Abstract
Much has been written about hepatic metastasis and animal models abound. In terms of the human experience, progress in treating this final common pathway, a terminal event of many human malignancies has been relatively slow. The current thinking is that primary prevention is best served by early detection of cancer and eradication of early stage cancers by screening. Some cancers spread early in their course and the role of screening may be limited. Until relatively recently there has not been a pathfinder model that makes the evasion of this unfortunate event a reality. This review discusses such an animal model and attempts to relate it to human disease in terms of intervention. Concrete proposals are also offered on how scientists may be able to intervene to prevent this deadly progression of the cancer process.
Keywords: Cotton top tamarin, Hepatic metastasis, Carcinoembryonic antigen, Fibulin-5, Common marmoset
Core tip: Hepatic metastasis is a terminal event. Avoiding this complication would prolong life and current understanding of inflammatory mediators allows possible secondary intervention. The cotton top tamarin (CTT), like humans develops inflammatory bowel disease complicated by colorectal cancer but avoids liver metastasis. We suggest 5 mechanisms by which CTT avoid liver spread. They involve changes in ICAMs and their receptors, carcinoembryonic antigen (CEA) family mediators of angiogenesis, post translational modifications of molecules like CEA, and increased expression of anti-proliferative agents such as fibulins. This changes our perception from “monkey see, monkey do” to “see what the monkeys do and do the same”. Possible avenues of intervention are suggested.
INTRODUCTION
There are many animal models for human cancer and most of these do not occur naturally but are designed to mimic human disease by introduction of mutagen and may also employ promoters. Natural models lack this human “design” element but historically the differences may enhance understanding of disease causality and management for example, the Roux Sarcoma virus in the Plymouth Barred Rock fowl (chickens) and Bittner milk factor in murine mammary carcinoma among others. It is however unusual for a single animal to provide more than 1 natural model for disease[1].
Enter the cotton top tamarin (CTT), a pint-sized lower order, New World primate of the order Callitrichidae[2]. This remarkable monkey provides a model for human disease in inflammatory bowel disease and potential mechanisms[3,4], cancer of the lymphatic system[5], large bowel cancer[6], and immune-altered states[7], and recently liver metastases[8]. While a review of these models apart from the latter is beyond the scope of this review, it is significant that the example of the liver metastasis model could best be described as a “negative” or “avoidance model” in that the CTT with colon cancer avoids liver metastasis with an estimated frequency of 97.2%.
The hypotheses of how the CTT achieves this are open for discussion and debate has been ongoing since the first symposium, reported in 1985[9] where anatomical blood vessel variance was advanced as an explanation. This was refuted by veterinarians since the anatomy is similar to most mammals including humans (N. Clapp- personal communication). Our group has been exploring hypotheses based on adhesion molecules known to be important in human liver metastasis. While the article will focus on these molecular families in tamarins, it is important to first discuss our understanding of their importance in human “metastogenesis” as the findings in the CTT may enable intervention in the human where over 70% of GI cancer victims succumb to death caused by hepatic metastases.
Hypotheses of hepatic metastases in humans
Cancers of the colon and rectum comprise 14.5% of all cancers diagnosed in the United States (149880) and 11.1% of cancer deaths (51710) each year[10]. The primary treatment for large bowel cancer is surgery. Patients who recur do so largely in the liver, lung or peritoneum. While combination chemotherapy can increase survival even in advanced disease it is rarely long term. Metastatic colorectal cancer is therefore a major public health problem. Colorectal cancer is associated with chronic inflammation[11-13]. Inflammatory cytokines can be induced by interaction of terminally differentiated macrophages with the cancer associated glycoprotein carcinoembryonic antigen (CEA)[14]. CEA (CEACAM5) is a large heavily glycosylated glycoprotein of indeterminate function in the normal tissue in which it is expressed[15,16]. Discovered in 1965 by Gold and Freedman[17] it has become the most used tumor marker for colorectal cancers but failed to live up to the early expectations for use as an early detector of cancer[15,18]. It is a glycoprotein that is a member of a large gene family that consists of 29 genes divided into three subgroups that include the CEA-like glycoproteins and the pregnancy-specific glycoproteins (PSGs)[19]. These CEA like proteins are members of the much larger immunoglobulin supergene family[15]. The nomenclature for the entire CEA and PSG families is published in Beauchemin et al[20].
CEA
CEA is a GPI anchored glycoprotein with an average MW of 180 kDa this varies depending on the degree of glycosylation of the native protein. CEA contains 651 amino acids and these are found in 7 immunoglobulin like domains, an IgV domain at the N-terminus and six disulfide bridge linked IgC domains[15,16]. There are 29 possible sites for N-linked glycosylation and they tend to be of the complex tetra antennary type[15]. Recently a large number of functions in cancer cells have been attributed to CEA. These include a role in cell-cell adhesion[21,22], apoptosis (anoikis)[23], inflammatory responses leading to increased hepatic and possibly lung metastasis[14] and angiogenesis[24].
CEA AND ITS IMPACT ON LIVER METASTASIS: INDUCTION OF INFLAMMATORY RESPONSES IN THE LIVER
Liver metastasis is the major cause of death in patients with colorectal cancers. There is good evidence that cytokines play a major role in preparing the liver for implantation and subsequent growth of cancer cells[14]. It appears that a relationship exists between the colon cancer derived glycoprotein CEA and macrophage related cytokine production particularly associated with CEA Kupffer cell interactions in metastasis of colorectal cancers to the liver. The discovery of CEA in 1965[17] marked a turning point in the study of cancer and led what might be called the age of tumor markers. CEA was the first commercially available tumor marker and may be considered the prototype[25]. Serum elevations of CEA (above 3 ng/mL) are seen in about 60% of colorectal cancer patients at presentation[26] and elevated levels are also seen in a number of other cancers including breast, gastric, lung and pancreas. Unfortunately, the early premise that CEA levels could be used as a screening test for colorectal cancer was not fulfilled. CEA was found not to be cancer specific and high false positive and false negative rates precluded its use in early detection of cancer. However, CEA is a useful marker in the determination of prognosis[27] and for detection of recurrence in the post-surgical follow up of colorectal cancer patients[28]. CEA has also been used for the detection of occult tumor by radioimmuno-detection[29] and as a target for treatment using radioimmuno-therapy[30]. In addition a number of studies have used CEA as the target antigen for immunotherapy[31]. Clinically CEA is very well understood, but its biological role is not clear and its function in the normal colonocyte still evades us. However over the years a number of functions mostly associated with tumor cell behavior have been attributed to CEA. We focused on the molecular interactions between CEA and its receptor CEAR in colorectal cancer cells and how disruption of these interactions and inhibition of cytokine production will affect the spread and growth of colorectal cancers. Designing an effective mimetic-based therapy requires the identification of the responsible molecules, mechanisms of action and regulation.
The most important functions of CEA are the effects associated with tumor cell survival whether it is at the primary site or at a distant metastasis. High levels of CEA are also associated with malignant ascites from colorectal cancer (CRC)[32]. CEA interacts with liver Kupffer cells through a cell surface receptor (CEAR) that we have identified as the heterogeneous RNA binding protein (hnRNP) M4[33]. The hnRNP group of proteins are multifunctional and are involved in many cellular events including alternative splicing, translational regulation and packaging of transcripts[34]. These proteins have also been implicated in the regulation of mRNA stability and translation in many cancers[35]. CEAR is a highly conserved protein that can bind both RNA and DNA and can transport mature RNA to the cytoplasm as well as acting as a splicing factor[36]. It can also be expressed both on the cell surface, in the cytoplasm and in the nucleus where it is most commonly found[37]. There are 4 isoforms of hnRNP M known[38]. Two of which we have shown bind CEA[33]. One form (hnRNP M4) which we originally identified as the CEA receptor has a 38 amino acid deletion between the first and second RNA binding domains. The longer form also binds CEA[33]. The two other forms have not been identified other than as proteins on 2D gels. CEAR will also bind to CEA in HT-29 colon cancer cells although the functional significance of this is not known. It has been suggested that it may be involved with the resistance afforded to anoikis[39,40]. Binding to CEAR occurs via a penta-peptide motif (PELPK) located at the hinge region between the CEA’s N-terminal and first immunoglobulin loop domain. This interaction produces cytokines by activating a signaling cascade and these cytokines alter the liver microenvironment such that it becomes more hospitable to the implantation and growth of the cancer cells[41,42]. Production of both IL-6 and IL-10 by CEA stimulated Kupffer cells improves the survival of highly metastatic human colorectal cancer cells in the nude mouse intra-splenic injection model for liver metastasis[43] (see Figure 1).
Figure 1.
