Abstract
Comorbidity is an issue of growing importance due to changing demographics and the increasing number of adults over the age of 65 with cancer. The best approach to the clinical management and decision-making in older adults with comorbid conditions remains unclear. In May 2015, the Cancer and Aging Research Group in collaboration with the National Cancer Institute and National Institute on Aging met to discuss the design and implementation of intervention studies in older adults with cancer. A presentation and discussion on comorbidity measurement, interventions, and future research was included. In this article we discuss the relevance of comorbidities in cancer, examine the commonly used tools to measure comorbidity, and discuss the future direction of comorbidity research. Incorporating standardized comorbidity measurement, relaxing clinical trial eligibility criteria, and utilizing novel trial designs are critical to developing a larger and more generalizable evidence base to guide the management of these patients. Creating or adapting comorbidity management strategies for use in older adults with cancer is necessary to define optimal care for this growing population.
Keywords: Comorbidity, Multimorbidity, Cancer, Geriatric Oncology, Aged
Introduction
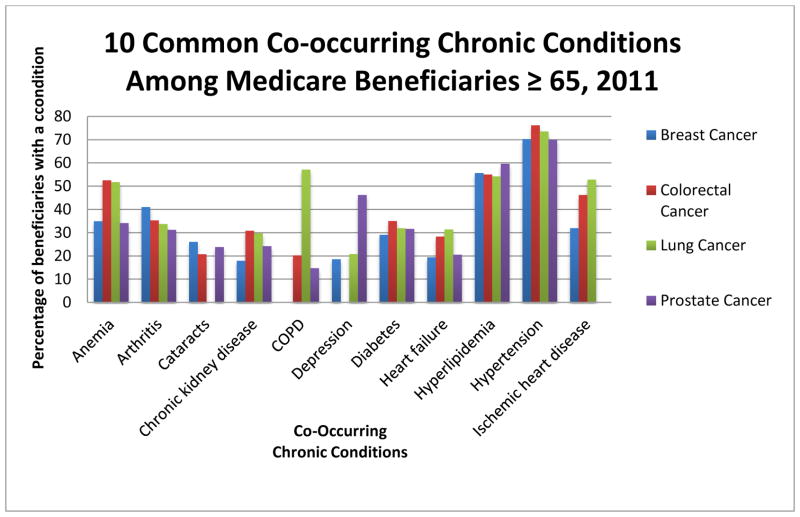
Comorbidity, defined as a medical condition that exists along with an index condition, is an issue of growing importance due to changing demographics and the increasing number of adults over the age of 65 with cancer.(1, 2) In a study of Medicare beneficiaries, over two-thirds had two or more medical conditions and nearly 25% of participants had four or more conditions.(3) Figure 1 shows the prevalence of 10 common co-occurring chronic conditions among Medicare beneficiaries 65 years of age or older with various cancer types. While the prevalence of multiple chronic illnesses in older adults is rapidly increasing in the United States,(3) older adults with cancer have an even higher prevalence of comorbidity than an age-matched control group without cancer.(4) More than half of all older adults with cancer have at least one comorbidity that may impact their cancer treatment.(5) The frequent practice of excluding patients with common comorbid illnesses and the lack of systematic measurement of comorbidities in cancer clinical trials limit the evidence base for making informed decisions regarding these patients. The best approach to the clinical management and decision-making in older adults with comorbid conditions remains unclear.(6) Most clinical practice guidelines, in cancer and elsewhere, are single disease focused, limiting their application in patients with multiple comorbid illnesses.(7) Recognizing and managing comorbidities in older adults with cancer will become an increasingly routine issue for oncologists and may in fact already be the rule rather than the exception.
Figure 1.
Prevalence of 10 common co-occurring chronic conditions among Medicare beneficiaries 65 years of age or older with various cancer types as the index condition. This figure includes the 10 most prevalent comorbidities, out of a set of 28 conditions, among beneficiaries with each individual cancer type as identified by diagnoses codes in Medicare claims data. This figure is based upon data previously published as part of ASCO Clinical Practice Guidelines.(72–75) (Intended for color on the web and black/white in print)
Although the term multimorbidity is frequently used interchangeably with comorbidity, we define multimorbidity as the presence of several comorbid illnesses in which one single condition is not the predominant focus. As this article is specifically addressing comorbidities within the context of cancer, we will be using the term comorbidity regardless of the number of comorbid illnesses. At the recent U-13 conference “Design and Implementation of Intervention Studies to Improve or Maintain Quality of Survivorship in Older and/or Frail Adults with Cancer”, a session was included on comorbidity measurement, interventions, and future research. Based on the discussion from the U13 conference, this article will:
Discuss the relevance of comorbidities in cancer
Examine the commonly used tools to measure comorbidity
Discuss the future direction of comorbidity research and key methodological considerations
Relevance in Cancer
Over the last decade, the importance of comorbidity in oncology has become clearer. Comorbid illnesses impact cancer in various ways. They serve as confounders that complicate the diagnosis and treatment of cancer, they mediate cancer or cancer treatment effects, and pose competing risks for morbidity and mortality.(2) The influence of comorbid conditions on cancer can vary dramatically as the comorbid illnesses themselves encompass a heterogeneous group of conditions ranging in number and severity. This multifaceted effect of comorbidities only further complicates the already complex decision-making in older patients with cancer. Incorporating comorbid conditions into cancer treatment decision-making has become a vital component of understanding the risks and benefits of treatments for an individual patient, especially an older one.
