Abstract
Inorganic arsenic, an environmental contaminant and a human carcinogen is associated with prostate cancer. Emerging evidence suggests cancer stem cells (CSCs) are the driving force of carcinogenesis. Chronic arsenic exposure malignantly transforms the human normal prostate stem/progenitor cell (SC) line, WPE-stem to arsenic-cancer SCs (As-CSCs), through unknown mechanisms. MicroRNAs (miRNAs) are small, non-coding RNAs that negatively regulate gene expression at the posttranscriptional level. In prior work, miR-143 was markedly downregulated in As-CSCs, suggesting a role in arsenic-induced malignant transformation. In the present study, we investigated whether loss of miR-143 expression is important in arsenic-induced transformation of prostate SCs. Restoration of miR-143 in As-CSCs was achieved by lentivirus-mediated miR-143 overexpression. Cells were assessed bi-weekly for up to 30 weeks to examine mitigation of cancer phenotype. Secreted matrix metalloproteinase (MMP) activity was increased by arsenic-induced malignant transformation, but miR-143 restoration decreased secreted MMP-2 and MMP-9 enzyme activities compared with scramble controls. Increased cell proliferation and apoptotic resistance, two hallmarks of cancer, were decreased upon miR-143 restoration. Increased apoptosis was associated with decreased BCL2 and BCL-XL expression. miR-143 restoration dysregulated the expression of SC/CSC self-renewal genes including NOTCH-1, BMI-1, OCT4 and ABCG2. The anticancer effects of miR-143 overexpression appeared to be mediated by targeting and inhibiting LIMK1 protein, and the phosphorylation of cofilin, a LIMK1 substrate. These findings clearly show that miR-143 restoration mitigated multiple cancer characteristics in the As-CSCs, suggesting a potential role in arsenic-induced transformation of prostate SCs. Thus, miR-143 is a potential biomarker and therapeutic target for arsenic-induced prostate cancer.
Keywords: Arsenic, Prostate, Stem cells, miRNA, Cancer
Introduction
Cancer stem cells (CSCs) are the driving force of tumor initiation, progression, and metastasis (Rosen and Jordan 2009). Accumulating evidence indicates that CSCs may be derived from normal stem cells (NSCs) (Iliopoulos et al. 2010; Wang 2010), or committed progenitors or even differentiated cells (Wang 2010). CSCs are typically a minor tumor subpopulation and share many characteristics with NSCs (Pardal et al. 2003). Like NSCs, CSCs have limitless self-renewal capacity, unlimited differentiation capacity, and reside in a niche (Borovski et al. 2011). However, unlike NSCs, CSCs show dysregulated self-renewal (Bomken et al. 2010).
Inorganic arsenic is a widespread environmental contaminant affecting millions of people worldwide, mainly through contaminated drinking water (IARC 2012). Arsenic is a human carcinogen (IARC 2012) with unclear underlying mechanisms. Arsenic causes cancers in the urogenital system in mice and humans (IARC 2012; Waalkes et al. 2007), and exposures in human populations suggest that the prostate is a possible target of arsenic carcinogenesis (Benbrahim-Tallaa and Waalkes 2008). In vitro, inorganic arsenic causes malignant transformation of the human prostate epithelial cell line RWPE-1 (Achanzar et al. 2002), and similarly, its isogenic stem cell derivative, WPE-stem (Tokar et al. 2010a) into a CSC-like phenotype. Both transformants produced highly aggressive tumors in mice (Achanzar et al. 2002; Tokar et al. 2010a). Various in vivo and in vitro data show that arsenic typically causes an overabundance of CSCs during the acquisition of a malignant phenotype in various models. For example, when RWPE-1 cells were malignantly transformed by arsenic exposure, there was a survival selection for, and overproduction of CSCs (Tokar et al. 2010b). In mice, arsenic exposure distorts skin SC dynamics, resulting in an overabundance of CSC (Waalkes et al. 2008), while in vitro, malignant transformation of the human skin keratinocytes by arsenic was associated with CSC overabundance (Sun et al. 2012). Similarly, there was a CSC overabundance in arsenic-induced lung and liver tumors in mice (Tokar et al. 2011). These data suggest that arsenic targets SCs as a central event in arsenic oncogenesis. Thus, SCs might serve as potential therapeutic targets in arsenic-induced carcinogenesis.
MicroRNAs (miRNAs) are a class of small non-coding RNAs widely expressed in plants and animals that predominantly inhibit gene expression at the post-transcriptional level by mRNA degradation or translational repression. Given their ability to regulate gene expression, miRNAs play critical roles in many cellular processes, and an aberration in expression can influence a variety of pathological events, including cancer (Calin and Croce 2006). In prostate cancer, there are several reports of aberrant miRNA expression in human tumors, cancer cell lines, or xenograft tumors (Calin and Croce 2006; Liu et al. 2012; Porkka et al. 2007; Volinia et al. 2006). Accumulating evidence indicates that miRNAs can also regulate properties of CSC, including prostate CSCs (Liu and Tang 2011). miRNAs can function as oncogenes or tumor suppressors, depending on their cellular context and target genes (Calin and Croce 2006). In our prior study (Ngalame et al. 2014b), we showed that arsenic-induced malignant transformation of the human prostate epithelial cells (CAsE-PE cells), and their isogenic prostate stem cells (As-CSCs) was associated with several dysregulated cancer-related miRNAs, suggesting a role for miRNAs in arsenic prostatic carcinogenesis. Among these dysregulated miRNAs was miR-143 which was downregulated 4.8-fold in the arsenic-transformed prostate SCs, As-CSCs (Ngalame et al. 2014b). The downregulation of miR-143 in As-CSCs is consistent with decreased expression of the miRNA in other prostate cancers (Ahmad et al. 2013; Clape et al. 2009; Xu et al. 2011) and other cancer types including cervical (Lipeng et al. 2012), lung (Ni et al. 2013), gastric (Takagi et al. 2009), leukemia (Akao et al. 2009; Shen et al. 2014), pancreatic (Pramanik et al. 2011), melanoma (Li et al. 2014), colorectal (Zhang et al. 2012), osteosarcoma (Osaki et al. 2011), and bladder (Noguchi et al. 2011). In some of the above-mentioned studies, miR-143 was shown to act as a tumor suppressor, as restoration of miR143 in these cancer cells significantly reduced cancer cell properties. Interestingly, the target and molecular mechanisms of miR-143 differed in each cancer type, indicating that miRNA targets and mechanisms can be cell-type dependent. While these studies indicate that miR-143 plays an important role in cancer, its role in arsenic-induced prostate SC transformation remains unclear.
