Abstract
Rationale
Asenapine is a second generation anti-psychotic approved in the USA in 2009 for the treatment of schizophrenia, but its efficacy has not been proven in Asian patients.
Objectives
The objectives of this study are to evaluate the efficacy and tolerability of asenapine in Asian patients experiencing an acute exacerbation of schizophrenia.
Methods
In this prospective, double-blind study, patients in Japan, Korea, and Taiwan were randomized (1:1:1) to asenapine 5 mg twice daily (bid), 10 mg bid or placebo for 6 weeks after a 3- to 7-day washout/screening period. The primary endpoint was the mean change in the positive and negative syndrome scale (PANSS) total score from baseline to day 42/treatment end.
Results
Of the 532 participants randomized, 530 received treatment. The primary endpoint was significantly greater with asenapine 5 and 10 mg bid than with placebo (−12.24 and −14.17 vs. −0.95; p < 0.0001). The results of secondary endpoints including PANSS negative subscale scores and PANSS responders at the end of treatment supported the results of the primary endpoint. There were no significant differences in the incidence of treatment-emergent adverse events reported with asenapine 5 and 10 mg bid and placebo (84.6, 80.7, and 81.6 %). There was a mean (± standard deviation) change in weight of −1.76 ± 2.45 kg for placebo, +0.42 ± 2.65 kg for asenapine 5 mg bid, and +0.81 ± 2.89 kg for asenapine 10 mg bid group.
Conclusions
Asenapine was effective and generally well tolerated when used for the treatment of acute exacerbations of schizophrenia in Asian patients.
Keywords: Schizophrenia, Asenapine, Efficacy, Tolerability
Introduction
Schizophrenia is a complex psychiatric disorder associated with variable degrees of functional impairment and social disability (Lublin et al. 2005; Tandon et al. 2009). It is a chronic relapsing disorder, with recurrent exacerbations of positive symptoms against a background of persistent negative symptoms, cognitive dysfunction, and depressive symptoms (Lublin et al. 2005; Tandon et al. 2009).
Anti-psychotic medication is the mainstay of treatment for schizophrenia (van Os and Kapur 2009). Historically, pharmacological treatments have focused on dopamine D2 receptor blockade to control the positive symptoms of schizophrenia, while negative and cognitive symptoms often persist (Abi-Dargham 2014; van Os and Kapur 2009). Second generation anti-psychotics, which also inhibit a range of other receptors, have been developed, but they vary in their effectiveness across mood domains (Lublin et al. 2005; Pompili et al. 2011). In addition, second generation anti-psychotics have differing levels of risk for weight gain and hyperprolactinemia (De Hert et al. 2012; Kane 2011; Tandon et al. 2010), which can negatively affect treatment adherence (Kane 2011; Lieberman et al. 2005).
Asenapine is a second generation anti-psychotic with a unique receptor-binding profile that has been approved in the USA for the treatment of schizophrenia (Citrome 2014a; Shahid et al. 2009). It has antagonistic activity at dopaminergic, serotonergic, α-adrenergic, and histaminic receptors but no appreciable affinity for muscarinic receptors (Shahid et al. 2009). The efficacy and safety of asenapine in schizophrenia has been established in a global clinical trial program (Citrome 2014b). In two 6-week fixed-dose trials, asenapine 5 mg twice daily (bid) was more effective than placebo in improving positive and negative syndrome scale (PANSS) total scores (Kay et al. 1987) in patients with acute exacerbations of schizophrenia, with only modest effects on weight and metabolic variables (Kane et al. 2010; Potkin et al. 2007).
In a dedicated study, the pharmacokinetic and safety profiles of asenapine were similar in healthy Japanese and Caucasian subjects (Merck Sharp & Dohme B.V. 2014), meaning no dosage adjustment is required on the basis of race. However, generally Asian subjects account for only a small percentage of the total populations enrolled in the phase II and III clinical studies of asenapine, and studies conducted in specific ethnic populations are still needed. Therefore, the study described herein was conducted to confirm the efficacy and safety of asenapine in Asian patients experiencing an acute exacerbation of schizophrenia.
Methods
Patients
The study was conducted from May 2010 to April 2014 at 112 centers: Japan, 81 sites; Taiwan, 15 sites; and Korea, 16 sites. Male and female patients aged 20–64 years with a Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) (American Psychiatric Association 2000) diagnosis of schizophrenia of paranoid (295.30), disorganized (295.10), catatonic (295.20), or undifferentiated (295.90) subtypes were eligible for randomization. The current acute exacerbation of schizophrenia had to be of ≤2 months duration, with symptoms that represented a dramatic and substantial change from the state prior to the exacerbation, and there was a requirement for a change in medication or dosage to treat new or worsened positive symptoms. Other key inclusion criteria were a PANSS total score ≥60, with scores of ≥4 in two or more of five items on the PANSS positive subscale (delusions, conceptual disorganization, hallucinatory behavior, grandiosity, suspiciousness/persecution) at the initial screening assessment and at baseline, and a score of ≥4 on the clinical global impressions-severity of illness (CGI-S) scale (Guy 1976) at baseline. Patients who had received previous anti-psychotic medication for a prior episode of acute exacerbation of schizophrenia were required to have had a positive response. The use of all prohibited concomitant medications (anti-psychotics, anti-depressants, mood stabilizers, anti-epileptics, monoamine oxidase inhibitors, St. John’s Wort, anti-emetics that are dopamine antagonists, and traditional herbal medication) had to be discontinued, with the last dose taken no later than the evening prior to the baseline visit, for inclusion in the trial (for depot neuroleptic, discontinuation must have occurred more than 3 months prior to randomization).
Patients with a diagnosis of schizoaffective disorder (295.70), schizophrenia of residual subtype (295.60), schizophreniform disorder (295.40), or a psychiatric disorder other than schizophrenia were excluded from the study. Women who were pregnant were ineligible for inclusion in the trial. Patients were also excluded if they had taken any experimental medication within 12 weeks before baseline, were defined as having treatment-refractory schizophrenia, had received treatment with ≥3 anti-psychotic drugs within the previous month, or had an uncontrolled, unstable clinically significant medical condition (e.g., renal, endocrine, hepatic, respiratory, cardiovascular, hematologic, immunologic or cerebrovascular disease, or malignancy) or abnormal laboratory, vital sign, physical examination, or electrocardiogram (ECG) findings at screening. Other exclusion criteria included the following: current (past 6 months) substance abuse; a body mass index (BMI) <16.0 or >35.0 at baseline; a diagnosis of Parkinson’s disease; a history of or current treatment for narrow angle glaucoma; a history of any seizure disorder beyond childhood; a history of allergy or sensitivity to drugs such as psychotropics and anti-psychotics; a ≥20 % decrease in PANSS total score from screening to baseline; imminent risk of self-harm or harm to others; current involuntary inpatient confinement; known diagnosis of borderline personality disorder, mental retardation, or organic brain disorder; and previous treatment with asenapine.
Patients classed as treatment-refractory (has been treated with at least two different atypical anti-psychotic agents at dosages equivalent to or greater than 600 mg/day of chlorpromazine [12 mg/day of haloperidol] for more than 4 weeks, each without clinical response, or has received clozapine for 12 weeks immediately preceding the screening) and those who received treatment with three or more anti-psychotic drugs, or dose equivalents higher than 18 mg/day of haloperidol (equivalent 900 mg/day of chlorpromazine) within 1 month prior to randomization were also excluded.
A number of measures were implemented to minimize the risks to patients randomized to placebo: hospitalization for a minimum 3 weeks, with the possibility of extending the hospitalization to 6 weeks if the patient was not clinically stable enough to be discharged; regular assessment of symptom severity; requirement that a caregiver oversees the patient’s compliance with trial medication during outpatient dosing; allowance of concomitant benzodiazepines to treat agitation and anxiety; and exclusion of high-risk subjects including those that are actively suicidal, homicidal, under involuntary commitment, or have a recent history of aggressive behavior.
Randomization and treatment
The study consisted of a 3- to 7-day washout/screening period, a 6-week double-blind treatment phase, and a follow-up period. Placebo was administered single blind for the duration of the screening period, and patients were tapered off their pre-study anti-psychotic medication, anti-depressant medication, and any anti-parkinsonian drugs to treat extrapyramidal symptoms (EPS).