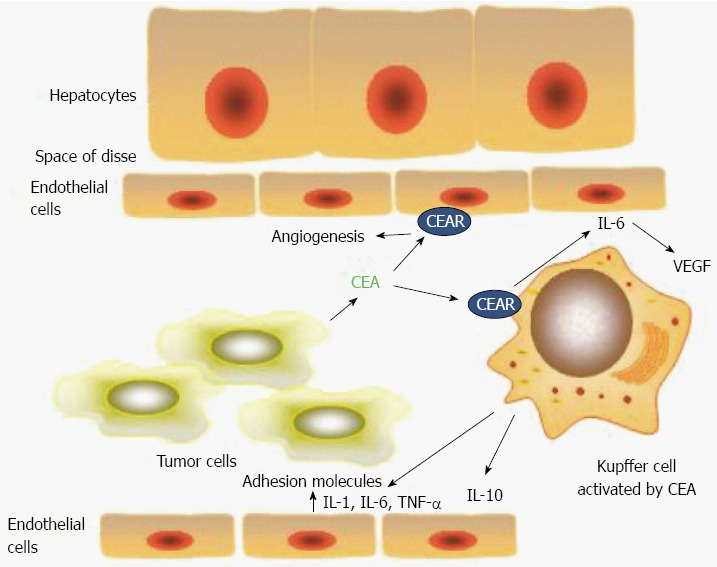
Schematic of the interactions of carcinoembryonic antigen in the hepatic sinusoid. Interactions of carcinoembryonic antigen (CEA) in the hepatic sinusoid. CEA released by the tumor cell binds with hnRNP M (CEAR) on the Kupffer cell surface resulting in release of the cytokines interleukin (IL)-1, IL-6, IL-10 and TNF-α. Effect of CEA induced cytokines on tumor cell interactions in the hepatic sinusoid. Cytokines IL-1, IL-6, IL-10 and TNF-α produced by Kupffer cells have a number of effects on the tumor cell microenvironment. These include up-regulation of adhesion molecules on hepatic sinusoidal endothelial cells. The most important of these seem to be E-selectin and ICAM-1. Cytokines such as IL-6 and IL-8 are pro-angiogenesis and they may also effect growth at the distant site[14,42].
CEA AS AN ADHESION MOLECULE
In 1989 Benchimol et al[22] demonstrated the first potential function for CEA. By transfecting tumor cells with the CEA gene they showed increased cell to cell adhesion and demonstrated that this could be inhibited using anti CEA antibodies. Adhesion was due to interactions between the N-terminal and the 5th and 6th (A3B3) immunoglobulin domains[44]. This suggested that these interactions may play a role in tumor cell behavior and in the development of metastases.
CEA AND ANOIKIS
Another important function that has been attributed to CEA expression is inhibition of apoptosis in particular the apoptosis caused by absence of attachment of cells to a substratum (anoikis)[23,40]. This would play an important role in metastases formation to a distant organ as unattached tumor cells in the circulation would be more susceptible to anoikis.
CEA AND ANGIOGENESIS
Here, we suggest a relationship between CEA, its receptor CEAR and angiogenesis. Others have also suggested a relationship between CEA and angiogenesis though they did not establish it as causal or identify potential mechanisms[45,46]. Recently, however they showed that secreted (soluble) CEA can directly activate endothelial cells via integrin β3 signaling[24,47]. We suggest that an alternate mechanism may also exist involving the relationship between CEA and its receptor CEAR. Interaction of CEAR with CEA in tumor associated stromal cells particularly macrophages is important. Disrupting that relationship may affect tumor growth and progression
It is significant that Low-Marchelli et al[48] have shown that CCL2 recruits macrophages to promote angiogenesis. We therefore suggest that colon cancer cells recruit macrophages by secreting MCP-1 and these macrophages are activated by tumor derived CEA to secrete IL-6 and IL-8 both known pro-angiogenic factors[49]. Anti angiogenic factors are also produced (IL-10) and an imbalance between these two competing factions will tend towards pro-angiogenesis.
TARGETING CEA/CEAR INTERACTIONS AS A MEANS OF INHIBITING CYTOKINE PRODUCTION AS AN ANTI-METASTATIC THERAPY IN COLORECTAL CANCER
Because transformed cells home into tissues from the circulation in a highly selective way through complex molecular mechanisms, it provides a template for targeted therapy. Designing an effective mimetic-based therapy requires the identification of the responsible molecules and their mechanisms of action along with their regulation. The investigation of methods to block or modulate CEAR function in vitro and in vivo and relating this to tumor growth and development will increase our understanding of the molecular and biological mechanisms involved in the progression of gastrointestinal cancers. The investigation of methods to block or modulate CEAR function in vitro and in vivo and relating this to tumor growth and development has increased our understanding of the molecular and biological mechanisms involved in the progression of colorectal cancers. We have shown that the binding peptide YPELPK will induce cytokine responses in macrophages activated by CEA[50]. We have shown that the binding peptide YPELPK will induce cytokine responses in PMA activated THP-1 macrophages. These types of study are important for the development of rational therapies that will enhance those currently available.
OTHER POTENTIALLY PRO/ANTI-METASTATIC MOLECULES STUDIED
Fibulin-5
During the initial development of metastasis, the adhesion of tumor cells is mainly mediated through binding of extracellular matrix components to cell surface receptors[51]. Interestingly, one study[52] found an association of fibulin-5, the newest member of the fibulin family of extracellular matrix glycoproteins, with hepatic metastasis of colorectal carcinoma. Since fibulin-5 plays an important role in antagonizing angiogenesis[51-53] in humans, we sought to confirm the expression of fibulin-5 expression level in the CTT.
Avoidance hypotheses in the CTT
Our understanding of cancer and metastases in primates began when colon cancer was described in old world monkeys as early as 1914 and then in the new world in inter alia, Goeldi’s monkey in the early 1960s. The latter, Callimico goeldii shares the Colombian habitat with the CTT and is a small black-furred New World lower order primate. At that time tens of thousands of CTT were imported by the pharmaceutical industry utilizing the “tamarin alarm reaction” to test the efficacy of anxiolytics although literature to document this is difficult to come by utilizing electronic search methods. Problems of CTT husbandry arose when the animals developed the “wasting marmoset syndrome”[54] postulated to be caused by a coronavirus but later effectively countered by improving the nutritional intake. The major challenge facing the CTT is the reduction of its numbers in its shrinking natural habitat in northwestern Colombia and most CTT are today found in zoos. As a result the species was listed as endangered in 1973 by the Convention on International Trade in Endangered Species of Wild Flora and Fauna (CITES)[55]. Since that time, the numbers of articles on the CTT had increased exponentially. This attests to the interest of the scientific community in the CTT specifically. Despite this interest, primate research in the United States is in decline with the closures of the ORAU (Oak Ridge Associated Universities) facility and that of the NEPC (New England Primate Center) which housed a significant proportion of CTT. The waning of public support and therefore funding opportunities combined with the expense of operations has largely been the cause of this decline. This is regrettable as it is generally not appreciated that monkey publication numbers are an extensive part of the scientific literature (678000 Medline™-listed publications) attesting to their usefulness and their command of the attention of the scientific community. Now, specifically CTT publications which were formerly about one tenth of non-human primate-themed publications (398 total CTT per National Library of Medicine ticket 28045-54653), are in decline in the example depicted graphically (Figure 2).
Figure 2.
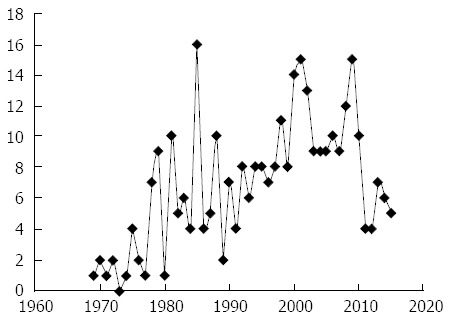
Graphic representation of published cotton top tamarin articles by year as estimated by a typical online search. The line chart is not necessarily inclusive of all cotton top tamarin (CTT) articles but merely confined to the search terms used. It is therefore used mainly to demonstrate the overall trends in annual publications devoted to this animal “CTT supermodel”.
In view of the importance of this animal model applicable to many models of human disease, a CTT symposium was convened resulting in the publication of numerous articles in a supplement published in the journal Digestive Diseases and Sciences in 1985[9]. Thereafter a comprehensive book on the CTT was edited by Dr. Neil K. Clapp and published in 1993 by CRC Press[2]. This review seeks to build on that experience specifically focusing on the ability of the CTT to avoid hepatic metastases even though a large proportion of the animals develop advanced colorectal cancer. This is a unique feature of the CTT not typically found in other marmosets. It would appear that CTT peritoneal metastasis and ascites is also not common as it is with terminal human disease.
CTT MODEL FOR HEPATIC METASTASIS
The CTT develops colitis in the juvenile years and about one third (34.5%) go on to develop colon cancer and die of the disease. An observational study showed only 1.2% of animals had hepatic metastases[56] (Figure 3).
Figure 3.
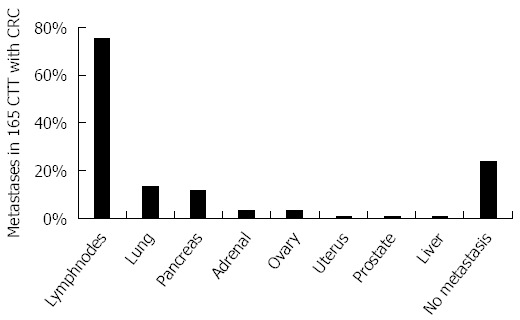
Schematic bar diagram depicting proportion of metastases in cotton top tamarin with colorectal cancer. The bar diagram shows the distribution of metastasis based on the ORAU colony cancer statistics. The paucity of liver metastases is remarkable. CTT: Cotton top tamarin; CRC: Colorectal cancer.
The etiology of the cancer seems to be the de novo inflammation-dysplasia-cancer variety although animals with adenomas have been described[56]. The risk of cancer deaths shows an increase from 3-8 years and then a gradual decline after age 12 similar to the recently described risk in humans with positive family history of CRC[57]. The etiology is still unknown but stress and micro-organisms may play a role[3,4]. Over half have multiple primaries diffusely distributed but the descending colon has the greatest proportion similar to the rectosigmoid colon predominance in ulcerative colitis cancer location.
