this index in animals subjected to stress. Analysis of MP-NK cell numbers also indicated that both the increase in MP-NK cytotoxicity by CpG-C, and its prevention by prolonged stress, occurred on a per NK cell basis, rather than through alterations in MP-NK cell numbers.
Ongoing stress also abolished the beneficial in vivo effects of CpG-C against MADB106 lung tumor retention (LTR). Under the stress condition, and in all three experiments conducted in both sexes, CpG-C had no beneficial effects on LTR, whereas in the absence of stress, it reduced LTR by approximately 50%. Blockade of GRs, β-adrenoceptors, or opioid receptors in this MADB106 model did not restore the efficacy of CpG-C in animals subjected to stress. Adrenalectomy was also ineffective, although this approach do not abolish the expected stress responses of catecholamines or opioids from non-adrenal sources. These findings suggest that none of our blockade strategies for ongoing stress responses were efficient. Among the many potential alternative (or additional) factors, recent findings have implicated neuropeptide Y (NPY), which is released from sympathetic nerve terminals upon sympathetic activation, in inhibition of NK cell activity and TH1 responses [43, 44]. Additionally, the neuropeptide ACTH, which is known to be secreted in response to stress, was shown to suppress various immune functions, including antibody and IFN-γ production, and lymphocyte proliferation [45]. However, in the absence of external prolonged stress, the combined blockade of GR and β-adrenoceptors (through RU-486 and nadolol) almost tripled the efficacy of CpG-C in improving the in vivo index of MADB106 LTR. These findings indicate the involvement of SNS and HPA reactivity in regulating the immunostimulatory effect of CpG-C through the systemic release of CAs and GCs. Stress responses are common in response to several immune-stimulatory agents (e.g., LPS [22]) and in natural responses to pathogens [46], and could constitute a self-limiting mechanism of TLR-9 activation by CpG-C. Interestingly, with respect to CpG-C, we were unable to observe systemic elevated levels of CORT at the time points studied herein and in previous studies in mice [5, 47]. Nevertheless, in vitro physiological CORT levels dose-dependently reduced the efficacy of CpG-C in elevating IL-12 levels. This effect of CORT was observed beginning at low physiological levels (10−9M) that characterized baseline (no stress) conditions. These in vitro findings correspond with the in vivo findings that blocking stress hormones in the absence of external stressor markedly potentiate the efficacy of CpG-C, although it is hard to extrapolate in vitro to in vivo outcomes [48].
Two additional tumor models were also used in mice to investigate the effects of ongoing stress on CpG-C efficacy – the CT-26 experimental hepatic metastasis model, and the B16 melanoma spontaneous metastasis model. Although these models are less characterized for specific immunological mechanisms controlling metastasis or mediating the effects of CpG-C, they both involve surgical procedures during immune stimulation with CpG-C, and thus provide additional evidence regarding the biological and clinical significance of the interaction between stress and immune stimulation.
Specifically, in the B16 melanoma spontaneous metastasis model in C57BL/6J mice, the development of spontaneous metastasis and survival rates are assessed following the excision of the primary tumor. As all mice are bearing a primary tumor and undergo its excision, stress responses are inherent to this model. Specifically, the presence of the primary tumor may induce nociception, pain, and other physiological responses that may activate neuroendocrine stress responses. In our previous work with this model we have shown that the excision of the primary tumor is a major stressor [15]. In addition to the classical stress responses (SNS and HPA), primary tumors and injured host tissue are known to release prostaglandins (PGs), which often cause immune suppression [49, 50]. Thus, in this model we utilized a COX2 inhibitor in addition to GR and β-adrenoceptor blockers. These three drugs and CpG-C were administered twice – a day before tumor excision, and simultaneously with tumor excision – covering the two perioperative days and their associated stress and surgical responses.
Our findings in this B16 model indicated that CpG-C, together with the three blockers, but not without them, significantly improved recurrence-free survival rates following tumor excision in both sexes. Because this model does not include a non-stress condition, we cannot directly implicate the above stress responses in abolishing the effects of CpG-C, but we can indicate a significant synergism between CpG-C and the blockade of these responses in the context of stress and surgery, a context that also characterizes treatment of cancer patients.