Association of comorbidity with survival
Most observational studies have found that patients with cancer and comorbidities have poorer survival than those without comorbidity.(8) While survival rates vary by cancer type, generally, patients with higher rates of comorbidity have 1.1 to 5.8-fold higher mortality risk.(8) In a recent cohort study of 6,325 older adults with cancer, the 5-year all-cause mortality increased with higher levels of comorbidity in a variety of different cancers including breast, lung, colorectal, prostate, and ovarian (e.g., hazard ratio for severe comorbidity in prostate cancer was 2.14, 95% CI 1.65–2.77).(4) The presence of moderate to severe comorbidities is of greatest prognostic importance among patients with localized and potentially curable cancer, such as early-stage breast or prostate cancer; in contrast, comorbidities have little impact on overall survival in more lethal and aggressive cancers where mortality is dominated by the primary disease.(9)
Impact of comorbidity on cancer risk, recurrence, and stage
Comorbid conditions impact cancer risk, cancer recurrence, and influence the stage at diagnosis. Patients with obesity, diabetes, end-stage renal disease, or immunodeficiencies are at an elevated risk for developing cancer.(10–13) Conversely, many of the treatments for common comorbidities, such as aspirin, statins, metformin, and anti-inflammatory agents, may help decrease cancer incidence and risk of recurrence.(14–18) Several studies have found a higher prevalence of comorbidity in early stage cancers.(8) This increase in comorbid conditions among patients with early stage cancer may be in part related to the frequent healthcare utilization and monitoring required for treatment of these conditions. A study using Surveillance, Epidemiology, and End Results (SEER)-Medicare data showed that comorbidity burden was associated with higher rates of mammography and with an earlier stage of diagnosis in breast cancer patients.(19) This same study also found that a group of more serious comorbid conditions, such as severe heart failure and end-stage pulmonary disease, were associated with less mammography screening and later stage at diagnosis.
Association of comorbidity with toxicity
Patients with cancer and comorbid conditions are at an increased risk of major toxicity and hospitalization due to chemotherapy-related toxicities.(20–22) In a study evaluating chemotherapy toxicities in older adults undergoing adjuvant chemotherapy for breast cancer, the risk of toxicity depended more on the presence of comorbidity than on age.(22) Older adults with a Charlson Comorbidity Index (CCI) score of ≥1 were three times more likely to experience grade 3 or 4 non-hematologic toxicities. Conversely, two recently developed chemotherapy toxicity calculators for use in older adults do not include comorbidities, as there was no significant association of comorbidities with the incidence of grade 3 or 4 toxicity when controlling for functional status, suggesting the impact of comorbidities may be via their effect on function.(23, 24) Similarly, in a recent secondary analysis of comorbidity in older adults with breast cancer, no association with toxicity was found.(25) In a study evaluating chemotherapy related hospitalizations, severe comorbid illness (CCI score ≥3) increased the risk of hospitalization nearly 10 fold and was the strongest predictor of hospitalization.(21) Moreover, patients with comorbidities are less likely to complete chemotherapy compared to patients without comorbidity.(26) Several studies have shown that comorbidity is associated with decreased rates of completing chemotherapy in several cancer types. However, whether this is a direct result of increased toxicity, decreased functional status, or is related to poor adherence is unclear.(8) The increased risk of toxicity in adults with comorbidities may in part be related to the increased use of medications for comorbid conditions and medication interactions; however, a recent study showed no association between the number of daily medications or potentially inappropriate medications in older adults undergoing chemotherapy.(27)
The differing rates of toxicity observed in patients with comorbid illnesses do not fully explain the differences in overall survival seen in some studies. In a large study of older adults with colorectal cancer undergoing adjuvant chemotherapy, the presence of diabetes had a significant impact on long-term survival (median survival of 6.0 versus 11.3 years) that was independent of treatment-related toxicity.(28) In addition, as cancer treatment can result in new comorbidities, such as cardiac toxicity, neuropathy, or renal impairment, it is important to distinguish pre-treatment comorbid conditions from those that develop as a consequence of toxicity.(29, 30)
Impact of comorbidities on cancer treatment decisions
As comorbidities alter both the risk and benefit from cancer treatments, they can impact the risk/benefit balance of many treatment decisions. This may be why patients with comorbidities are less likely to receive adjuvant chemotherapy.(31–34) In a study using SEER-Medicare database, older adults with resected stage III colorectal cancer with comorbidities were less likely to be referred to a medical oncologist for consideration of adjuvant chemotherapy and less likely to be given chemotherapy when seen by an oncologist.(33) The presence of comorbidities is one of the most frequent reasons for non-receipt of cancer treatment cited in the medical chart.(35, 36) Although chronic conditions appear to be a strong barrier to the receipt of adjuvant chemotherapy, some studies have shown a similar survival benefit with or without many common comorbid illnesses.(37) Reduced chemotherapy treatment and decreased adjuvant referral patterns may represent thoughtful and appropriate treatment decisions on the part of both physicians and/or patients based on the presence of comorbidities and limited life expectances. However, these decisions may also represent an inappropriate reflection of physician bias based on chronologic age.
Measuring Comorbidity
Given the overall impact of comorbidities in cancer care, there is a compelling need to measure comorbidities in research and clinical settings. Standardized comorbidity assessment should be considered as a common data element for every patient. Without the systematic incorporation of comorbid conditions in cancer decision-making, oncologists may overestimate cancer risk, overestimate the benefits of cancer treatment, and will be unable to distinguish toxicity from comorbidity. Over the last decade a growing number of studies have included measures of comorbidity, but with little consistency in measurement and a resulting lack of comparability across research settings.(38) Due to the diverse nature of possible comorbid diseases, a systematic assessment of every conceivable comorbid illness and degree of severity would easily create an unmanageable amount of information; therefore, some selection and pooling of information is necessary.(39) Many methods for assessing comorbid illnesses are available and no gold standard approach exists (see table 1 for a selected list of available instruments).
