Abstract
Goal
The objective of this study is to design and develop a portable tool consisting of a disposable biochip for measuring electro-thermo-mechanical (ETM) properties of breast tissue.
Methods
A biochip integrated with a microheater, force sensors and electrical sensors is fabricated using microtechnology. The sensor covers the area of 2mm and the biochip is 10mm in diameter. A portable tool capable of holding tissue and biochip is fabricated using 3D printing. Invasive ductal carcinoma (IDC) and normal tissue blocks are selected from cancer tissue bank in Biospecimen Repository Service at Rutgers Cancer Institute of New Jersey. The ETM properties of the normal and cancerous breast tissues (3mm thickness and 2mm diameter) are measured by indenting the tissue placed on the biochip integrated inside the 3D printed tool.
Results
Integrating microengineered biochip and 3D printing we have developed a portable cancer diagnosis device. Using this device, we have shown a statistically significant difference between cancerous and normal breast tissues in mechanical stiffness, electrical resistivity, and thermal conductivity.
Conclusion
The developed cancer diagnosis device is capable of simultaneous ETM measurements of breast tissue specimens and can be a potential candidate for delineating normal and cancerous breast tissue cores.
Significance
The portable cancer diagnosis tool could potentially provide a deterministic and quantitative information about the breast tissue characteristics, as well as the onset and disease progression of the tissues. The tool can be potentially used for other tissue-related cancers.
Index Terms: Biomedical microelectromechanical systems, breast cancer diagnosis, tissue engineering
I. INTRODUCTION
According to American Cancer Society, this year about 40,290 deaths would occur in USA due to breast cancer [1]. Thus, breast cancer diagnosis and treatments are the topic of utmost importance. The mechanical properties (stiffness or elasticity) of the breast tissue change with the progression of the disease and act as a bio-marker for detecting and studying cancerous breast tissues. The change in stiffness can be directly linked to cancer progression [2,3]. The mechanical property that is known to be linked to the tumor formation is due to the modified structure of extracellular matrix (ECM) proteins which are found near breast cancer cells [4–10]. Based on several studies using Atomic Force Microscopy (AFM) and micro-sensors, it is known that the stiffness of the epithelial and stromal layer in cancerous tissue significantly differs from the normal breast tissue [3, 11]. An important current area of cancer research is to study the ETM properties of the breast tissue simultaneously and provide more accurate diagnostic information about the breast cancer. In addition, interest also lies in developing a portable device for quick and accurate analysis of the breast cancer. However, no significant effort has been made towards the development of a portable device that is capable of measuring ETM properties of the breast tissue simultaneously.
Microelectromechanical systems (MEMS) consists of electrical and mechanical components ranging from few microns to few hundred microns. MEMS-based devices are broadly classified as actuators or sensors. MEMS-based sensors and flexible devices have been used in biomedical application [12–18] and recently few groups used MEMS-based sensors to study the mechanical property of the breast tissue and cells as it has the capability of analyzing and palpating the biological materials at microscale and nanoscale [2, 3, 19]. The possibility of incorporating MEMS into portable lab-on-a-chip devices makes the MEMS-based sensor a potential candidate for a portable diagnostic device. Since MEMS dice interfaces with the environment for sensing, acutating and interconnection, MEMS packaging needs extra care [20–22]. The packaging of MEMS is application specific and should not only be economical but also provide ease of replacing biochips/integrated circuits/MEMS-based device [22]. In the present work, the tool is designed in a way to have press-fit contacts for taking out electrical connection from the biochip for analyzing the data. This design overcomes the challenges of soldering or wire bonding at a small scale and makes the device portable. Through the design of this platform, we can achieve a reliable mechanical and electrical contact from the biochip or MEMS to the external circuitry for data collection and analysis, along with a built in digital display. For each tissue measurement, a new biochip needs to be used so as to avoid any cross contamination (blood or tissue specimens left behind from previous measurements). In our present design, each 4-inch silicon wafer can yield twelve biochips. The number of biochips fabricated per wafer can be increased by using silicon wafer of larger diameter to increase the throughput in a single microfabrication batch process.