After the baseline assessments were completed, patients considered eligible by an investigator were randomized (1:1:1) to receive sublingual asenapine 5 mg bid, 10 mg bid, or placebo. The allocation of patients was performed using permuted-block randomization (block size of 6 and allocation ratio of 1:1:1) using SAS. Participants and investigators remained blinded to treatment assignment, in accordance with the double-blind study design. The asenapine and placebo tablets were identical in appearance and characteristics (i.e., all tablets were fast dissolving) and had identical packaging. During the treatment period, participants received their allocated dosage of asenapine or placebo twice a day every day for 6 weeks. Tablets were administered sublingually without water.
Participants were hospitalized during screening and for the first 3 weeks of the treatment period, with the possibility of extending hospitalization to 6 weeks in the absence of a responsible caregiver to provide support and ensure compliance with study medication. Compliance during inpatient treatment was recorded by hospital staff and monitored by returned tablet counts during outpatient treatment.
Concomitant lorazepam and short-acting benzodiazepines were permitted to treat agitation and anxiety, and concomitant EPS medication was permitted if EPS worsened or appeared.
An interim analysis was built into the protocol to assess whether the trial should be discontinued (in case of lack of efficacy). In May 2012, an independent data-monitoring committee was convened to review the efficacy data for half of the planned number of participants collected up to the day of database lock (April 26, 2012). Upon review of the data, the committee concluded that continuation of the trial was appropriate.
Efficacy measurement
The primary efficacy outcome was change in the PANSS total score from baseline to day 42 (end of treatment). Secondary efficacy outcomes included changes from baseline to end of treatment in PANSS subscale scores (positive symptoms, negative symptoms and general psychopathology), PANSS Marder factor scores (positive symptoms, negative symptoms, disorganized thought, hostility/excitement, anxiety/depression), and CGI-S scores. Additional secondary variables were the percentage of PANSS responders (those with ≥30 % decrease in the total PANSS total score from baseline to end of treatment) and the percentage of CGI-global improvement (CGI-I) responders (those who are assessed as very much improved, much improved or minimally improved by the investigator) at day 42. Post-baseline efficacy assessments were obtained on day 4 and 7 and weekly thereafter (day 14, 21, 28, 35, and 42). Efficacy assessments were performed by an investigator or a trained rater assigned by the investigator. Using the same rater for assessment of a subject throughout their participation in the study was strongly encouraged.
Safety assessments
Key safety parameters were weight gain, body mass index (BMI), EPS, glycosylated hemoglobin (HbA1c), fasting glucose, insulin, and prolactin. For prolactin, only clinically relevant abnormal values were reported. Other safety variables were the frequency of the onset of treatment-emergent adverse events (TEAEs) and clinically relevant changes in physical findings, vital signs, electrocardiogram parameters, laboratory values, and use of anti-parkinsonian drugs.
Laboratory, ECG, and BMI assessments were made at baseline and on days 14, 28, and 42. Assessments of vital signs were conducted at baseline and on days 14, 21, 28, and 42. Physical examinations were conducted at screening and at end of treatment (day 42). The incidence, nature, and severity of AEs were recorded throughout the screening and treatment periods until 7 days after the last dose of study drug. EPS were reported as AEs and rated according to the drug-induced extrapyramidal symptoms scale (DIEPSS) (Inada 2009) at baseline and on days 21 and 42.
Sample size calculations
Based on the results of previous phase II and III clinical trials of asenapine in patients with schizophrenia (Minassian and Young 2010; Potkin et al. 2007), the range of the difference in the change in PANSS total score from baseline to end of treatment at 42 days between the asenapine 5 mg bid and placebo groups was between −3.4 and −9.7. Referring to the study by Kane et al. (2010), we assumed that the change from baseline in PANSS total score would be −6, and the standard deviation (SD) of the total of change in PANSS score would be 20 (effect size = 0.3). The sample size required to show superiority of asenapine over placebo was estimated be 176 per group, with a two sided significance level of 5 and 80 % power. Therefore, approximately 530 participants were expected to be randomized.
Statistical analysis
Several sets were defined for the analysis of outcomes. The subjects as treated (i.e., safety analysis set; All Subjects Treated, AST) consisted of all participants enrolled in the study who received at least part of one dose of the study drug, while the full analysis set (FAS) consisted of all participants in the AST who had a baseline and at least one post-baseline PANSS measurement.
The primary efficacy outcome was analyzed in the FAS using analysis of covariance (ANCOVA) with dropout or missing data imputed by using the last observation carried forward (LOCF). No patient had ≥6 missing PANSS items; for 5 or fewer missing PANSS items, the PANSS score was calculated by multiplying the total for the non-missing items by the total number of items and then dividing by the number of non-missing items.
The model included change from baseline in PANSS total score as a response variable, baseline PANSS total score as a covariate, and treatment groups and regions (Japan, Taiwan, and Korea) as explanatory variables. Pairwise comparisons for the primary efficacy outcome were performed in the following order: (1) asenapine 5 mg bid vs. placebo and (2) asenapine 10 mg bid vs. placebo. The result of the second comparison was considered only when the result of asenapine 5 mg bid vs. placebo was statistically significant. Further analysis using a mixed model for repeated measures (MMRM) was performed using change from baseline in PANSS total score at each visit from 7 days as the response variables and treatment groups, regions, visits, and treatment by visit interaction as the fixed effect variables and baseline PANSS total score as the covariates. Changes from baseline to end of treatment in selected secondary efficacy outcomes (PANSS subscale, Marder factor, and CGI-S scores) were also assessed using ANCOVA with LOCF. The MMRM method was also performed for PANSS subscale and Marder factor scores. PANSS and CGI-I responder rates in the placebo versus each asenapine group were compared using the Cochran-Mantel-Haenszel (CMH) test with an adjustment for region. Regarding the analysis of secondary endpoints, these were exploratory in nature and no adjustment for multiplicity was made. Safety variables in the AST were summarized using descriptive statistics. TEAEs and categorical variables for demographics were compared the placebo and each asenapine group using Fisher’s exact test. Continuous variables for demographics were compared the placebo and each asenapine group using ANOVA. A p value less than 0.05 was considered statistically significant (two-tailed).
Results
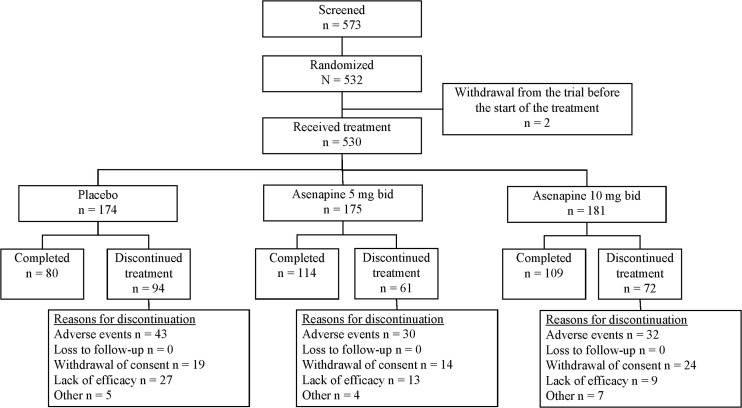
Of a total of 573 patients screened, 532 participants were randomized to treatment. The AST population included 530 participants (asenapine 5 mg bid: n = 175; asenapine 10 mg bid: n = 181; placebo: n = 174) and the FAS included 525 participants (asenapine 5 mg bid: n = 173; asenapine 10 mg bid: n = 178; placebo: n = 174). The flow of participants throughout the study is presented in Fig. 1. A total of 303 participants completed treatment, with a greater percentage of those in the asenapine 5 mg bid (65 %) or 10 mg bid (60 %) groups completing the trial compared with those receiving placebo (46 %). The incidence of withdrawal due to lack of efficacy was lower for participants receiving asenapine 5 mg bid (7.4 % of treated participants) or 10 mg bid (5.0 %) than for those receiving placebo (15.5 %).
Fig. 1.
Patient disposition: numbers of patients who were screened, randomized to treatment, received treatment, and completed treatment, with reasons for discontinuation shown
The key baseline characteristics of the AST population are presented in Table 1. No meaningful differences in baseline demographics or clinical characteristics including PANSS total scores were noted between the treatment groups. Of the concomitantly used medications, all the groups (71.3 % in the placebo, 62.9 % in the asenapine 5 mg bid, and 70.2 % in the asenapine 10 mg bid.