In this study, we aimed to investigate the role of miR-143 in the malignant transformation of prostate SCs by arsenic, and thus in arsenic-induced prostate carcinogenesis. The investigation was done by restoring (overexpressing) miR-143 in the As-CSCs using precursor miR-143. Stably miR-143 overexpressing cells were isolated and the effects on cancer characteristics were investigated. The targets and pathways of miR-143 were also assessed.
Materials and Methods
Chemicals and reagents
Keratinocyte serum-free medium (K-SFM), bovine pituitary extract (BPE), epidermal growth factor (EGF), and 100 × antibiotic-antimycotic mixture were purchased from Life Technologies, Inc. (Grand Island, NY). miR-143 precursor vector construct and scramble miRNA vectors were purchased from SBI System Biosciences (Mountain View, CA). Puromycin was purchased from Cellgro (Manassas, VA). Rabbit anti-LIMK1 was purchased from Cell Signaling Technology (Beverly, MA). Mouse anti-phospho-Cofilin was purchased from Santa Cruz Biotech. Inc. (Santa Cruz, CA), and mouse anti-β-actin was purchased from Sigma Aldrich (St. Louis, MO). Horseradish peroxidase-conjugated mouse and rabbit secondary antibodies were purchased from Santa Cruz Biotech. Inc. (Santa Cruz, CA), and Bradford Protein Assay came from Bio-Rad Laboratories (Hercules, CA).
Cells and cell culture
As-CSC is a prostate cancer SC line derived by chronic exposure of the normal prostate SC line, WPE-stem to inorganic arsenic (5 μM, 18 wk) (Tokar et al. 2010a). As-CSC cells show multiple malignant characteristics in vitro and produced highly aggressive, pleiomorphic tumors in nude mice (Tokar et al. 2010a). As-CSC cells were grown in K-SFM containing 50μg/mL BPE and 5ng/mL EGF, supplemented with antibiotic/antimycotic mixture. The cells were incubated at 37°C in a humidified atmosphere containing 5% carbon dioxide until pre-confluence. Due to poor attachment and poor spreading of the SCs on plastic surface, As-CSC cells were cultured in flasks coated with a mixture of type IV collagen and fibronectin (2.5 μg each /cm2) (Tokar et al. 2005).
Transduction of lentiviral particles into arsenic-transformed SCs
Stable overexpression of miR-143 in As-CSCs was performed using transduction of precursor miR-143. The human miR-143 precursor sequence (catalog # PMIRH143PA-1; from SBI System Biosciences) was sub-cloned into SBI vector CD513B-1 resulting in a construct that contains puromycin selectable marker. The construct was received as bacterial streaks. Plasmid DNA from bacterial streaks was prepared using Qiafilter plasmid Maxi Kit (Qiagen, Valencia, CA). Lentiviral particles were packaged in HEK293T/17 cells (ATCC # CRL-11268) by the NIEHS Viral Vector Core according to a published protocol (Salmon and Trono 2006). Briefly, 293T cells were transiently transfected with pMD2G, psPAX2 and transfer vector containing the miR-143 precursor sequence or scramble miRNA using Lipofectamine 2000. Supernatant was collected 48 hours post transfection and concentrated by centrifugation at 50,000 g for 2 hours. Pellets were resuspended in PBS and used for infection. All titers were determined by quantitative PCR to measure the number of lentiviral particles that integrated into the host genome. In addition to quantitative PCR, biological titration of viruses that co-expressed fluorescent moieties was determined by flow cytometry. A Multiplicity of Infection (MOI) of 10 was used for infection of As-CSC cells. Stable cells were selected with 3 μg/mL puromycin. Cells were harvested and maintained in selection media, and analyzed for miR-143 overexpression 2 weeks post transduction. A stable miR-143 overexpression phenotype was maintained throughout the experiment (up to 30 weeks) by inclusion of puromycin. Puromycin selects only for cells that overexpress miR-143, and not selecting for a change in cell phenotype. It is noteworthy that the inclusion of puromycin in the medium did not have an effect on cell survival. In vitro biomarkers of malignant phenotype were assessed bi-weekly to determine the effects of miR-143 restoration on the malignant phenotype of these As-CSC cells.
Zymographic analysis of matrix metalloproteinase (MMP) activity
Secreted MMP activity generally correlates well with arsenic-induced malignant transformation (Achanzar et al. 2002; Tokar et al. 2010a). After miR-143 overexpression, cells were plated in 6 well plates for zymographic analysis of MMP-9 and MMP-2, as described previously (Tokar et al. 2005).
Cell proliferation
Cell proliferation was assessed in miR-143 overexpressing As-CSCs using The Cell Titer 96 Aqueous One Solution Cell proliferation Assay (MTS) kit (Promega, Madison, WI) and trypan blue as previously described (Ngalame et al. 2014a). Cell proliferation was assessed both in the presence and absence of growth factors in the media.
Apoptosis assay
Apoptosis was measured using Annexin V apoptosis detection kit (Molecular Probes, Eugene, OR). Briefly, floating and attached cells were collected, washed with PBS and resuspended in binding buffer and stained with Annexin V-350 conjugate and propidium iodide (PI). After 15 min of incubation at room temperature, samples were analyzed on a BD LSR II flow cytometer (BD Bioscience), and results were processed with a BD FACSDiva Software Version 6.1.3 to determine the percentage of apoptotic cells.
RNA extraction and quantitative Real-Time PCR (qRT-PCR)
Gene expression levels were determined using quantitative real time reverse transcription polymerase chain reaction (qRT-PCR) as described (Ngalame et al. 2014a). Total RNA including miRNAs was isolated from the transduced cells using miRNeasy kit (Qiagen Inc., Valencia, CA) according to manufacturer’s instructions. Total RNA concentration was measured using a NanoDrop 2000 spectrophotometer (ThermoFisher Scientific, Rochester, NY). For the analysis of mRNA expression, RNA was reverse transcribed with Moloney murine leukemia virus (MuLV) reverse transcriptase and oligo-d (T) primers. The primers for selected genes were designed with ABI Primer Express 3.0 Software (Applied Biosystems, Foster City, CA). Absolute SYBR Green ROX Mix (ThermoFisher Scientific, Rochester, NY) was used for RT-PCR analysis. Data were analyzed using the ΔΔCt method of relative quantification in which cycle times were normalized with GAPDH from the same sample, and then expressed as percentage of scramble miRNA control. To confirm miR-143 overexpression in transduced As-CSC cells, cDNA was generated using the miScript II RT kit (Qiagen Inc., Valencia, CA), and used as the template for real-time PCR with the miScript SYBR Green PCR Kit and miScript Primer Assays for miR-143 and RNU6-2 (Qiagen Inc., Valencia, CA). Real-time fluorescence detection was performed on an iCycler (Bio-Rad, Hercules, CA). Cycle times were normalized with RNU6-2 internal control, and then expressed as percentage of scramble miRNA control. qRT-PCR and real-time fluorescence detection were performed on an MyiQ2 Real Time-PCR Detection System (Bio-Rad, Hercules, CA)
Western blot analysis
Protein level was assessed by western blot analysis as previously described (Ngalame et al. 2014a). Membranes were incubated with LIMK1 and phospho-Cofilin primary antibodies, followed by horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary as appropriate.