In this work, the electro-thermo-mechanical properties of breast tissue sample from normal and cancerous regions are measured. To the best of our knowledge, we are the first group to design and fabricate portable cancer diagnostic tool integrated with multifunctional sensors for cancer diagnostic studies such as the one described in this manuscript, namely the simultaneous measurement of electrical, mechanical, and thermal properties of tissues. The schematic diagram and the process flow for biochip fabrication is shown in Fig. 1.
Fig. 1.
(a) Schematic diagram of biochip, (b) Process flow for fabricating the biochip.
II. MATERIALS AND METHODS
A. Sensor Fabrication
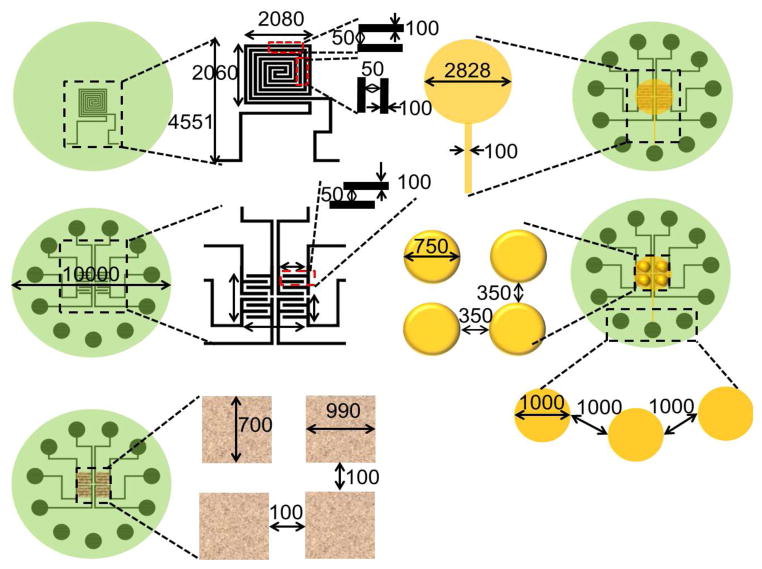
The biochip integrated with microheater and piezoresistive sensor array is fabricated on oxidized silicon substrate using a seven mask process. The sensor array covers 2000μm × 2000μm area while the complete device is 10mm in diameter. The fabrication process is as follows (see Fig. 1(b)): (i) a 4-inch, (100) orientation silicon (Si) wafer (500μm thick) is used as a substrate, (ii) 1μm silicon dioxide (SiO2) is grown using thermal evaporation, (iii) a microheater is fabricated on oxidized silicon substrate by patterning sputtered deposited nichrome (NiCr) (0.3μm thick). (iv) SiO2 (0.5μm) is deposited using plasma enhanced chemical vapor deposition (PECVD) over microheater. SiO2 is etched from the contact area of the microheater and Cr/Au (0.02μm/0.5μm) is deposited using e-beam evaporation and patterned to form interdigitated electrodes, (v) Germanium (1.5μm) is deposited over Cr/Au electrodes using e-beam evaporation and patterned using lift off technique to form sensing layer. SiO2 (1.8μm) is deposited using PECVD over the sensing layer and etched from the contact pads. Cr/Au (0.02μm/0.5μm) film is deposited and patterned to form the contact pad for the electrical connection to the tissue. (vi) SU-8 pillars (750μm diameter and 100μm height) are patterned over this contact pad and coated with metal to make them electrically conductive. These pillars serve a dual purpose: (a) they act as the force transmitters to the breast tissue during tissue indentation and (b) they are electrically conductive electrodes (E2). (vii) Front-to-Back alignment is used to open the window from the backside of silicon wafer and SiO2 layer is etched followed by silcon etching (350μm) to form 150μm diaphragm. The fabricated biochip is realized by dicing it from silicon wafer using a dicing saw from MicroAutomation®. Figure 2 shows the dimensions of the elements used in fabricating the biochip.
