Abstract
BACKGROUND
Bait formulations are considered the most effective method for reducing German cockroach infestations. An important property of some bait formulations is secondary kill, whereby active ingredient is translocated in insect-produced residues throughout the cockroach population, especially affecting relatively sedentary early instar nymphs.
RESULTS
Blattella germanica was collected from a location where baits containing hydramethylnon, fipronil, or indoxacarb became ineffective, and these AIs were topically applied to adult males. Results revealed the first evidence for hydramethylnon resistance, moderate resistance to fipronil and extremely high resistance to indoxacarb. Insecticide residues excreted by field-collected males that ingested commercial baits effectively killed nymphs of an insecticide-susceptible laboratory strain of B. germanica but failed to kill most nymphs of the field-collected strain.
CONCLUSIONS
We report three novel findings: 1) The first evidence for hydramethylnon resistance in any insect; 2) extremely high levels of indoxacarb resistance in a field population; and 3) reduced secondary mortality in an insecticide-resistant field-collected strain of B. germanica. We suggest that while secondary mortality is considered to be advantageous in cockroach interventions, the ingestion of sublethal doses of AI by nymphs may select for high insecticide resistance by increasing the frequency of AI resistance alleles within the population.
Keywords: Blattella germanica, fipronil, indoxacarb, hydramethylnon, insecticide resistance, bait, secondary kill
1 Introduction
The German cockroach (Blattella germanica) is a widespread urban pest of significant health concern, mainly because it produces asthma-triggering allergens1,2 and can vector pathogenic microorganisms.3–5 It is generally recognized that the most effective way to control German cockroach populations is with bait formulations.6 These products include nutrients that stimulate feeding and a toxic active ingredient (AI). The targeted nature of bait formulations reduces insecticide exposure to humans and their pets.6,7 Several bait formulations have secondary8,9 and even tertiary10 kill properties through various mechanisms, including coprophagy,8 cannibalism,11 and emetophagy.12 Deposition of insecticide-containing feces within harborages and consumption of feces by early instar nymphs via coprophagy can make the insecticide more accessible to the cockroach population.8,13–15
Though bait formulations are largely effective, several B. germanica field populations have been reported to be resistant to some bait AIs (sulfluramid;16 fipronil;17,19,20 indoxacarb;18 abamectin;19,20 imidacloprid20). Hydramethylnon21 has been a very effective AI in bait products against the German cockroach. Despite its longstanding use since the 1980s, no hydramethylnon-resistant populations of any insect have been found, and the highest resistance ratio (RR) reported in the German cockroach was 1.5.16,22–25
In August 2012, we collected B. germanica from an apartment in Puerto Rico where performance of Advion® (AI – indoxacarb), Maxforce FC Magnum® (AI – fipronil) and Maxforce Pro Roach Killer® (AI – hydramethylnon) gel baits was poor. We conducted both laboratory bait efficacy studies and topical assays with fipronil, indoxacarb, and hydramethylnon to determine whether insecticide resistance in this strain (PR 712) might explain, in part, the observed poor bait performance. Moreover, we investigated secondary kill in this strain. All reports to date on cockroach secondary kill had been performed with longstanding insecticide-susceptible laboratory colonies. Yet, the efficacy of baits may be further compromised in resistant populations by poor secondary kill performance. Our goals were to 1) characterize the collected field strain for resistance through topical application of AI and ingestion of formulated bait and 2) compare the toxicity of adult excreta to nymphs from the field-collected strain and from a laboratory-susceptible strain of B. germanica.
2 Experimental Methods
2.1 Insect strains and rearing conditions
We compared two Blattella germanica strains: 1) Orlando Normal, an insecticide susceptible strain maintained in the laboratory for over 70 years, 2) PR-712, collected in August 2012 from a single apartment in Monseratte Tower 1, Carolina, Puerto Rico. The cockroach population in this unit could not be controlled with a range of commercial bait products. We propagated newly collected PR 712 for two to three generations then allocated these insects to four treatments: 1) unselected, 2) fipronil-selected (Maxforce FC Magnum gel bait), 3) indoxacarb-selected (Advion gel bait), and 4) hydramethylnon-selected (Maxforce Pro Roach Killer). Insecticide selection was accomplished by placing approximately two grams of bait in a rearing container for three days then removing any remaining bait. After bait exposure, living insects were moved to a clean container. Food (Purina 5001 Rodent Diet, PMI Nutrition International, St. Louis, MO, USA) and water were provided ad libitum. This process was repeated every two months for two years, after which time the experiments detailed below were performed. Laboratory conditions for insect rearing and all experiments were 25°C (± 1°C), 37 ± 5% RH and LD 12:12.