Statistical analysis
All data are presented as mean ± SEM from three or more independent experiments. Statistical analyses were performed using an unpaired Student’s t-test. A two-tailed p < 0.05 was considered significant in all cases.
RESULTS
Stable overexpression of miR-143 in As-CSCs
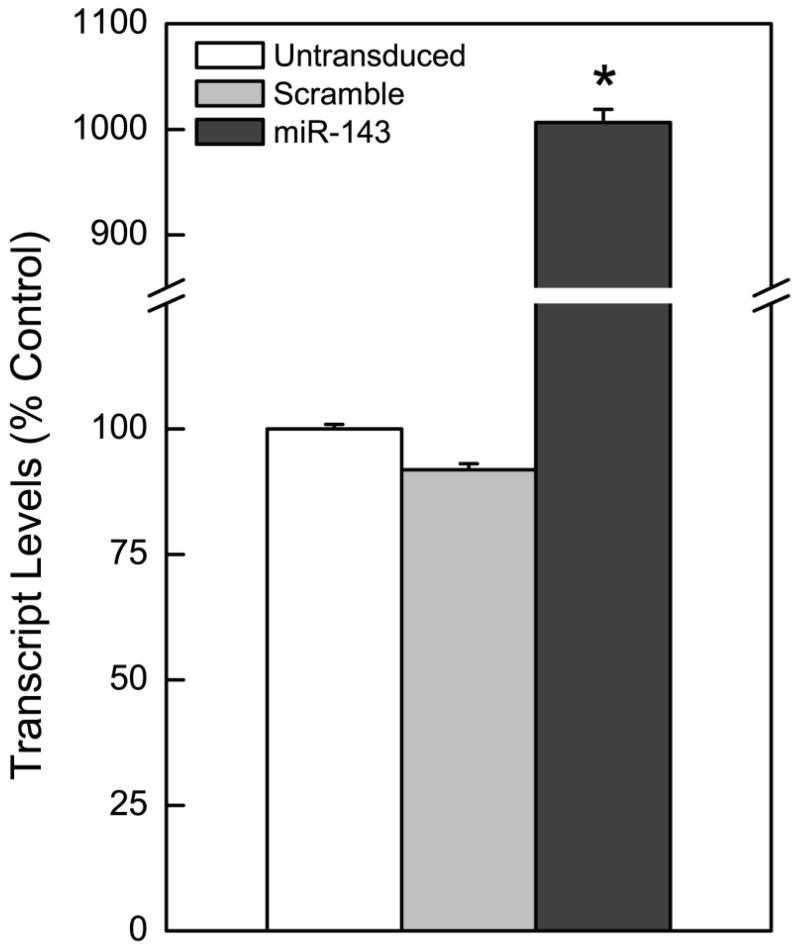
Stable re-expression of miR-143 into As-CSC cells by lentiviral transduction was performed to determine whether loss of this miRNA plays a role in initiating or maintaining arsenic-induced malignant phenotype. miR-143 overexpression was confirmed by qRT-PCR (Fig.1). Phenotype was stably maintained through the remainder of the experiment by continuous inclusion of puromycin in the media (up to 30 weeks).
Figure 1.
Overexpression of miR-143 in arsenic-transformed SCs, As-CSCs. miR-143 overexpression compared to scramble miRNA (negative control) or untransduced cells.
miR-143 overexpression mitigates acquired cancer cell characteristics in As-CSC cells
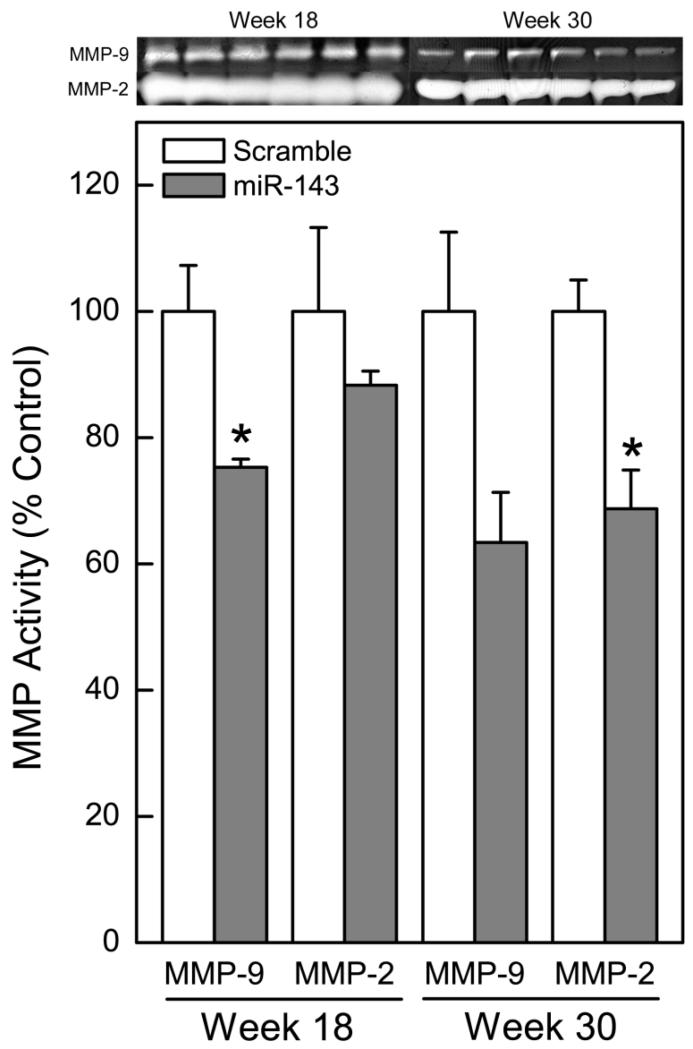
Secreted MMP enzyme activity typically increases with arsenic transformation (Achanzar et al. 2002; Tokar et al. 2010a). Thus, the effect of miR-143 overexpression on secreted MMP enzyme activity was determined by zymography. As expected, the overexpression of miR-143 markedly decreased secreted MMP-9 (by week 18) and MMP-2 (by week 30) enzyme activities in the transformed SCs (Fig. 2) This decrease in MMP activity in the miR-143 overexpressing cells indicates mitigation of cancer phenotype.