Table 1.
Baseline characteristics and demographics (all patients as treated population)
| Characteristic | Placebo (n = 174) | Asenapine 5 mg bid (n = 175) | Asenapine 10 mg bid (n = 181) | Total (n = 530) | p Value |
|---|---|---|---|---|---|
| Gender, n (%) | |||||
| Male | 81 (46.6) | 75 (42.9) | 99 (54.7) | 255 (48.1) | 0.0722a |
| Female | 93 (53.4) | 100 (57.1) | 82 (45.3) | 275 (51.9) | |
| Age, years | 41.11 ± 12.27 | 41.41 ± 11.00 | 41.72 ± 11.10 | 41.42 ± 11.45 | 0.8844b |
| Bodyweight, kg | 62.58 ± 12.62 | 62.51 ± 14.15 | 64.28 ± 13.18 | 63.14 ± 13.33 | 0.3670b |
| BMI, kg/m2 | 23.49 ± 3.88 | 23.64 ± 4.05 | 24.15 ± 4.42 | 23.76 ± 4.13 | 0.2895b |
| Region, n (%) | |||||
| Japan | 91 (52.3) | 96 (54.9) | 87 (48.1) | 274 (51.7) | 0.7846a |
| Taiwan | 50 (28.7) | 49 (28.0) | 57 (31.5) | 156 (29.4) | |
| Korea | 33 (19.0) | 30 (17.1) | 37 (20.4) | 100 (18.9) | |
| Schizophrenia (DSM-IV-TR) diagnosis, n (%) | |||||
| Paranoid | 137 (78.7) | 139 (79.4) | 138 (76.2) | 414 (78.1) | 0.9850a |
| Disorganized | 13 (7.5) | 14 (8.0) | 14 (7.7) | 41 (7.7) | |
| Catatonic | 4 (2.3) | 3 (1.7) | 4 (2.2) | 11 (2.1) | |
| Undifferentiated | 20 (11.5) | 19 (10.9) | 25 (13.8) | 64 (12.1) | |
| Duration of current episode, n (%) | |||||
| <2 weeks | 47 (27.0) | 34 (19.4) | 32 (17.7) | 113 (21.3) | 0.1969a |
| ≥2 weeks and <1 month | 47 (27.0) | 59 (33.7) | 55 (30.4) | 161 (30.4) | |
| ≥1 month and <2 months | 80 (46.0) | 81 (46.3) | 94 (51.9) | 255 (48.1) | |
| ≥2 months | 0 | 1 (0.6) | 0 | 1 (0.2) | |
| PANSS total score | 94.51 ± 17.26 | 94.15 ± 17.97 | 92.74 ± 17.34 | 93.79 ± 17.51 | 0.6049b |
| Concomitant medicationc, n (%) | |||||
| Present | 172 (98.9) | 171 (97.7) | 179 (98.9) | ||
| A02 drugs for acid related disorders | |||||
| Magnesium oxide | 24 (13.8) | 16 (9.1) | 27 (14.9) | ||
| A06 drugs for constipation | |||||
| Sennoside a + b calcium | 23 (13.2) | 27 (15.4) | 33 (18.2) | ||
| Sodium picosulfate | 26 (14.9) | 13 (7.4) | 18 (9.9) | ||
| N02 analgesics | |||||
| Paracetamol | 24 (13.8) | 20 (11.4) | 21 (11.6) | ||
| N03 anti-epileptics | |||||
| Clonazepam | 20 (11.5) | 10 (5.7) | 7 (3.9) | ||
| Lorazepam | 124 (71.3) | 110 (62.9) | 127 (70.2) | ||
| N05 psycholeptics | |||||
| Olanzapine | 26 (14.9) | 21 (12.0) | 19 (10.5) | ||
| Aripiprazole | 18 (10.3) | 18 (10.3) | 10 (5.5) | ||
| Risperidone | 28 (16.1) | 25 (14.3) | 15 (8.3) | ||
| Etizolam | 22 (12.6) | 27 (15.4) | 21 (11.6) | ||
| Brotizolam | 51 (29.3) | 51 (29.1) | 53 (29.3) | ||
| Flunitrazepam | 18 (10.3) | 13 (7.4) | 15 (8.3) | ||
| Zolpidem | 31 (17.8) | 29 (16.6) | 44 (24.3) | ||
| Zopiclone | 28 (16.1) | 28 (16.0) | 33 (18.2) | ||
| N06 anti-depressants | |||||
| Escitalopram | 0 (0.0) | 1 (0.6) | 0 (0.0) | ||
| Escitalopram oxalate | 0 (0.0) | 1 (0.6) | 0 (0.0) | ||
| Fluoxetine hydrochloride | 1 (0.6) | 0 (0.0) | 0 (0.0) | ||
| Fluvoxamine maleate | 0 (0.0) | 0 (0.0) | 1 (0.6) | ||
| Sertraline hydrochloride | 0 (0.0) | 0 (0.0) | 1 (0.6) | ||
| Bupropion | 1 (0.6) | 0 (0.0)) | 1 (0.6) | ||
| Mirtazapine | 2 (1.1) | 0 (0.0) | 0 (0.0) | ||
| Setiptiline maleate | 0 (0.0) | 1 (0.6) | 0 (0.0) | ||
| Trazodone | 2 (1.1) | 0 (0.0) | 2 (1.1) | ||
| Trazodone hydrochloride | 1 (0.6) | 0 (0.0) | 1 (0.6) | ||
All data are mean ± SD unless otherwise stated
BMI body mass index, DSM-IV Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision, PANSS positive and negative syndrome scale
aFisher’s exact test
bANOVA
cDrugs administered from the start of the double-blind treatment period to 7 days after the end of the study treatment (coded by WHO Drug Dictionary)
Primary efficacy outcome: PANSS total score
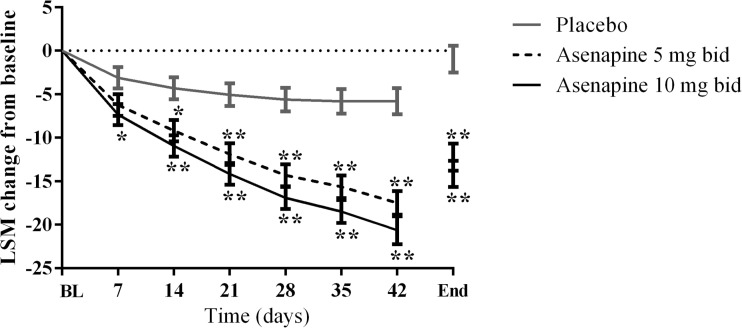
Mean PANSS total scores at baseline and at treatment end (day 42) are shown in Table 2. The least squares mean (LSM) changes from baseline in the PANSS total score at end of treatment (day 42) in the FAS were −12.24 (95 % confidence interval [CI] −15.28, −9.20), −14.17 (95 % CI −17.12, −11.22) and −0.95 (95 % CI −3.95, 2.06) in the asenapine 5 mg bid, asenapine 10 mg bid, and placebo groups, respectively. The improvements from baseline in PANSS total score were significantly greater in participants receiving asenapine 5 mg bid or asenapine 10 mg bid, compared with placebo from days 14 and 7, respectively. Overall, the efficacy profile of the asenapine 5 and 10 mg groups were similar (Table 2). Analysis of the change in PANSS total score from baseline over time using MMRM showed that improvements from baseline in PANSS total score were significantly larger in the asenapine 5 and 10 mg bid groups compared with placebo from day 14 and 7, respectively (p < 0.05) and were sustained through to day 42 (Fig. 2). Table 3 describes the proportion of responders using different thresholds to define response (i.e., ≥20, ≥30, ≥40, and ≥50 % decrease in PANSS total score).
Table 2.