2.2 Topical application of insecticides
Technical insecticides were serially diluted with acetone and 0.5 μL of a dilution was applied to the ventral surface of the cockroach between the metacoxae with a repeating micro-pipette (Hamilton Company, Reno, NV, USA). At least 30 individuals were treated with each concentration. Following treatment, cockroaches were maintained in three groups of 10 in 10 cm diameter petri dishes (Fisher Scientific, Pittsburgh, PA) and provisioned with rat chow and water. Cockroaches topically treated with fipronil or indoxacarb were monitored for mortality daily for two days; those treated with hydramethylnon for five days. The greatest range of mortality for the AIs tested at given doses occurred at these two time points. Insects that could not right themselves within 30 seconds when flipped, and would exhibit erratic appendage movements were considered dead. Values for LD50 and LD90, and their respective fiducial limits, were estimated from Probit analysis in Polo Plus (LeOra Software Company, Petaluma, CA, USA).26
2.3 Effect of bait formulations on adult male survival
We provided male German cockroaches ~0.5 g of one of three baits in a vial cap (Maxforce FC Magnum, 0.05% Fipronil; Advion, 0.06% Indoxacarb; Maxforce Pro Roach Killer, 2.15% Hydramethylnon), plus rodent chow and water for one week. The cockroaches were housed in glass jars (Diam: 88 mm; Ht: 95 mm), with the inside rim coated with a thin layer of petroleum jelly/mineral oil to prevent escape. We recorded mortality daily and removed dead cockroaches. Five replicates, with 20 insects per replicate were performed for each of the treatments.
2.4 Secondary toxicity to nymphs
After one week of bait exposure, all adults and bait were removed from the jars. We then placed 20 first-instar nymphs in each jar: 10 of an insecticide-susceptible orange-body variant of Orlando Normal27 plus 10 PR-712 nymphs from the same selection regime cohort initially evaluated in the bait experiment with adult males. The orange-body variant enabled us to distinguish effects on insecticide-susceptible and insecticide-resistant nymphs exposed to the same residues (feces, regurgitate), and thus served as a within-jar control. As a control for this color variant, both black-body (wild type) and orange-body Orlando Normal nymphs were exposed to deposits produced by black-body adult male Orlando Normal. Rodent chow and water were provided ad libitum.
2.5 Statistical analysis
LD50 and LD90 values for each strain-AI pair were calculated and compared using the lethal dose ratio test, whereby LD50 and LD90 values are significantly different from one another if the upper and lower 95% confidence intervals of the ratio do not contain 1 (PoloPlus program, LeOra Software Company, Petaluma, CA, USA).26 We used a log-rank test to compare strains in the bait primary and secondary kill assays. A Sidak-adjustment was used to account for multiple-comparisons in primary kill (SAS 9.3, SAS Institute, Cary, NC). No adjustment was needed for comparisons of secondary kill because only two strains were compared. Resistance ratios (RR) were calculated by dividing the LD50 and LD90 values of the PR-712 strain by the respective LD50 and LD90 values of the Orlando Normal susceptible strain.
3 Results
3.1 Topical application of insecticides
Acetone alone did not cause any mortality. The PR-712 strain was significantly more resistant to fipronil, indoxacarb, and hydramethylnon than the Orlando Normal susceptible strain (LD50 RR: 5.60, 23.21, 3.89; LD90 RR: 9.78, 391.3, 8.74, respectively; Table 1). Continued lab selection of PR-712 increased fipronil, indoxacarb, and hydramethylnon resistance (LD50 RR: 15.92, 13,375, 19.31; LD90 RR: 20.20, ~54,619, 350.9, respectively; Table 1). LD90 could not be accurately estimated for the PR-712 lab-selected cockroaches treated with indoxacarb, because only 16% of the individuals died at the highest topical dose (150 μg 0.5 μL−1), so an approximation was made based on log10 dose and probit value regression.
Table 1.