Figure 2.
miR-143 overexpression decreases secreted MMP enzyme activity. Zymographic analysis of secreted MMP-9 and MMP-2 enzyme activity in miR-143 overexpressing As-CSC cells. Data represent mean ± SEM (n = 3). * p<0.05 compared with controls.
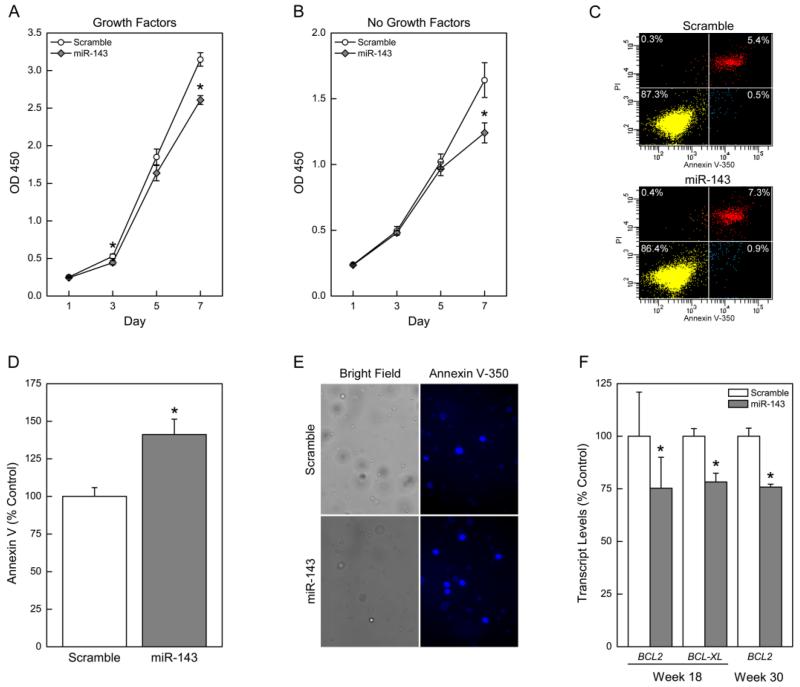
Uncontrolled proliferation and evasion of apoptosis are two hallmarks of cancer cells (Hanahan and Weinberg 2000). Therefore, a loss of cancer phenotype would be associated with decreased cell proliferation and increased apoptosis. The restoration of miR-143 significantly decreased proliferation of the arsenic-transformed CSCs over 1 week as assessed by the MTS assay (Fig. 3 A and B) and confirmed by vital stain trypan blue (data not shown). Decreased proliferation was consistent in the presence and absence of growth factors. These results suggest that miR-143 negatively regulates cell proliferation in these cells. A concurrent increase in apoptosis following miR-143 restoration was also seen (Fig. 3 C-E). This increase in apoptosis was associated with decreased expression of anti-apoptotic genes BCL2 and BCL-XL (Fig. 3F), suggesting these genes help mediate the apoptotic process in these cells. These results demonstrate an ability of miR-143 to induce apoptosis, resulting in repressed cancer cell proliferation.
Figure 3.
miR-143 overexpression decreases cancer cell proliferation and induces apoptosis in arsenic-transformed SCs. MTS assay over 7 days to assess cell growth in the presence (A) and absence (B) of growth factors. Data represent mean ± SEM (n = 6). * p<0.05 compared with controls. Apoptosis was determined by flow cytometry: (C) Distribution of cells after flow cytometry. Lower left quadrant is viable cells, and lower right quadrant is apoptotic cells (D) Quantification of apoptotic cells expressed as percentage scramble control; (E) Microscopy of stained cells showing apoptotic cells. (F) Gene expression of anti-apoptotic genes BCL2 and BCL-XL. Data represent mean ± SEM (n = 3). * p<0.05
miR-143 overexpression activates and then suppresses SC self-renewal genes in As-CSCs
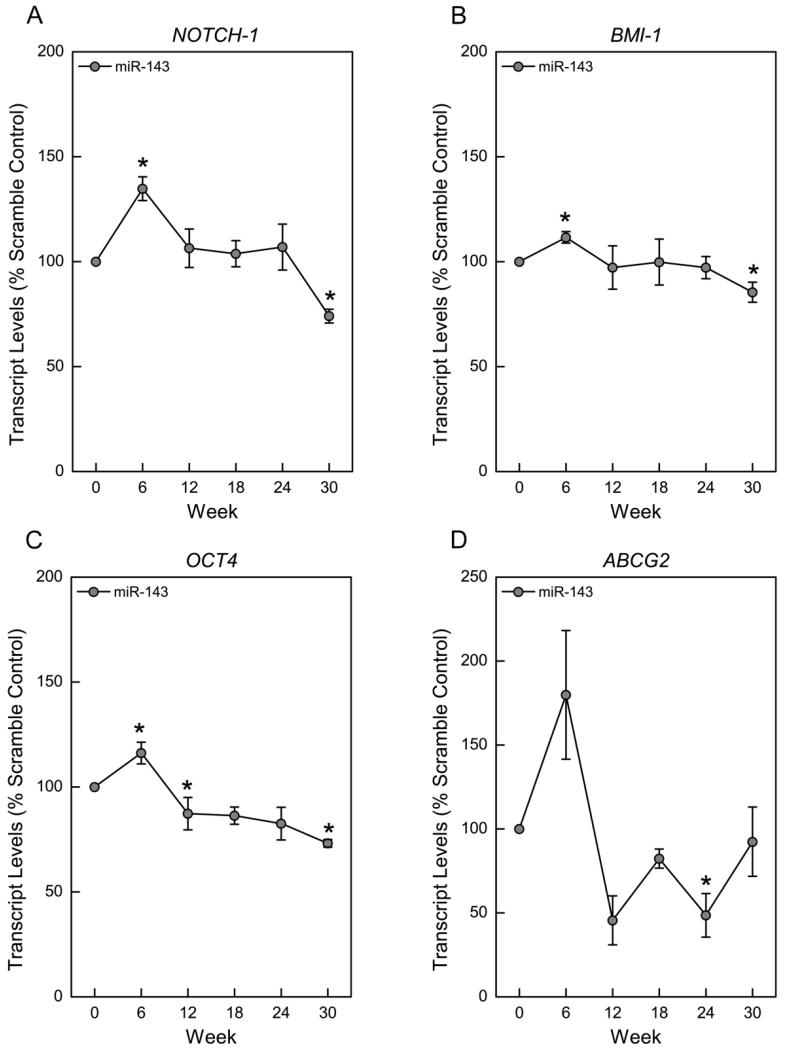
Emerging evidence suggests that miRNAs can regulate SC and CSC characteristics (Tay et al. 2008). During arsenic-induced CSC phenotype acquisition, the expression pattern of many SC self-renewal genes was dysregulated (Tokar et al. 2010a). We therefore examined the effect of miR-143 overexpression on the expression of SC self-renewal-related genes. The results show a consistent temporal pattern of initial gene activation, and subsequent suppression (Fig. 4). Approximately 6 weeks after miR-143 overexpression, several SC self-renewal genes including NOTCH-1, BMI-1, OCT4 and ABCG2, were markedly increased. This initial increase was followed by a gradual suppression as the cells showed a diminished cancer phenotype by 30 weeks. These results suggest that miR-143 can modulate CSC properties in As-CSCs by regulating NOTCH-1, BMI-1, OCT4 and ABCG2.