Positive and negative syndrome scale total scores during the study (full analysis set, last observation carried forward)
| Placebo (n = 174) | Asenapine 5 mg bid (n = 173) | Asenapine 10 mg bid (n = 178) | Asenapine 5 mg bid − placebo | Asenapine 10 mg bid − placebo | |
|---|---|---|---|---|---|
| At baseline | |||||
| Mean | 94.51 | 94.23 | 92.83 | ||
| SD | 17.26 | 18.06 | 17.42 | ||
| End of treatment (day 42) | |||||
| Mean | 93.38 | 81.84 | 78.60 | ||
| SD | 25.30 | 26.10 | 25.01 | ||
| Change from baseline to end of treatment | |||||
| LSM | −0.95 | −12.24 | −14.17 | −11.29 | −13.22 |
| SE | 1.53 | 1.55 | 1.50 | 2.10 | 2.09 |
| 95 % CI | −3.95, 2.06 | −15.28, −9.20 | −17.12, −11.22 | −15.42, −7.16 | −17.33, −9.12 |
| p valuea | – | – | – | <0.0001 | <0.0001 |
aFor between-group comparisons
bid twice daily, CI confidence interval, LSM least squares mean, SD standard deviation, SE standard error
Fig. 2.
Primary efficacy outcome: change from baseline in PANSS total score over time (full analysis set population). BL, baseline, LSM least squares mean. *p < 0.05; **p < 0.01 vs. placebo
Table 3.
Responder rates for change in positive and negative syndrome scale (PANSS) total scores, across different threshold values. Responder categories are separated by set limits for the magnitude of decrease in the total PANSS score from baseline to day 42/end of treatment
| Placebo (n = 174) | Asenapine 5 mg bid (n = 173) | Asenapine 10 mg bid (n = 178) | Asenapine 5 mg bid − placebo | Asenapine 10 mg bid − placebo | |
|---|---|---|---|---|---|
| ≥20 % decrease | |||||
| Responder, n (%) | 52 (29.9) | 92 (53.2) | 91 (51.1) | 23.3 | 21.2 |
| 95 % CI (%) | 23.2, 37.3 | 45.5, 60.8 | 43.5, 58.7 | 13.2, 33.4 | 11.2, 31.2 |
| ≥30 % decrease | |||||
| Responder, n (%) | 36 (20.7) | 68 (39.3) | 78 (43.8) | 18.6 | 23.1 |
| 95 % CI (%) | 14.9, 27.5 | 32.0, 47.0 | 36.4, 51.4 | 9.2, 28.1 | 13.7, 32.6 |
| ≥40 % decrease | |||||
| Responder, n (%) | 21 (12.1) | 41 (23.7) | 56 (31.5) | 11.6 | 19.4 |
| 95 % CI (%) | 7.6, 17.9 | 17.6, 30.7 | 24.7, 38.8 | 3.7, 19.6 | 11.0, 27.8 |
| ≥50 % decrease | |||||
| Responder, n (%) | 8 (4.6) | 25 (14.5) | 40 (22.5) | 9.9 | 17.9 |
| 95 % CI (%) | 2.0, 8.9 | 9.6, 20.6 | 16.6, 29.3 | 3.8, 15.9 | 11.0, 24.8 |
bid twice daily, CI confidence interval
Secondary efficacy outcomes
Positive and negative syndrome scale subscale scores and responders
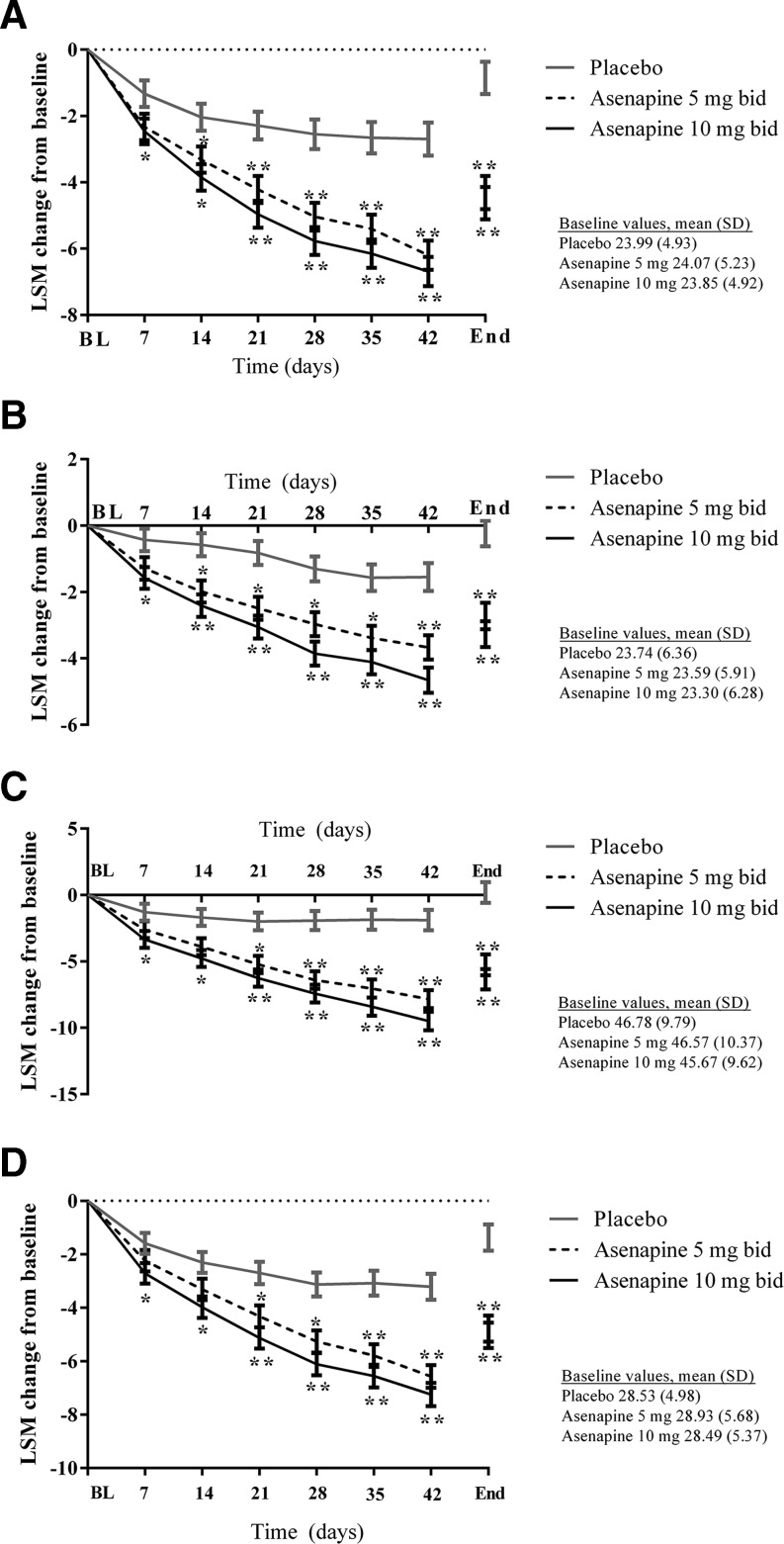
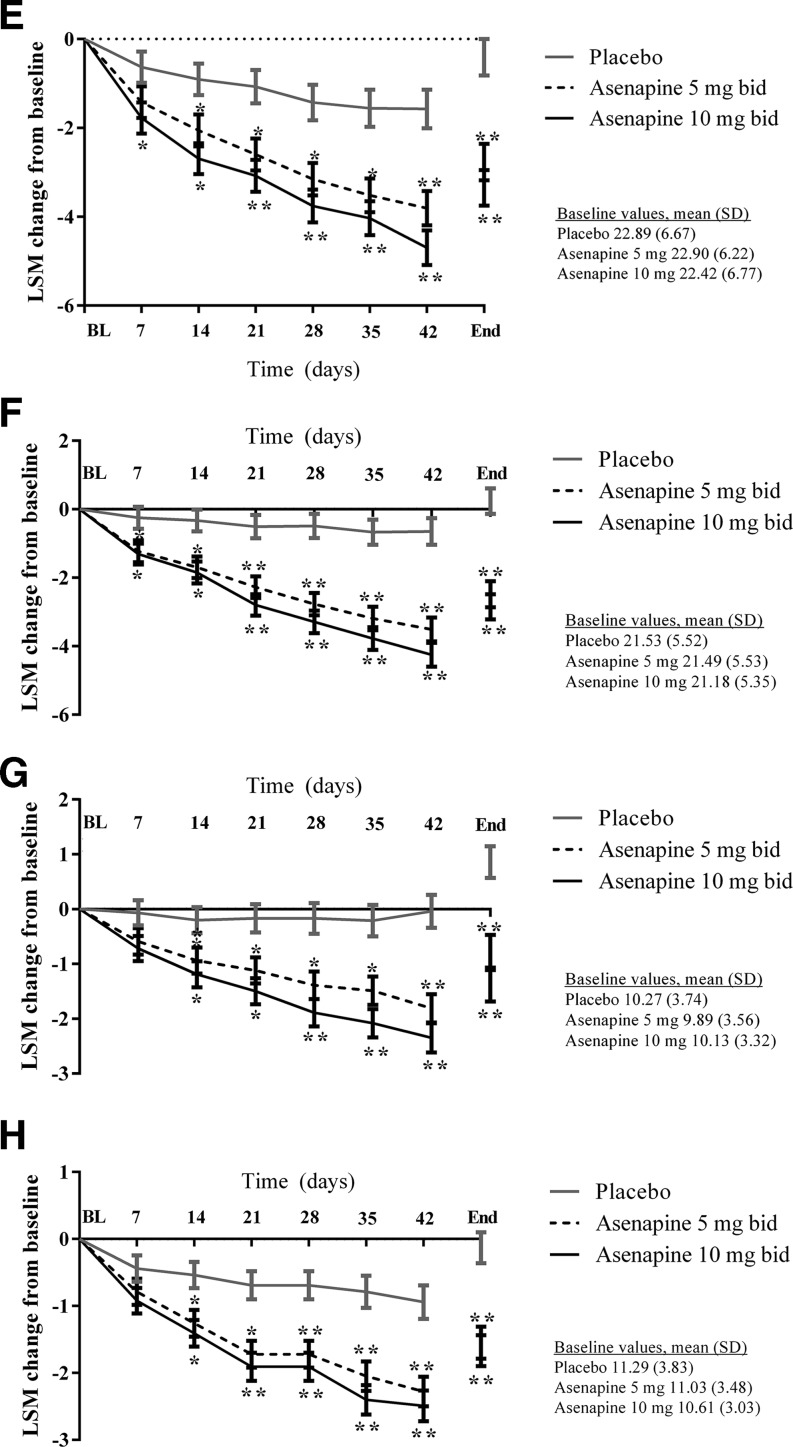
Changes in the PANSS subscale scores and PANSS Marder factor scores supported the results of the primary efficacy outcome analysis (Fig. 3a–h), whereby significantly more participants were classified as PANSS responders (≥30 % decrease in score) at the end of treatment in the asenapine 5 mg bid (p = 0.0001) and 10 mg bid (p < 0.0001) groups compared with the placebo group. This was evident for PANSS positive, negative, and general scores and all PANSS Marder subscores.
Fig. 3.
Secondary efficacy outcomes: changes from baseline in a PANSS positive symptom factor, b PANSS negative symptom factor, c PANSS general psychopathology score, d PANSS Marder positive symptom factor, e PANSS Marder negative symptom factor, f PANSS Marder disorganized thought factor, g PANSS Marder Hostility/Excitement Factor, and h PANSS Marder anxiety/depression factor. BL baseline, End end of treatment, LSM least squares mean, SD standard deviation. *p < 0.05; **p < 0.01 vs. placebo
Clinical global impressions-severity of illness scores and responders
CGI-S scores also supported the results of the primary efficacy outcome analysis. The percentage of CGI-I responders at end of treatment was significantly higher in both asenapine treatment groups than in the placebo group (both p < 0.0001).
Safety and tolerability