Table 1.
Selected list of comorbidity scales discussed in this article organized by scale type.
| Type | Index | Items and rating | How constructed | References |
|---|---|---|---|---|
| Summative | ||||
| Elixhauser | 30 dichotomous conditions | Length of stay, total charges, and in-hospital mortality of hospitalized patients | (40) | |
| Weighted | ||||
| Charlson Comorbidity Index (CCI) | 19 conditions weighted 1 to 6 | 1 year mortality in hospitalized internal medicine patients | (42) | |
| NCI Comorbidity Index | 16 conditions weighted by empirically derived weights | 2 year non-cancer mortality in prostate, breast, lung, and colorectal cancer patients | (46, 47) | |
| Systems-Based | ||||
| Cumulative Illness Rating Scale (CIRS) | 13 or 14 organ system categories, each rated 0–4 | Comprehensive listing of conditions weighted by physician’s judgement, unclear original population used to derive | (51) | |
| Kaplan-Feinstein Index (KFI) | 12 conditions, each rated 0–3 | Survival in 188 male patients with diabetes | (53) | |
| Index of Coexistent Disease (ICED) | Disease severity sub-index: 14 diseases (rated 0–4). Functional severity sub-index: 12 conditions (rated 0–2). Total: 0–3 | Anticipated outcomes 2 years after hospitalization in breast cancer patients | (56) | |
| Cancer Specific | ||||
| Washington University Head and Neck Comorbidity (WUHNCI) | 7 conditions, each rated 0–4 | 5 year survival in patients with head and neck cancer | (49) | |
| Hematopoietic cell transplantation-comorbidity index (HCT-CI) | 17 conditions, each rated 0–3 | 2 year non-relapse mortality in patients undergoing HCT | (50) | |
In a recent review of methods used to measure comorbidity in cancer populations, 21 distinct approaches were identified.(38) These methods varied from simple counts of individual conditions to weighted indices. The approach of measuring the prevalence of individual conditions and either using them separately or summing the number of conditions, is the simplest method.(40, 41) This method has been used in the development of a range of comorbidity indexes in several different cancer types with a variable number of conditions included, ranging from 7 to 102.(38) Inclusion and exclusion of comorbid conditions were based on clinical judgement, on literature in the area, on empirical analysis, or on a combination thereof. Notably, many of these exclude obesity as an identified comorbidity. As many of these indices combine conditions into a single unweighted measure of comorbidity, the implicit assumption is made that all conditions are equally important in relationship to outcomes.
In contrast, a weighted index assigns importance to individual conditions according to their relative impact on key outcomes and attempts to account for the differential impact of certain conditions on outcomes. The most frequently cited weighted index in the literature and the first example of this approach was the Charlson Comorbidity Index (CCI).(42) The CCI was developed by Mary Charlson and colleagues in 1987 using data from hospital notes on an internal medicine service. Charlson et al analyzed mortality at 1 year as a function of various comorbidities. The resulting Index was a list of 19 conditions that are weighted from 1 to 6 according to their relative risk of death. The total score is calculated and can be then collapsed into four categories: 0, 1–2, 3–4, and ≥5. Algorithms have been developed to calculate Charlson scores from the use of administrative data and the index has been reweighted using administrative claims to account for advances in medical care.(43, 44) Questionnaires have also been developed that utilize patient self-report to calculate Charlson scores.(45) The CCI is the basis of other comorbidity indices including the NCI Comorbidity Index that utilizes a subset of the same conditions and weights conditions using beta coefficients (rather than relative risk used by the CCI).(46, 47)
As some comorbid conditions are more prevalent in certain cancers — for instance, COPD in lung cancer — and the impact of comorbid illnesses is often specific to the cancer type, weighted indices have been developed for use in particular cancers.(48–50) For example, the Washington University Head and Neck Comorbidity Index (WUHNCI) was developed for use in patients with head and neck cancer and includes seven comorbid conditions that were significantly related to survival in these patients.(49) Similarly, a hematopoietic cell transplant (HCT)-specific comorbidity index has been developed to improve the sensitivity and specificity for predicting risks of non-relapse mortality after HCT.(50)
Another commonly used approach to comorbidity measurement is to assess the impact of comorbid illnesses on the function of body organs or systems.(38) The first example of this method was the Cumulative Illness Rating Scale (CIRS) that classifies comorbidities by organ system affected and rates comorbidities according to their severity from 0 to 4.(51) Scores can be summarized as a total score, as a total number of categories involved, as a mean score, or as a number of grade 3 or 4 diseases.(52) The Kaplan-Feinstein Index (KFI) also groups conditions into 12 categories each with a severity rating of 0–3.(53) Although the KFI was originally developed in a group of 188 men with diabetes, it has subsequently been used in a number of cancer-related studies.(54, 55)
The Index of Coexistent Diseases (ICED) combines two dimensions of comorbid illness: a measure of comorbid disease severity assessed by organ system and the degree of physical impairment.(56) The ICED has been most widely used to study the correlation of comorbidity with treatment patterns and survival.(52) Lastly, the Older Americans Resources and Services Questionnaire (OARS) Physical Health subscale obtains information on 14 specific comorbid conditions and includes the degree that each interferes with the participant’s activities.(57)
Choosing the “right” comorbidity measurement tool depends on the specific research question and/or the clinical setting. Some tools are better suited for use in the clinical setting while others are more useful for retrospective review or for use with administrative claims data. Some comorbidity measures are developed and validated to predict mortality, which may or may not be helpful in a study of healthcare utilization. Ultimately, what is most important is not how comorbid illnesses are measured, but that the comorbidities are measured and considered in some way (although, as we discuss below, standardization of comorbidity assessment is an important goal for clinical trials). Furthermore, the timing of the measurement is also important as comorbidities can also be a direct consequence of cancer treatment.(58) The method of measurement used must also be transparent in reports of research. Several detailed reviews of comorbidity measurement can be consulted for an examination of available measures.(38, 52)
In the context of prospective clinical trial design, a concerted effort is needed to standardize comorbidity measurement. The lack of comorbidity measurement in ongoing clinical trials tremendously limits our understanding of the impact of comorbid illnesses. An estimate of the burden of comorbidity is needed to better characterize study participants, and analyses of trial results in subgroups of individuals with comorbidities would improve our ability to extrapolate findings to older patients. Even in the trials that incorporate comorbidity measurement, the lack of standardization limits our ability to compare across trials. A minimum data set that includes some measure of comorbidity is necessary across oncology trials.