Figure 4.
miR-143 overexpression dysregulates SC maintenance and self-renewal gene expression in arsenic-transformed SCs. Transcript levels for NOTCH-1 (A), BMI-1 (B), OCT4 (C), and ABCG2 (D). Data represent mean ± SEM (n = 3). * p<0.05
LIM Domain Kinase 1 (LIMK1) is a likely target of miR-143
To investigate the potential downstream targets of miR-143 that could be implicated in arsenic-induced prostate SC transformation, we used the bioinformatics tools Target Scan 6.2 (http://www.targetscan.org/) and microRNA.org (http://www.microrna.org), as well as conducted a literature search for experimentally shown targets of miR-143. This search produced 5 lead candidate genes associated with carcinogenesis: KRAS, LIMK1, ERK5, PKCε, and Syn-1. Amongst these gene targets, only LIMK1 gene expression showed a clear inverse correlation with miR-143 overexpression in the As-CSCs. A significant decrease in LIMK1 protein level was observed 12 weeks after miR-143 precursor transduction, and this decrease persisted to the end of the experiment (30 weeks) (Fig. 5A). These data suggest that LIMK1 is a likely target of miR-143 in these As-CSC cells, consistent with reports of miR-143 directly targeting LIMK1in non-small cell lung cancer (NSCLC) (Xia et al. 2014). To ensure that the decrease in LIMK1 protein levels is not caused by other upstream regulators of LIMK1 such as Rac, ROCK and PAK1 (Manetti 2012), we examined the protein levels of these regulators. RAC, ROCK, and PAK1 levels were not changed with miR-143 overexpression, thus indicating that the observed decrease in LIMK1 is a result of miR-143 targeting. It is worth mentioning that KRAS (Xu et al. 2011) and ERK5 (Clape et al. 2009), which have been shown to be direct targets of miR-143 in prostate cancer cell lines, were not altered in the miR-143 overexpressing arsenic-transformed prostate SCs. This observation clearly indicates that miRNA targets are not only cell-type specific, but may also be specific to the type of inducing carcinogen.
Figure 5.
miR-143 overexpression suppresses LIMK1 expression and activity. (A) Time course showing LIMK1 protein level; (B) Protein level of p-Cofilin, a reflection of LIMK1 activity. Data represent mean ± SEM (n = 3). * p<0.05
To investigate whether changes in the level of LIMK1 protein also resulted in changes in its activity, we determined the level of phosphorylation of cofilin, the endogenous substrate of LIMK1. As expected, miR-143 overexpressing As-CSCs showed a significant decrease in phosphorylated Cofilin at week 30 (Fig. 5B). The activity of LIMK1 as judged by the level of phosphorylated Cofilin inversely correlated with miR-143 overexpression.
Discussion
Accumulating evidence shows that miRNAs play a role in cancer development and progression (Calin and Croce 2006). Dysregulated expression of miRNAs has been linked to prostate carcinogenesis, with some miRNAs acting like oncogenes and some like tumor suppressors (Calin and Croce 2006). Prior work showed that arsenic-induced transformation of prostate SCs was associated with dysregulation of several cancer-related miRNAs amongst which miR-143 was markedly downregulated, suggesting a role in arsenic-induced transformation of these cells (Ngalame et al. 2014b). In the present study, we have clearly shown that loss of miR-143 is important for the malignant transformation of the prostate SCs by arsenic. Restoration of miR-143 in the arsenic-transformed prostate SCs by lentivirus-mediated miR-143 overexpression decreased multiple cancer characteristics, including MMP-9 and MMP-2 enzyme activities, cell proliferation, apoptotic resistance and SC self-renewal capabilities. We further showed that miR-143 overexpression might be mediating its anti-cancer effects by targeting LIMK1, persistently inhibiting its expression and phosphorylation activity on cofilin. These findings show that miR-143 may act as a tumor suppressor in the As-CSCs and play a key role in the transformation of these cells.
MMPs are a family of enzymes that have a wide variety of roles in carcinogenesis including extracellular matrix digestion, metastasis and migration of cancer cells (Bachmeier et al. 2000; Stetler-Stevenson et al. 1996), epithelial to mesenchymal transition, tumor progression (Radisky and Radisky 2010), and regulation of signaling pathways that control inflammation, cell growth or angiogenesis, (Kessenbrock et al. 2010). Increased activity of MMPs is a characteristic of cancer cells and hypersecretion of MMP-2 and MMP-9 is positively correlated with arsenic-induced malignant transformation (Achanzar et al. 2002; Tokar et al. 2010a). Indeed, MMP-9 activity was highly elevated in the As-CSC cells during malignant transformation (Tokar et al. 2010a). However, upon miR-143 overexpression in these As-CSC cells, there was a marked inhibition of MMP-9 and MMP-2 activities. Considering the important roles of MMPs in carcinogenesis as modulators of the tumor microenvironment (Bachmeier et al. 2000; Kessenbrock et al. 2010; Radisky and Radisky 2010; Stetler-Stevenson et al. 1996), the observed decrease in MMP-9 and MMP-2 activities in response to miR-143 overexpression is indicative of a diminished cancer phenotype in the As-CSCs.