There were no differences in the overall incidence of TEAEs across the treatment groups; TEAEs were reported in 81.6 % of participants receiving placebo, 84.6 % of participants receiving asenapine 5 mg bid, and 80.7 % of participants receiving asenapine 10 mg bid. Serious adverse events (SAEs) were reported in 5.7 and 2.8 % of participants in the asenapine 5 mg bid and 10 mg bid groups, and in 7.5 % of those receiving placebo. No SAE occurred in more than one subject, apart from aggravated schizophrenia, which occurred in 4.6 and 2.2 % of participants receiving asenapine 5 mg bid and 10 mg bid, respectively, and 4.6 % of the placebo group. As per withdrawals for lack of efficacy, the incidence of withdrawal due to adverse events (AEs) was lower for those receiving asenapine 5 mg bid (17.1 % of treated participants) or 10 mg bid (17.7 %), compared with those receiving placebo (24.7 %).
Disease exacerbation and extrapyramidal symptoms
TEAEs occurring in ≥5 % of participants in any treatment group are shown in Table 4. The most commonly reported TEAEs for asenapine were aggravated schizophrenia, akathisia, oral hypoesthesia, and somnolence. As shown in Table 4, aggravated schizophrenia occurred in at least 10 % of subjects in each treatment group but was less frequent in the asenapine groups than in the placebo group. In contrast, rates of EPS and akathisia were higher in participants receiving asenapine 5 mg (5.1 and 11.4 %) and 10 mg (7.7 and 10.5 %) bid than in those receiving placebo (1.7 and 5.2 %). The incidence rates of dizziness and increase in blood creatine phosphokinase were higher in the asenapine 10 group, but there were no differences in the other TEAEs between the treatment groups (Table 4).
Table 4.
Incidence of treatment-emergent adverse events occurring in ≥5 % of patients in at least one treatment group (all patients as treated population)
| Adverse event, n (%) | Placebo (n = 174) | Asenapine 5 mg bid (n = 175) | Asenapine 10 mg bid (n = 181) | p Valuea |
|---|---|---|---|---|
| Any adverse event | 142 (81.6) | 148 (84.6) | 146 (80.7) | 0.4018 |
| Aggravated schizophrenia | 49 (28.2) | 23 (13.1) | 28 (15.5) | 0.5488 |
| Hypoaesthesia oral | 6 (3.4) | 19 (10.9) | 17 (9.4) | 0.7261 |
| Akathisia | 9 (5.2) | 20 (11.4) | 19 (10.5) | 0.8657 |
| Extrapyramidal symptoms | 3 (1.7) | 9 (5.1) | 14 (7.7) | 0.3906 |
| Somnolence | 3 (1.7) | 17 (9.7) | 22 (12.2) | 0.5004 |
| Headache | 11 (6.3) | 11 (6.3) | 10 (5.5) | 0.8243 |
| Constipation | 11 (6.3) | 10 (5.7) | 13 (7.2) | 0.6683 |
| Dizziness | 5 (2.9) | 7 (4.0) | 17 (9.4) | 0.0560 |
| Sedation | 2 (1.1) | 4 (2.3) | 9 (5.0) | 0.2588 |
| Vomiting | 6 (3.4) | 8 (4.6) | 9 (5.0) | 1.0000 |
| Blood creatine phosphokinase increased | 4 (2.3) | 3 (1.7) | 12 (6.6) | 0.0318 |
| Insomnia | 21 (12.1) | 17 (9.7) | 14 (7.7) | 0.5748 |
| Nasopharyngitis | 8 (4.6) | 13 (7.4) | 11 (6.1) | 0.6755 |
Comparison between the 5 and 10 mg groups
aFisher’s exact test
Weight
There was a mean (± standard deviation [SD]) change in weight of −1.76 ± 2.45 kg for placebo, +0.42 ± 2.65 kg for asenapine 5 mg bid, and +0.81 ± 2.89 kg for asenapine 10 mg bid. Clinically significant weight gain (≥7 % of baseline body weight) was reported in 4.7 to 7.3 % of participants in the asenapine groups and none in the placebo group. BMI decreased by 0.66 ± 0.91 kg/m2 with placebo and increased by 0.16 ± 1.03 and 0.32 ± 1.09 kg/m2 with asenapine 5 mg bid and 10 mg bid.
Laboratory values and vital signs
There were no clinically significant differences in laboratory values and vital signs between the treatment groups. Mean ± SD changes from baseline in insulin were 2.37 ± 14.08, 2.24 ± 13.78, and 0.22 ± 20.34 μIU/mL with asenapine 5 mg bid and 10 mg bid and placebo, respectively; mean ± SD changes in fasting glucose were −0.03 ± 0.85, 0.11 ± 0.77, and 0.18 ± 0.97 mmol/L; and mean ± SD changes in HbA1c were −0.01 % ± 0.41 %, −0.01 ± 0.49 % and −0.04 ± 0.45 %.