Fig. 2.
Dimensions of the biochip and integrated elements. All dimensions are in microns. Drawings are not to scale.
The SEM images of microheater, interdigitated electrodes, sensing layer, gold electrode for electrical contact, SU-8 pillars over gold electrode, and diaphragm is shown in Fig. 3(a) to Fig. 3(e). Figure 3(f) shows the photograph of the biochip.
Fig. 3.
SEM images of: (a) Microheater, (b) Cr/Au interdigitated electrodes over microheater, (c) Sensing layer, (d) Gold coated SU-8 pillars over Cr/Au electrode over silicon dioxide over sensing layer, (e) Backside of silicon diaphragm, (f) A photograph of the biochip.
B. Breast Tissue Preparation
Invasive ductal carcinoma (IDC) and normal tissue blocks are selected from cancer tissue bank in Biospecimen Repository Service at Rutgers Cancer Institute of New Jersey. Guided by a hematoxylin and eosin (H&E) stained tissue section, the technician carefully extracted each tissue cylinder by inserting a needle with 2mm inner diameter into the selected region of the tissue block using a Manual Tissue Arrayer (Beecher Instruments). The tissue cylinders, which are visually confirmed to contain enough length of embedded tissue, are placed into color-coded Eppendorf tube and deparaffinized (xylene 5min × 3, 100% alcohol 5min × 3, 95 % alcohol 5min × 1, 75 % alcohol 3min x1, keep in PBS) before the experiment. Compared to our previous work [23, 24], the tissue specimens used in this study are larger in size (2mm diameter and 3–3.8mm thickness). The reasons for using larger tissues are: 1) to make it a possible candidate for diagnostic device since larger sampling area helps in reducing the potential repetition of sampling needed for generating reliable results, 2) larger tissue sample facilitates the use of multiple sensors simultaneously, 3) although AFM measurements on tissue specimens often displayed variation over even a small sampling area on the surface of the specimen [3, 16], our experimental data on piezo-resistive assessment of tissue sample had been relatively stable, both over the small spatial area and across specimens of same disease group [23]. Therefore, we considered it is appropriate to increase the sampling distance between the sensors while maintaining signal sensitivity, and 4) having larger tissue specimen may better mimic the scenario of detecting tumor or tumor margin on the surgical surface. Figure 4(a) shows the schematic diagram of the breast tissue core preparation, while Fig. 4(b) and 4(c) show the photographs of the paraffinized and deparaffinized breast tissue cores respectively.
Fig. 4.
(a) Schematic diagram of breast tissue core preparation, (b) and (c) Photographs of paraffinized tissue and deparaffinized breast tissue.
The scanning electron microscopy (SEM) images of the normal and IDC breast tissue is shown in Fig. 5. Figure 5(a) and 5(b) shows top view and magnified view of the surface of normal and IDC tissue, respectively. The tumor specimen appeared rough and coarse whereas normal tissue displayed a refined and delicate structure. Figure 5(c) and (d) shows the H&E images of normal and IDC breast tissue cores. We digitized tissue sections from the FFPE blocks before and after extracting the experimental piece to closely monitor tissue histology on the exact tissue pieces that were examined to include only the most typical histology to represent the disease group where the actual location of sampling is highlighted in yellow. The normal specimen captured a typical terminal ductal lobular unit, which is the basic functional and histological unit of the breast. A segment of terminal duct was shown to branch and form organized clusters of small ductules, where each cluster was called a lobule. The ductules displayed a typical two-layered structure with myothelial cells as the outer layer. Intra-lobule connective tissue and inter-lobule connective tissue is also captured in this specimen. The tumor specimen contained large groups of infiltrating tumor cells separated by thin layers of desmoplastic connective tissue and lymphocytes. The breast tumor cells lost the normal structure and displayed mitotic figures.