The ability of cancer cells to expand in number is dependent on both the rate of cell proliferation and cell death, mainly by apoptosis. Acquired apoptotic resistance is a hallmark of most cancer cells. Indeed, arsenic-induced malignant transformation of the prostate SCs was associated with increased resistance to apoptosis, and increased expression of the anti-apoptotic genes BCL2 and BCL-XL (Ngalame et al. 2014b; Tokar et al. 2010b). When miR-143 expression was restored in As-CSCs, they exhibited decreases in cell proliferation and apoptotic resistance. Therefore, a decrease in cell proliferation and increase in apoptosis in miR-143 overexpressing transformed cells point toward a diminished cancer phenotype, and suggest a tumor suppressive role of miR-143 in these cells. These data are consistent with other reports showing that miR-143 restoration decreased the proliferation of the prostate cancer cell lines DU145, PC3, LNCaP and C4-2 enhancing their sensitivity to docetaxel (Xu et al. 2011), and abrogating tumor growth in mice (Clape et al. 2009). Similarly, miR-143 is downregulated in various cancer cells including leukemia cells (Shen et al. 2014), non-small cell lung cancer (NSCLC) (Xia et al. 2014), cervical cancer (Lipeng et al. 2012), and melanoma cells (Li et al. 2014). Restoration of miR-143 in these cancer cells inhibited cell proliferation and induced apoptosis.
Self-renewal capacity is an important property of normal and cancer SCs, and is frequently dysregulated in carcinogenesis (Pardal et al. 2003; Reya et al. 2001). During the arsenic-induced transformation of As-CSC cells, the expression of several SC/CSC self-renewal- and differentiation-associated genes (NOTCH-1, BMI-1, OCT4, ABCG2, SHH, K5) was dysregulated (Tokar et al. 2010a). Expression of each of these genes was initially suppressed but subsequently reactivated upon malignant transformation to a CSC-like phenotype. Interestingly, upon overexpression of miR-143 in these As-CSC cells, four of these SC self-renewal genes (NOTCH-1, BMI-1, OCT4 and ABCG2) showed a consistent expression pattern opposite to the “U-shaped” pattern seen during transformation, consisting of an initial gene increase and then suppression. Notch-1 is a marker for SC/progenitor cells that is required for normal development, and in prostate carcinogenesis, can act as an oncogene and tumor suppressor (Leong and Gao 2008). BMI-1 is required for self-renewal of both SCs and CSCs in the prostate (Lessard and Sauvageau 2003; Pardal et al. 2003). ABCG2 plays a role in SC population maintenance and is a marker for prostate SCs and CSCs (Huss et al. 2005; Zhou et al. 2001). OCT-4 is a pluripotency gene required for self-renewal and maintenance of SCs and is often activated in carcinogenesis (Hochedlinger et al. 2005). Together, the present data suggest that miR-143 can regulate CSC characteristics, pointing towards a diminished CSC self-renewal capacity in the As-CSC cells. These data are consistent with reports of decreased SC self-renewal capacity of PC3 cells following miR-143 and miR-145 overexpression (Huang et al. 2012).
LIMK1 is a serine/threonine kinase that plays a key role in actin cytoskeleton dynamics and regulation. One of the major activities of LIMK1 is the phosphorylation and inactivation of its substrate Cofilin, an actin depolymerization factor. When phosphorylated, Cofilin is unable to bind to and sever filamentous actin (F-actin) into shorter actin (G-actin), resulting in the accumulation of actin polymers and a consequent dysregulation of actin-mediated cytoskeletal changes (Manetti 2012; Scott and Olson 2007). Actin dynamics contribute to many cellular processes including motility, differentiation, morphology, cell cycle, apoptosis and metastasis (Manetti 2012; Scott and Olson 2007). Hence, dysregulation of LIMK1 expression and/or signaling is associated with many disorders and diseases (Scott and Olson 2007). LIMK1 is overexpressed in many cancer cells including those of the prostate (Davila et al. 2003), breast (Yoshioka et al. 2003), and skin (Okamoto et al. 2005). In prostate cancer cells, LIMK1 overexpression correlates with tumor progression and invasion, and decreased expression of LIMK1 retards cell proliferation by arresting cells at the G2/M phase (Davila et al. 2007). Thus, the observed decrease in cell proliferation in the current study might be mediated by the decreased LIMK1 expression. LIMK1 expression has also been implicated in cancer cell migration and invasiveness. However, the activity of LIMK1 depends on the cellular context and signaling pathway, either promoting or inhibiting cell mobility (Ahmed et al. 2008; Borensztajn et al. 2010; Davila et al. 2003; Yoshioka et al. 2003). In this study, miR-143 overexpression did not alter the invasive ability of the As-CSC cells (data not shown), suggesting that this ability is not associated with LIMK1 activity in these cells.
It is unclear at this time why it took approximately 30 weeks to see significant effects on some results. It is possible that the delayed effects may be a function of the production of miR-143, and it may have taken ~30 weeks to reach levels that could induce these changes. It is also possible that several other miR-143-associated factors may be involved. It is well-known that one miRNA can affect multiple genes either by having a direct effect on the gene or an indirect effect further downstream the signaling pathway of the gene. Thus, the results seen early after transduction might be due to a more direct effect of miR-143 on those genes, whereas the delayed results may be due to a more indirect effect involving several additional genes/factors and/or signaling pathways.
In conclusion, our findings have established a causal role for loss of miR-143 expression during the arsenic-induced formation of prostate CSCs (As-CSCs). Restoration of miR-143 expression in these CSCs led to marked decreases in multiple cancer characteristics. These findings suggest that miR-143 is a potential biomarker for arsenic transformation of SCs, at least in the prostate. Moreover, considering that CSCs are considered by many to be the driving force of carcinogenesis, restoration of miR-143 expression may be a potential therapeutic target for arsenic-induced prostate cancer.
Research Highlights.
-
➢
Chronic arsenic exposure malignantly transforms the human normal prostate stem/progenitor cell (SC) line, WPE-stem to arsenic-cancer SCs (As-CSCs), through unknown mechanisms.
-
➢
miR-143 was several fold downregulated in the As-CSCs, suggesting a likely role in transformation.
-
➢
miR-143 restoration reduced multiple cancer characteristics in the As-CSC cells, suggesting a potential role in arsenic-induced transformation of the prostate SCs.
-
➢
We further showed that miR-143 appears to exert its anticancer effect by targeting and inhibiting the expression and activity of LIMK1, its predicted gene target.
-
➢
Findings suggest miR-143 is a potential biomarker and therapeutic target for arsenic-induced prostate cancer.