CTT COLON CANCER BIOLOGY VIS A VIS HUMAN
Despite similarities in cancer biology between CTT and humans it can be seen from Table 1 that the anticipated mutations are either not described or characterization has not been attempted.
Table 1.
Comparison of cancer genetics in the cotton top tamarin and human
| Stage of neoplasia | Sporadic human CRC | Human IBD-associated CRC | CTT colitis-CRC |
| Normal-appearing mucosa | APC initiator | Inflammation leading to mutations listed below | No cell line mutations in APC exon 4 and 15[58] |
| Early adenoma/indefinite dysplasia | Aneuploidy, methylation, Sialyl Tn | Aneuploidy, methylation, Sialyl Tn, MSI | 90%diploid; no methylation[59] |
| Early promotor | MSI; kRAS, COX-2 | DCC | kRAS absent[60] |
| Intermediate adenoma/low-grade dysplasia | c-src | c-src | c-src ND |
| Intermediate promoter | DCC/DPC4 | kRAS | DCC/DPC4 ND; kRAS absent[60] |
| Late promoter | p53 | APC | p53 ND; as above for APC exon 4 and 15[58] |
Adapted from Itzkowitz and Harpaz 2004[61]; and Mattar MC, Lough D, Pishvaian MJ, Charabaty A[62]. APC: Adenomatous polyposis coli gene; MSI: Microsatellite instability; DCC: Deleted in colon cancer; kRAS: V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog; COX-2: Cyclooxygenase 2 gene; c-src: Rous sarcoma gene that encodes for usual C-terminal inhibitory phosphorylation site (tyrosine-527); DPC4: Deleted in pancreatic carcinoma, locus 4; p53: Protein 53 kilodaltons; ND: Not done.
When considering changes associated with metastases[63] no CXC, CXCR4, CXCL12 (fusins), CCR7 (chemokine receptor 7) chemokines thought to be important in metastases have been described as yet in the CTT. Interestingly CXCL12 has been described in the common marmoset[64] and thus likely to be expressed in the CTT but is not necessarily a given. The human kallikrein zymogens (hK2) closely related to prostate specific antigen (PSA) have been studied in the CTT[65]. They concluded that the CTT has no functional hK2 or PSA. Since these moieties regulate cancer cell survival and growth the CTT may be a natural knock-out model for the study of this topic. We see in these findings yet another potential mechanism by which the CTT impedes cancer proliferation. Likely Toll-like receptors (TLR) also remain to be discovered in the CTT and may have likely similar functions to the human.
Since lectins[66] and adhesion molecules have been described in the CTT we have focused our attention on these molecules, particularly CEA. We also explored extracellular matrix proteins as the interaction between these proteins and cells play an important role in tumorigenesis and metastases. One such protein, fibulin-5[51-53] has calcium-binding EGF-like domains and associate with vascular and vascular structures. It is thought therefore to have anti-angiogenic actions and be an inhibitor of metastasis. It has not as yet been studied in the CTT.
CEA HOMOLOGUES IN THE CTT AND HUMAN
Cross reactive CEA species in monkeys were known when we published our seminal paper in 1994[67]. Haagensen et al[68] had reported CEA in higher order non-human primates, including the chimpanzee and gorilla. A mouse analogue had also been described by Abraham Fuks’ group in 1989[69]. Our initial experiments used a number of antibodies to CEA including the T84.66 antibody developed by Dr. J. Shively’s laboratory group at the Beckman Institute at the City of Hope which was used in the CEA kit commercialized by Roche™. This antibody when used in immunohistochemistry produced consistently negative results when using colon CTT fixed tissues and later native antigen in CTT colon tissue extracts in early experiments[70]. We tested other antigens such as organ specific neoantigen[71], thought to be the mediator of the lymphocyte adherence inhibition test for cancer and found reactivity[72]. In the early 1990’s Karel Kithier of the Department of Pathology at Wayne State University was beta-testing a CEA kit produced by Tosoh Medics (CEA-AIA PACK kit™) which seemed to have a greater proportion of positive results when compared to another CEA kit marketed by Abbot. These results were expanded and confirmed and found to yield similar levels in CTT and humans with IBD[60,73] which opened a pathway to possible intervention[33] in addition to other cancer related moieties such as telomerase[60]. In order to show the CEA molecular moieties recognized by the kit we obtained both solid phase and tracer antibodies from Hybritech Inc., from Dr. Harry Rittenhouse. The resultant Western blotting[67] showed a specific 50 kDa band shared by CTT and humans (Figure 4).
Figure 4.
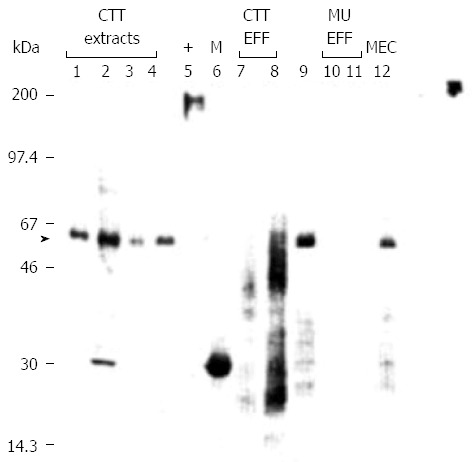
Specific carcinoembryonic antigen bands shared by cotton top tamarin and humans. Western blot using solid phase anti-CEA monoclonal antibody. lmmunoblotting was performed using 5.6 pg protein/ml antibody after electrophoresis of a 12% SDS-polyacrylamide gel run under reducing conditions. Relative mobility (M) is shown on the left, and the type of samples loaded at 10 pg protein/lane are shown above the numbered lanes. Cotton-top tamarin extracts are in lanes 1 to 4 and effluent samples in 7 and 8. An extract from a patient with histologically proven rectal cancer and IBD is in lane 9, with human effluent samples in lanes I0 and 11 and human meconium in lane 12. Lane 5 contains the positive human CEA control, and the M, markers (M) are in lane 6. An arrow indicates the M, - 50000 band evident also in human extract (lane 5) likely a deglycosylated moiety. (Published with permission of Elsevier[67] and modified). CEA: Carcinoembryonic antigen.
A control blot using T84.1EC monoclonal which reacts with both NCA and CEA did not recognize the 50 kDa shared antigen in the same samples. Based on the known epitopes on the antibodies concerned we were able to speculate that the shared antigen included the Ig-like loop domains however the exact structure of this moiety remains unknown. In this publication we did hypothesize that “the smaller CTT CEA molecule might lack the critical peptides necessary for uptake and hence explain the paucity of liver involvement in CTTs with CRC”.
EXPRESSION OF THE CEA GENE FAMILY OF PROTEINS IN THE CTT AND HUMANS
The next question we needed to answer was if an entire repertoire of CEA-family adhesion molecules were expressed by the CTT and if this subspecies was unique in this respect in the Callitrichidae family. In a later paper in 2000[73] we explored these questions by expanding the repertoire of antigens and extending our observations to include a close cousin of the CTT, the common marmoset (Callithrix jacchus - CM) which is not endangered but does develop colitis uncomplicated by cancer. Aware that the changes likely to influence CEA uptake were at the N-terminal end of the CEA molecule we used antibodies directed at epitope in that region, including the aforementioned Tosoh Medics™ kit and T84.66 antibody to quantify the CEA in colonic washings.
We found that the concentration of CEA in washings was sevenfold times higher than in washings from humans with IBD. T84.66 antibody was able to demonstrate a faint high MW band in a specimen of colonic washing from a CTT by immunoblot. In the Western blot using T84.66 we could now also demonstrate specific bands (immunohistochemistry had been consistently negative) in CTT washings and tissue extract of Mr ≥ 90 kDa which is the size of the non-glycosylated protein core of the CEA molecule[74]. CEA levels assayed by the Tosoh™ kit in CTT extracts were significantly higher than those from CM extracts (P < 0.005). Consistent with this finding was a high mean value in sera of CTT (134 ng/mL) compared to undetectable levels in the CM. NCA levels tended to be the lowest in the CTT as compared to humans and CM. Both animals’ samples were low when reacted with a CEA-family antibody. In contrast binding with the anti-BGP was seen in both animals. We concluded that the similarity between human and tamarin native CEA is greater than previously believed and that it was likely that the N-terminal epitopes were conserved in the tamarins.
OTHER ANTIGENS SHARED BY CTT AND HUMANS
By the end of 2000 we published an expanded group of antibody assays involving 7 additional common epitopes representing most colonic cell types and blood group/carbohydrate antigens, some of them accepted tumor markers[60,70-76]. Mucin antigens, epidermal growth factor (EGFR) and telomerase were shared by CTT and humans but k-ras p21 and adenoma antigen (Adnab-9) were not. This supported the notion that carcinogenesis was likely de novo and did not progress through the adenoma-carcinoma sequence. Our finding of EGFR reactivity in the CTT provided a basis[76] for fibulin-5 role in the anti-metastatic armamentarium and OSN (by reactivity of BAC 18 monoclonal) suggesting similarities in anti-cancer immune responsiveness via the lymphocyte migration reaction. We were able to demonstrate also significant levels of EGFR epitope in the urine of 5 CTT (0.152 ± 0.053 absorbance OD-background at 405 μm) by ELISA. CEA reactivity with various antibodies in these samples were low (mean < 0.05). Blood Group Substances (BGS) were detectable [Span 0.063; FBB 0.103; CaCo 0.054) as were Bac18.1 (anti-OSN antibody) at 0.06]. Wild-type (Table 1) Ras p21; src, common membrane antigen (CMA), were negative corresponding to the data derived from CTT tissue. The only pieces of the puzzle missing were the questions of CEA molecular homology and how to tie the available data together to explain how the CTT dodges liver metastases.