Fig. 5.
SEM images of (a) normal (27959), (b) invasive ductal carcinoma (24353) breast tissue cores, (c) and (d) H&E images of normal and invasive ductal carcinoma breast tissue cores, respectively.
C. Experimental Setup
Figure 6(a) shows the schematic diagram of a portable cancer diagnosis tool. The tool consists of a MP-285 micromanipulator attached with an indenter and a disposable sensor module integrated with the biochip for measuring ETM properties of the breast tissue. Figure 6(b) shows the blown up schematic diagram of the disposable sensor module. The system is a combination of microfabrication technology, 3D printing technology, reliable packaging, and multi-functional tissue characterization techniques. The sensor module consists of a room for placing tissue sample, a biochip, and connecting pins facilitating the incorporation of the biochip output to the data acquisition card. The tissue sample is placed in the cylindrical space inside the sensor module. The indenter connected to MP-285 micromanipulator also works as an electrically conducting electrode (E1) when it comes in contact with the tissue. A temperature sensor is attached to the end of the indenter to measure the temperature at the top surface of the tissue. With the assumption that the sample tissues are regarded as uniform structures, the measured temperature values are converted to thermal conductivity using:
| (1) |
where, q[W] is the rate of heat transfer through the tissue along the length of the tissue, k[W/mK] is the thermal conductivity, A[m2] is the cross-sectional area of the tissue, ΔT [K] is the temperature difference between bottom and top surface of the tissue, and Lt [m] is the thickness of the tissue.
Fig. 6.
(a) Schematic diagram of the experimental setup, (b) Blown-up schematic diagram of the packaging system.
III. RESULTS AND DISCUSSION
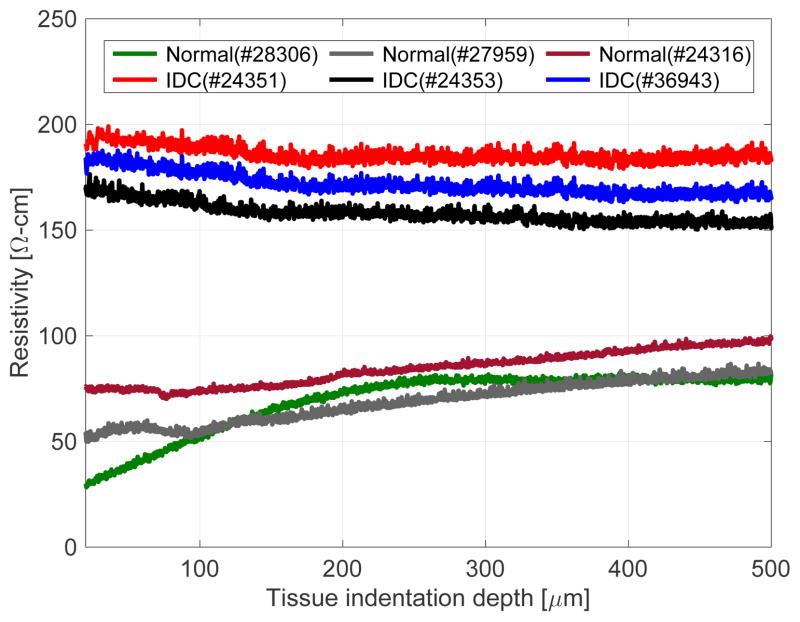
To measure the electrical conductivity of the tissue, a constant voltage is applied between the top electrode (E1) and the bottom electrode (E2) on the sensor chip. The electrical path is complete when the electrode E1 touches the tissue sample and current passes through the top electrode E1 through the tissue to the bottom electrode E2. The output voltage from the voltage divider varies with respect to the resistivity of the tissue, which correspondingly depends on the type of the tissue (normal or cancerous (IDC)). From the scanning electron microscopic image, we observed that the normal tissues show a smooth topography compared to cancerous tissues, which show ruptured structures [8]. We hypothesize that: (i) when the current passes through the tissue, the resistance fluctuates depending on the composition of the tissue, (ii) the cancerous tissues being coarse in nature provides a higher resistance path for current to flow compared to smooth structures of the normal tissues. Figure 7 shows the electrical resistivity plot of the breast tissues (three normal and three IDC). Cancerous tissue (IDC) shows higher resistivity during indentation compared to normal tissue. The resistivity values obtained from both tissue cores agree with the resistivity of human breast tissues which ranges from 149–463Ω-cm [25]. Breast tissues can be categorized as gland tissue, connective tissue, and subcutaneous fatty tissue according to the morphology of the breast. It is known that there is a significant difference in electrical resistivity among them (For example, fatty tissue has a couple of times higher electrical resistivity than gland tissue). The range (149–463Ω-cm) includes the results from all kind of breast tissues. Since breast cancer is detected in gland tissue most frequently, we used gland tissue in the experiment. Therefore, it should vary within smaller range than entire range of resistivity. Of course, narrowing down the range does not guarantee successful differentiation. The literature about electrical resistivity of cancerous breast tissue shows statistical difference between normal and cancer tissue groups instead of suggesting a specific threshold due to overlapping data.
