Abstract
The fibrosis of liver cirrhosis was considered to be irreversible before the anti-viral drugs showed that it is reversible when they lead to continuous suppression of viral replication and inflammation. However, several reports previously showed that fibrosis of type B liver cirrhosis was almost completely absorbed after the natural remission of chronic inflammation. This phenomenon might not be limited to exceptional patients, but rather occur commonly, considering the dynamic clinical features of chronic hepatitis B (CHB), where inactive carrier stage normally follows aggravation of hepatitis and progression of fibrosis at the time of HBeAg seroconversion. Thus, fibrosis levels of CHB as a hepatocellular carcinoma (HCC)-surveillance marker, particularly those of the inactive stage, could be underestimated, because some of them might have been (pre)cirrhotic in the past and recovered with the natural regression of fibrosis. We argue that cirrhosis-induced HCC mechanisms, rather than direct action of viral genome, may be more common than generally considered in CHB patients. This may have some impact on reconsidering the surveillance rationale for HCC in CHB, from where advanced HCCs tended to be missed. In addition, a molecular marker to assess the cancer-prone characteristics of the liver will definitely be needed to resolve the issue.
Keywords: Chronic hepatitis B, Cirrhosis, Spontaneous remission, Regression of fibrosis, Occult hepatitis B infection, Hepatocellular carcinoma surveillance of hepatitis B virus
Core tip: The fibrosis of liver cirrhosis may be reversible. Regression of fibrosis in hepatitis B virus (HBV) patients with (pre)cirrhosis might be a more common phenomenon than generally considered. This might cause the underestimation of fibrosis levels in chronic hepatitis B, suggesting a difficulty with the surveillance system of HBV-hepatocellular carcinoma (HCC). That is, some HCC patients with non-cirrhotic liver might have been cirrhotic in the past, after which spontaneous regression of fibrosis occurred. Cirrhosis-HCC mechanisms, compared to the direct action of the viral genome, might be more prevalent than generally considered in HBV patients.
INTRODUCTION
Continuous inflammation of the liver induces deposition of extracellular matrix (ECM) and results in liver cirrhosis. Although several reports showed regression of fibrosis in animal models with liver cirrhosis[1,2], and in human case reports[3-5], established fibrosis of cirrhosis was generally considered to be irreversible[6,7]. However, reversibility of fibrosis in the liver became commonly-known recently because elimination of viruses with anti-hepatitis B virus (HBV) or hepatitis C virus (HCV) drugs caused regression of fibrosis even in cirrhotic cases[8,9].
The natural history of chronic HBV infection pursues a dynamic process caused by the conflict between virus and immune system, which results in the seroconversion of HBeAg to anti-HBe, followed by that of HBsAg to anti-HBs[10]. Long-standing liver injury in the process of HBeAg-seroconversion induces particularly rapid progression of fibrosis that frequently results in cirrhosis. However, due to the dynamic course of the disease, stable clinical remission with low HBV DNA and transaminase levels follows, and the majority of patients become inactive carriers. Natural regression of fibrosis induced by this clinical remission has been reported[3,11].
In general, hepatocellular carcinoma (HCC) with HBV arises more frequently in non-cirrhotic liver than in HCV infection[12,13]. This is considered mainly due to the direct mutagenesis effect of the HBV genome that integrates into the host genome or the direct hepatocarcinogenic action of viral genes such as hepatitis B virus X (HBx)[14]. However, if HCCs have arisen in the liver in which fibrosis naturally regressed from advanced liver fibrosis, they might be misinterpreted as having arisen from a non-cirrhotic liver.
Consequently, it should always be kept in mind that fibrosis levels of chronic hepatitis B (CHB) always have the possibility of being underestimated in terms of HCC-risk, because they might have regressed from a more advanced stage of fibrosis[15]. We attempt to spotlight the natural regression of fibrosis in chronic HBV infection and re-evaluate the clinical background of HBV-induced HCC in this review.
CHRONIC HBV DISEASE AND PROGRESSION OF FIBROSIS
About 350 million people in the world have chronic HBV infection[16]. Chronic HBV infection is a significant cause of liver cirrhosis, which bears a high risk of HCC. The annual rate of development of HCC has been reported to be 10% to 17% in HBV-induced liver cirrhosis[17]. In general, fibrosis levels correlate well with the risk of HCC, as in the case with HCV infection.
The immunological attack against hepatocytes infected with HBV induces a dynamic clinical course which is represented by seroconversion of HBeAg to anti-HBe. Almost no or very mild liver injury is observed in the HBeAg-positive immunotolerant phase[18]. The severity of liver injury and the progression of fibrosis depend on how long and severe the immunological attacks continue in the phase of HBeAg seroconversion. A rapid transition to anti-HBe normally results in a silent clinical course, referred to as the inactive carrier state, while a difficult transition that is accompanied by aggravation of chronic hepatitis result in liver cirrhosis or HBeAb-positive chronic active hepatitis, which frequently bears core-promoter mutations. Importantly, the majority of patients become inactive carriers afterwards, irrespective of their clinical course[10,19].