Mean ± SD changes from baseline in prolactin were −17.92 ± 45.31, −13.27 ± 43.93 and −27.79 ± 46.25 μg/L with asenapine 5 mg bid and 10 mg bid and placebo, respectively. The percentage of participants who had normal prolactin levels at baseline but levels above the reference range at the end of treatment (day 42) was higher in those receiving asenapine 5 mg bid (27.9 %) and asenapine 10 mg bid (31.7 %) than in those receiving placebo (10.3 %).
No adverse event related to QT prolongation (SMQ) was observed during the monitoring of the current trial; however, the data are not shown.
Stratified analyses
Given that the study was performed over a number of different sites and countries, the potential for a country-by-treatment interaction was examined, but no significant influences were found (i.e., results were p > 0.05). Furthermore, stratified analyses were performed for sex, disease duration, and schizophrenia subtypes; the results of each indicated that there were no demographic or disease subtype factors that influenced the overall results.
Discussion
In this randomized, double-blind clinical trial, asenapine 5 and 10 mg bid was significantly more effective than placebo in treating acute schizophrenia in Asian patients, as demonstrated by statistically significant improvements from baseline in PANSS total scores. In addition, asenapine 5 and 10 mg bid was effective in controlling both positive and negative symptoms, providing statistically significant improvements from baseline in PANSS positive and negative scores compared with placebo (by 11–13 points), as well as significant improvements in general scores and PANSS Marder 5 factor scores. Moreover, the asenapine treatment groups showed significant improvements versus placebo in other secondary efficacy variables (though it should be noted that these secondary variables were only part of an exploratory analysis); overall the efficacy profiles of the asenapine 5 and 10 mg groups appeared to be similar. Asenapine was safe and well tolerated, with minimal impact on body weight and no notable effects on other metabolic parameters. The incidence of discontinuation because of AEs or lack of efficacy was greater in the placebo group than in the asenapine treatment groups. With the exception of elevated blood creatine phosphokinase and increased dizziness, there were no differences between the 5 and 10 mg groups for TEAEs.
Results of the current study, conducted in Asian countries, complement the robustly positive results of two similarly designed 6-week studies conducted in North America and Europe in patients with an acute exacerbation of schizophrenia (Kane et al. 2010; Potkin et al. 2007). While the study by Kane et al. found significantly greater reductions in mean PANSS total scores, no significant difference from placebo was observed in negative scores, or hostility/excitement subscores (Kane et al. 2010). However, in our study, asenapine significantly reduced the scores on the negative factor and hostility/excitement factor, suggesting that asenapine has efficacy across a broad range of symptoms in schizophrenia. Two other short-term trials have been conducted with asenapine in the 5–10 mg bid dose range: one was negative (active control, but not asenapine, separated from placebo) and one failed (neither asenapine nor active control separated from placebo) (Citrome 2014b; Szegedi et al. 2012). A meta-analysis of pooled data from all four trials found asenapine to be superior to placebo with regard to mean change in PANSS total score (Szegedi et al. 2012). Both asenapine doses were safe and well tolerated, with minimal effects on weight and no notable effects on metabolic parameters, in all studies.
In one of the previous asenapine studies, a high placebo response rate was reported; the authors suggested that this may have contributed to the finding that asenapine 10 mg bid did not have an advantage over placebo in that trial (Kane et al. 2010). Low effect sizes versus placebo are also illustrated in the previously mentioned failed and negative studies, reflecting the finding that placebo response tended to be larger than historically expected (Szegedi et al. 2012). High placebo response rates underscore the need to demonstrate that active treatment can separate from placebo (Kane et al. 2010). In order to exclude initial placebo responders in the present study, oral placebo tablets were administered during the screening period. Restriction of the duration of the current acute exacerbation of schizophrenia to ≤2 months may have contributed to the low placebo response rate and efficacy separation of the asenapine treatment groups from placebo seen in this trial. In the present trial, asenapine 5 mg bid and 10 mg bid were both found to be significantly effective in reducing PANSS scores compared with placebo, which is clearly in line with the previous pooled analysis of placebo-controlled trials of asenapine in acute schizophrenia showing that the two doses had similar efficacy (Friberg et al. 2009).
The results of the present study demonstrate that asenapine is well tolerated, with rates of SAEs and withdrawals due to AEs lower in both active treatment groups compared with placebo. The AE profile of asenapine seen in this study was as expected; reviews of the literature show that asenapine treatment is associated with EPS and akathisia, increases in prolactin versus placebo, oral hypoesthesia, and weight gain (a known class effect) (Citrome 2014b), and the results reported herein confirm that asenapine recipients experience these AEs more frequently than those receiving placebo. However, other active controlled studies suggest that the rates of EPS and akathisia vary depending on the drug, and that asenapine induces these events at a lower rate than some anti-psychotics (Citrome 2014b). Similarly, the effect of asenapine on weight has been reported as modest compared with other anti-psychotics such as olanzapine (Citrome 2014b). In the present study, only modest weight gain was observed in the asenapine group. Asenapine’s unique functional activities at diverse neurotransmitter receptors, including various serotonin receptors, may contribute to the relatively benign effects on body weight (Tarazi and Neill 2013).
Limitations of this study include the absence of an active control arm and ethical questions regarding the use of placebo in patients with acute schizophrenia. Another limitation of this study was the reliance on pill counts to monitor adherence rather than more reliable measures, such as measurement of blood drug levels.
In conclusion, results of this double-blind, placebo-controlled 6-week study showed that asenapine 5 mg bid and 10 mg bid are effective and well tolerated in the treatment of Asian patients with an acute exacerbation of schizophrenia.
Acknowledgments
The authors would like to thank Therese Chapman who provided medical writing assistance on behalf of Springer Healthcare Communications. This assistance was funded by MSD K.K. (Japan) and Meiji.Seika Pharma Co., Ltd.