DEFINITIVE STUDIES ON THE HOMOLOGY OF THE CEA MOLECULE
A comparative study of the C-terminus of the CEA molecule by the Stanners’ group looked at multiple primate and prosimian species. The membrane linkage in CTT and other monkeys was found to be GPI anchorage rather than transmembrane (TM) seen in mice and rats[77]. This paper showed that the more versatile glycophosphatidyl inositol (GPI) linkage with a greater functionality constellation likely confers a tendency to tumorigenesis. Mutational packages evolved over time, one applicable to most primates including humans, and the second confined to the Cebidae radiation of New World monkeys leading to inhibition of cell differentiation. The rate of Ka (indicator of selective pressure acting on a protein-coding gene) nonsynonymous mutations for this radiation is 7X higher than the average Ka in primates. This led us to postulate that if such mutations occur at the C-terminus, it is quite feasible that similar mutations occur at the N-terminus where they may affect uptake of CEA, inhibiting hepatic metastases. Having also found common CEA-family and fibulin-5 ligand antigens we resolved to also investigate for parallel pathways of metastasis inhibition.
SUMMARY: SPECIFIC METASTASIS-TARGETED STUDIES IN CTT AND HUMANS
Using DNA extracted from the CTT tissues a Hot start PCR was performed and (1) sequences around the hinge region between the N-terminal and the first loop domain of CEA obtained using a Big Dye Terminator™ sequencing kit. We also immunolabeled human and CTT liver tissue using: (2) BGP (CEACAM1) antibody kindly supplied by Christophe Wagener (University of Freiberg, Germany); (3) CEAR (CEA receptor) polyclonal antibody; and (4) an antibody directed to fibulin-5 supplied by W. P. Schiemann (Department of Pharmacology, University of Colorado, Aurora, CO, United States). Finally, (5) we deglycosylated CTT CEA to determine the degree of N-glycosylation, as glycosylation may correlate with hepatic metastases. We thus examined 5 potential ways in which the CTT may evade hepatic metastases in 10 CTT and 25 humans.
To briefly summarize the observed[8] results (Table 2) we enumerate: (1) 63% of the CTT had non-synonymous PELPK pentapeptide mutations shown to be essential for hepatic uptake of numerous groups of protein including CEA and stromelysin, laminin, complement protein etc. CM had the PELPK mutations to a lesser extent (73% vs 29%, P < 0.05). Sequence changes in the PELPK binding motif are found in the CTT that prevent binding to CEAR and do not allow activation of Kupffer cells and thus secretion of pro-inflammatory cytokines.
Table 2.
Representative somatic sequences from the PELPK region of carcinoembryonic antigen in humans, cotton top tamarin and common marmoset
| HCEA-A1 PELPKPSISSNNSKPVEDKDAVAFTCEPETQDA |
| CTT-WT PELPKPSISSNNSKPVEDKDAGAFTCEPETQDA |
| CM-WT PELPKPSISSINSKPVEDKDAGAFTCEPETQDA |
| M1-A1 (CTT) PEVPKPSISSNNSKPGGDKDAGAFTWEPETQDA |
| M2-A1 (CTT) PEVSKPSISSNNSKPGGDKDAGAFTWEPETQDA |
| M3-A1 (CTT) PEVSKPSISSNNSKPVGDKDAGAFTWEPETQDA |
| M4-A1 (CTT) PEVSKPSISSNNSKPVGDKDAGAFTWEPETQDA |
| M5-A1 (CTT) PEVSKPYISSNNSNPVENKDAGAFTWEPETQDA |
| M6-A1 (CTT) PEVSKPFIFSNNSKPGGDKDAGAFTWEPETQDA |
| M8-A1 (CTT) PEVSKPFIFRNNSKPGGDKDAGAFTWEPGTQDA |
| M10-A1 (CTT) PELPKPFIFSNNFKPVEDKDAVAFTCEPETQDT |
| M14-A1 (CTT) PELPKPFIFSNNFKPVEDKDAVAFTCEPETQDA |
| M15-A1 (CTT) PELPKPFIFSNNFKPVEDKDAVAFTCEPETQDA |
| M16-A1 (CTT) PELPKPSILSNNSKPVEDKDAVAFTCEPETQDA |
| M17-A1 (CTT) PELPKPSIPSNNSNPVEDKDAVGLTCEPDTQNT |
| M19-A1 (CM) PELPKPSISSYNSKPVEDKDAGAFTCEPETQDA |
| M20-A1 (CM) PELPKPSISSYNSKPVEDKDAGAFTCEPETQDA |
| M21-A1 (CM) PELPKPFISSYNFKPVEDKDAGAFTCEPETQDA |
| M24-A1 (CM) PELPKPSIFSNNSKPVEDKDAVAFTCEPETQDA |
| M27-A1 (CM) PELPKPSISSNNSNPVEDKDAVAFTCEPETQDA |
| M31-A1 (CM) PEVSKPFIFSNNSKPVGDKDAGAFTCEPETQDA |
| M40-A1 (CM) PGVPKPFIFRINFKPVGDKDAGAFTCEPETQDA |
Sequences changes from those of wild type (germ line) are shown in bold. Published with permission of Springer[8].
This could account for the high CEA serum levels in the CTT as compared to CM and humans and contribute to the lack of liver metastasis. Zimmer and Thomas in 2001 showed in a minority of patients with advanced colon cancer all of whom had high CEA levels, changes in the PELPK sequence. CEA from these patients could not bind to Kupffer cells. These patients did not have liver metastasis though peritoneal carcinomatosis was common[78]. Serendipitously: (2) Normal sections of human livers bearing metastatic disease (our intended “negative” controls) showed translocation of the BGP to the cytosol of the hepatocyte whereas CTT livers did not have detectable BGP except in the gallbladder wall. BGP is usually a structural protein found in the bile canaliculus but conceivably, if translocated to the cytosol, may act as a downstream mediator of VEGF, facilitating the progression of liver metastasis. Human cirrhotic and hepatitis livers demonstrated orthotopic BGP compared to human livers bearing metastases (P < 0.02) where the BGP had translocated to the cytoplasm (Figure 5).
Figure 5.
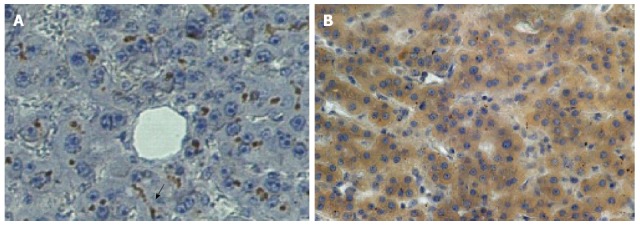
Distribution of CEACAM1 (BGP) in human liver. Photomicrographs of CEACAM1 staining with monoclonal antibody 4D1/C2 showing very intense brown staining mainly in the distribution of the biliary canaliculus in normal human liver (A).In contrast, in the normal portion of a liver from a patient with hepatic metastasis (B), dark staining is seen in the cytoplasm of the hepatocytes with no canalicular staining evident. Published with permission of Springer[8]. The arrow points to the typical distribution of bound ant-BGP in the bile canaliculus in the normal liver (A) at center, bottom.
This may answer the age-old clinical question as to why patients with cirrhosis rarely get hepatic metastases from extrahepatic primary cancers.We also observed: (3) a diminution of CEAR staining (Figure 6) in the CTT liver sections compared to humans (P < 0.02), a further barrier to CEA hepatic uptake in the CTT as compared to the human liver. Surprisingly: (4) Extremely robust fibulin-5 hepatocyte labeling was detected in CTT livers (Figure 7) and finally: (5) Minimal glycosylation of CTT CEA was demonstrated as opposed to human CEA. 80% of CTT vs 60% of CM were positive for fibulin-5 binding with functions of the various fibulins contrasted in Table 3.
Figure 6.
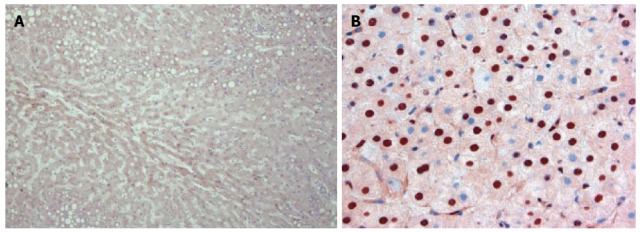
Distribution of carcinoembryonic antigen receptor in cotton top tamarin and human. The distribution of CEAR in the CTT (A) at low power can be seen in the cytoplasm of the hepatocyte by the light brown stain. In humans (B) at a higher power the increased intensity of staining in hepatocyte nuclei can be clearly seen. Published with permission of Springer[8]. CEA: Carcinoembryonic antigen; CTT: Cotton top tamarin; CEAR: Carcinoembryonic antigen receptor.
Figure 7.