MECHANISM OF HBV-INDUCED HCC AND CLINICAL BACKGROUND CHARACTERISTICS
Chronic inflammation induces a progression of fibrosis resulting in liver cirrhosis in which HCC frequently develops. This cirrhosis-HCC mechanism is common to both HBV and HCV infections and is considered to play the main role in hepatocarcinogenesis, to which a process of accumulation of genetic mutations and epigenetic events contributes. In addition to this common carcinogenetic mechanism, each virus infection has its own carcinogenic mechanism.
On the other hand, HBV, which is related to retroviruses, integrates into the host genome during the process of replication and acts as a mutagen. In addition, the HBx gene, which is a pleiotropic transactivator of many genes, has been known to be directly involved in hepatocarcinogenesis[20]. Because “cirrhosis to HCC” (indirect) and these direct mechanisms are not mutually exclusive, it is unknown how much a role each mechanism plays in real hepatocarcinogenesis, especially in individual HCC patients with variable clinical backgrounds.
DIFFICULTY OF HCC SURVEILLANCE IN CHRONIC HBV PATIENTS
Because the carcinogenic processes of HBV and HCV are different, the rationale to perform effective cancer surveillance should be confirmed based on the difference in the specific clinical courses of these infections. Age, sex, fibrosis levels, the presence of HBeAg, HBV viral load, HBsAg levels accounts for the risks of HBV-HCC[21]. In reports from Japan, HCC patients with HBV tended to be younger and have advanced tumor when diagnosed, as well as lower fibrosis levels with good liver function when compared to those with HCV[22,23]. Our result also showed that patients with HBV were younger, with lower fibrosis levels and higher platelet counts than those with HCV (Table 1). Notably, the clinical stages of HBV-HCC were more advanced. These clinical features suggest that cancer surveillance of HBV is much more difficult than that of HCV infection, where continuous elevation of transaminase values induces gradually increasing fibrosis levels, and a low platelet count may be associated with advanced fibrosis levels[24]. In general, a direct hepatocarcinogenic mechanism of HBV, such as the integration of the genome or a direct role of an HBV gene such as HBx, may be considered to be involved in the occurrence of HCC in these “unexpected” patients. However, our question is “Is this the full explanation?”
Table 1.
A comparison of clinic-pathological data between type-B and type-C hepatocellular carcinoma
| Type B (n = 97) | Type C (n = 81) | P value | |
| Age (yr) | 56.1 ± 11.9 | 66.7 ± 9.8 | < 0.0001 |
| Sex (M:F) | 71.1%:28.9% | 60.5%:39.5% | 0.18 |
| AST (IU/L) | 82.2 ± 98.6 | 81.8 ± 44.1 | 0.18 |
| ALT (IU/L) | 67.7 ± 70.9 | 83.2 ± 56.9 | 0.11 |
| T.Bil (mg/dL) | 1.2 ± 1.1 | 1.0 ± 0.6 | 0.36 |
| Alb (g/dL) | 4.0 ± 0.6 | 3.9 ± 0.5 | 0.22 |
| Plt (/mm3) | 13.9 ± 8.1 | 10.8 ± 5.5 | < 0.005 |
| Histology | |||
| F1:2:3:4 | 2:20:3:32 | 1:6:8:21 | < 0.005 |
| A1:2:3 | 17:25:12 | 1:14:17 | < 0.005 |
| Clinical stage (I:II:III:IV) | 12:29:25:31 | 15:32:29:5 | < 0.0005 |
It seems very paradoxical that HBeAg-positive carriers in the immunotolerant phase with high viral loads and little inflammation, who are supposed have abundant viral gene expression and integration event, seldom suffer from HCC[18]. On the other hand, most HCC patients who were younger than 20 years old were already seroconverted to anti-HBe, and the majority of them were cirrhotic[25-27]. Thus, the cirrhosis to HCC mechanism might play a central role even in these young HCC patients. These facts render the HCC surveillance system for HBV more complex.
REVERSIBILITY OF FIBROSIS WITH LIVER CIRRHOSIS WITH ANTI-HBV TREATMENT AND NATURAL COURSE
The generally accepted idea was that the fibrosis of liver cirrhosis is an irreversible process, and established fibrosis could not regress. However, the development of innovative anti-viral drugs for HBV and HCV changed this long-lasting concept[8]. Fibrosis is reversed when inflammation is suppressed by effective anti-viral agents, and even cirrhosis can be cured. Dienstag et al[28] reported that the fibrosis of cirrhosis was reversed during 3.5 years lamivudine treatment. Several reports also confirmed the improvement of fibrosis levels with anti-HBV drugs[29,30].