The potential reduction of the efficacy of CpG-C immune stimulation by stress is further generalized by the evidence observed herein employing the CT-26 hepatic metastasis model in Balb/C mice. Whereas CpG-C markedly reduced metastatic development, simultaneous exposure to stress profoundly impaired its beneficial effects, but did not eliminate it, as evidenced by the number of liver metastases and liver weight. Specifically, in the HCC condition CpG-C markedly reduced tumor retention to 17% of non-treated animals, whereas under the wet cage condition CpG-C reduced it only to 44% of the non-treated animals. Thus, at the doses of CpG-C used in mice (approximately 4-fold higher than in rats) CpG-C was still effective under stress conditions. Clearly, different tumor models have different and multiple interactions with various aspects of host physiology, and thus one cannot expect the exact same findings when employing the three models used in this study.
In conclusion, the current study and our previous studies[5, 6] indicate that therapies based on immune stimulation should be tested in the context of stress, as now there is clear evidence for deleterious impact of various stressors on the immune-enhancing and tumor-reducing effects of such immune-stimulating agents. Our current study clearly indicates the biological significance of such deleterious effects of stress, through testing in vivo outcomes of metastatic progression in different tumor models, including the study of survival rates following the excision of a primary tumor. GCs, CAs, and potentially PGs responses, play a role in limiting the beneficial effects of CpG-C, at least under some circumstances. The need to further identify specific neuroendocrine or paracrine mediators of this interaction warrants additional research, which may yield a better mechanistic understanding and suggest potential prophylactic approaches. Such insights would be of clinical significance, as patients who might be treated with immune-stimulatory agents are commonly in medical situations characterized by various psychological and physiological stressors.
Supplementary Material
Highlights.
Ongoing stress impaired the efficacy of CpG-C in potentiating NK activity
Perioperative stress responses reduced the beneficial anti-metastatic effects of CpG-C
Blocking HPA and SNS responses can potentiate the efficacy of CpG-C
Outcomes are generalized in several tumor models and species
Acknowledgements
This work was supported by NIH/NCI grant # CA125456 and CA172138 (SBE) and by the Israel-USA bi-national Science Foundation # 2005331 (SBE & GGP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
All authors declare that there are no conflicts of interest.
References
- 1.Cortes JE, et al. A pilot study of interleukin-2 for adult patients with acute myelogenous leukemia in first complete remission. Cancer. 1999;85(7):1506–13. [PubMed] [Google Scholar]
- 2.Steiner A, Wolf C, Pehamberger H. Comparison of the effects of three different treatment regimens of recombinant interferons (r-IFN alpha, r-IFN gamma, and r-IFN alpha + cimetidine) in disseminated malignant melanoma. J Cancer Res Clin Oncol. 1987;113(5):459–65. doi: 10.1007/BF00390040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13(2):155–68. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 4.Hurteau JA, et al. Evaluation of recombinant human interleukin-12 in patients with recurrent or refractory ovarian cancer: a gynecologic oncology group study. Gynecol Oncol. 2001;82(1):7–10. doi: 10.1006/gyno.2001.6255. [DOI] [PubMed] [Google Scholar]
- 5.Goldfarb Y, et al. CpG-C immunotherapeutic efficacy is jeopardized by ongoing exposure to stress: potential implications for clinical use. Brain, Behavior, and Immunity. 2011;25(1):67–76. doi: 10.1016/j.bbi.2010.07.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levi B, et al. Continuous stress disrupts immunostimulatory effects of IL-12. Brain, Behavior, and Immunity. 2011;25(4):727–35. doi: 10.1016/j.bbi.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bode C, et al. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011;10(4):499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holtick U, et al. Toll-like receptor. 9 agonists as cancer therapeutics. Expert Opin Investig Drugs. 2011;20(3):361–72. doi: 10.1517/13543784.2011.553187. [DOI] [PubMed] [Google Scholar]