Acknowledgments
The authors wish to thank the NIEHS Viral Vector Core for the packaging of the lentiviral particles, and Matt Bell for his assistance in preparation of the graphics. This work was totally supported by Intramural Program funds from National Institute of Environmental Health Sciences, Division of the National Toxicology Program (ES102925).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None
References
- Achanzar WE, Brambila EM, Diwan BA, Webber MM, Waalkes MP. Inorganic arsenite-induced malignant transformation of human prostate epithelial cells. J Natl Cancer Inst. 2002;94:1888–1891. doi: 10.1093/jnci/94.24.1888. [DOI] [PubMed] [Google Scholar]
- Ahmad I, Singh LB, Yang ZH, Kalna G, Fleming J, Fisher G, et al. Mir-143 expression inversely correlates with nuclear Erk5 immunoreactivity in clinical prostate cancer. Br J Cancer. 2013;108:149–154. doi: 10.1038/bjc.2012.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed T, Shea K, Masters JR, Jones GE, Wells CM. A pak4-limk1 pathway drives prostate cancer cell migration downstream of HGF. Cell Signal. 2008;20:1320–1328. doi: 10.1016/j.cellsig.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Akao Y, Nakagawa Y, Iio A, et al. Role of miRNA-143 in Fas-mediated apoptosis in human T-cell leukemia Jurkat cells. Leuk Res. 2009;33:1530–1538. doi: 10.1016/j.leukres.2009.04.019. [DOI] [PubMed] [Google Scholar]
- Bachmeier BE, Boukamp P, Lichtinghagen R, Fusenig NE, Fink E. Matrix metalloproteinases-2,-3,-7,-9 and-10, but not MMP-11, are differentially expressed in normal, benign tumorigenic and malignant human keratinocyte cell lines. Biol Chem. 2000;381:497–507. doi: 10.1515/BC.2000.064. [DOI] [PubMed] [Google Scholar]
- Benbrahim-Tallaa L, Waalkes MP. Inorganic arsenic and human prostate cancer. Environ Health Perspect. 2008;116:158–164. doi: 10.1289/ehp.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomken S, Fiser K, Heidenreich O, Vormoor J. Understanding the cancer stem cell. Br J Cancer. 2010;103:439–445. doi: 10.1038/sj.bjc.6605821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borensztajn K, Peppelenbosch MP, Spek CA. Coagulation Factor Xa inhibits cancer cell migration via Limk1-mediated cofilin inactivation. Thromb Res. 2010;125:e323–328. doi: 10.1016/j.thromres.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Borovski T, De Sousa EMF, Vermeulen L, Medema JP. Cancer stem cell niche: The place to be. Cancer Res. 2011;71:634–639. doi: 10.1158/0008-5472.CAN-10-3220. [DOI] [PubMed] [Google Scholar]
- Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- Clape C, Fritz V, Henriquet C, Apparailly F, Fernandez PL, Iborra F, et al. Mir-143 interferes with Erk5 signaling, and abrogates prostate cancer progression in mice. PLoS ONE. 2009;4:e7542. doi: 10.1371/journal.pone.0007542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila M, Frost AR, Grizzle WE, Chakrabarti R. Lim kinase 1 is essential for the invasive growth of prostate epithelial cells: Implications in prostate cancer. J Biol Chem. 2003;278:36868–36875. doi: 10.1074/jbc.M306196200. [DOI] [PubMed] [Google Scholar]
- Davila M, Jhala D, Ghosh D, Grizzle WE, Chakrabarti R. Expression of Lim kinase 1 is associated with reversible G1/S phase arrest, chromosomal instability and prostate cancer. Mol Cancer. 2007;6:40. doi: 10.1186/1476-4598-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–477. doi: 10.1016/j.cell.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Huang S, Guo W, Tang Y, Ren D, Zou X, Peng X. Mir-143 and Mir-145 inhibit stem cell characteristics of PC-3 prostate cancer cells. Oncol Rep. 2012;28:1831–1837. doi: 10.3892/or.2012.2015. [DOI] [PubMed] [Google Scholar]
- Huss WJ, Greenberg NM, Mohler JL, Smith GJ. Breast cancer resistant protein-mediated efflux of androgen in putative benign and malignant prostate stem cells. Cancer Res. 2005;65:6640–6650. doi: 10.1158/0008-5472.CAN-04-2548. [DOI] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) arsenic and arsenic compounds. IARC Monogr Eval Carcinog Risk Hum. 2012;100C:41–94. [Google Scholar]
- Iliopoulos D, Hirsch HA, Wang G, Struhl K. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via Il6 secretion. Proc Natl Acad Sci. 2010;108:1397–1402. doi: 10.1073/pnas.1018898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong KG, Gao W-Q. The Notch pathway in prostate development and cancer. Differentiation. 2008;76:699–716. doi: 10.1111/j.1432-0436.2008.00288.x. [DOI] [PubMed] [Google Scholar]
- Lessard J, Sauvageau G. BMI-1 determines the proliferative capacity of normal and leukemic stem cells. Nature. 2003;423:255–260. doi: 10.1038/nature01572. [DOI] [PubMed] [Google Scholar]
- Li R, Zhang L, Jia L, Duan Y, Li Y, Wang J, et al. MicroRNA-143 targets Syndecan-1 to repress cell growth in melanoma. PLoS One. 2014;9:e94855. doi: 10.1371/journal.pone.0094855. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lipeng L, Xiaohua Y, Xiaofang G, Zhi T, Min S, Yu L, et al. Mir-143 is downregulated in cervical cancer and promotes apoptosis and inhibits tumor formation by targeting Bcl-2. Molecular Medicine Reports. 2012;5:753–760. doi: 10.3892/mmr.2011.696. [DOI] [PubMed] [Google Scholar]
- Liu C, Tang DG. MicroRNA regulation of cancer stem cells. Cancer Research. 2011;71:5950–5954. doi: 10.1158/0008-5472.CAN-11-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Kelnar K, Vlassov AV, Brown D, Wang J, Tang DG. Distinct microRNA expression profiles in prostate cancer stem/progenitor cells and tumor-suppressive functions of let-7. Cancer Research. 2012;72:3393–3404. doi: 10.1158/0008-5472.CAN-11-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manetti F. Lim kinases are attractive targets with many macromolecular partners and only a few small molecule regulators. Med Res Rev. 2012;32:968–998. doi: 10.1002/med.20230. [DOI] [PubMed] [Google Scholar]
- Ngalame NN, Tokar EJ, Person RJ, Waalkes MP. Silencing Kras overexpression in arsenic-transformed prostate epithelial and stem cells partially mitigates malignant phenotype. Toxicol Sci. 2014a;142:489–496. doi: 10.1093/toxsci/kfu201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngalame NN, Tokar EJ, Person RJ, Xu Y, Waalkes MP. Aberrant microRNA expression likely controls ras oncogene activation during malignant transformation of human prostate epithelial and stem cells by arsenic. Toxicol Sci. 2014b;138:268–277. doi: 10.1093/toxsci/kfu002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Z, Yunshu S, Lijun X. Targeting PKCe by mir-143 regulates cell apoptosis in lung cancer. FEBS Letters. 2013;587:3661–3667. doi: 10.1016/j.febslet.2013.09.018. [DOI] [PubMed] [Google Scholar]