However, the regression of fibrosis occurs even in the natural course of CHB, where remission of inflammation usually follows the aggravation of hepatitis and progression of fibrosis that occurred during the HBeAg-positive stage[31]. In the past, several reports showed the improvement of fibrosis during the natural course of CHB. Fong et al[3] showed the improvement of histology comparing before and after the seroclearance of HBsAg. Waneless et al[32] reported that most cirrhotic livers showed some findings indicating the regression of fibrosis, irrespective of etiology. Natural regression of fibrosis consists of three steps, that is, thinning of fibrous septa, regeneration of hepatocytes and recovery of acinal structure. The regression of fibrosis occurs at a particularly early stage of improvement[6].
Recently, Jang et al[33] reported improvement of the long-term prognosis with anti-HBV drugs for patients with HBV-cirrhosis. However, suppression of HCC development was not observed in these cirrhotic patients, while it has been confirmed in patients with chronic hepatitis[8]. These results strongly suggest that liver cirrhosis still provides a strong risk for HCC, even after the eradication of virus and suppression of inflammation.
THE LESSONS FROM THE HCC CASES THAT OCCURRED AFTER HBSAG SEROCLEARANCE
We previously reported several cases with HCC that developed after seroconvesion from HBsAg to anti-HBs[4,34]. They progressed to liver cirrhosis or precirrhosis when they had HBeAg-positive chronic hepatitis several decades earlier and became inactive carriers with low viral load and long-standing normal transaminase levels after the seroconversion to anti-HBe. HCC developed further after the seroconversion to anti-HBs. Hepatic fibrosis was dramatically reversed and found to become only thin septa. Changes in liver histology of one patient[4] are shown in Figure 1. Similarly, Bortolotti et al[5] reported 2 pediatric patients with HBV-induced liver cirrhosis, whose fibrosis was almost completely resolved after normalization of transaminase levels. Similar to our cases, these results suggested that fibrosis of cirrhosis could be dramatically resolved when viral replication and inflammation were suppressed[31,35]. These cases, considering the dynamic natural course of HBV infection, give us some important suggestions about the clinical background characteristics of HBV-induced hepatocarcinogenesis.
Figure 1.
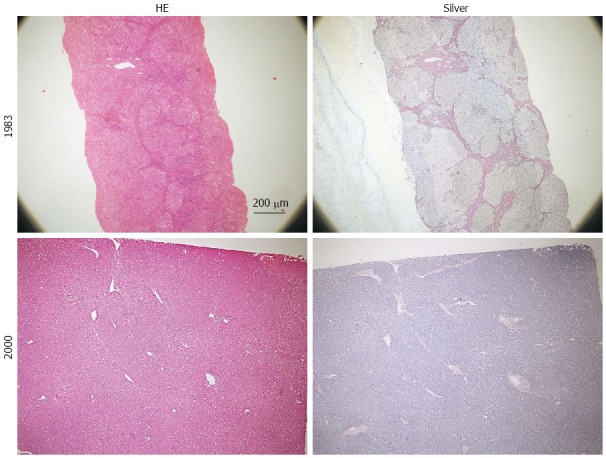
Liver histology of a patient obtained in 1983 (58 year old, needle biopsy) and 2000 (surgical resection of hepatocellular carcinoma). Slides stained with hematoxylin-eosin are shown on the left and those stained with silver are on the right. Marked reduction of fibrosis with improvement of inflammation is observed (This patient was patient 1 in our previous report[4]).
First, as with anti-HBV treatment, these cases are typical examples showing that advanced fibrosis could be absorbed even in the natural course of CHB. After HBeAg-positive chronic hepatitis progresses to liver cirrhosis, the majority of patients follow the clinical course of long-standing remission with a low viral load. Considering this typical clinical course of CHB, natural regression of fibrosis might not be an exceptional phenomenon limited to these case reports, but it might occur commonly. This also suggests that some population of HBV-HCC patients with non-cirrhotic liver might have regressed fibrosis that was once cirrhotic or pre-cirrhotic. Consequently, our hypothesis is that the incidence and role of cirrhosis-derived HCC might be underestimated in HBV-HCC, and the roles of direct carcinogenesis due to HBV integration or viral gene function with a slight involvement of necroinflammation in non-cirrhotic HCC are overestimated.
HBV-cirrhosis is “macronodular” and has thinner septa than HCV[32]. Micronodular cirrhosis changes to macronodular with the suppression of inflammation[36]. This morphological observation might reflect the clinical course of these viruses, where HCV takes a continuous progressive course, and HBV is characterized by the alternating increases and normalization of transaminase levels.