Toxicity, by topical application, of fipronil, indoxacarb, or hydramethylnon to adult males of a field-collected (PR-712) and a laboratory insecticide-susceptible strain of Blattella germanica.
| Strain | Insecticide | n | Slope (±SE) | LD50 (95% CI)(μg·g−1)a | LD90 (95% CI)(μg·g−1)b | Χ2 (d.f.) | RR50c | RR90d |
|---|---|---|---|---|---|---|---|---|
| Orlando Normal | Fipronil | 150 | 7.59 (±1.4) | 0.04 (0.04–0.05)e | 0.06 (0.05–0.07)e | 1.999 (2) | – | – |
| PR-712 unselected | Fipronil | 180 | 3.07 (±0.41) | 0.22 (0.12–0.33)f | 0.60 (0.39–1.81)f | 9.749 (4) | 5.6 | 9.78 |
| PR-712 Fipronil selected | Fipronil | 180 | 4.59 (±0.72) | 0.64 (0.50–0.99)g | 1.21 (0.84–4.61)g | 4.491 (3) | 15.93 | 20.2 |
|
| ||||||||
| Orlando Normal | Indoxacarb | 330 | 6.21 (±0.91) | 3.8 (3.2–4.5)e | 6.1 (5–14.3)e | 28.659 (8) | – | – |
| PR-712 unselected | Indoxacarb | 210 | 0.9 (±0.12) | 88.2 (9.3–323.2)f | 2387.2 (559.7–442224.3)f | 11.760 (4) | 23.21 | 391.34 |
| PR-712 Indoxacarb selected | Indoxacarb | 210 | 0.66 (±0.22) | 50839 (9318–134000000)g | ~33317865 (147493.7–NA)g | 3.008 (4) | 13374.73 | ~54619.45h |
|
| ||||||||
| Orlando Normal | Hydramethylnon | 180 | 2.82 (±0.3) | 19 (14.2–24.8)e | 54.3 (39.8–85.9)e | 6.830 (6) | – | – |
| PR-712 unselected | Hydramethylnon | 210 | 1.59 (±0.19) | 74 (23.7–186.2)f | 474.7 (188.2–5512.5)f | 11.099 (4) | 3.9 | 8.74 |
| PR-712 Hydramethylnon selected | Hydramethylnon | 180 | 0.75 (±0.16) | 367 (186.4–793.2)g | 19054 (4961.9–422880)g | 1.989 (3) | 19.32 | 350.9 |
LD50a,LD90b values with 95% confidence intervals. Values in μg active ingredient g−1 insect.
Resistance ratios at LD50c and LD90d. RR50 = LD50 value of PR712 strain/LD50 value of Orlando Normal strain.
Significant based on non-overlap of 95% confidence intervals among strains within insecticide applied.
LD90 value cannot be accurately determined due to high-level resistance.
Strain weights: Orlando Normal 52.95 mg male−1, PR-712 unselected 54.27 mg male−1, PR-712 fipronil selected 53.72 mg male−1, PR-712 indoxacarb selected 51.90 mg male−1, PR-712 hydramethylnon selected 56.82 mg male−1.
3.2 Effect of bait formulations on adult male mortality
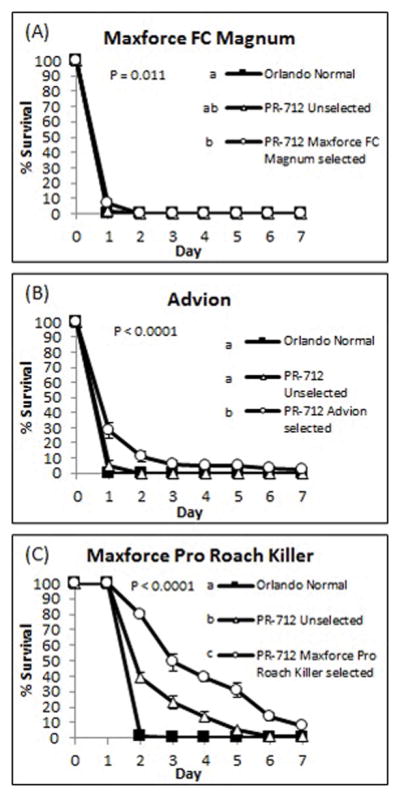
Unselected PR-712 males survived longer than Orlando Normal males when exposed to hydramethylnon bait (Log rank test: Χ2 = 14.6813, P = 0.0004) but not Maxforce FC Magnum (Χ2 = 0.6850, P = 0.7924) or Advion (Χ2 = 0.5389, P = 0.8450; Figure 1).