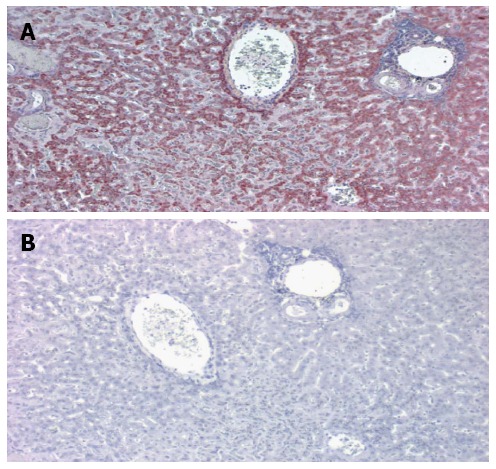
Immune labeling of CTT liver by anti fibulin-5 monoclonal antibody (A) and corresponding negative control (B). A: A central vein is seen in the upper center and a portal triad to the right. The dark red staining denotes the distribution of the antibody which is particularly intense surround the central vein. This is a low power magnification; B: In the negative control no staining is evident.
Table 3.
| Attribute | Fibulin-1 | Fibulin-2 | Fibulin-3 | Fibulin-4 | Fibulin-5 |
| Invasion of endothelium | Decrease | Increase | Decrease | Unknown | Decrease |
| Binding of tropoelastin | Moderate | High | low | Moderate | High |
| Effect on angiogenesis | Unknown | Unknown | Reduced | Unknown | Reduce |
| MMP/fibronectin change | Increase | Unknown | Reduced | Unknown | Reduced |
Hepatocytes were the predominant cell types expressing fibulin-5 in both types of animals but tended to be more intense in the CTT (0.75 ± 0.289 and 0.4 ± 0.418 respectively) and also seen to be of the same distribution and intensity in the human liver controls. Kupffer cells did not stain (P = 0.033 CTT and P = 0.09 CM) but occasional bile duct cells were positive but of lesser intensity in the CTT (0.125 ± 0.25 and 0.375 ± 0.479 respectively - Figure 8). Vascular staining was seen only in 2/5 CTT sections at a mean intensity of (0.3 ± 0.447). No appreciable uptake was noted in white blood cells in CTT and minimal in CM.
Figure 8.
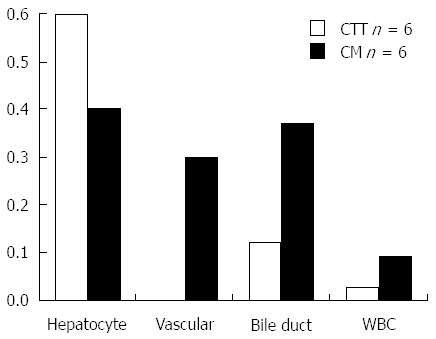
Summary of fibulin-5 immune labeling in cotton top tamarin, common marmoset and humans. Intensity of labeling: CTT and CM of Hepatocyte, Bile Ducts, Vascular Cells and White Blood Cells with Anti-fibulin-5. CTT: Cotton top tamarin; CM: Common marmoset.
We therefore postulate 5 possible pathways of spontaneous hepatic metastasis evasion in the CTT, 4 of which we have previously reported[8], summarized in Table 4.
Table 4.
Summarization of our findings in this study
| Parameter | CTT | CM | Human |
| PELPK Change | + + + + | + + | - |
| CEAR expression | ± | ± | + + + + |
| CEA glycosylation | ± | ± | + + + + |
| CEACAM1 expression | - | - | + + + + |
| Fibulin-5 expression | + + + | + + + | + +1 |
Human data have limited staining data; Modified from and published with permission of Springer[8]. CTT: Cotton top tamarin; CM: Common marmoset; CEA: Carcinoembryonic antigen.
OTHER POTENTIAL ANTI-METASTATIC AND METASTOGENIC MOIETIES IN THE CTT
Lastly using the Gelco Diagnostics antibody kit to POA we determined a low level of POA[79] in extracts from CTT non-cancerous tissues 1.603 ± 1.656 μg/mL (mean ± SD). In contrast to serum in 13 humans with pancreatic cancer (9.622 ± 6.424) these levels were modest.Since normal serum levels in humans are < 15 U/mL even an order of magnitude increase in the CTT is unlikely to increase levels where they might influence the tumor invasiveness. In the study cited, a significant correlation was found between POA and CEA levels classified by stage of CRC (P = 0.003). Although further study is required it would appear that POA is not a major determinant in the CTT of the metastatic process to the extent that it is in humans making this another potential discouraging factor for the development of hepatic metastasis.
Ultimately, we would need to show that the cytokine environment milieu is similar in the CTT as described above for humans. Wilson et al[80] using 32 monoclonal antibodies has shown MHC Class 1 and II proteins on lymphocytic B cells (CD20-21, 23) and CD16-56 on NK cells; CD2 and CD3 and CD4/8 helper T cells. In addition IL-2 receptor CD25 receptor is present as well as B chain LFA-1 CD 18. There was however poor reactivity for CD11a; and ICAM-1 (CD54) was negative. 14 years later with more reagents available, Kap et al[81] confirmed much of the above findings with IL1, 2, 4, 6, 10, 12, and 17a found. In addition they found all 4 clones against CD11a to be positive in contrast to the above paper. CD11a and CD18 and CD29 are important factors for adhesion and are expressed in the CTT. In addition, CD40 of the TNF family is present thus demonstrating that the CTT has almost all the necessary repertoire of proteins as is present in humans to develop hepatic metastasis. Although ICAM-1 was not included in the repertoire, by virtue of CTT colitis to respond to an anti-integrin monoclonal[82], we can conclude that selectins adhesion molecules are also likely expressed. This being the case, it would appear that the aforementioned inhibitory mechanisms are sufficient to abrogate this phenomenon almost completely.
We cannot rule out additional mechanisms and would encourage such research efforts. While ICAM1 may not have been confirmed in the above review (because it does not seem that it was included in the battery of monoclonals described above), E-selectin and integrins certainly have been convincingly demonstrated to be actionable by therapeutic intervention in the CTT[80-82].
Certainly, the above hypotheses are not established scientific facts but they are testable and applicable to a particularly vexing problem presented by hepatic metastases. The ability to intervene using novel approaches based on these ideas will constitute the final section of this review and may represent an encouraging future vision of hope for myriads of “terminal” cancer patients.
In quoting the final chapter of the late Neal K. Clapp’s book on this topic: “Our research opportunities using the cotton-top tamarins to study colonic diseases are truly only limited by our ingenuity”.
HOW MIGHT WE EXPLOIT THESE ADVANCES FOR THE BETTERMENT OF THE CANCER PATIENT?
Hepatic metastasis of CRC is a very common clinical situation in oncology with prevalence at the time of diagnosis of approximately 20%-25%. The liver has been shown to be the most common site of metastatic spread of colorectal cancer. The prognosis of colorectal hepatic metastases has improved in the last few years owing to surgical resection of liver metastases for patients with no extrahepatic disease; however, liver metastases are resectable in only 15% of the cases[83,84]. Often, factors such as location, size and number of hepatic metastases make it difficult to resect these tumors[83,85]. Thus early prevention of metastasis is crucial in improving the prognosis of patients diagnosed with primary colorectal cancer. As earlier documented by Smedsrød et al[86] colorectal hepatic metastasis involve four steps which are interrelated; the cancer cells must establish micrometastasis in the liver by passing through microvasculature which is guarded by Kupffer acting in concert with NK cells. To ensure nutritional supply and establish clinically evident metastatic disease, these cells engage growth factors and adhesion molecules such as VEGF, VCAMs etc. As can be appreciated from both clinical and experimental evidence, Kupffer cells are involved with all the rate limiting step of this macrometastatic disease process which has been established in experimental systems but this role is not proven in human cancers[86]. These phases represent target points of recent therapeutic interventional designs.
USE OF GADOLINIUM BLOCKADE TO DEPLETE KUPFFER CELLS
In our earlier study using CTT[8], we showed that although they have CEA receptors similar to humans, they are able to evade CRC hepatic metastasis via two possible means; defective CEA receptors with much reduced receptor capacity or by sequence changes in the PELPK binding motif that is required for receptor activation (Table 2). We hypothesize exploiting methods to prevent CEA/CEAR binding in humans may prove effective in reducing the occurrence of hepatic and possibly lung metastasis. This hypothesis was tested earlier using gadolinium to block uptake of CEA by sinusoidal cells. Although the result was promising, only a transient blockage of CEA was achieved mostly due to the short wash out period for gadolinium (Figure 9). Therefore, using animal models this approach can be refined to optimize our earlier findings using gadolinium as blocking agent for Kupffer cells and the CEA/CEAR interaction.
Figure 9.
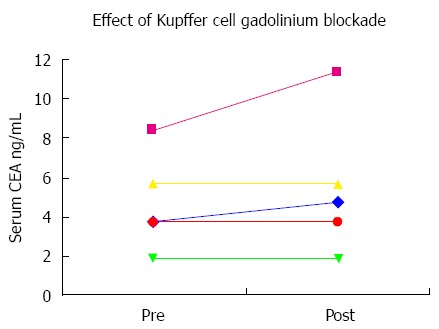
Carcinoembryonic antigen concentrations before and after gadolinium injection for magnetic resonance imaging. Five human patients had serial CEA levels measured before and after MRI with gadolinium contrast administration. No significant changes are seen after gadolinium for patients with normal or near-normal baseline levels. However the patient with a baseline CEA elevation showed a sizeable increase in the CEA level designated by the pink line. CEA: Carcinoembryonic antigen.