- 9.Krieg AM. Antitumor applications of stimulating toll-like receptor 9 with CpG oligodeoxynucleotides. Curr Oncol Rep. 2004;6(2):88–95. doi: 10.1007/s11912-004-0019-0. [DOI] [PubMed] [Google Scholar]
- 10.Krieg AM. Therapeutic potential of Toll-like receptor. 9 activation. Nat Rev Drug Discov. 2006;5(6):471–84. doi: 10.1038/nrd2059. [DOI] [PubMed] [Google Scholar]
- 11.Goldfarb Y, et al. CpG-C oligodeoxynucleotides limit the deleterious effects of beta-adrenoceptor stimulation on NK cytotoxicity and metastatic dissemination. J Immunother. 2009;32(3):280–91. doi: 10.1097/CJI.0b013e31819a2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldfarb Y, et al. CpG-C immunotherapeutic efficacy is jeopardized by ongoing exposure to stress: potential implications for clinical use. Brain Behav Immun. 2011;25(1):67–76. doi: 10.1016/j.bbi.2010.07.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldfarb Y, et al. Improving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg. 2011;253(4):798–810. doi: 10.1097/SLA.0b013e318211d7b5. [DOI] [PubMed] [Google Scholar]
- 14.Ben-Eliyahu S, et al. Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and beta- adrenoceptors. Neuroimmunomodulation. 2000;8(3):154–64. doi: 10.1159/000054276. [DOI] [PubMed] [Google Scholar]
- 15.Glasner A, et al. Improving survival rates in two models of spontaneous postoperative metastasis in mice by combined administration of a beta-adrenergic antagonist and a cyclooxygenase-2 inhibitor. J Immunol. 2010;184(5):2449–57. doi: 10.4049/jimmunol.0903301. [DOI] [PubMed] [Google Scholar]
- 16.Shakhar G, Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J Immunol. 1998;160(7):3251–8. [PubMed] [Google Scholar]
- 17.Yakar I, et al. Prostaglandin e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Annals of Surgical Oncology. 2003;10(4):469–79. doi: 10.1245/aso.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Benish M, et al. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Annals of Surgical Oncology. 2008;15(7):2042–52. doi: 10.1245/s10434-008-9890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenne E, et al. In vivo suppression of NK cell cytotoxicity by stress and surgery: glucocorticoids have a minor role compared to catecholamines and prostaglandins. Brain Behav Immun. 2014;37:207–19. doi: 10.1016/j.bbi.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaashua L, et al. Plasma IL-12 levels are suppressed in vivo by stress and surgery through endogenous release of glucocorticoids and prostaglandins but not catecholamines or opioids. Psychoneuroendocrinology. 2014;42:11–23. doi: 10.1016/j.psyneuen.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallejo R, de Leon-Casasola O, Benyamin R. Opioid therapy and immunosuppression: a review. Am J Ther. 2004;11(5):354–65. doi: 10.1097/01.mjt.0000132250.95650.85. [DOI] [PubMed] [Google Scholar]
- 22.Naor R, et al. Metastatic-promoting effects of LPS: Sexual dimorphism and mediation by catecholamines and prostaglandins. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levi B, et al. Continuous stress disrupts immunostimulatory effects of IL-12. Brain Behav Immun. 2011;25(4):727–35. doi: 10.1016/j.bbi.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dengler HG, Hengstmann JH. Metabolism and pharmacokinetics of orciprenaline in various animal species and man. Arch Int Pharmacodyn Ther. 1976;223(1):71–87. [PubMed] [Google Scholar]
- 25.Rosenne E, et al. Inducing a mode of NK-resistance to suppression by stress and surgery: a potential approach based on low dose of poly I-C to reduce postoperative cancer metastasis. Brain Behav Immun. 2007;21(4):395–408. doi: 10.1016/j.bbi.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matzner P, et al. Perioperative treatment with the new synthetic TLR- 4 agonist GLA-SE reduces cancer metastasis without adverse effects. Int J Cancer. 2015 doi: 10.1002/ijc.29885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melamed R, et al. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain, Behavior, & Immunity. 2005;19(2):114–26. doi: 10.1016/j.bbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Ben-Eliyahu S, et al. Stress-induced suppression of natural killer cell cytotoxicity in the rat: a naltrexone-insensitive paradigm. Behav Neurosci. 1990;104(1):235–8. doi: 10.1037//0735-7044.104.1.235. [DOI] [PubMed] [Google Scholar]