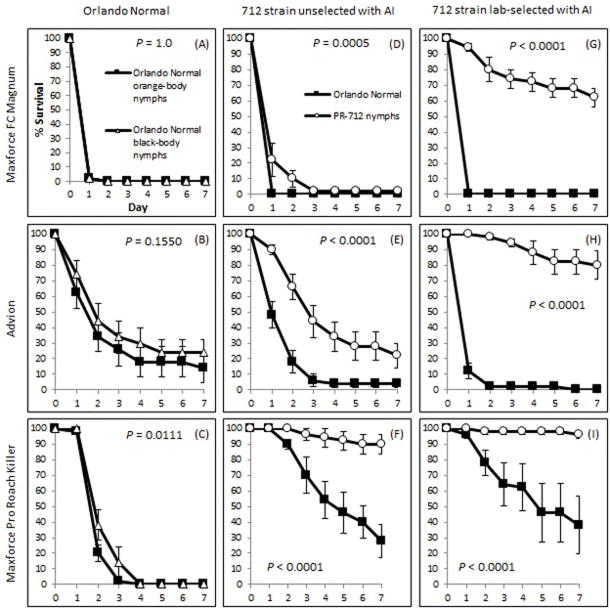
Figure 1.
Survival of adult male B. germanica on three commercial bait products. (A) Maxforce FC Magnum cockroach bait (0.05% fipronil); (B) Advion cockroach bait (0.6% indoxacarb); (C) Maxforce pro roach killer (2.15% hydramethylnon). P–values determined with Log-rank test, plus-Sidak adjustment. P-values represent overall differences among strains. Strains not sharing lower case letter (adjacent to legend) are significantly different from each other.
Continuous laboratory selection with each bait extended the survival of PR-712 relative to Orlando Normal (fipronil bait: Χ2 = 8.3912, P = 0.0113, indoxacarb: Χ2 = 42.6871, P < 0.0001, hydramethylnon: Χ2 = 127.2, P < 0.0001) and unselected PR-712 (indoxacarb bait: Χ2 = 29.4652, P < 0.0001, hydramethylnon: Χ2 = 34.2846, P < 0.0001; Figure 1B,C) but not for fipronil (Χ2 = 4.2812, P = 0.1112; Figure 1A).
3.3 Secondary toxicity to nymphs
The orange-body within-jar controls did not differ from the black-bodied insecticide-susceptible strain when exposed to secondary excretions from black-body Orlando Normal adult males fed fipronil- or indoxacarb-containing cockroach bait (Χ2 = 0, P = 1.0; Χ2 = 2.0222, P = 0.1550; Figure 2A, B respectively) but the two lab susceptible genotypes differed in their secondary response to hydramethylnon (Χ2 = 6.4451, P = 0.0111; Figure 2C). Nevertheless, the absolute differences between black and orange body cockroaches were relatively minor and justified the use of the latter in subsequent assays.
Figure 2.
Survival of Orlando normal and PR-712 nymphs continuously exposed to excreta from Orlando normal or PR-712 males that were fed one of three commercial baits. (A–C) Survival of Orlando normal black-body and orange-body nymphs on excreta from black-body Orlando normal adult males. (D–F) Survival of Orlando normal orange-body nymphs and unselected PR-712 nymphs on the excreta of unselected PR-712 adult males. (G–I) Survival of Orlando normal orange-body nymphs and bait selected PR-712 nymphs on the excreta of selected PR-712 adult males. P–values determined with Log-rank test.
In all subsequent assays nymphs were exposed to residues of adults of the same strain, so different amounts of residues might have been available in different treatments. Therefore, comparisons should be limited mainly to the two strains cohabiting the same jar. Nymphs of the PR-712 unselected strain survived significantly longer than nymphs of the Orlando Normal susceptible strain when exposed to secondary excretions from PR-712 unselected adults that had been fed fipronil-, indoxacarb-, or hydramethylnon-containing bait (Log rank test: Χ2 = 12.2360, P = 0.0005; Χ2 = 27.2406, P < 0.0001; Χ2 = 40.4296, P < 0.0001; Figure 2D,E,F, respectively).
Laboratory selection with baits further decreased mortality of PR-712 nymphs exposed to residues of adult males; these nymphs always survived significantly longer than Orlando Normal nymphs, regardless of bait (Maxforce FC Magnum: Χ2 = 87.7925, P < 0.0001; Advion: Χ2 = 106.5, P < 0.0001; Hydramethylnon bait: Χ2 = 38.8107, P < 0.0001; Figure 2G,H,I, respectively).