As a novel approach to anti-metastatic therapy, gadolinium may be encapsulated in smart nano-devices that will prolong the residence time significantly. Using PEGylated-gellan-gum as the polymer of choice, gadolinium can be encapsulated in nanoparticles with average size of 250 nm[87].
Gellan gum is an exocellular microbial polysaccharide with a natural propensity to absorb biological fluid in vivo thus regulating the rate of drug/agent release. It consists of repeating tetrasaccharide units of glucose, glucuronic acid and rhamnose in a molar ratio of 2:1:1[88,89]. In addition to being used as a food additive, gellan gum has a wide range of applications in the pharmaceutical industry. In this area, its use has been mainly concentrated in ophthalmic drug delivery and oral sustained-release preparations, in which its ability to undergo cation-induced gelation is utilized as the main fabrication platform[87]. Gellan gum has a number of advantages as it can undergo temperature-dependent as well as cation-induced gelation, which may be of importance for in vivo slow release of encapsulated gadolinium. It also has proven non-toxicity in humans[90]. The FDA has also approved gellan gum as a food additive for human consumption[90], thus, its safety in humans is established. To improve the effectiveness of this targeting system, a three-dimensional matrix via covalent crosslinking with an end-reactive polyethylene glycol (PEG) through amide linkage can be used. Such modification provides a stronger gellan gum matrix that is nontoxic to normal cells and possesses the ability to deliver biological molecules such as gadolinium to target cells[91] in a controlled manner as illustrated in Figure 10. There is some experimental animal model work that suggests that gadolinium in the form of metallofullerenol nanoparticles inhibit cancer metastases[92] but the metastases observed were mainly pulmonary.
Figure 10.
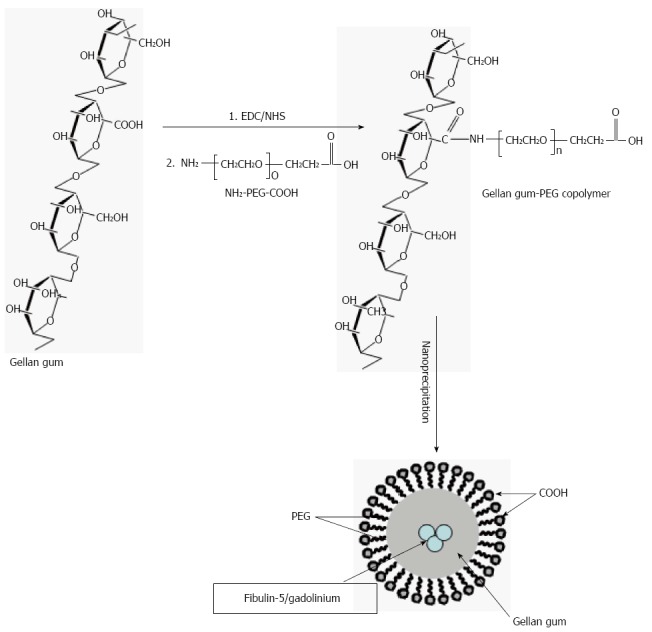
Schematic representation of the technique used for the synthesis of gadolinium encapsulated Gellan-gum-block-Polyethylene glycol co-polymeric nanoparticles. The above figure is a representation of the technical steps to generate nanoparticles of gadolinium encapsulated by Gellan-gum-block-Polyethylene glycol copolymeric micelle nanoparticles. This should ensure delivery and advantageous dwell time in order to block Kupffer functions of uptake of CEA and reduce the pro-metastogenic role of these cells. CEA: Carcinoembryonic antigen.
FIBULIN AS A TREATMENT FOR HEPATIC METASTASIS
The process of nanoprecipitation described by the schematics in Figure 10 can potentially be applied to protein encapsulation provided the formulation parameters are optimized as described earlier by our group[91]. Fibulin-5 plays an important role in antagonizing angiogenesis in humans; furthermore its overexpression has been shown to inhibit HCC cell migration and invasion in vitro[93], thus fibulin-5 could also be encapsulated in gellan gum nanoparticles to increase their in vivo residence time. Nanoparticles formulation should be optimized as described earlier[91] prior to encapsulation of fibulin-5. We would expect fibulin-5 to be the most effective of the fibulins in reducing angiogenesis but fibulin-3 may also be considered (Table 3). While fibulin-5 has been shown to suppress metastasis to the liver and lung[94], it has yet to be used directly in a nanotechnology formulation as we propose. A report that human oncogenic fibulin-5 may promote nasopharyngeal cancer metastasis[95] would suggest that we proceed cautiously with this approach with both agents.
TARGETING CEA/CEAR INTERACTIONS AS A THERAPEUTIC APPROACH TO LIVER METASTASIS
CEA has been shown to be a mediator of metastasis for colorectal cancer by causing changes in the tumor microenvironment that increase the potential for tumor cell implantation and growth[14]. CEA interaction with its receptor therefore suggests a targeted approach to therapy. A number of methods could be used to achieve disruption of CEA/CEAR including the use of antibodies specific for CEA or its receptor. In general this type of approach has worked well against various cancers and growth receptors e.g., EGFR. Other approaches using small molecule inhibitors of ligand receptor interactions are also being used, In particular RNA aptamers hold great promise as therapeutic agents[96]. These aptamers are now being used in studies involving CEA[97]. Lee et al[98] have described the use of a RNA aptamer directed against the PELPK motif of CEA to prevent liver metastasis from the mucinous colon cancer cell line LS174T. They suggest this occurred by disruption of CEA/CEAR interaction. LS174T produces large amounts of CEA and has a high metastatic potential to the liver[99]. Recently, aptamers have also been used against the homotypic adhesion sites of CEA to block tumor cell/cell interactions[100] further emphasizing the feasibility of this approach[101]. Aptamers are also being used for radiolocalization of CEA producing tumors and may even supplant the use of antibodies for this purpose[102]. The use of CEA antagonists to reduce the metastatic potential has now become feasible especially with the advent of small molecule inhibitors. More pre-clinical trials are needed before these approaches can be used in cancer patients at high risk for metastases.
CONCLUSION
We thus have the means to potentially interfere with the uptake of CEA from the hepatic sinusoid Kupffer cell receptor and hopefully disrupt the inflammatory cascade and well as intervening in the fibulin pathways in delivery of fibulin-5 to shore up hepatic defenses against tumor spread to the liver. In time, additional interventions will be designed to make this a reality based on the lessons learned from the cotton top tamarin- the artful liver metastasis dodger.
ACKNOWLEDGMENTS
Were we to mention every contributor to this substantial body of work this section would undoubtedly be the most extensive.We hope that the names listed in the references will be a curtain call for us to applaud their hard work and sincere efforts. At the same time there were a number of individuals without whom this work would not have been done. We recognize our mentors Norman Zamcheck and Neil Clapp of blessed memory. The guidance and generosity of Phil Gold, Abraham Fuks, Jack Shively, Young S Kim, Jim Fox and Sen-Itoh Hakamori, Clifford Stanners, Zvi Bentwich, Steven Itzkowitz and James Allard was peerless. Their mention here should not be construed to imply their approval of this work but we hope that they do. The tenacity and attention to detail by Karel Kithier and James Hatfield were legend. To the many students, resident and Fellows who appear in many meeting abstracts we are most grateful. Our work could not have progressed without various grants from National Institute of Health, Veterans Health Administration, Kaiser Permanente and Hybritech™ Inc. Thanks also to S. Jatczak and the library staff at the Saginaw VAMC. All previously published material that is used in this manuscript by the principal author emanates from work done as a US Government employee and is therefore in the public domain. However, this work does not necessarily reflect the views of the US Government. As a courtesy, permissions were sought from the relevant publishing houses and graciously granted. This paper is dedicated to the memory of Mendel Tobi whose love for the animal world was boundless.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest related to the manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 22, 2016
First decision: April 14, 2016
Article in press: May 23, 2016
P- Reviewer: Berg T S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
References
- 1.Weiss RA, Vogt PK. 100 years of Rous sarcoma virus. J Exp Med. 2011;208:2351–2355. doi: 10.1084/jem.20112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clapp NK, Nardi RV, Tobi M. Future Directions for Colon Disease Research Using Cotton-Top Tamarins. In: Clapp NK, editor. A Primate Model for the Study of Colitis and Colon Carcinoma The Cotton Top Tamarin Saguinus oedipus. Boca Raton: CRC Press; 1993. pp. 319–324. [Google Scholar]