Another important suggestion relates to the assessment of fibrosis in CHB patients for the establishment of an effective HCC surveillance system. Recently, not only liver biopsy which is a gold standard for the evaluation of fibrosis, but a handful of methods to assess fibrosis levels have been used clinically. However, these methods may not discriminate these cases of “regressed fibrosis from cirrhosis”, which might have more risk of HCC than “ongoing fibrosis”. Thus, it might be possible to underestimate the risk of HCC in these patients with regressed fibrosis levels using these current methods. Indeed, needle biopsy might give the diagnosis of an almost normal liver with completely regressed fibrosis[32].
Cirrhosis-HCC mechanisms can be glimpsed in studies that examined the incidence of HCC after the seroclearance of HBsAg, which normally has a good prognosis[10,37,38]. Yuen et al[39] reported that the risk factors of HCC at this stage were the presence of liver cirrhosis and old age. Liver cirrhosis was also counted as a significant factor for the seroclearance of HBsAg[40]. This indicates that the strong immune reaction that caused liver cirrhosis eventually contributed to the seroclearance of HBsAg, providing some risk of HCC.
Similar suggestions could be applied to HCC cases with occult HBV infection (OHB). Some patients might have overt chronic HBV infection before entering the stage of OHB[41]. These patients might have “regressed fibrosis from advanced fibrosis” during the process of becoming seronegative for HBsAg. They can easily be missed during clinical surveillance of HCC, unless they were recognized as HBV carriers, possibly in early life[15]. Integration of HBV DNA or the direct action of an HBV gene such as HBx from long-standing viral replication is considered to play a role in the occurrence of HCC with OHB[42]. However, cirrhosis-HCC mechanism might play a central role in more number of patients with OHB-HCC than generally considered.
Liver biopsy has been a gold standard of evaluation for liver fibrosis[43]. Non-invasive methods for evaluation of fibrosis are widely used in clinical settings. They have been primarily used for the assessment of HCV-related fibrosis, and their application to HBV-induced fibrosis has always been under constant debate[44]. Transient elastography (TE) is one of the most reliable recent modalities to assess the fibrosis level without biopsy[45]. Several reports evaluated the efficacy of TE in both CHB and CHC patients and reported that diagnostic accuracy was almost equal between them[46,47]. However, a lower liver stiffness (LS) cut-off in CHB than in CHC in the advanced fibrosis stage was reported[48]. The interpretation of this was that fibrous septa were thinner in CHB than in CHC. Moreover, because CHB tended to have macronodular cirrhosis, TE waves go through liver parenchyma and produce a low stiffness value[49]. Likewise, Castéra et al[50] reported that the cut off values of TE in cirrhosis were higher in HCV-LC than that of HBV-LC and this difference came from the high prevalence of macronodular LC in HBV. Thus, although fibrosis markers are considered to be useful in the evaluation of fibrosis levels of CHB in general[49], there have been no studies attempting to assess the fibrosis levels following CHB patients from aggravation of hepatitis to the inactive stage.
APPLICATION OF MOLECULAR ANALYSIS TO THE “HCC FROM REGRESSED FIBROSIS”
Innovative advances of molecular analysis recently allow comprehensive cancer genome analysis in a short time, resulting in the accumulation of an overwhelming body of information about the cancer genome[51]. Schulze et al[52] analyzed the whole exon of 243 HCC tissues and reported that genomic pathways centered on three signaling abnormalities, β-catenin (CTNNB1), TP53, and AXIN1-related. They also suggested that they can distinguish HCC that arose from cirrhosis from those derived from non-cirrhosis using the difference in incidence of CTNNB1 TP53, a mutation of telomerase reverse transcriptase (TERT). In addition, mutations of TERT promoter have been noted as a characteristic genomic change of HBV-HCC, in which integration of the HBV genome frequently occurs[53,54]. It is interesting to know whether HCC that arose from “liver with regressed fibrosis from cirrhosis” is close to a cirrhotic or a non-cirrhotic pattern.
Telomere shortening is a general genetic marker of liver cirrhosis causing senescence of hepatocytes that is associated with the development of HCC[55-57]. Recently, TERT promotor mutations were observed already in premalignant nodules in cirrhotic liver[58]. In addition, Hartmann et al[59] reported that TERT gene mutations were frequently detected in liver cirrhosis irrespective of etiology, suggesting that they may be used to evaluate cancer-prone characteristics of liver. This result might be applicable in the evaluation of whether the liver with regressed fibrosis has oncogenic potential close to that of real cirrhosis, helping the understandings of the carcinogenic potential of that state. Such molecular markers that can assess cancer-prone characteristics of cirrhotic or non-cirrhotic liver are much needed to prove that a cirrhosis-HCC mechanism might be involved in the development of HCC than currently considered, even in low-fibrosis cases.