4 Discussion
The PR-712 strain was collected from an apartment where repeated treatments with insecticidal baits failed to provide adequate cockroach control. We report three novel findings from experiments with this field-collected strain of B. germanica: 1) The first evidence for hydramethylnon resistance in any insect, 2) rapid elevation in both hydramethylnon and indoxacarb resistance in response to selection, and 3) reduced hydramethylnon, indoxacarb, and fipronil secondary mortality in nymphs, suggesting a novel mechanism of selection for insecticide resistance.
4.1 Resistance to active ingredients
Our findings with topical applications of insecticides indicate that control failures were, at least in part, attributable to resistance to a broad-spectrum of AIs. Cockroaches have developed resistance to every organic insecticide within several years of intensive usage, going back to DDT, regardless of its formulation.6 Resistance has been documented even to the most recent introductions of new AIs.25 Surprisingly, however, despite its widespread and intensive usage in commercial cockroach baits for over 30 years, resistance to hydramethylnon has remained low, with a RR less than 1.5.16,22–25 The field-collected PR-712 strain exhibited RR50 and RR90 of 4-fold and 9-fold, respectively, and after artificial selection these values increased to 19-fold and 351-fold, respectively (Table 1). This rapid increase in resistance following two years of continuous artificial selection indicates that the allele(s) underlying hydramethylnon resistance were present in this population and selection elevated their frequency. Moreover, stability of hydramethylnon resistance in the PR-712 strain suggests that reversion to susceptibility in the absence of selection would be slow. Although specific records of hydramethylnon use are unavailable, hydramethylnon-based baits presumably selected on this population sometime prior to 2010.
The PR-712 population was exposed to intensive applications of Advion (indoxacarb) and Maxforce FC Magnum (fipronil) gel baits between 2010 and 2012, and not surprisingly, this strain exhibited resistance to both AIs (Table 1), showing that this is a multi-resistant strain. As with hydramethylnon, topical assays revealed that continuous artificial selection on PR-712 significantly increased resistance (RR50) to fipronil from 6-fold to 16-fold and to indoxacarb from 23-fold to >10,000-fold compared to the susceptible strain. Such high levels of resistance, based on topical applications, would be expected to impede pest control efforts.
However, bait formulations may be efficacious even in moderately resistant populations. The difference in mortality between insecticide-susceptible Orlando Normal and PR-712 was much less in bait feeding tests than from topical application, as most adult PR-712 died by the end of the bait feeding trials. The disparity between topical application and ingestion results may be related to two major issues: First, many modern bait AIs are much more toxic by ingestion than when topically applied, and some are activated by gut enzymes. Gondhalekar et al. (2010) reported a lower RR to indoxacarb when a B. germanica field strain ingested the AI compared to topical exposure, possibly a consequence of post-ingestive activation.28 Second, ingestion can deliver a massive dose of AI that often can overcome low to moderate resistance. For example, fipronil’s LD50 was 2.12 ng per Orlando Normal male (LD90 = 3.18 ng). Ingesting 2.5 mg of bait (0.05% AI), the typical daily intake of adult males,29 would deliver 1,250 ng fipronil, or almost 600-fold the LD50 and nearly 400-fold the LD90. This amount of AI would be sufficient to overcome the 16-fold resistance even of our artificially selected PR-712 strain. Likewise, the corresponding Orlando Normal estimates for hydramethylnon are: topical LD50 = 1.0 μg (LD90 = 2.9 μg); bait (2.15% AI) would deliver 53.8 μg AI, which is much more than necessary to kill the hydramethylnon selected PR-712 cockroaches. For indoxacarb, however, these estimates reveal that ingestion of even large amounts of AI are not likely to overcome indoxacarb resistance. The topical LD50 for Orlando Normal is 201 ng indoxacarb per male (LD90 = 323 ng); the Advion bait (0.06% AI) would deliver 1,500 ng AI, ~7.5-fold the LD50 dose. Thus, it is unlikely that the ingested dose would overcome a RR50 of 23.2 in the unselected strain, and >10,000 in the artificially selected PR-712 strain. We emphasize two important points regarding comparisons of topical applications and ingestion: First, we provided bait in excess, allowing continued ad libitum ingestion for the 7 day duration of the experiment. Thus, whereas faster-acting AIs (e.g., fipronil, indoxacarb) incapacitate the cockroach and probably limit it to a single meal, slower acting AIs (e.g., hydramethylnon) may allow multiple meals – and more AI ingested – before death. Second, these comparisons assume similar toxicodynamics for ingested and topically applied AIs, but more of the ingested than surface applied AI is expected be metabolized in vivo, leaving a smaller percentage of ingested AI to reach the target site, compared to topical application where AI bypasses the harsh digestive tract. Nevertheless, the huge amounts of ingested AIs compared to topically applied AIs, discussed above, are expected to compensate for any losses due to AI metabolism. Overall, these estimates predict that under field conditions, where cockroach populations are high and bait is often limited, highly or moderately resistant cockroaches may not ingest sufficient bait to succumb to a lethal dose of insecticide.