- 29.Melamed R, et al. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19(2):114–26. doi: 10.1016/j.bbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 30.Zagron G, Weinstock M. Maternal adrenal hormone secretion mediates behavioural alterations induced by prenatal stress in male and female rats. Behav Brain Res. 2006;175(2):323–8. doi: 10.1016/j.bbr.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 31.Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci U S A. 1999;96(3):1059–64. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barlozzari T, et al. Direct evidence for the role of LGL in the inhibition of experimental tumor metastases. J Immunol. 1985;134(4):2783–9. [PubMed] [Google Scholar]
- 33.Ben-Eliyahu S, Page GG. In vivo assessment of antural killer activity in rats. Prog Neuroendocrineimmunol. 1992;5:199–214. [Google Scholar]
- 34.Ben-Eliyahu S, et al. Acute alcohol intoxication suppresses natural killer cell activity and promotes tumor metastasis. Nat Med. 1996;2(4):457–60. doi: 10.1038/nm0496-457. [DOI] [PubMed] [Google Scholar]
- 35.Corbett TH, et al. Tumor induction relationships in development of transplantable cancers of the colon in mice for chemotherapy assays, with a note on carcinogen structure. Cancer Res. 1975;35(9):2434–9. [PubMed] [Google Scholar]
- 36.Ben-Eliyahu S, et al. Increased susceptibility to metastasis during pro-oestrus/oestrus in rats: possible role of oestradiol and natural killer cells. Br J Cancer. 1996;74(12):1900–7. doi: 10.1038/bjc.1996.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ottenhof PC, Morales A, Baines MG. Quantitation of a whole blood assay for human natural killer cell activity. J Immunol Methods. 1981;42(3):305–18. doi: 10.1016/0022-1759(81)90159-9. [DOI] [PubMed] [Google Scholar]
- 38.Melamed R, et al. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: Suppression by surgery and the prophylactic use of a -adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. doi: 10.1016/j.bbi.2004.07.004. In Press. [DOI] [PubMed] [Google Scholar]
- 39.Chambers WH, et al. Monoclonal antibody to a triggering structure expressed on rat natural killer cells and adherent lymphokine-activated killer cells. Journal of Experimental Medicine. 1989;169(4):1373–89. doi: 10.1084/jem.169.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ben-Eliyahu S, Page GG. In vivo assessment of natural killer cell activity in rats. Progress in Neuroendocrineimmunology. 1992;5:199–214. [Google Scholar]
- 41.Ben-Eliyahu S, et al. Increased susceptibility to metastasis during pro-oestrus/oestrus in rats: possible role of oestradiol and natural killer cells. British Journal of Cancer. 1996;74(12):1900–7. doi: 10.1038/bjc.1996.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shakhar G, Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. Journal of Immunology. 1998;160(7):3251–8. [PubMed] [Google Scholar]
- 43.Bedoui S, et al. Relevance of neuropeptide Y for the neuroimmune crosstalk. J Neuroimmunol. 2003;134(1-2):1–11. doi: 10.1016/s0165-5728(02)00424-1. [DOI] [PubMed] [Google Scholar]
- 44.Wheway J, et al. A fundamental bimodal role for neuropeptide Y1 receptor in the immune system. J Exp Med. 2005;202(11):1527–38. doi: 10.1084/jem.20051971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plaut M. Lymphocyte hormone receptors. Annu Rev Immunol. 1987;5:621–69. doi: 10.1146/annurev.iy.05.040187.003201. [DOI] [PubMed] [Google Scholar]
- 46.Ader R. Psychoneuroimmunology. 4 ed. Academic Press; San Diego: 2007. [Google Scholar]
- 47.Matzner P, et al. Resilience of the Immune System in Healthy Young Students to 30-Hour Sleep Deprivation with Psychological Stress. Neuroimmunomodulation. 2013;20(4):194–204. doi: 10.1159/000348698. [DOI] [PubMed] [Google Scholar]
- 48.Gotlieb N, et al. The misleading nature of in vitro and ex vivo findings in studying the impact of stress hormones on NK cell cytotoxicity. Brain Behav Immun. 2015;45:277–86. doi: 10.1016/j.bbi.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benish M, et al. Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann Surg Oncol. 2008;15(7):2042–52. doi: 10.1245/s10434-008-9890-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yakar I, et al. Prostaglandin e(2) suppresses NK activity in vivo and promotes postoperative tumor metastasis in rats. Ann Surg Oncol. 2003;10(4):469–79. doi: 10.1245/aso.2003.08.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.