Studying Comorbidity
Many significant research gaps exist in the cancer care of individuals with comorbid conditions. Incorporating and developing standardized comorbidity assessments in clinical trials, as described above, is an important first step. Reducing the common eligibility criteria that limit the participation of older adults with comorbid conditions is necessary to improve the evidence base for treating these patients. The use of innovative trial designs can help to better incorporate comorbidities into clinical trials. Lastly, the development and adaption of comorbidity interventions and management strategies is necessary to elucidate best practices.
Common eligibility criteria used in clinical trials lead to the exclusion of older adults, particularly those with comorbid illnesses.(59) Due to these strict eligibility criteria, study populations rarely are representative of the population at large with the given condition. The response of older adults to cancer treatment is heterogeneous and may be different from younger patients, due not only to age-related changes in organ function, but also to the higher prevalence of comorbidity and the use of concomitant medications. There is increasing evidence that relaxed eligibility criteria can be implemented without reducing scientific rigor.(60) Less stringent criteria will ultimately decrease barriers to accrual and lead to more generalizable research.(61) Future studies should include populations that reflect the age and health profiles of the patients with the disease,(62) and this can only be achieved with more inclusive eligibility criteria.
Innovative trial designs allow investigators to include larger numbers of patients with high levels of comorbidities. Clinical trial designs have progressed from testing of two treatment options in homogenous cohorts to more innovative and pragmatic designs that purposefully allow the inclusion of greater numbers of complex patients. Randomization balances the observed and unobserved characteristics (including comorbidities) across treatment groups; however, these complex, heterogeneous groups have greater variability for the treatment effect, which effectively requires greater sample sizes for the same power to detect differences. The advantage of adaptive randomization techniques is that patients may be allocated at a higher probability to treatment arms that are more likely to be favorable, particularly in trials with early endpoints (e.g. biomarkers).(63) This approach assumes that the trial is testing for the most favorable therapy regardless of comorbidities. If there is knowledge a priori that therapeutic options might differ by the presence of specific comorbidities, then trials should be designed for patients with and without those comorbidities.
Platform trials focus on studying the disease for which new therapies are continually developed and tested. This new type of trial design belongs to the family of adaptive randomization designs and is intended to continue beyond the evaluation of the initial treatments to investigate treatment combinations, as well as to quantify differences in treatment effects in subgroups while treating patients as effectively as possible within the trial.(64) The i-SPY2 (Investigation of Serial Studies to Predict Your Therapeutic Response With Imaging and Molecular Analysis) breast cancer trial is an example of a trial that utilizes an adaptive, modular design process for the purpose of screening phase II agents concurrently in women with early-stage breast cancer.(65) Sequential, multiple assignment, randomized trial (SMART) designs also are being used to construct adaptive interventions and allow augmentation of initial treatment for non-responders.(66) This approach may be modified for cohorts with comorbidities and does not require endpoints to occur as quickly as adaptive randomization designs. Lastly, standardly-tailored designs have been used in geriatrics for decades to evaluate multicomponent interventions.(67, 68) These designs randomize participants into active treatment or usual care and do not require that all subjects be eligible for every interventional component. Those in the active treatment arm receive per protocol therapies for all conditions present that the trial is testing. This approach yields an overall intervention effect and is fully flexible to heterogeneous cohorts.
Another effective avenue for studying comorbidities is through the use of comparative-effectiveness research (CER). CER utilizes existing health care information to evaluate which treatments or interventions work best, for whom, and in what circumstances. Many large databases exist or are planned with information on a diverse range of older adults with cancer and comorbidities, such as the ORIEN network or the SEER-Medicare databases. These administrative databases provide a forest view of comorbidities and often lack granularity about comorbid conditions (such as severity and impact on function) and other important geriatric assessment variables. Although CER data are not always collected systematically and must be interpreted with caution to minimize the risk of drawing erroneous conclusions, CER can be a powerful tool to produce inexpensive and quick results using data from larger, more diverse populations.(59)
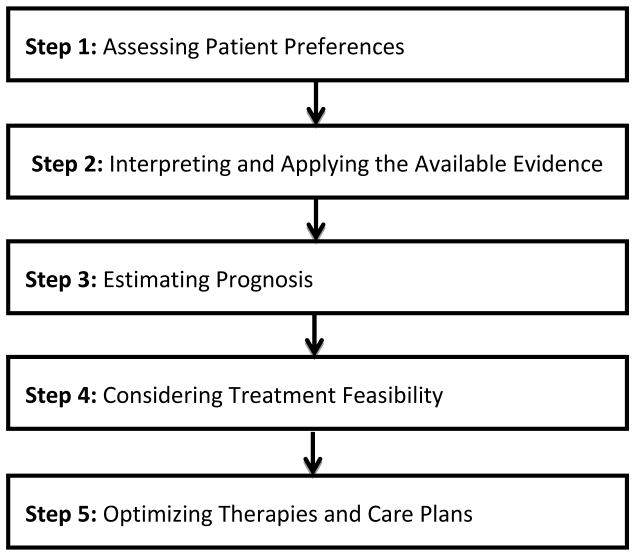
Not only are more clinical trials needed that include patients with comorbid conditions and incorporate systematic comorbidity measurement, there is also a need to develop and study comorbidity interventions. Research to date on comorbidities in oncology has primarily focused on descriptive epidemiology and impact assessment with limited exploration of potential interventions. There are a limited number of studies on comorbidities even in non-cancer patients.(69) Implementing conceptual frameworks such as the American Geriatrics Society’s Guiding Principles for the Care of Older Adults with Multimorbidity in oncology patients may be an ideal first step.(70) These five guiding principles (see figure 2) were developed from a structured review of the literature and expert consensus and provide a stepwise approach to management of older patients with multiple comorbidities. The challenge in older oncology patients, in particular, is operationalizing these principles and adapting them to guide oncology decision-making.(71) Developing new care models and flexible coordinated care strategies is also necessary given the breadth of potential comorbid illnesses present in older adults with cancer. Systematically testing these new models and strategies in older cancer patients with comorbidity is required to ultimately provide the evidence to change cancer care for these complex patients.