CONCLUSION
The clinical course of chronic HBV infection is dynamic and complex, with changes of serological markers, which is distinct from HCV infection that takes a more continuous clinical course. Because of this clinical profile, long-standing remission after established cirrhosis, result in the regression of fibrosis, where only thin septa are recognized. On the other hand, the integration of the HBV genome or a direct gene function, such as HBx, may play an important role in so-called direct hepatocarcinogenesis. HCCs from regressed fibrosis and those via direct hepatocarcinogenesis might be categorized together into “HCC from non-cirrhotic liver”. Thus, these mechanisms render the assessment of fibrosis as a risk factor for HCC very complex. Therefore, there is an urgent need to establish effective cancer surveillance system that addresses these complex issues. In addition, molecular markers to evaluate the risk of HCC in non-cirrhotic as well as cirrhotic liver should be clarified in a future analysis.
Footnotes
Supported by A Grant-in-Aid for Scientific Research (C) (25461012 to Ohkoshi S) from the Japan Society for the Promotion of Science.
Conflict-of-interest statement: The authors do not have any commercial affiliation or consultancy that could be construed as a conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 7, 2016
First decision: April 14, 2016
Article in press: May 4, 2016
P- Reviewer: Sunbul M S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
References
- 1.Iredale JP, Thompson A, Henderson NC. Extracellular matrix degradation in liver fibrosis: Biochemistry and regulation. Biochim Biophys Acta. 2013;1832:876–883. doi: 10.1016/j.bbadis.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Issa R, Zhou X, Constandinou CM, Fallowfield J, Millward-Sadler H, Gaca MD, Sands E, Suliman I, Trim N, Knorr A, et al. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2004;126:1795–1808. doi: 10.1053/j.gastro.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 3.Fong TL, Di Bisceglie AM, Gerber MA, Waggoner JG, Hoofnagle JH. Persistence of hepatitis B virus DNA in the liver after loss of HBsAg in chronic hepatitis B. Hepatology. 1993;18:1313–1318. [PubMed] [Google Scholar]
- 4.Okoshi S, Igarashi M, Suda T, Iwamatsu H, Watanabe K, Ishihara K, Ogata N, Nomoto M, Takahashi T, Ichida T, et al. Remote development of hepatocellular carcinoma in patients with liver cirrhosis type B serologically cured for HBs antigenemia with long-standing normalization of ALT values. Dig Dis Sci. 2002;47:2002–2006. doi: 10.1023/a:1019608509402. [DOI] [PubMed] [Google Scholar]
- 5.Bortolotti F, Guido M, Cadrobbi P, Crivellaro C, Bartolacci S, Rugge M, Gatta A. Spontaneous regression of hepatitis B virus-associated cirrhosis developed in childhood. Dig Liver Dis. 2005;37:964–967. doi: 10.1016/j.dld.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Bedossa P. Reversibility of hepatitis B virus cirrhosis after therapy: who and why? Liver Int. 2015;35 Suppl 1:78–81. doi: 10.1111/liv.12710. [DOI] [PubMed] [Google Scholar]
- 7.Calvaruso V, Craxì A. Regression of fibrosis after HBV antiviral therapy. Is cirrhosis reversible? Liver Int. 2014;34 Suppl 1:85–90. doi: 10.1111/liv.12395. [DOI] [PubMed] [Google Scholar]
- 8.Liaw YF. Impact of therapy on the outcome of chronic hepatitis B. Liver Int. 2013;33 Suppl 1:111–115. doi: 10.1111/liv.12057. [DOI] [PubMed] [Google Scholar]
- 9.Shiratori Y, Imazeki F, Moriyama M, Yano M, Arakawa Y, Yokosuka O, Kuroki T, Nishiguchi S, Sata M, Yamada G, et al. Histologic improvement of fibrosis in patients with hepatitis C who have sustained response to interferon therapy. Ann Intern Med. 2000;132:517–524. doi: 10.7326/0003-4819-132-7-200004040-00002. [DOI] [PubMed] [Google Scholar]
- 10.McMahon BJ. The natural history of chronic hepatitis B virus infection. Hepatology. 2009;49:S45–S55. doi: 10.1002/hep.22898. [DOI] [PubMed] [Google Scholar]