The authors thank the following investigators who participated in this trial: Hajime Arakaki (Arakaki Hospital, Okinawa, Japan); Masaaki Takahashi (Itoman Seimei Hospital, Okinawa, Japan); Naoki Taira (Amekudai Hospital, Okinawa, Japan); Naohiko Asato (Hirayasu Hospital, Okinawa, Japan); Nobuo Shimizu (Medical Corporation Koshokai Aino Hanazono Hospital, Osaka, Japan); Masashi Takakura (Hotei Hospital, Aichi, Japan); Hitoshi Sato (Goshikidai Hospital, Kagawa, Japan); Yutaka Fujiwara (Takaoka Mental Hospital, Hyogo, Japan); Yasushi Horikiri (Kagoshima Prefectural Aira Hospital, Kagoshima, Japan); Ryuzo Nakatani (Kita Odawara Hospital, Kanagawa, Japan); Kimihiro Nakajima (Goryokai Medical Corporation, Hokkaido, Japan); Tadayuki Hayashishita (Hayashishita Hospital, Hokkaido, Japan); Makoto Nakayama (Nakae Hospital, Hokkaido, Japan); Tadashi Murakami (Sanmaibashi Hospital, Gunma, Japan); Hisashi Tanaka (Tanaka Hospital, Gunma, Japan); Yuichiro Tsutsumi (Ongata Hospital, Tokyo, Japan); Nobutomo Yamamoto, Ataru Inagaki, Hideo Oda (Seiwa Hospital, Neuropsychiatry Research Institute, Tokyo, Japan); Hiroki Nakagawa (Medical Corporation Fukujinkai Hospital, Fukui, Japan); Tomotaka Yoshida (Medical Corporation Seitaikai Mental Support Soyokaze Hospital, Nagano, Japan); Seiichi Sugawara (NHO Higashiowari Hospital, Aichi, Japan); Hajime Ohga (Mikawa Hospital, Aichi, Japan); Takao Maeda (Medical Corporation Issokai Ichinokusa Hospital, Aichi, Japan); Kiyoshi Fujita (Okehazama Hospital Fujita Kokoro Care Center, Aichi, Japan); Takeshi Shimoyama (Matsusaka Kosei Hospital, Mie, Japan); Toshiaki Kachi (Hinaga General Center for Mental Care, Mie, Japan); Hideki Tanaka (Asakayama General Hospital, Osaka, Japan); Kazuhisa Maeda (Medical Welfare Center Kurayoshi Hospital, Tottori, Japan); Masahiro Shono (Yuge Hospital, Kumamoto, Japan); Seiichi Higuchi, Takanori Kubota, Hiroaki Hanada (National Hospital Organization Beppu Medical Center, Oita, Japan); Masatsugu Nagao (Hoyu Hospital, Horoshima, Japan); Hiroshi Yoneda (Osaka Medical College Hospital, Osaka, Japan); Tsuyoshi Miyaoka (Shimane University Hospital, Shimane, Japan); Mahito Kimura (Nippon Medical School Chiba Hokusoh Hospital, Department of Mental Health, Chiba, Japan); Shin Kashiwakura (Ashiribetsu Hospital, Hokkaido, Japan); Teruaki Ikeda (Soen Hospital, Hokkaido, Japan); Takeshi Ashizawa, Takeshi Iwasaki (Asahiyama Hospital, Hokkaido, Japan); Koubun Imai (Center Hospital of the National Center for Global Health and Medicine, Tokyo, Japan); Miyuki Sadamatsu, Motoasa Kou, Jun Kosaka (Nara Medical University, Nara, Japan); Hideki Takeyoshi (Uenohara Hospital, Gunma, Japan); Takashi Inada (Koura Hospital, Hyogo, Japan); Ichiro Kusumi (Hokkaido University Hospital, Hokkaido, Japan); Motohiro Ozone, Norifumi Tsuno (Jikei University School of Medicine Hospital, Tokyo, Japan); Hiroki Tao (Oyachi Hospital, Hokkaido, Japan); Minoru Honda (Honda Memorial Hospital, Hokkaido, Japan); Toshihide Kuroki, Hideyuki Iwanaga (National Hospital Organization Hizen Psychiatric Center, Saga, Japan); Kimihiko Asahi (Asahi Hospital, Tochigi, Japan); Shuzo Hoshino (Takeda General Hospital, Fukushima, Japan); Naohiro Kurotaki (Nagasaki University Hospital, Nagasaki, Japan); Tsuruhei Sukegawa (National Hospital Organization Tottori Medical Center, Tottori, Japan); Mitsumoto Onaya (National Hospital Organization Shimofusa Psychiatric Center, Chiba, Japan); Masanori Ishikawa, Mitsutoshi Okazaki (National Center of Neurology and Psychiatry, Tokyo, Japan); Mitsuhiro Tada (National Hospital Organization Kurihama Medical and Addiction Center, Kanagawa, Japan); Yoshiyuki Takaishi, Hiroyuki Yamaguchi (National Hospital Organization Kamo Psychiatry Medical Center, Horoshima, Japan); Hideaki Ninomiya (Dazaifu Hospital, Fukuoka Prefectural Psychiatric Center, Fukuoka, Japan); Yoko Iwasaki (Medical Corporation Kouseikai Kusatsu Hospital, Hiroshima, Japan); Jun Shiraishi (Hokuriku National Hospital, Toyama, Japan); Hiroyuki Takagi (Seimou Hospital, Gunma, Japan); Koichi Amano (Kakamigahara Hospital, Gifu, Japan); Takao Nishimura, Kenji Narushima (Tokyo Metropolitan Tama Medical Center, Tokyo, Japan); Koji Suzuki (Negishi Hospital, Tokyo, Japan); Hiroaki Shinoda (Shinodanomori Hospital, Chiba, Japan); Buichiro Toyonaga (Iizuka Memorial Hospital, Fukuoka, Japan); Kazuhisa Hayashida, Hisashi Yamada (The Hospital of Hyogo College of Medicine, Hyogo, Japan); Tomonobu Shirasaka (Ishibashi Hospital, Hokkaido, Japan); Yoshiharu Honda (Shichiyama Hospital, Osaka, Japan); Katsuhisa Ando (Kyowa Hospital, Aichi, Japan); Kenji Miyazu (Minamitoyama Nakagawa Hospital, Toyama, Japan); Ryutaro Nakajo (Medical Corporation Shuhokai Hokushin Hospital, Saitama, Japan); Ken Eguchi (Okute Hospital, Gifu, Japan); Yohko Yoshinaga (Hasegawa Hospital, Tokyo, Japan); Kimio Nishiura, Hiroyuki Nishiura (Keihan Hospital Nishiurakai Medical Corporation, Osaka, Japan); Kenji Kuroda (Hannan Hospital, Osaka, Japan); Akihiko Yamagata (Ibaraki Prefectural Medical Center of Pscychiatry, Ibaraki, Japan); Taro Shindo (Rainbow & Sea Hospital, Saga, Japan); Kunio Tamanaha (Riverside Hospital, Oita, Japan); Takashi Kobayashi (Tasaki Hospital, Okinawa, Japan); Kazuhisa Tomita (Saiseikai Kounosu Hospital, Saitama, Japan); Kenshiro Miyamoto (Yatsushiro Kousei Hospital, Kumamoto, Japan); Yoshie Tamura (Shiunkai Yokohama Hospital, Kanakawa, Japan); Masaki Kato (Kansai Medical University Takii Hospital, Osaka, Japan); Young-Chul Chung, Kil Sang Yoon, EunJi Kim, Jin Mahn Jang, In Ho Hwang, Seonguk Yeo, Chul Hyun Park, Nam In Gang (Chonbuk National University Hospital, Jeollabukdo, Korea); Do-Hoon Kim, Yong Nam Kim, Yeo-Jin Hong, So Yeon Kim, Kyu Ho Kim (Chuncheon Sacred Heart Hospital, Gangwon-do, Korea); Kyung Joon Min, Doug Hyun Han, Hyung Woo Park, Woo Hyun Song, Sunmi Kim, Gi Jung Hyun, JiHyuan Park (Chung-ang University Hospital, Seoul, Korea); Jong-Hoon Kim, Seong-Jin Cho, Sunsik Joo, Joong-il Kwon; YeonWoo Lee, SeogJu Kim, Yun mi Kim (Gachon University Gil Medical Center, Incheon, Korea); Chul Eung Kim, Young-seon Moon, HeeWon Lee, Jihoon Ahn, Hee Yun Kim, Young Soo Lee (Inha University Hospital, Incheon, Korea); Do-Un Jung, Joo-Cheol Shim, Min-Kyung Park, Tae-Hong Song, Ji Seop Lim, Hyun Jung Kim (Inje University Busan Paik Hospital, Busan, Korea); Min-Soo Lee, Byung-Joo Ham, Eun-Soo Won, Kyu-Man Han, Hwa Young Lee, Jae Byung Lee, In Kwang Choi, JongHa Lee (Korea University Anam Hospital, Seoul, Korea); Bo-Hyun Yoon, Ji-Seon Lee, Chi-Ho Lee, Jin-hyeong Park, Hye-young Min, Neung-Se Lee, Kyung Min Kim, Seok Hyun Nam, Haran Jung, Hyunsoo Lee, YuRan Jeong, Yun Ki Kim, June Su Ha, Kyung Mi Lee, Hyung Geun Choi, Kam-Doo Kang, Hocheol Shin, Hun Shin (Naju National Hpsital, Jeollanam-do, Korea); Jong Il Lee, Minah Soh, Hyundo Kim, Joon Noh Lee, Hwan Bin Lee (Seoul National Hospital, Seoul, Korea); Won-Myong Bahk, Young Sup Woo, Hoorim Song, Hee Ryung Wang, InWoo Kim, Hyun Jun Kim, Hye Rim Hwang, Ga Young Lee, Mi Jin Yi, Miha Kyoung, Je Hee Han, Hyun Jung Kwak, MinKyu Song, Inhee Shim, Se Yeun Jang, Mina Lee (The Catholic University of Korea Yeouido St. Mary’s Hospital, Seoul, Korea); Seunghee Won, Seung Jae Lee, Do Hoon Kim, Ji Eun Kim, Ji Young Kim, Byunggu Jan, JiWoo Kim, Soljee Kim (Kyungpook National University Hospital, Daegu, Korea); Tae-Kyou Choi, Sang Hyuk Lee, Ki Hwan Yook, Borah Kim (CHA Bundang Medical Center, CHA University, Gyeonggi-do, Korea); Hyun Kim, Dohung Kim, Ram Hwangbo, Yeon Kyung Jung, Jimin Lee, Kang-Joon Lee, Han Seo (Inje University, Ilsan-Park Hospital, Gyeonggi-do, Korea); Sun Woo Lee, SeungMin Cha, Jaeheon Kim, Jaemin Lee, SoHyun Ahn, JinHun Choi (Chungnam National University Hospital, Daejeon, Korea); Sang-Eun Shin, Bo-Ra Kim, Jung-Eun Choi, Woon Jin Jeong, Kyungjin An, Hee Jung Nam, Nam Hee Kim, Hyo Jung Ko, Sang Hyun Koh (Seoul Metropolitan Eunpyeong Hospital, Seoul, Korea); Tak Youn, YounSik Kim, In-Won Chung, NamYoung Lee, Yoong Lee, SanYi Shin, Hyesung Kim, Sangwon Park, (DongGuk University Ilsan Hospital, Gyeonggi-do, Korea); Chang-Jer Tsai, Ding-Lieh Liao, Cheng-Yi Hung, An-Sheng Lin, Chia-Chia Pao (Bali Psychiatric Center, Taipei City, Taiwan); San-Yuan Huang, Yeh-Yi Wei (Tri-Service General Hospital, Taiwan); Nan-Ying Ciu, Wen-Yu Hsu, Mo Geng-han (Changhua Christian Hospital, Taiwan); Tsung-Ming Hu, Fu-Chen Liang, Jen-Yeu Chen, Tzu-Ting Chen, Shaw-Ji Chen, Te Chung (YuLi Veterans Hospital VACRS, Taiwan); Ching-Hua Lin, Cheng-Chung Chen, Hin-Yeung Tsang, Shih-Chi Lin, Chao-Chan Kuo, Mei-Feng Huan (Kaohsiung Kai-Suan Psychiatric Hospital, Taiwan); Chun-Hung Lee, Wen-Chen Ouyang, Chieh-Nan Lin, Kun-Chia Chang, Yu-Chung Lin, Chen-Pang Wang, Ming-Shun Chung (Jianan Mental Hospital, Tainan, Taiwan); Bo-Jian Wu, Chuan-Hsun Yu, Hsun-Yi Liao, Hsing-Kang Chen (YuLi Hospital, Hualian County, Taiwan); Tzu-Ting Chen, Yi-Ting Lin, Li-Ren Chang, Yi-Yung Hung, Meng-Chang Tsai, Chin-Chuen Lin, Taoyuan County, Taiwan).