Figure 2.
Guiding principles for the care of older adults with multimorbidity.
Conclusions
As the population continues to age and the prevalence of comorbidity in oncology grows, developing a better understanding of the impact of comorbid illnesses on cancer care and management strategies will be vital. Although oncologists and patients are primarily focused on cancer and its treatment, the implications and management of comorbidities will become increasingly important. Incorporating standardized comorbidity measurement, relaxing clinical trial eligibility criteria, and utilizing novel trial designs are critical to developing a larger, more generalizable evidence base to guide the management of these patients. Creating comorbidity management strategies for use in older adults with cancer is necessary to provide optimal care for this growing population.
Acknowledgments
Supported by Grant No. U13 AG038151 from the National Institute on Aging and National Cancer Institute (NCI; National Institutes of Health) and written on behalf of the Cancer and Aging Research Group. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yancik R, Ries LAG. Cancer and aging in America - Demographic and epidemiologic perspectives. Hematol Oncol Clin N. 2000;14(1):17. doi: 10.1016/s0889-8588(05)70275-6. [DOI] [PubMed] [Google Scholar]
- 2.Ritchie CS, Kvale E, Fisch MJ. Multimorbidity: an issue of growing importance for oncologists. Journal of oncology practice / American Society of Clinical Oncology. 2011;7(6):371–4. doi: 10.1200/JOP.2011.000460. Epub 2012/03/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162(20):2269–76. doi: 10.1001/archinte.162.20.2269. Epub 2002/11/07. [DOI] [PubMed] [Google Scholar]
- 4.Jorgensen TL, Hallas J, Friis S, Herrstedt J. Comorbidity in elderly cancer patients in relation to overall and cancer-specific mortality. British journal of cancer. 2012;106(7):1353–60. doi: 10.1038/bjc.2012.46. Epub 2012/02/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koroukian SM, Murray P, Madigan E. Comorbidity, disability, and geriatric syndromes in elderly cancer patients receiving home health care. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(15):2304–10. doi: 10.1200/JCO.2005.03.1567. Epub 2006/05/20. [DOI] [PubMed] [Google Scholar]
- 6.Guiding principles for the care of older adults with multimorbidity: an approach for clinicians: American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Journal of the American Geriatrics Society. 2012;60(10):E1–E25. doi: 10.1111/j.1532-5415.2012.04188.x. Epub 2012/09/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA : the journal of the American Medical Association. 2005;294(6):716–24. doi: 10.1001/jama.294.6.716. Epub 2005/08/11. [DOI] [PubMed] [Google Scholar]
- 8.Sogaard M, Thomsen RW, Bossen KS, Sorensen HT, Norgaard M. The impact of comorbidity on cancer survival: a review. Clinical epidemiology. 2013;5(Suppl 1):3–29. doi: 10.2147/CLEP.S47150. Epub 2013/11/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Read WL, Tierney RM, Page NC, Costas I, Govindan R, Spitznagel EL, et al. Differential prognostic impact of comorbidity. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22(15):3099–103. doi: 10.1200/JCO.2004.08.040. Epub 2004/07/31. [DOI] [PubMed] [Google Scholar]
- 10.Maisonneuve P, Agodoa L, Gellert R, Stewart JH, Buccianti G, Lowenfels AB, et al. Cancer in patients on dialysis for end-stage renal disease: an international collaborative study. Lancet. 1999;354(9173):93–9. doi: 10.1016/s0140-6736(99)06154-1. Epub 1999/07/17. [DOI] [PubMed] [Google Scholar]
- 11.Forte V, Pandey A, Abdelmessih R, Forte G, Whaley-Connell A, Sowers JR, et al. Obesity, Diabetes, the Cardiorenal Syndrome, and Risk for Cancer. Cardiorenal medicine. 2012;2(2):143–62. doi: 10.1159/000337314. Epub 2012/08/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hemminki K, Liu X, Ji J, Sundquist J, Sundquist K. Effect of autoimmune diseases on risk and survival in histology-specific lung cancer. The European respiratory journal. 2012;40(6):1489–95. doi: 10.1183/09031936.00222911. Epub 2012/02/11. [DOI] [PubMed] [Google Scholar]
- 13.Sainz J, Rudolph A, Hoffmeister M, Frank B, Brenner H, Chang-Claude J, et al. Effect of type 2 diabetes predisposing genetic variants on colorectal cancer risk. The Journal of clinical endocrinology and metabolism. 2012;97(5):E845–51. doi: 10.1210/jc.2011-2565. Epub 2012/03/16. [DOI] [PubMed] [Google Scholar]
- 14.Bardia A, Olson JE, Vachon CM, Lazovich D, Vierkant RA, Wang AH, et al. Effect of aspirin and other NSAIDs on postmenopausal breast cancer incidence by hormone receptor status: results from a prospective cohort study. Breast cancer research and treatment. 2011;126(1):149–55. doi: 10.1007/s10549-010-1074-x. Epub 2010/07/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan AT, Ogino S, Fuchs CS. Aspirin use and survival after diagnosis of colorectal cancer. JAMA : the journal of the American Medical Association. 2009;302(6):649–58. doi: 10.1001/jama.2009.1112. Epub 2009/08/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sassano A, Platanias LC. Statins in tumor suppression. Cancer letters. 2008;260(1–2):11–9. doi: 10.1016/j.canlet.2007.11.036. Epub 2008/01/09. [DOI] [PubMed] [Google Scholar]