- 11.Sharma SK, Saini N, Chwla Y. Hepatitis B virus: inactive carriers. Virol J. 2005;2:82. doi: 10.1186/1743-422X-2-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lok AS. Hepatitis B: liver fibrosis and hepatocellular carcinoma. Gastroenterol Clin Biol. 2009;33:911–915. doi: 10.1016/j.gcb.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Bralet MP, Régimbeau JM, Pineau P, Dubois S, Loas G, Degos F, Valla D, Belghiti J, Degott C, Terris B. Hepatocellular carcinoma occurring in nonfibrotic liver: epidemiologic and histopathologic analysis of 80 French cases. Hepatology. 2000;32:200–204. doi: 10.1053/jhep.2000.9033. [DOI] [PubMed] [Google Scholar]
- 14.Neuveut C, Wei Y, Buendia MA. Mechanisms of HBV-related hepatocarcinogenesis. J Hepatol. 2010;52:594–604. doi: 10.1016/j.jhep.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 15.Bortolotti F, Guido M, Bartolacci S, Cadrobbi P, Crivellaro C, Noventa F, Morsica G, Moriondo M, Gatta A. Chronic hepatitis B in children after e antigen seroclearance: final report of a 29-year longitudinal study. Hepatology. 2006;43:556–562. doi: 10.1002/hep.21077. [DOI] [PubMed] [Google Scholar]
- 16.Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053–2063. doi: 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- 17.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–352. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Hui CK, Leung N, Yuen ST, Zhang HY, Leung KW, Lu L, Cheung SK, Wong WM, Lau GK. Natural history and disease progression in Chinese chronic hepatitis B patients in immune-tolerant phase. Hepatology. 2007;46:395–401. doi: 10.1002/hep.21724. [DOI] [PubMed] [Google Scholar]
- 19.Hsu YS, Chien RN, Yeh CT, Sheen IS, Chiou HY, Chu CM, Liaw YF. Long-term outcome after spontaneous HBeAg seroconversion in patients with chronic hepatitis B. Hepatology. 2002;35:1522–1527. doi: 10.1053/jhep.2002.33638. [DOI] [PubMed] [Google Scholar]
- 20.Feitelson MA, Lee J. Hepatitis B virus integration, fragile sites, and hepatocarcinogenesis. Cancer Lett. 2007;252:157–170. doi: 10.1016/j.canlet.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Wong VW, Janssen HL. Can we use HCC risk scores to individualize surveillance in chronic hepatitis B infection? J Hepatol. 2015;63:722–732. doi: 10.1016/j.jhep.2015.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa T. Clinical features of hepatitis B virus-related hepatocellular carcinoma. World J Gastroenterol. 2010;16:2463–2467. doi: 10.3748/wjg.v16.i20.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanizaki H, Ryu M, Kinoshita T, Kawano N, Konishi M, Cho A, Nakatsura T, Natsume T, Takahashi S, Sugita M, et al. Comparison of clinical features and survival in patients with hepatitis B and C virus-related hepatocellular carcinoma. Jpn J Clin Oncol. 1997;27:67–70. doi: 10.1093/jjco/27.2.67. [DOI] [PubMed] [Google Scholar]
- 24.Shiratori Y, Omata M. Predictors of the efficacy of interferon therapy for patients with chronic hepatitis C before and during therapy: how does this modify the treatment course? J Gastroenterol Hepatol. 2000;15 Suppl:E141–E151. doi: 10.1046/j.1440-1746.2000.02116.x. [DOI] [PubMed] [Google Scholar]
- 25.Komatsu H, Inui A. Hepatitis B virus infection in children. Expert Rev Anti Infect Ther. 2015;13:427–450. doi: 10.1586/14787210.2015.1019867. [DOI] [PubMed] [Google Scholar]
- 26.Tazawa Y, Nishinomiya F, Noguchi H, Tsuchiya S, Hayashi Y, Abukawa D, Watabe M, Nakagawa M, Imaizumi M, Ohi R. Hepatocellular carcinoma in children with hepatitis B surface antigen. Tohoku J Exp Med. 1992;167:47–55. doi: 10.1620/tjem.167.47. [DOI] [PubMed] [Google Scholar]
- 27.Zhang XF, Liu XM, Wei T, Liu C, Li MX, Long ZD, Lv Y. Clinical characteristics and outcome of hepatocellular carcinoma in children and adolescents. Pediatr Surg Int. 2013;29:763–770. doi: 10.1007/s00383-013-3334-4. [DOI] [PubMed] [Google Scholar]
- 28.Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, Gray DF, Schiff ER. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105–117. doi: 10.1053/gast.2003.50013. [DOI] [PubMed] [Google Scholar]
- 29.Chang TT, Liaw YF, Wu SS, Schiff E, Han KH, Lai CL, Safadi R, Lee SS, Halota W, Goodman Z, et al. Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology. 2010;52:886–893. doi: 10.1002/hep.23785. [DOI] [PubMed] [Google Scholar]