Compliance with ethical standards
This was a phase III, multicentre, randomized, double-blind, placebo-controlled, parallel-group fixed-dose study conducted in accordance with ICH Guidelines for Good Clinical Practice. The protocol was approved by the appropriate institutional review board or independent ethics committee at each center, and written informed consent was obtained from all patients prior to participating in the trial. The study has been registered with ClinicalTrials.gov (record number: NCT01098110) and was financially supported by MSD K.K.
Conflict of interest
Toshihiko Kinosita has received research support from Otsuka Pharmaceutical, Daiichi Sankyo, Eisai, Shionogi; consultant fees from Otsuka Pharmaceutical, Takeda Pharmaceutical, Meiji Seika Pharma; speaker’s honoraria from Otsuka Pharmaceutical, Janssen Pharmaceutical, Meiji Seika Pharma, Takeda Pharmaceutical, Dainippon Sumitomo Pharma, MSD; and manuscript fees from Otsuka Pharmaceutical. Jong-Hoon Kim received research funds from Merck, Otsuka, and Roche.
Ya-Mei Bai has received speaker’s honorarium from Pfizer, Eli Lilly, AstraZeneca, GlaxoSmithKline, Johnson & Johnson, Otsuka, Sanofi-Aventis, Pfizer, and Astellas Pharmaceuticals.
Mutsuo Miyake and Nobuyuki Oshima have no conflicts to declare.
References
- Abi-Dargham A. Schizophrenia: overview and dopamine dysfunction. J Clin Psychiatry. 2014;75:e31. doi: 10.4088/JCP.13078tx2c. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2000) Diagnostic and Stastical Manual of Mental Disorders: DSM-IV-TR. Washington, DC.
- Citrome L. Asenapine review, part I: chemistry, receptor affinity profile, pharmacokinetics and metabolism. Expert Opin Drug Metab Toxicol. 2014;10:893–903. doi: 10.1517/17425255.2014.908185. [DOI] [PubMed] [Google Scholar]
- Citrome L. Asenapine review, part II: clinical efficacy, safety and tolerability. Expert Opin Drug Saf. 2014;13:803–830. doi: 10.1517/14740338.2014.908183. [DOI] [PubMed] [Google Scholar]
- De Hert M, Yu W, Detraux J, Sweers K, van Winkel R, Correll CU. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs. 2012;26:733–759. doi: 10.2165/11634500-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Merck Sharp & Dohme B.V. (2014) Saphris (asenapine) sublingual tablets. Prescribing information, revised November 2014. Available from: http://pi.actavis.com/data_stream.asp?product_group=1908&p=pi&language=E. Accessed 28 Feb 2015
- Friberg LE, de Greef R, Kerbusch T, Karlsson MO. Modeling and simulation of the time course of asenapine exposure response and dropout patterns in acute schizophrenia. Clin Pharmacol Ther. 2009;86:84–91. doi: 10.1038/clpt.2009.44. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Washington, DC: Department of HEW Publication; 1976. [Google Scholar]
- Inada T (2009) A second-generation rating scale for antipsychotic-induced extrapyramidal symptoms: Drug-induced Extrapyrammidal Symptoms Scale
- Kane JM. Addressing side effects from antipsychotic treatment in schizophrenia. J Clin Psychiatry. 2011;72:e07. doi: 10.4088/JCP.9101tx3c. [DOI] [PubMed] [Google Scholar]
- Kane JM, Cohen M, Zhao J, Alphs L, Panagides J. Efficacy and safety of asenapine in a placebo- and haloperidol-controlled trial in patients with acute exacerbation of schizophrenia. J Clin Psychopharmacol. 2010;30:106–115. doi: 10.1097/JCP.0b013e3181d35d6b. [DOI] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Lublin H, Eberhard J, Levander S. Current therapy issues and unmet clinical needs in the treatment of schizophrenia: a review of the new generation antipsychotics. Int Clin Psychopharmacol. 2005;20:183–198. doi: 10.1097/00004850-200507000-00001. [DOI] [PubMed] [Google Scholar]
- Minassian A, Young J. Evaluation of the clinical efficacy of asenapine in schizophrenia. Expert Opin Pharmacother. 2010;11:2107–2115. doi: 10.1517/14656566.2010.506188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompili M, Serafini G, Innamorati M, Ambrosi E, Telesforo L, Venturini P, Giordano G, Battuello M, Lester D, Girardi P. Unmet treatment needs in schizophrenia patients: is asenapine a potential therapeutic option? Expert Rev Neurother. 2011;11:989–1006. doi: 10.1586/ern.11.82. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Cohen M, Panagides J. Efficacy and tolerability of asenapine in acute schizophrenia: a placebo- and risperidone-controlled trial. J Clin Psychiatry. 2007;68:1492–1500. doi: 10.4088/JCP.v68n1004. [DOI] [PubMed] [Google Scholar]
- Shahid M, Walker GB, Zorn SH, Wong EH. Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol. 2009;23:65–73. doi: 10.1177/0269881107082944. [DOI] [PubMed] [Google Scholar]
- Szegedi A, Verweij P, van Duijnhoven W, Mackle M, Cazorla P, Fennema H. Meta-analyses of the efficacy of asenapine for acute schizophrenia: comparisons with placebo and other antipsychotics. J Clin Psychiatry. 2012;73:1533–1540. doi: 10.4088/JCP.11r07596. [DOI] [PubMed] [Google Scholar]
- Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110:1–23. doi: 10.1016/j.schres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 5. Treatment and prevention. Past, present, and future. Schizophr Res. 2010;122:1–23. doi: 10.1016/j.schres.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Neill JC. The preclinical profile of asenapine: clinical relevance for the treatment of schizophrenia and bipolar mania. Expert Opin Drug Discov. 2013;8:93–103. doi: 10.1517/17460441.2013.738193. [DOI] [PubMed] [Google Scholar]
- van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–645. doi: 10.1016/S0140-6736(09)60995-8. [DOI] [PubMed] [Google Scholar]