- 17.Takkouche B, Regueira-Mendez C, Etminan M. Breast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysis. Journal of the National Cancer Institute. 2008;100(20):1439–47. doi: 10.1093/jnci/djn324. Epub 2008/10/09. [DOI] [PubMed] [Google Scholar]
- 18.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, et al. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer prevention research. 2010;3(11):1451–61. doi: 10.1158/1940-6207.CAPR-10-0157. Epub 2010/10/16. [DOI] [PubMed] [Google Scholar]
- 19.Yasmeen S, Xing G, Morris C, Chlebowski RT, Romano PS. Comorbidities and mammography use interact to explain racial/ethnic disparities in breast cancer stage at diagnosis. Cancer. 2011;117(14):3252–61. doi: 10.1002/cncr.25857. Epub 2011/01/20. [DOI] [PubMed] [Google Scholar]
- 20.Gronberg BH, Sundstrom S, Kaasa S, Bremnes RM, Flotten O, Amundsen T, et al. Influence of comorbidity on survival, toxicity and health-related quality of life in patients with advanced non-small-cell lung cancer receiving platinum-doublet chemotherapy. Eur J Cancer. 2010;46(12):2225–34. doi: 10.1016/j.ejca.2010.04.009. Epub 2010/05/18. [DOI] [PubMed] [Google Scholar]
- 21.Hassett MJ, Rao SR, Brozovic S, Stahl JE, Schwartz JH, Maloney B, et al. Chemotherapy-related hospitalization among community cancer center patients. The oncologist. 2011;16(3):378–87. doi: 10.1634/theoncologist.2010-0354. Epub 2011/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zauderer M, Patil S, Hurria A. Feasibility and toxicity of dose-dense adjuvant chemotherapy in older women with breast cancer. Breast cancer research and treatment. 2009;117(1):205–10. doi: 10.1007/s10549-008-0116-0. Epub 2008/07/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2012;118(13):3377–86. doi: 10.1002/cncr.26646. Epub 2011/11/11. [DOI] [PubMed] [Google Scholar]
- 24.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting Chemotherapy Toxicity in Older Adults With Cancer: A Prospective Multicenter Study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 doi: 10.1200/JCO.2011.34.7625. Epub 2011/08/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klepin HD, Pitcher BN, Ballman KV, Kornblith AB, Hurria A, Winer EP, et al. Comorbidity, chemotherapy toxicity, and outcomes among older women receiving adjuvant chemotherapy for breast cancer on a clinical trial: CALGB 49907 and CALGB 361004 (alliance) Journal of oncology practice / American Society of Clinical Oncology. 2014;10(5):e285–92. doi: 10.1200/JOP.2014.001388. Epub 2014/07/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neugut AI, Matasar M, Wang X, McBride R, Jacobson JS, Tsai WY, et al. Duration of adjuvant chemotherapy for colon cancer and survival among the elderly. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006;24(15):2368–75. doi: 10.1200/JCO.2005.04.5005. Epub 2006/04/19. [DOI] [PubMed] [Google Scholar]
- 27.Maggiore RJ, Dale W, Gross CP, Feng T, Tew WP, Mohile SG, et al. Polypharmacy and potentially inappropriate medication use in older adults with cancer undergoing chemotherapy: effect on chemotherapy-related toxicity and hospitalization during treatment. Journal of the American Geriatrics Society. 2014;62(8):1505–12. doi: 10.1111/jgs.12942. Epub 2014/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB, 3rd, et al. Impact of diabetes mellitus on outcomes in patients with colon cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2003;21(3):433–40. doi: 10.1200/JCO.2003.07.125. Epub 2003/02/01. [DOI] [PubMed] [Google Scholar]
- 29.Colombo A, Cipolla C, Beggiato M, Cardinale D. Cardiac toxicity of anticancer agents. Current cardiology reports. 2013;15(5):362. doi: 10.1007/s11886-013-0362-6. Epub 2013/03/21. [DOI] [PubMed] [Google Scholar]
- 30.Abbas A, Mirza MM, Ganti AK, Tendulkar K. Renal Toxicities of Targeted Therapies. Targeted oncology. 2015 doi: 10.1007/s11523-015-0368-7. Epub 2015/04/30. [DOI] [PubMed] [Google Scholar]
- 31.Bradley CJ, Given CW, Dahman B, Fitzgerald TL. Adjuvant chemotherapy after resection in elderly Medicare and Medicaid patients with colon cancer. Arch Intern Med. 2008;168(5):521–9. doi: 10.1001/archinternmed.2007.82. Epub 2008/03/12. [DOI] [PubMed] [Google Scholar]
- 32.Dy SM, Sharkey P, Herbert R, Haddad K, Wu AW. Comorbid illnesses and health care utilization among Medicare beneficiaries with lung cancer. Critical reviews in oncology/hematology. 2006;59(3):218–25. doi: 10.1016/j.critrevonc.2006.04.001. Epub 2006/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo R, Giordano SH, Freeman JL, Zhang D, Goodwin JS. Referral to medical oncology: a crucial step in the treatment of older patients with stage III colon cancer. The oncologist. 2006;11(9):1025–33. doi: 10.1634/theoncologist.11-9-1025. Epub 2006/10/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasetto LM, Falci C, Basso U, Gasparini G, D’Andrea M, Bonginelli P, et al. Adjuvant treatment for elderly patients with colon cancer. An observational study. Anticancer research. 2008;28(4C):2513–8. Epub 2008/08/30. [PubMed] [Google Scholar]