4.2 Secondary kill of nymphs
Our secondary-kill assay was designed to expose both Orlando Normal and PR-712 first instar nymphs to equal amounts of insecticide residues produced by either Orlando Normal or PR-712 males. To distinguish these two co-habiting strains, we used orange-body mutants of the susceptible Orlando Normal strain. Both orange-body and black-body nymphs responded similarly to fipronil- and indoxacarb-containing residues. Surprisingly, however, black-body nymphs exhibited significantly delayed mortality relative to orange-body nymphs on hydramethylnon-containing excretions from adult males. Reasons for this disparity, including the possibility that orange-body nymphs consume more feces than black-body nymphs, will be investigated in future research.
Nevertheless, the differences between orange- and black-body nymphs are minor compared to the differences between orange-body nymphs and PR-712 nymphs. In all instances we examined, mortality of Orlando Normal orange-body nymphs was significantly faster and greater than unselected and selected PR-712. Although this pattern is largely attributable to multi-resistance of PR-712 nymphs to fipronil, indoxacarb and hydramethylnon, we cannot rule out that the two strains differed in their consumption of the AI-containing adult excretions or their post-ingestive processing.
Commercial baits and AIs can also vary in secondary kill characteristics. Translocation of hydramethylnon and indoxacarb largely occurs via coprophagy,8,10 whereas fipronil acts secondarily via emetophagy and contact.12 In our study, all adults were removed before the nymphs were introduced, so horizontal translocation of AI through mechanisms other than coprophagy was most likely very limited, though surface contact of nymphs with toxic excretions was possible. Metabolic differences and differences in pre-ingestive sensory preferences of nymphs could also alter the lethality of excretions to nymphs. It is possible that coprophagy is more pronounced in laboratory colonies, and German cockroaches in the field (including PR-712) rely less on coprophagy, resulting in PR-712 nymphs ingesting less feces (AI) than Orlando Normal nymphs. Additionally, the two strains may differ in their qualitative preferences of adult feces. First instar nymphs offered equal amounts of adult male and female feces perform better (more likely to molt to the second stadium) on female feces.15 Although no study has yet determined whether nymphs exhibit preferences for ingesting male vs. female feces, it is conceivable that differential preferences of the two strains contributed to differences in mortality.
We found that only ~70% of the insecticide susceptible nymphs died within 7 days on residues from PR-712 adults fed hydramethylnon bait (Figure 2F,I), whereas 100% of these nymphs died when exposed to residues from Orlando Normal adults fed the same bait (Figure 2C). It is possible that PR-712 adults ingested less bait than Orlando Normal during the same time period, suggesting either lower general food intake, or a sensory avoidance of the bait. Glucose-averse cockroaches avoid glucose-containing baits,30 and our preliminary evidence suggests that a small fraction of the PR-712 population is sugar-averse,31 possibly contributing to lower ingestion of baits. In addition, it is also possible that one of several mechanisms that underlie hydramethylnon resistance in PR-712 cockroaches is the catabolism and inactivation of hydramethylnon in the digestive tract. Both mechanisms would result in less AI in the adult feces, and lower mortality of nymphs exposed to adult feces. Nevertheless, the striking differences between the Orlando Normal and PR-712 nymphs, and especially of the artificially selected PR-712 line, support the conclusion that multi-AI resistance was the primary factor that significantly lessened secondary kill in PR-712 nymphs.