- 30.Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R, et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468–475. doi: 10.1016/S0140-6736(12)61425-1. [DOI] [PubMed] [Google Scholar]
- 31.Shah U, Kelly D, Chang MH, Fujisawa T, Heller S, González-Peralta RP, Jara P, Mieli-Vergani G, Mohan N, Murray KF. Management of chronic hepatitis B in children. J Pediatr Gastroenterol Nutr. 2009;48:399–404. doi: 10.1097/MPG.0b013e318197196e. [DOI] [PubMed] [Google Scholar]
- 32.Wanless IR, Nakashima E, Sherman M. Regression of human cirrhosis. Morphologic features and the genesis of incomplete septal cirrhosis. Arch Pathol Lab Med. 2000;124:1599–1607. doi: 10.5858/2000-124-1599-ROHC. [DOI] [PubMed] [Google Scholar]
- 33.Jang JW, Choi JY, Kim YS, Woo HY, Choi SK, Lee CH, Kim TY, Sohn JH, Tak WY, Han KH. Long-term effect of antiviral therapy on disease course after decompensation in patients with hepatitis B virus-related cirrhosis. Hepatology. 2015;61:1809–1820. doi: 10.1002/hep.27723. [DOI] [PubMed] [Google Scholar]
- 34.Tsuboi Y, Ohkoshi S, Yano M, Suzuki K, Tsubata SS, Ishihara K, Ichida T, Sugitani S, Shibazaki K, Aoyagi Y. Common clinicopathological features of the patients with chronic hepatitis B virus infection who developed hepatocellular carcinoma after seroconversion to anti-HBs--a consideration of the pathogenesis of HBV-induced hepatocellular carcinoma and a strategy to inhibit it. Hepatogastroenterology. 2006;53:110–114. [PubMed] [Google Scholar]
- 35.Bortolotti F, Guido M. Reversal of liver cirrhosis: a desirable clinical outcome and its pathogenic background. J Pediatr Gastroenterol Nutr. 2007;44:401–406. doi: 10.1097/MPG.0b013e318032069a. [DOI] [PubMed] [Google Scholar]
- 36.Fauerholdt L, Schlichting P, Christensen E, Poulsen H, Tygstrup N, Juhl E. Conversion of micronodular cirrhosis into macronodular cirrhosis. Hepatology. 1983;3:928–931. doi: 10.1002/hep.1840030607. [DOI] [PubMed] [Google Scholar]
- 37.Liaw YF. Natural history of chronic hepatitis B virus infection and long-term outcome under treatment. Liver Int. 2009;29 Suppl 1:100–107. doi: 10.1111/j.1478-3231.2008.01941.x. [DOI] [PubMed] [Google Scholar]
- 38.Liu J, Yang HI, Lee MH, Lu SN, Jen CL, Wang LY, You SL, Iloeje UH, Chen CJ. Incidence and determinants of spontaneous hepatitis B surface antigen seroclearance: a community-based follow-up study. Gastroenterology. 2010;139:474–482. doi: 10.1053/j.gastro.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 39.Yuen MF, Wong DK, Sablon E, Tse E, Ng IO, Yuan HJ, Siu CW, Sander TJ, Bourne EJ, Hall JG, et al. HBsAg seroclearance in chronic hepatitis B in the Chinese: virological, histological, and clinical aspects. Hepatology. 2004;39:1694–1701. doi: 10.1002/hep.20240. [DOI] [PubMed] [Google Scholar]
- 40.Liaw YF, Sheen IS, Chen TJ, Chu CM, Pao CC. Incidence, determinants and significance of delayed clearance of serum HBsAg in chronic hepatitis B virus infection: a prospective study. Hepatology. 1991;13:627–631. [PubMed] [Google Scholar]
- 41.Huang Y, Wang W, Chen Y, Huang Y, Zhang J, He S, Tan Y, Qiang F, Li A, Røe OD, et al. The opposite prognostic significance of nuclear and cytoplasmic p21 expression in resectable gastric cancer patients. J Gastroenterol. 2014;49:1441–1452. doi: 10.1007/s00535-013-0900-4. [DOI] [PubMed] [Google Scholar]
- 42.Pollicino T, Saitta C. Occult hepatitis B virus and hepatocellular carcinoma. World J Gastroenterol. 2014;20:5951–5961. doi: 10.3748/wjg.v20.i20.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495–500. doi: 10.1056/NEJM200102153440706. [DOI] [PubMed] [Google Scholar]