- 3.Wood JD, Peck OC, Tefend KS, Stonerook MJ, Caniano DA, Mutabagani KH, Lhoták S, Sharma HM. Evidence that colitis is initiated by environmental stress and sustained by fecal factors in the cotton-top tamarin (Saguinus oedipus) Dig Dis Sci. 2000;45:385–393. doi: 10.1023/a:1005485215128. [DOI] [PubMed] [Google Scholar]
- 4.Mansfield KG, Lin KC, Xia D, Newman JV, Schauer DB, MacKey J, Lackner AA, Carville A. Enteropathogenic Escherichia coli and ulcerative colitis in cotton-top tamarins (Saguinus oedipus) J Infect Dis. 2001;184:803–807. doi: 10.1086/322990. [DOI] [PubMed] [Google Scholar]
- 5.Hofmann P, Kahnt K, Mätz-Rensing K, Brack M, Kaup FJ. Three spontaneous lymphomas in a colony of cotton-top tamarins (Saguinus oedipus) J Med Primatol. 2001;30:322–327. doi: 10.1034/j.1600-0684.2001.300606.x. [DOI] [PubMed] [Google Scholar]
- 6.Lushbaugh CC, Humason GL, Swartzendruber DC, Richter CB, Gengozian N. Spontaneous colonic adenocarcinoma in marmosets. Primates Med. 1978;10:119–134. [PubMed] [Google Scholar]
- 7.Watkins DL, Letvin NL. Immunobiology of the Cotton Top Tamarin. In: Clapp NK, editor. A primate Model for the Study of Colitis and Colon Carcinoma The Cotton Top Tamarin Saguinus oedipus. Boca Raton: CRC Press; 1993. pp. 299–307. [Google Scholar]
- 8.Tobi M, Kim M, Zimmer R, Hatfield J, Kam M, Khoury N, Carville A, Lawson MJ, Schiemann WP, Thomas P. Colorectal cancer in the cotton top tamarin (Saguinus oedipus): how do they evade liver metastasis? Dig Dis Sci. 2011;56:397–405. doi: 10.1007/s10620-010-1314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lushbaugh C, Humason G, Clapp N. Histology of colitis: Saguinus oedipus oedipus and other marmosets. Dig Dis Sci. 1985;30:45S–51S. doi: 10.1007/BF01296974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 11.Tarcic O, Pateras IS, Cooks T, Shema E, Kanterman J, Ashkenazi H, Boocholez H, Hubert A, Rotkopf R, Baniyash M, et al. RNF20 Links Histone H2B Ubiquitylation with Inflammation and Inflammation-Associated Cancer. Cell Rep. 2016;14:1462–1476. doi: 10.1016/j.celrep.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas P, Forse RA, Bajenova O. Carcinoembryonic antigen (CEA) and its receptor hnRNP M are mediators of metastasis and the inflammatory response in the liver. Clin Exp Metastasis. 2011;28:923–932. doi: 10.1007/s10585-011-9419-3. [DOI] [PubMed] [Google Scholar]
- 15.Thomas P, Toth CA, Saini KS, Jessup JM, Steele G. The structure, metabolism and function of the carcinoembryonic antigen gene family. Biochim Biophys Acta. 1990;1032:177–189. doi: 10.1016/0304-419x(90)90003-j. [DOI] [PubMed] [Google Scholar]
- 16.Hammarström S. The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 17.Gold P, Freedman SO. Specific carcinoembryonic antigens of the human digestive system. J Exp Med. 1965;122:467–481. doi: 10.1084/jem.122.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beauchemin N, Arabzadeh A. Carcinoembryonic antigen-related cell adhesion molecules (CEACAMs) in cancer progression and metastasis. Cancer Metastasis Rev. 2013;32:643–671. doi: 10.1007/s10555-013-9444-6. [DOI] [PubMed] [Google Scholar]
- 19.Paxton RJ, Mooser G, Pande H, Lee TD, Shively JE. Sequence analysis of carcinoembryonic antigen: identification of glycosylation sites and homology with the immunoglobulin supergene family. Proc Natl Acad Sci USA. 1987;84:920–924. doi: 10.1073/pnas.84.4.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beauchemin N, Draber P, Dveksler G, Gold P, Gray-Owen S, Grunert F, Hammarström S, Holmes KV, Karlsson A, Kuroki M, et al. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res. 1999;252:243–249. doi: 10.1006/excr.1999.4610. [DOI] [PubMed] [Google Scholar]
- 21.Aarons CB, Bajenova O, Andrews C, Heydrick S, Bushell KN, Reed KL, Thomas P, Becker JM, Stucchi AF. Carcinoembryonic antigen-stimulated THP-1 macrophages activate endothelial cells and increase cell-cell adhesion of colorectal cancer cells. Clin Exp Metastasis. 2007;24:201–209. doi: 10.1007/s10585-007-9069-7. [DOI] [PubMed] [Google Scholar]
- 22.Benchimol S, Fuks A, Jothy S, Beauchemin N, Shirota K, Stanners CP. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989;57:327–334. doi: 10.1016/0092-8674(89)90970-7. [DOI] [PubMed] [Google Scholar]
- 23.Ordoñez C, Screaton RA, Ilantzis C, Stanners CP. Human carcinoembryonic antigen functions as a general inhibitor of anoikis. Cancer Res. 2000;60:3419–3424. [PubMed] [Google Scholar]
- 24.Bramswig KH, Poettler M, Unseld M, Wrba F, Uhrin P, Zimmermann W, Zielinski CC, Prager GW. Soluble carcinoembryonic antigen activates endothelial cells and tumor angiogenesis. Cancer Res. 2013;73:6584–6596. doi: 10.1158/0008-5472.CAN-13-0123. [DOI] [PubMed] [Google Scholar]
- 25.Zamcheck N. The expanding field of colorectal cancer markers: CEA, the prototype. Cancer Bull. 1981;33:141–151. [Google Scholar]
- 26.Thomas P, Zamcheck N. Role of the liver in clearance and excretion of circulating carcinoembryonic antigen (CEA) Dig Dis Sci. 1983;28:216–224. doi: 10.1007/BF01295116. [DOI] [PubMed] [Google Scholar]
- 27.Zamcheck N, Liu P, Thomas P, Steele G. Search for useful biomarkers of early malignant tumors. In: Steele G, Burt RW, Winawer SJ, Karr JP, editors. Basic and Clinical Perspectives of Colorectal Polyps and Cancer (Progress in Clinical and Biological Research) New York: Clinical and Biological Research; 1988. pp. 251–275. [PubMed] [Google Scholar]
- 28.Goldstein MJ, Mitchell EP. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Invest. 2005;23:338–351. doi: 10.1081/cnv-58878. [DOI] [PubMed] [Google Scholar]
- 29.Goldenberg DM, Larson SM. Radioimmunodetection in cancer identification. J Nucl Med. 1992;33:803–814. [PubMed] [Google Scholar]
- 30.Behr TM, Sharkey RM, Juweid ME, Dunn RM, Vagg RC, Ying Z, Zhang CH, Swayne LC, Vardi Y, Siegel JA, et al. Phase I/II clinical radioimmunotherapy with an iodine-131-labeled anti-carcinoembryonic antigen murine monoclonal antibody IgG. J Nucl Med. 1997;38:858–870. [PubMed] [Google Scholar]
- 31.Morse MA, Nair SK, Mosca PJ, Hobeika AC, Clay TM, Deng Y, Boczkowski D, Proia A, Neidzwiecki D, Clavien PA, et al. Immunotherapy with autologous, human dendritic cells transfected with carcinoembryonic antigen mRNA. Cancer Invest. 2003;21:341–349. doi: 10.1081/cnv-120018224. [DOI] [PubMed] [Google Scholar]
- 32.O’Brien MJ, Bronstein B, Zamcheck N, Saravis C, Burke B, Gottlieb LS. Cholestasis and hepatic metastases: a factor contributing to extreme elevations of carcinoembryonic antigen. J Natl Cancer Inst. 1980;64:1291–1294. doi: 10.1093/jnci/64.6.1291. [DOI] [PubMed] [Google Scholar]
- 33.Bajenova OV, Zimmer R, Stolper E, Salisbury-Rowswell J, Nanji A, Thomas P. Heterogeneous RNA-binding protein M4 is a receptor for carcinoembryonic antigen in Kupffer cells. J Biol Chem. 2001;276:31067–31073. doi: 10.1074/jbc.M104093200. [DOI] [PubMed] [Google Scholar]
- 34.Han SP, Tang YH, Smith R. Functional diversity of the hnRNPs: past, present and perspectives. Biochem J. 2010;430:379–392. doi: 10.1042/BJ20100396. [DOI] [PubMed] [Google Scholar]
- 35.Han N, Li W, Zhang M. The function of the RNA-binding protein hnRNP in cancer metastasis. J Cancer Res Ther. 2013;9 Suppl:S129–S134. doi: 10.4103/0973-1482.122506. [DOI] [PubMed] [Google Scholar]
- 36.Marko M, Leichter M, Patrinou-Georgoula M, Guialis A. hnRNP M interacts with PSF and p54(nrb) and co-localizes within defined nuclear structures. Exp Cell Res. 2010;316:390–400. doi: 10.1016/j.yexcr.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 37.Bajenova O, Stolper E, Gapon S, Sundina N, Zimmer R, Thomas P. Surface expression of heterogeneous nuclear RNA binding protein M4 on Kupffer cell relates to its function as a carcinoembryonic antigen receptor. Exp Cell Res. 2003;291:228–241. doi: 10.1016/s0014-4827(03)00373-2. [DOI] [PubMed] [Google Scholar]
- 38.Datar KV, Dreyfuss G, Swanson MS. The human hnRNP M proteins: identification of a methionine/arginine-rich repeat motif in ribonucleoproteins. Nucleic Acids Res. 1993;21:439–446. doi: 10.1093/nar/21.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soeth E, Wirth T, List HJ, Kumbhani S, Petersen A, Neumaier M, Czubayko F, Juhl H. Controlled ribozyme targeting demonstrates an antiapoptotic effect of carcinoembryonic antigen in HT29 colon cancer cells. Clin Cancer Res. 2001;7:2022–2030. [PubMed] [Google Scholar]