The horizontal transfer of AIs has potential advantages and disadvantages.7 In the short-term, it results in secondary kill and presumably amplifies the direct effects of the AI, although it is important to note that all the evidence for these secondary effects come from laboratory and mesocosm studies and not from efficacy trials with field populations. On the other hand, we provide empirical support for the idea that translocation of AIs can expose low- or moderately resistant populations to sublethal doses of AIs, selecting for and causing a rapid increase in the frequency of resistance alleles, as discussed by Gressel (2010).32 Coupled with other mechanisms that produce sublethal exposure to AIs in moderately resistant cockroaches (e.g., ingestion of less bait, glucose- and other nutrient aversions), exposure through contact and coprophagy to sublethal amounts of AI in conspecific feces may constitute an important mechanism that accelerates the development of insecticide resistance. This mechanism may counteract or even negate the advantages of secondary kill inherent to some bait products.
Acknowledgments
This study was supported in part by the Blanton J. Whitmire Endowment at North Carolina State University and grants from the Pest Management Foundation (2013-2258), the U.S. Department of Housing and Urban Development Healthy Homes program (NCHHU0001-11 and NCHHU0017-13), and the Alfred P. Sloan Foundation (2013-5-35 MBE), and the National Institute of Environmental Health Sciences of the National Institutes of Health (P30ES025128).
REFERENCES CITED
- 1.Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D, Malveaux F. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 2.Arruda LK, Vailes LD, Ferriani VPL, Santos ABR, Pomes A, Chapman MD. Cockroach allergens and asthma. J Allergy Clin Immunol. 2001;107:419–428. doi: 10.1067/mai.2001.112854. [DOI] [PubMed] [Google Scholar]
- 3.Zurek L, Schal C. Evaluation of the German cockroach (Blatella germanica) as a vector for verotoxigenic Escherichia coli F18 in confined swine production. Vet Microbiol. 2004;101:263–267. doi: 10.1016/j.vetmic.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Ahmad A, Ghosh A, Schal C, Zurek L. Insects in confined swine operations carry a large antibiotic resistant and potentially virulent enterococcal community. BMC Microbiol. 2011;11:1471–2180. doi: 10.1186/1471-2180-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jalil N, Amir K, Hasan MKS, Mahdi M, Monireh M, Atefeh B. Cockroaches’ bacterial infections in wards of hospitals, Hamedan city, west of Iran. Asian Pac J Trop Dis. 2012:381–384. [Google Scholar]
- 6.Rust MK, Owens JM, Reierson DA. Understanding and controlling the German cockroach. Oxford University Press; New York: 1995. [Google Scholar]
- 7.Schal C. Cockroaches. In: Hedges S, Moreland D, editors. Handbook of Pest Control. GIE Media. Mallis Handbook; 2011. pp. 150–291. [Google Scholar]
- 8.Silverman J, Vitale GI, Shapas TJ. Hydramethylnon uptake by Blattella germanica (Orthoptera: Blattellidae) by coprophagy. J Econ Entomol. 1991;84:176–180. doi: 10.1093/jee/84.1.176. [DOI] [PubMed] [Google Scholar]
- 9.Bayer BE, Pereira RM, Koehler PG. Differential consumption of baits by pest blatted and blattellid cockroaches and resulting direct and secondary effects. Entomol Exp Appl. 2012;145:250–259. [Google Scholar]
- 10.Buczkowski G, Scherer CW, Bennett GW. Horizontal transfer of bait in the German cockroach: Indoxacarb causes secondary and tertiary mortality. J Econ Entomol. 2008;101:894–901. doi: 10.1603/0022-0493(2008)101[894:htobit]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 11.Gahlhoff JE, Miller DM, Koehler PG. Secondary kill of adult male German cockroaches (Dictyoptera: Blattellidae) via cannibalism of nymphs fed toxic baits. J Econ Entomol. 1999;92(5):1133–1137. [Google Scholar]
- 12.Buczkowski G, Schal C. Emetophagy: Fipronil-induced regurgitation of bait and its dissemination from German cockroach adults to nymphs. Pest Biochem Physiol. 2001;71:147–155. [Google Scholar]