Fig. 7.
Experimental results for electrical characterization of tissues.
A number of related experiments in the literature show higher resistivity of breast cancer tissue compared to normal mammary glands or normal cases. Morimoto et al. [26] reported the resistance value of breast tissues as 1445 ± 586Ω for cancerous tissue (n = 31) and 780 ± 148Ω for normal tissue. Maecka-Massalska et al. [27] also reported that cancerous breast tissue (684.06 ± 15.83Ω (n = 34)) showed higher resistance value than normal breast tissue (580.42 ± 12.71Ω (n = 34)). Jossinet [28] showed that the electrical resistance of cancerous breast tissue (n = 23) is higher than mammary gland (n = 28) normal breast tissue. This observation is confirmed by our previous study as well [24]. We believe our localized measurements were related to the disturbance in tissue structure and loss of inter-cellular junctions between cancer cells. Though our experiments had limited number of samples, we were able to find the result consistent with the earlier studies. To measure the mechanical properties of the tissue, the tissue placed inside the sensor module is pressed on the SU-8 pillars using an indenter. These pillars are used to transfer the force to the sensing layer. The output signal from the sensor array depends on the magnitude of force sensed. The amount of force sensed by the sensor depends on the elasticity of the tissue. Thus, the change in signal from the sensor corresponds to the elasticity of the tissue. Figure 8(a) shows the calibration result with the commercial load cell (MDB-2.5, Transducer Techniques, USA). The change in resistance values of the sensing layer are measured by the voltage divider. On applying the compressive load normal to the sensor, the output voltage changes linearly with R2-value of 0.9830. Fig. 8(b) shows the compressive force measured during the tissue indentation experiment with 100μm/s indentation speed. It is known that resistance of p-type piezoresistive material decreases when compression force is applied on it [29–30]. The fabricated sensor is connected in the voltage divider circuit. As resistance of the sensor decreases due to the applied compressional force, the output voltage from the voltage divider also decreases. The difference between normal and IDC tissues is small for indentations upto 200μm, however, the difference gets larger on indenting the tissue above 200μm. This trend corresponds with the published result [25, 31], which shows that the difference in the elasticity values between normal and cancerous breast tissues are higher for larger value of the strains compared to the smaller strain values. Cancerous tissue shows higher elasticity value, since it contains harder inclusion. At small strain, viscoelastic characteristics of biological tissue hide the effect of inclusion (i.e. tissue can be deformed slightly with very small reaction force whether it contains harder inclusion or not). Based on our experiment, the optimal indention depth is around 400μm which is the point that gives maximum value from the objective function:
| (2) |
where, DE and DR is the difference of mean elasticity [kPa] and mean resistivity [Ω-cm] between the two groups, respectively.