- 44.Xu XY, Kong H, Song RX, Zhai YH, Wu XF, Ai WS, Liu HB. The effectiveness of noninvasive biomarkers to predict hepatitis B-related significant fibrosis and cirrhosis: a systematic review and meta-analysis of diagnostic test accuracy. PLoS One. 2014;9:e100182. doi: 10.1371/journal.pone.0100182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fraquelli M, Rigamonti C, Casazza G, Conte D, Donato MF, Ronchi G, Colombo M. Reproducibility of transient elastography in the evaluation of liver fibrosis in patients with chronic liver disease. Gut. 2007;56:968–973. doi: 10.1136/gut.2006.111302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Afdhal NH, Bacon BR, Patel K, Lawitz EJ, Gordon SC, Nelson DR, Challies TL, Nasser I, Garg J, Wei LJ, et al. Accuracy of fibroscan, compared with histology, in analysis of liver fibrosis in patients with hepatitis B or C: a United States multicenter study. Clin Gastroenterol Hepatol. 2015;13:772–9.e1-772-9.e3. doi: 10.1016/j.cgh.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 47.Cardoso AC, Carvalho-Filho RJ, Stern C, Dipumpo A, Giuily N, Ripault MP, Asselah T, Boyer N, Lada O, Castelnau C, et al. Direct comparison of diagnostic performance of transient elastography in patients with chronic hepatitis B and chronic hepatitis C. Liver Int. 2012;32:612–621. doi: 10.1111/j.1478-3231.2011.02660.x. [DOI] [PubMed] [Google Scholar]
- 48.Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54:650–659. doi: 10.1016/j.jhep.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 49.Branchi F, Conti CB, Baccarin A, Lampertico P, Conte D, Fraquelli M. Non-invasive assessment of liver fibrosis in chronic hepatitis B. World J Gastroenterol. 2014;20:14568–14580. doi: 10.3748/wjg.v20.i40.14568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Castéra L, Bernard PH, Le Bail B, Foucher J, Trimoulet P, Merrouche W, Couzigou P, de Lédinghen V. Transient elastography and biomarkers for liver fibrosis assessment and follow-up of inactive hepatitis B carriers. Aliment Pharmacol Ther. 2011;33:455–465. doi: 10.1111/j.1365-2036.2010.04547.x. [DOI] [PubMed] [Google Scholar]
- 51.Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic Landscape and Biomarkers of Hepatocellular Carcinoma. Gastroenterology. 2015;149:1226–1239.e4. doi: 10.1053/j.gastro.2015.05.061. [DOI] [PubMed] [Google Scholar]
- 52.Schulze K, Imbeaud S, Letouzé E, Alexandrov LB, Calderaro J, Rebouissou S, Couchy G, Meiller C, Shinde J, Soysouvanh F, et al. Exome sequencing of hepatocellular carcinomas identifies new mutational signatures and potential therapeutic targets. Nat Genet. 2015;47:505–511. doi: 10.1038/ng.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buendia MA, Neuveut C. Hepatocellular carcinoma. Cold Spring Harb Perspect Med. 2015;5:a021444. doi: 10.1101/cshperspect.a021444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sung WK, Zheng H, Li S, Chen R, Liu X, Li Y, Lee NP, Lee WH, Ariyaratne PN, Tennakoon C, et al. Genome-wide survey of recurrent HBV integration in hepatocellular carcinoma. Nat Genet. 2012;44:765–769. doi: 10.1038/ng.2295. [DOI] [PubMed] [Google Scholar]
- 55.Kojima H, Yokosuka O, Imazeki F, Saisho H, Omata M. Telomerase activity and telomere length in hepatocellular carcinoma and chronic liver disease. Gastroenterology. 1997;112:493–500. doi: 10.1053/gast.1997.v112.pm9024303. [DOI] [PubMed] [Google Scholar]
- 56.Suda T, Isokawa O, Aoyagi Y, Nomoto M, Tsukada K, Shimizu T, Suzuki Y, Naito A, Igarashi H, Yanagi M, et al. Quantitation of telomerase activity in hepatocellular carcinoma: a possible aid for a prediction of recurrent diseases in the remnant liver. Hepatology. 1998;27:402–406. doi: 10.1002/hep.510270213. [DOI] [PubMed] [Google Scholar]
- 57.Wiemann SU, Satyanarayana A, Tsahuridu M, Tillmann HL, Zender L, Klempnauer J, Flemming P, Franco S, Blasco MA, Manns MP, et al. Hepatocyte telomere shortening and senescence are general markers of human liver cirrhosis. FASEB J. 2002;16:935–942. doi: 10.1096/fj.01-0977com. [DOI] [PubMed] [Google Scholar]
- 58.Nault JC, Calderaro J, Di Tommaso L, Balabaud C, Zafrani ES, Bioulac-Sage P, Roncalli M, Zucman-Rossi J. Telomerase reverse transcriptase promoter mutation is an early somatic genetic alteration in the transformation of premalignant nodules in hepatocellular carcinoma on cirrhosis. Hepatology. 2014;60:1983–1992. doi: 10.1002/hep.27372. [DOI] [PubMed] [Google Scholar]
- 59.Hartmann D, Srivastava U, Thaler M, Kleinhans KN, N’kontchou G, Scheffold A, Bauer K, Kratzer RF, Kloos N, Katz SF, et al. Telomerase gene mutations are associated with cirrhosis formation. Hepatology. 2011;53:1608–1617. doi: 10.1002/hep.24217. [DOI] [PubMed] [Google Scholar]