- 13.Kopanic RJ, Schal C. Relative significance of direct ingestion and adult-mediated translocation of bait to German cockroach (Dictyoptera: Blattellidae) nymphs. J Econ Entomol. 1997;90:1073–1079. [Google Scholar]
- 14.Kopanic RJ, Schal C. Coprophagy facilitates horizontal transmission of bait among cockroaches (Dictyoptera: Blattellidae) Environ Entomol. 1999;28:431–438. [Google Scholar]
- 15.Kopanic RJ, Holbrook GL, Sevala V, Schal C. An adaptive benefit of facultative coprophagy in the German cockroach (Blattella germanica) Ecol Entomol. 2001;26:154–162. [Google Scholar]
- 16.Schal C. Sulfluramid resistance and vapor toxicity in field-collected German cockroaches (Dictyoptera: Blattellidae) J Med Entomol. 1992;29(2):207–215. doi: 10.1093/jmedent/29.2.207. [DOI] [PubMed] [Google Scholar]
- 17.Holbrook GL, Roebuck J, Moore CB, Waldvogel MG, Schal C. Origin and extent of resistance to Fipronil in the German cockroach, Blattella germanica (L.) (Dictyoptera: Blattellidae) J Econ Entomol. 2003;96:1548–1558. doi: 10.1093/jee/96.5.1548. [DOI] [PubMed] [Google Scholar]
- 18.Gondhalekar AD, Scharf ME. Mechanisms underlying Fipronil resistance in a multiresistant field strain of the German cockroach (Blattodea: Blattellidae) J Med Entomol. 2012;49:122–131. doi: 10.1603/me11106. [DOI] [PubMed] [Google Scholar]
- 19.Wang C, Scharf ME, Bennett GW. Behavioral and physiological resistance of the German cockroach to gel baits (Blattodea: Blattellidae) J Econ Entomol. 2004;97:2067–2072. doi: 10.1093/jee/97.6.2067. [DOI] [PubMed] [Google Scholar]
- 20.Wang C, Scharf ME, Bennett GW. Genetic basis for resistance to gel baits, Fipronil, and sugar-based attractants in German cockroaches (Dictyoptera: Blattellidae) J Econ Entomol. 2006;99:1761–1767. doi: 10.1603/0022-0493-99.5.1761. [DOI] [PubMed] [Google Scholar]
- 21.Hollingshaus JG. Inhibition of mitochondrial electron transport by hydramethylnon: a new amidinohydrazone insecticide. Pestic Biochem Physiol. 1987;27:61–70. [Google Scholar]
- 22.Koehler PG, Patterson RS. Toxicity of hydramethylnon to laboratory and field strains of German cockroach (Orthoptera: Blattellidae) Fla Entomol. 1991;74(2):345–349. [Google Scholar]
- 23.Ajjan I, Robinson WH. Measuring hydramethylnon resistance in the German cockroach, Blattella germanica (L.). Proceedings of the Second International Conference on Urban Pests; 1996. [Google Scholar]
- 24.Valles SM, Brenner RJ. Variation in hydramethylnon susceptibility among insecticide-resistant German cockroaches (Blattodea: Blattellidae) J Econ Entomol. 1999;92:617–623. [Google Scholar]
- 25.Gondhalekar AD, Song C, Scharf ME. Development of strategies for monitoring indoxacarb and gel bait susceptibility in the German cockroach (Blattodea: Blattellidae) Pest Manag Sci. 2010;67:262–270. doi: 10.1002/ps.2057. [DOI] [PubMed] [Google Scholar]
- 26.Robertson JL, Savin N, Preisler HK, Russell RM. Bioassays with Arthropods. CRC Press; Boca Raton, FL, USA: 2007. [Google Scholar]
- 27.Ross MH, Cochran DG. A body colour mutation in the German cockroach. Nature. 1962;165:518–519. [Google Scholar]
- 28.Wing KD, Andaloro JT, McCann SF. Indoxacarb and the sodium channel blocker insecticides: chemistry, physiology, and biology. In: Gilbert LI, Gill SS, editors. Insect Control. Academic Press; 2010. pp. 35–53. [Google Scholar]
- 29.Hamilton RL, Schal Effects of dietary protein levels on reproduction and food consumption in the German cockroach (Dictyoptera: Blattellidae) Ann Entomol Soc Am. 1988;81(6):969–976. [Google Scholar]
- 30.Silverman J, Bieman DN. Glucose aversion in the German cockroach. J Insect Physiol. 1993;39:925–933. [Google Scholar]
- 31.Wada-Katsumata A, Silverman J, Schal C. Changes in taste neurons support the emergence of an adaptive behavior in cockroaches. Science. 340:972–975. doi: 10.1126/science.1234854. [DOI] [PubMed] [Google Scholar]
- 32.Gressel J. Low pesticide rates may hasten the evolution of resistance by increasing mutation frequencies. Pest Manag Sci. 2010;67:253–257. doi: 10.1002/ps.2071. [DOI] [PubMed] [Google Scholar]