- Noguchi S, Mori T, Hoshino Y, et al. MicroRNA-143 functions as a tumor suppressor in human bladder cancer T24 cells. Cancer Lett. 2011;307:211–220. doi: 10.1016/j.canlet.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Okamoto I, Pirker C, Bilban M, Berger W, Losert D, Marosi C, et al. Seven novel and stable translocations associated with oncogenic gene expression in malignant melanoma. Neoplasia. 2005;7:303–311. doi: 10.1593/neo.04514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaki M, Takeshita F, Sugimoto Y, et al. MicroRNA-143 regulates human osteosarcoma metastasis by regulating matrix metalloprtease-13 expression. Mol Ther. 2011;19:1123–1130. doi: 10.1038/mt.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3:895–902. doi: 10.1038/nrc1232. [DOI] [PubMed] [Google Scholar]
- Porkka KP, Pfeiffer MJ, Waltering KK, Vessella RL, Tammela TL, Visakorpi T. MicroRNA expression profiling in prostate cancer. Cancer Research. 2007;67:6130–6135. doi: 10.1158/0008-5472.CAN-07-0533. [DOI] [PubMed] [Google Scholar]
- Pramanik D, Campbell NR, Karikari C, et al. Restitution of tumor suppressor microRNAs using a systemic nanovector inhibits pancreatic cancer growth in mice. Mol Cancer Ther. 2011;10:1470–1480. doi: 10.1158/1535-7163.MCT-11-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radisky ES, Radisky DC. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. Journal of mammary gland biology and neoplasia. 2010;15:201–212. doi: 10.1007/s10911-010-9177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weismann IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Rosen JM, Jordan CT. The increasing complexity of the cancer stem cell paradigm. Science. 2009;324:1670–1673. doi: 10.1126/science.1171837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon P, Trono D. Production and titration of lentiviral vectors. Curr Protoc Neurosci. 2006 doi: 10.1002/0471142301.ns0421s37. Chapter 4:Unit 4.21. [DOI] [PubMed] [Google Scholar]
- Scott RW, Olson MF. Lim kinases: Function, regulation and association with human disease. J Mol Med (Berl) 2007:85. doi: 10.1007/s00109-007-0165-6. [DOI] [PubMed] [Google Scholar]
- Shen JZ, Zhang YY, Fu HY, Wu DS, Zhou HR. Overexpression of microRNA-143 inhibits growth and induces apoptosis in human leukemia cells. Oncol Rep. 2014;31:2035–2042. doi: 10.3892/or.2014.3078. [DOI] [PubMed] [Google Scholar]
- Stetler-Stevenson WG, Hewitt R, Corcoran M. Matrix metalloproteinases and tumor invasion: From correlation and causality to the clinic. Semin Cancer Biol. 1996;7:147–154. doi: 10.1006/scbi.1996.0020. [DOI] [PubMed] [Google Scholar]
- Sun Y, Tokar EJ, Waalkes MP. Overabundance of putative cancer stem cells in human skin keratinocyte cells malignantly transformed by arsenic. Toxicol Sci. 2012;125:20–29. doi: 10.1093/toxsci/kfr282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi T, Iio A, Nakagawa Y. Decreased expression of microRNA-143 and -145 in human gastric cancers. Oncology. 2009;77:12–21. doi: 10.1159/000218166. [DOI] [PubMed] [Google Scholar]
- Tay YM, Tam WL, Ang YS, Gaughwin PM, Yang H, Wang W, et al. MicroRNA-134 modulates the differentiation of mouse embryonic stem cells, where it causes post-transcriptional attenuation of Nanog and LRH1. Stem Cells. 2008;26:17–29. doi: 10.1634/stemcells.2007-0295. [DOI] [PubMed] [Google Scholar]
- Tokar EJ, Ancrile BB, Cunha GR, Webber MM. Stem/progenitor and intermediate cell types and the origin of human prostate cancer. Differentiation. 2005;73:463–473. doi: 10.1111/j.1432-0436.2005.00047.x. [DOI] [PubMed] [Google Scholar]
- Tokar EJ, Diwan BA, Waalkes MP. Arsenic exposure transforms human epithelial stem/progenitor cells into a cancer stem-like phenotype. Environ Health Perspect. 2010a;118:108–115. doi: 10.1289/ehp.0901059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar EJ, Qu W, Liu J, Liu W, Webber MM, Phang JM, et al. Arsenic-specific stem cell selection during malignant transformation. J Natl Cancer Inst. 2010b;102:638–649. doi: 10.1093/jnci/djq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar EJ, Diwan BA, Ward JM, Delker DA, Waalkes MP. Carcinogenic effects of “whole-life” exposure to inorganic arsenic in CD1 mice. Toxicol Sci. 2011;119:73–83. doi: 10.1093/toxsci/kfq315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Diwan BA. Transplacental arsenic carcinogenesis in mice. Toxicol Appl Pharmacol. 2007;222:271–280. doi: 10.1016/j.taap.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Germolec DR, Trempus CS, Cannon RE, Tokar EJ, et al. Arsenic exposure in utero exacerbates skin cancer response in adulthood with contemporaneous distortion of tumor stem cell dynamics. Cancer Research. 2008;68:8278–8285. doi: 10.1158/0008-5472.CAN-08-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JC. Good cells gone bad: The cellular origins of cancer. Trends in molecular medicine. 2010;16:145–151. doi: 10.1016/j.molmed.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Xia H, Sun S, Wang B, Wang T, Liang C, Li G, et al. Mir-143 inhibits NSCLC cell growth and metastasis by targeting Limk1. Int J Mol Sci. 2014;15:11973–11983. doi: 10.3390/ijms150711973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B, Niu X, Zhang X, Tao J, Wu D, Wang Z, et al. Mir-143 decreases prostate cancer cells proliferation and migration and enhances their sensitivity to docetaxel through suppression of KRAS. Mol Cell Biochem. 2011;350:207–213. doi: 10.1007/s11010-010-0700-6. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Foletta V, Bernard O, Itoh K. A role for Lim kinase in cancer invasion. Proc Natl Acad Sci U S A. 2003;100:7247–7252. doi: 10.1073/pnas.1232344100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang Z, Chen M, et al. MicroRNA-143 targets MACCI to inhibit cell invasion and migration in colorectal cancer. Mol Cancer. 2012;11:23. doi: 10.1186/1476-4598-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, et al. The ABC transporter bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med. 2001;7:1028–1034. doi: 10.1038/nm0901-1028. [DOI] [PubMed] [Google Scholar]