Fig. 8.
(a) Force calibration curve of the fabricated sensor, (b) Force curves obtained from indenting normal and cancerous (IDC) breast tissues.
To measure the response of the microheater, a DC voltage from 0–1.8V with increment of 0.2V step is applied and the temperature is measured. The steady state values of the temperature at each voltage is measured and plotted (Fig. 9(a)). The best fit R2-value obtained is 0.9966. It is observed that the difference between the surface temperature of the tissue and the measured temperature at the top of the tissue is very small (Fig. 9(a)). In order to understand the plot more clearly, the measured temperature values from the top surface of the tissue are converted to thermal conductivity values as shown in Fig. 9(b).
Fig. 9.
(a) Calibration plot of the microheater, (b) Temperature and thermal conductivity measurement of normal and cancerous (IDC) breast tissues.
The breast tissue placed on the biochip is heated from 25 °C to 50 °C with 5 °C step increment using the integrated microheater and the thermal conductivity of the normal and IDC tissues is measured using heat conduction equation (using Eqn. 1). The thermal conductivity of normal and IDC breast tissues is plotted by measuring the temperature at the top end of tissue using the thermistor placed on the indenter (Fig. 9(b)). We conducted the two-sample t-test for the thermal conductivity data of normal and IDC groups to analyze data statistically. Apart from the first temperature range (20–25 °C), rest of the data falls within p-value of 0.05. The p-value for the entire data set was calculated as 0.000027 which shows statistically significant difference in thermal conductivity between normal and cancer tissue groups. This implies the thermal conductivity of breast tissue can be used as a biomarker to differentiate normal tissue from cancerous breast tissue, once we have enough number of measurement points. In the case of cancerous tissue, the thermal conductivity of the tissue increases with increase in temperature while in normal tissues, the change in the thermal conductivity does not show a particular trend. However, from the t-test it is observed that the thermal conductivity values obtained are statistically different.
The device fabricated for the present study uses 4-inch silicon wafer with the combination of microtechnology and low-cost rapid prototyping. The estimated cost of the device including micro-fabrication, additive manufacturing, testing and evaluation would be around 500 USD. However, the cost can be reduced by fabricating biochips on 12-inch silicon wafer presently used in industry instead of using 4-inch wafer.
IV. CONCLUSION
The novelty of this work is the design and fabrication of a complete system for measurement of multiple tissue parameters, which can potentially provide a deterministic and quantitative information of the tissue characteristics, includes mechanical, electrical, and thermal characteristic of the normal tissue as well as the onset and disease progression of the tissue. This device for cancer diagnosis comprises of a disposable single-use components as well as a base platform, which can be used in several studies. In the present work, we use a micromanipulator for micro-scale indentation of the tissue placed on the base of the biochip. The device shown in present work could delineate the normal and diseased [invasive ductal carcinoma (IDC)] breast tissues by measuring the electro-thermo-mechanical changes in the tissue sample. In our future work, large number of samples would be investigated using this tool. Our focus is on building a portable automated device which can serve as an indicator to measure the false positive or negative in breast cancer and help the physician to determine the stage of cancer.
Acknowledgments
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R01CA161375.
We acknowledge the support of Maryland Nanocenter for SEM images and sensor fabrication, as well as Histopathology and Imaging Shared Resources of the Rutgers Cancer Institute of New Jersey for tissue archive and specimen preparation.
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contributor Information
Hardik J. Pandya, Department of Mechanical Engineering, University of Maryland, College Park, MD, USA. He is now with Brigham and Women’s Hospital - Harvard Medical School, Cambridge, MA, USA.
Kihan Park, Department of Mechanical Engineering, University of Maryland, College Park, MD, USA.
Wenjin Chen, Department of Pathology and Laboratory Medicine, Rutgers Robert Wood Johnson Medical School, Rutgers, The State University of New Jersey, New Brunswick, NJ, USA.