- 35.O’Grady MA, Slater E, Sigurdson ER, Meropol NJ, Weinstein A, Lusch CJ, et al. Assessing compliance with national comprehensive cancer network guidelines for elderly patients with stage III colon cancer: the Fox Chase Cancer Center Partners’ initiative. Clinical colorectal cancer. 2011;10(2):113–6. doi: 10.1016/j.clcc.2011.03.007. Epub 2011/08/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vinod SK, Sidhom MA, Gabriel GS, Lee MT, Delaney GP. Why do some lung cancer patients receive no anticancer treatment? Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2010;5(7):1025–32. doi: 10.1097/JTO.0b013e3181da85e4. Epub 2010/05/11. [DOI] [PubMed] [Google Scholar]
- 37.Gross CP, McAvay GJ, Guo Z, Tinetti ME. The impact of chronic illnesses on the use and effectiveness of adjuvant chemotherapy for colon cancer. Cancer. 2007;109(12):2410–9. doi: 10.1002/cncr.22726. Epub 2007/05/19. [DOI] [PubMed] [Google Scholar]
- 38.Sarfati D. Review of methods used to measure comorbidity in cancer populations: no gold standard exists. Journal of clinical epidemiology. 2012;65(9):924–33. doi: 10.1016/j.jclinepi.2012.02.017. Epub 2012/06/29. [DOI] [PubMed] [Google Scholar]
- 39.Extermann M. Measurement and impact of comorbidity in older cancer patients. Critical reviews in oncology/hematology. 2000;35(3):181–200. doi: 10.1016/s1040-8428(00)00090-1. Epub 2000/08/29. [DOI] [PubMed] [Google Scholar]
- 40.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. Epub 1998/02/07. [DOI] [PubMed] [Google Scholar]
- 41.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Annals of internal medicine. 1994;120(2):104–10. doi: 10.7326/0003-4819-120-2-199401150-00002. Epub 1994/01/15. [DOI] [PubMed] [Google Scholar]
- 42.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. Epub 1987/01/01. [DOI] [PubMed] [Google Scholar]
- 43.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. Journal of clinical epidemiology. 1992;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. Epub 1992/06/01. [DOI] [PubMed] [Google Scholar]
- 44.Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–82. doi: 10.1093/aje/kwq433. Epub 2011/02/19. [DOI] [PubMed] [Google Scholar]
- 45.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. Epub 1996/01/01. [DOI] [PubMed] [Google Scholar]
- 46.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. Journal of clinical epidemiology. 2000;53(12):1258–67. doi: 10.1016/s0895-4356(00)00256-0. Epub 2001/01/09. [DOI] [PubMed] [Google Scholar]
- 47.Klabunde CN, Legler JM, Warren JL, Baldwin LM, Schrag D. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Annals of epidemiology. 2007;17(8):584–90. doi: 10.1016/j.annepidem.2007.03.011. Epub 2007/05/29. [DOI] [PubMed] [Google Scholar]
- 48.Colinet B, Jacot W, Bertrand D, Lacombe S, Bozonnat MC, Daures JP, et al. A new simplified comorbidity score as a prognostic factor in non-small-cell lung cancer patients: description and comparison with the Charlson’s index. British journal of cancer. 2005;93(10):1098–105. doi: 10.1038/sj.bjc.6602836. Epub 2005/10/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piccirillo JF, Lacy PD, Basu A, Spitznagel EL. Development of a new head and neck cancer-specific comorbidity index. Archives of otolaryngology--head & neck surgery. 2002;128(10):1172–9. doi: 10.1001/archotol.128.10.1172. Epub 2002/10/09. [DOI] [PubMed] [Google Scholar]
- 50.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912–9. doi: 10.1182/blood-2005-05-2004. Epub 2005/07/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. Journal of the American Geriatrics Society. 1968;16(5):622–6. doi: 10.1111/j.1532-5415.1968.tb02103.x. Epub 1968/05/01. [DOI] [PubMed] [Google Scholar]
- 52.Extermann M. Measuring comorbidity in older cancer patients. Eur J Cancer. 2000;36(4):453–71. doi: 10.1016/s0959-8049(99)00319-6. Epub 2000/03/16. [DOI] [PubMed] [Google Scholar]
- 53.Kaplan MH, Feinstein AR. The importance of classifying initial co-morbidity in evaluating the outcome of diabetes mellitus. Journal of chronic diseases. 1974;27(7–8):387–404. doi: 10.1016/0021-9681(74)90017-4. Epub 1974/09/01. [DOI] [PubMed] [Google Scholar]
- 54.Hall SF, Rochon PA, Streiner DL, Paszat LF, Groome PA, Rohland SL. Measuring comorbidity in patients with head and neck cancer. The Laryngoscope. 2002;112(11):1988–96. doi: 10.1097/00005537-200211000-00015. Epub 2002/11/20. [DOI] [PubMed] [Google Scholar]
- 55.Newschaffer CJ, Bush TL, Penberthy LT. Comorbidity measurement in elderly female breast cancer patients with administrative and medical records data. Journal of clinical epidemiology. 1997;50(6):725–33. doi: 10.1016/s0895-4356(97)00050-4. Epub 1997/06/01. [DOI] [PubMed] [Google Scholar]
- 56.Greenfield S, Blanco DM, Elashoff RM, Ganz PA. Patterns of care related to age of breast cancer patients. JAMA : the journal of the American Medical Association. 1987;257(20):2766–70. Epub 1987/05/22. [PubMed] [Google Scholar]
- 57.Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. 1981;36(4):428–34. doi: 10.1093/geronj/36.4.428. Epub 1981/07/01. [DOI] [PubMed] [Google Scholar]
- 58.Boyd CM, Ritchie CS, Tipton EF, Studenski SA, Wieland D. From Bedside to Bench: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Comorbidity and Multiple Morbidity in Older Adults. Aging clinical and experimental research. 2008;20(3):181–8. doi: 10.1007/bf03324775. Epub 2008/07/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hurria A, Levit LA, Dale W, Mohile SG, Muss HB, Fehrenbacher L, et al. Improving the Evidence Base for Treating Older Adults With Cancer: American Society of Clinical Oncology Statement. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015 doi: 10.1200/JCO.2015.63.0319. Epub 2015/07/22. [DOI] [PubMed] [Google Scholar]
- 60.Van Spall HG, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA : the journal of the American Medical Association. 2007;297(11):1233–40. doi: 10.1001/jama.297.11.1233. Epub 2007/03/22. [DOI] [PubMed] [Google Scholar]
- 61.George SL. Reducing patient eligibility criteria in cancer clinical trials. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1996;14(4):1364–70. doi: 10.1200/JCO.1996.14.4.1364. Epub 1996/04/01. [DOI] [PubMed] [Google Scholar]
- 62.Levit L, Balogh E, Nass S, Ganz PA, editors. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington (DC): 2013. [PubMed] [Google Scholar]
- 63.Kuehn BM. Industry, FDA warm to “adaptive” trials. JAMA : the journal of the American Medical Association. 2006;296(16):1955–7. doi: 10.1001/jama.296.16.1955. Epub 2006/10/26. [DOI] [PubMed] [Google Scholar]
- 64.Berry SM, Connor JT, Lewis RJ. The platform trial: an efficient strategy for evaluating multiple treatments. JAMA : the journal of the American Medical Association. 2015;313(16):1619–20. doi: 10.1001/jama.2015.2316. Epub 2015/03/24. [DOI] [PubMed] [Google Scholar]
- 65.Esserman LJ, Woodcock J. Accelerating identification and regulatory approval of investigational cancer drugs. JAMA : the journal of the American Medical Association. 2011;306(23):2608–9. doi: 10.1001/jama.2011.1837. Epub 2011/12/22. [DOI] [PubMed] [Google Scholar]
- 66.Murphy SA, Collins LM, Rush AJ. Customizing treatment to the patient: adaptive treatment strategies. Drug and alcohol dependence. 2007;88(Suppl 2):S1–3. doi: 10.1016/j.drugalcdep.2007.02.001. Epub 2007/03/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allore HG, Murphy TE. An examination of effect estimation in factorial and standardly-tailored designs. Clinical trials. 2008;5(2):121–30. doi: 10.1177/1740774508089278. Epub 2008/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Allore HG, Tinetti ME, Gill TM, Peduzzi PN. Experimental designs for multicomponent interventions among persons with multifactorial geriatric syndromes. Clinical trials. 2005;2(1):13–21. doi: 10.1191/1740774505cn067oa. Epub 2005/11/11. [DOI] [PubMed] [Google Scholar]
- 69.Smith SM, Soubhi H, Fortin M, Hudon C, O’Dowd T. Interventions for improving outcomes in patients with multimorbidity in primary care and community settings. The Cochrane database of systematic reviews. 2012;4:CD006560. doi: 10.1002/14651858.CD006560.pub2. Epub 2012/04/20. [DOI] [PubMed] [Google Scholar]
- 70.American Geriatrics Society Expert Panel on the Care of Older Adults with M. Patient-centered care for older adults with multiple chronic conditions: a stepwise approach from the American Geriatrics Society: American Geriatrics Society Expert Panel on the Care of Older Adults with Multimorbidity. Journal of the American Geriatrics Society. 2012;60(10):1957–68. doi: 10.1111/j.1532-5415.2012.04187.x. Epub 2012/09/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thompson K, Dale W. How do I best manage the care of older patients with cancer with multimorbidity? Journal of geriatric oncology. 2015;6(4):249–53. doi: 10.1016/j.jgo.2015.05.005. Epub 2015/07/08. [DOI] [PubMed] [Google Scholar]
- 72.CMS administrative claims data. Chronic Condition Warehouse (CCW); [Google Scholar]
- 73.Basch E, Loblaw DA, Oliver TK, Carducci M, Chen RC, Frame JN, et al. Systemic therapy in men with metastatic castration-resistant prostate cancer:American Society of Clinical Oncology and Cancer Care Ontario clinical practice guideline. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(30):3436–48. doi: 10.1200/JCO.2013.54.8404. Epub 2014/09/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burstein HJ, Temin S, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline focused update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32(21):2255–69. doi: 10.1200/JCO.2013.54.2258. Epub 2014/05/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Masters GA, Temin S, Azzoli CG, Giaccone G, Baker S, Jr, Brahmer JR, et al. Systemic Therapy for Stage IV Non-Small-Cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015 doi: 10.1200/JCO.2015.62.1342. Epub 2015/09/02. [DOI] [PMC free article] [PubMed] [Google Scholar]