Lauri A. Goodell, Department of Pathology and Laboratory Medicine, Rutgers Robert Wood Johnson Medical School, Rutgers, The State University of New Jersey, New Brunswick, NJ, USA
David J. Foran, Department of Pathology and Laboratory Medicine, Rutgers Robert Wood Johnson Medical School, Rutgers, The State University of New Jersey, New Brunswick, NJ, USA
Jaydev P. Desai, Department of Mechanical Engineering, University of Maryland, College Park, MD, USA.
References
- 1.American Cancer Society. Breast Cancer Facts & Figures 2015. American Cancer Society; Atalanta, GA: 2015. [Online]. Available: http://www.cancer.org/acs/groups/content/@editorial/documents/document/acspc-044552.pdf. [Google Scholar]
- 2.Cross SE, et al. Nanomechanical Analysis of Cells from Cancer Patients. Nat Nanotechnol. 2007 Dec;2:780–783. doi: 10.1038/nnano.2007.388. [DOI] [PubMed] [Google Scholar]
- 3.Roy R, et al. Microarray Facilitated Mechanical Characterization of Breast Tissue Pathology Samples Using Atomic Force Microscopy (AFM)”, in. Proc IEEE RAS/EMBS BioRob. 2010:710–715. [Google Scholar]
- 4.Keese CR, Giaever I. A Biosensor that Monitors Cell Morphology with Electrical Fields. IEEE Eng Med Biol. 1994 Jun;13(3):402–408. [Google Scholar]
- 5.Deryugina EI, Quigley JP. Extracellular Matrix Degradation. Berlin: Springer-Verlag; 2011. The Role of Matrix Metalloproteinases in Cellular Invasion and Metastasis; pp. 145–191. [Google Scholar]
- 6.Lu P, et al. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb Perspect Biol. 2011 Dec;3(12):a005058. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mangia A, et al. Tissue Remodelling in Breast Cancer: Human Mast Cell Tryptase as an Initiator of Myofibroblast Differentiation. Histopathology. 2011 Jun;58(7):1096–1106. doi: 10.1111/j.1365-2559.2011.03842.x. [DOI] [PubMed] [Google Scholar]
- 8.Lu P, et al. The Extracellular Matrix: A Dynamic Niche in Cancer Progression. J Cell Biol. 2012 Feb;196(4):395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wegener J, et al. Electric Cell-substrate Impedance Sensing (ECIS) as a Noninvasive Means to Monitor the Kinetics of Cell Spreading to Artificial Surfaces. Exp Cell Res. 2000 Aug;259(1):158–166. doi: 10.1006/excr.2000.4919. [DOI] [PubMed] [Google Scholar]
- 10.Mamishev AV, et al. Interdigital Sensors and Transducers. Proc IEEE. 2004 May;92(5):808–845. [Google Scholar]
- 11.Samani A, et al. Elastic Moduli of Normal and Pathological Human Breast Tissues: An Inversion technique-based Investigation of 169 Samples. Phys Med Biol. 2007 Feb;52(6):1565–1576. doi: 10.1088/0031-9155/52/6/002. [DOI] [PubMed] [Google Scholar]
- 12.Sekitani T, et al. A Rubber like Stretchable Active Matrix Using Elastic Conductors. Science. 2008 Sep;321(5895):1468–1472. doi: 10.1126/science.1160309. [DOI] [PubMed] [Google Scholar]
- 13.Kim DH, et al. Stretchable and Foldable Silicon Integrated Circuits. Science. 2008 Apr;320(5875):507–511. doi: 10.1126/science.1154367. [DOI] [PubMed] [Google Scholar]
- 14.Someya T, et al. A Large-area, Flexible Pressure Sensor Matrix with Organic Field-effect Transistors for Artificial Skin Applications. Proc Natl Acad Sci USA. 2004 Jul;101(27):9966–9970. doi: 10.1073/pnas.0401918101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Someya T, et al. Conformable, Flexible, Large-area Networks of Pressure and Thermal Sensors with Organic Transistor Active Matrixes. Proc Natl Acad Sci USA. 2005 Aug;102(35):12321–12325. doi: 10.1073/pnas.0502392102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suresh S. Biomechanics and Biophysics of Cancer Cells. ACTA Mater. 2007 Jul;55(12):3989–4014. doi: 10.1016/j.actbio.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swift J, et al. Nuclear Lamin-A Scales with Tissue Stiffness and Enhances Matrix-directed Differentiation. Science. 2013 Aug;341(6149):1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pandya HJ, et al. Accurate Characterization of Benign and Cancerous Breast Tissues: A specific Patient Studies Using Piezoresistive Microcantilevers. Biosens Bioelectron. 2015 Jan;63(1):414–424. doi: 10.1016/j.bios.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Plodinec M, et al. The Nanomechanical Signature of Breast Tissue. Nat Nanotechnol. 2012 Nov;7(11):757–765. doi: 10.1038/nnano.2012.167. [DOI] [PubMed] [Google Scholar]
- 20.Hvims HL. Conductive Adhesives for SMT and Potential Applications. Microelectron Reliab. 1996 Apr;36(4):554–555. [Google Scholar]
- 21.Rusanen O, Lenkkeri J. Reliability Issues of Replacing Solder With Conductive Adhesives in Power Modules. IEEE Trans Compon Pack B. 1995 May;18(2):320–325. [Google Scholar]
- 22.Monk DJ, et al. Media Compatible Packaging and Environmental Testing of Barrier Coating Encapsulated Silicon Pressure Sensors. SOLSEN. 1996:36–41. [Google Scholar]
- 23.Pandya HJ, et al. Mechanical Phenotyping of Breast Cancer Using MEMS: A Method to Demarcate Benign and Cancerous Breast Tissues. Lab Chip. 2014 Sep;14(23):4523–4532. doi: 10.1039/c4lc00594e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pandya HJ, et al. Design and Fabrication of a Flexible MEMS-based Electro-mechanical Sensor Array for Breast Cancer Diagnosis. J Micromech Microeng. 2015 Jun;25(7):075025. doi: 10.1088/0960-1317/25/7/075025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faes TJC, et al. The Electric Resistivity of Human Tissues (100 Hz-10 MHz): A Meta-analysis of Review Studies. Physiol Meas. 1999 Nov;20(4):R1. doi: 10.1088/0967-3334/20/4/201. [DOI] [PubMed] [Google Scholar]
- 26.Morimoto T, et al. A Study of the Electrical Bioimpedance of Tumors. J Invest Surg. 1993 Jan;6(1):25–32. doi: 10.3109/08941939309141189. [DOI] [PubMed] [Google Scholar]
- 27.Massalska M, et al. Altered Tissue Electrical Properties in Women with Breast Cancer - Preliminary Observations. Ann Agr Env Med. 2013;20(3):523–527. [PubMed] [Google Scholar]
- 28.Jossinet J. Variability of Impedivity in Normal and Pathological Breast Tissue. Med Biol Eng Comput. 1996 Sep;34(5):346–350. doi: 10.1007/BF02520002. [DOI] [PubMed] [Google Scholar]
- 29.Rowe ACH. Piezoresistance in Silicon and Its Nanostructures. J Mater Res. 2014 Mar;29(6):731–744. [Google Scholar]
- 30.Shahrjerdi D, et al. Low Temperature Stress-induced Crystallization of Germanium on Plastic. Thin Solid Films. 2003 Mar;427(1):330–334. [Google Scholar]
- 31.Wellman PS, et al. Breast Tissue Stiffness in Compression is Correlated to Histological Diagnosis. Harvard BioRobotics Laboratory; Cambridge, MA: 1999. [Online]. Available: https://biorobotics.harvard.edu/pubs/1999/mechprops.pdf. [Google Scholar]