- 40.Samara RN, Laguinge LM, Jessup JM. Carcinoembryonic antigen inhibits anoikis in colorectal carcinoma cells by interfering with TRAIL-R2 (DR5) signaling. Cancer Res. 2007;67:4774–4782. doi: 10.1158/0008-5472.CAN-06-4315. [DOI] [PubMed] [Google Scholar]
- 41.Gangopadhyay A, Bajenova O, Kelly TM, Thomas P. Carcinoembryonic antigen induces cytokine expression in Kuppfer cells: implications for hepatic metastasis from colorectal cancer. Cancer Res. 1996;56:4805–4810. [PubMed] [Google Scholar]
- 42.Gangopadhyay A, Lazure DA, Thomas P. Adhesion of colorectal carcinoma cells to the endothelium is mediated by cytokines from CEA stimulated Kupffer cells. Clin Exp Metastasis. 1998;16:703–712. doi: 10.1023/a:1006576627429. [DOI] [PubMed] [Google Scholar]
- 43.Jessup JM, Laguinge L, Lin S, Samara R, Aufman K, Battle P, Frantz M, Edmiston KH, Thomas P. Carcinoembryonic antigen induction of IL-10 and IL-6 inhibits hepatic ischemic/reperfusion injury to colorectal carcinoma cells. Int J Cancer. 2004;111:332–337. doi: 10.1002/ijc.20264. [DOI] [PubMed] [Google Scholar]
- 44.Zhou H, Fuks A, Alcaraz G, Bolling TJ, Stanners CP. Homophilic adhesion between Ig superfamily carcinoembryonic antigen molecules involves double reciprocal bonds. J Cell Biol. 1993;122:951–960. doi: 10.1083/jcb.122.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bramswig KH, Prager GW, Kallinowska W, Zielinski C, Prager G. Carcinoembryonic antigen (CEA) affects tumor angiogenesis in colon carcinomas. Denver, CO. Philadelphia (PA): AACR; 2009. p. Abstract 3183. [Google Scholar]
- 46.Bramswig KH, Prager GW, Martel A, Heinze G, Binder BR, Brodowicz T, Kornek G, Scheithauer W, Zielinski C. Predictive role of carcinoembryonic antigen (CEA) for therapeutic efficacy of angiogenesis-targeting therapy in colorectal cancer: Novel clinical observation based on recently discovered CEA biology. J Clin Oncol. 2010;28:3574. [Google Scholar]
- 47.Prager GW, Braemswig KH, Martel A, Unseld M, Heinze G, Brodowicz T, Scheithauer W, Kornek G, Zielinski CC. Baseline carcinoembryonic antigen (CEA) serum levels predict bevacizumab-based treatment response in metastatic colorectal cancer. Cancer Sci. 2014;105:996–1001. doi: 10.1111/cas.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Low-Marchelli JM, Ardi VC, Vizcarra EA, van Rooijen N, Quigley JP, Yang J. Twist1 induces CCL2 and recruits macrophages to promote angiogenesis. Cancer Res. 2013;73:662–671. doi: 10.1158/0008-5472.CAN-12-0653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271:736–741. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- 50.Palermo NY, Thomas P, Murphy RF, Lovas S. Hexapeptide fragment of carcinoembryonic antigen which acts as an agonist of heterogeneous ribonucleoprotein M. J Pept Sci. 2012;18:252–260. doi: 10.1002/psc.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Albig AR, Schiemann WP. Fibulin-5 antagonizes vascular endothelial growth factor (VEGF) signaling and angiogenic sprouting by endothelial cells. DNA Cell Biol. 2004;23:367–379. doi: 10.1089/104454904323145254. [DOI] [PubMed] [Google Scholar]
- 52.Albig AR, Neil JR, Schiemann WP. Fibulins 3 and 5 antagonize tumor angiogenesis in vivo. Cancer Res. 2006;66:2621–2629. doi: 10.1158/0008-5472.CAN-04-4096. [DOI] [PubMed] [Google Scholar]
- 53.Sullivan KM, Bissonnette R, Yanagisawa H, Hussain SN, Davis EC. Fibulin-5 functions as an endogenous angiogenesis inhibitor. Lab Invest. 2007;87:818–827. doi: 10.1038/labinvest.3700594. [DOI] [PubMed] [Google Scholar]
- 54.Barnard D, Knapka JJ. Callitrichid Nutrition. In: Clapp NK, editor. A Primate Model for the Study of Colitis and Colon Carcinoma The Cotton Top Tamarin Saguinus oedipus. Boca Raton: CRC Press; 1993. pp. 55–79. [Google Scholar]
- 55.Mast RB, Rodriguez JV, Mittermeier RA. The Colombian Cotton Top Tamarin in the Wild. In: Clapp NK, editor. A primate Model for the Study of Colitis and Colon Carcinoma The Cotton Top Tamarin Saguinus oedipus. Boca Raton: CRC Press; 1993. pp. 4–43. [Google Scholar]
- 56.Clapp NK, Henke MA. Spontaneous colonic carcinoma observations in the Oak Ridge Associated Universities’ 26-year-old Cotton-Top Tamarin (Saguinus Oedipus) Colony. In: Clapp NK, editor. A primate Model for the Study of Colitis and Colon Carcinoma The Cotton Top Tamarin Saguinus oedipus. Boca Raton: CRC Press; 1993. pp. 171–185. [Google Scholar]
- 57.Murff HJ. Cohort analysis finds that the proportion of people who meet high risk criteria for colorectal, breast or prostate cancer screening based on family history increases between age 30 and 50. Evid Based Med. 2012;17:50–51. doi: 10.1136/ebm.2011.100190. [DOI] [PubMed] [Google Scholar]
- 58.Mao X, McGuire S, Hamoudi RA. Molecular and cytogenetic analysis of lymphoblastoid and colon cancer cell lines from cotton-top tamarin (Sagiunus oedipus) Cancer Genet Cytogenet. 2000;120:6–10. doi: 10.1016/s0165-4608(99)00237-x. [DOI] [PubMed] [Google Scholar]
- 59.Fuhr JE, Van Meter S, Andrews RB, Clapp NK. Tamarin colon cancer and flow cytometry. In: Clapp NK, editor. A primate Model for the Study of Colitis and Colon Carcinoma The Cotton Top Tamarin Saguinus oedipus. Boca Raton: CRC Press; 1993. pp. 231–238. [Google Scholar]
- 60.Tobi M, Chintalapani S, Kithier K, Clapp N. Gastrointestinal tract antigenic profile of cotton-top tamarin, Saguinus oedipus, is similar to that of humans with inflammatory bowel disease. Dig Dis Sci. 2000;45:2290–2297. doi: 10.1023/a:1005622521294. [DOI] [PubMed] [Google Scholar]
- 61.Itzkowitz SH, Harpaz N. Diagnosis and management of dysplasia in patients with inflammatory bowel diseases. Gastroenterology. 2004;126:1634–1648. doi: 10.1053/j.gastro.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 62.Mattar MC, Lough D, Pishvaian MJ, Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Cancer Res. 2011;4:53–61. [PMC free article] [PubMed] [Google Scholar]
- 63.Murphy PM. Chemokines and the molecular basis of cancer metastasis. N Engl J Med. 2001;345:833–835. doi: 10.1056/NEJM200109133451113. [DOI] [PubMed] [Google Scholar]
- 64.Westernströer B, Langenstroth D, Kliesch S, Troppmann B, Redmann K, Macdonald J, Mitchell R, Wistuba J, Schlatt S, Neuhaus N. Developmental expression patterns of chemokines CXCL11, CXCL12 and their receptor CXCR7 in testes of common marmoset and human. Cell Tissue Res. 2015;361:885–898. doi: 10.1007/s00441-015-2164-1. [DOI] [PubMed] [Google Scholar]
- 65.Olsson AY, Valtonen-André C, Lilja H, Lundwall A. The evolution of the glandular kallikrein locus: identification of orthologs and pseudogenes in the cotton-top tamarin. Gene. 2004;343:347–355. doi: 10.1016/j.gene.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 66.Boland CR, Clapp NK. Glycoconjugates in the colons of New World monkeys with spontaneous colitis. Association between inflammation and neoplasia. Gastroenterology. 1987;92:625–634. doi: 10.1016/0016-5085(87)90010-2. [DOI] [PubMed] [Google Scholar]
- 67.Tobi M, Memon M, Kithier K, Clapp N. A putative CEA moiety is shared by the cotton-top tamarin (Saguinus oedipus) and humans. Cancer Lett. 1994;77:7–13. doi: 10.1016/0304-3835(94)90341-7. [DOI] [PubMed] [Google Scholar]
- 68.Haagensen DE, Metzgar RS, Swenson B, Dilley WG, Cox CE, Davis S, Murdoch J, Zamcheck N, Wells SA. Carcinoembryonic antigen in nonhuman primates. J Natl Cancer Inst. 1982;69:1073–1076. [PubMed] [Google Scholar]
- 69.Beauchemin N, Turbide C, Afar D, Bell J, Raymond M, Stanners CP, Fuks A. A mouse analogue of the human carcinoembryonic antigen. Cancer Res. 1989;49:2017–2021. [PubMed] [Google Scholar]
- 70.Tobi M, Kaila V, Chintalapani S, Kithier K, Henke MA, Clapp NK. An antigenic profile in cotton-top tamarins (Saguinus oedipus): a model for human inflammatory bowel disease and colorectal cancer. In: Clapp NK, editor. A primate Model for the Study of Colitis and Colon Carcinoma The Cotton Top Tamarin Saguinus oedipus. Boca Raton: CRC Press; 1993. pp. 113–125. [Google Scholar]
- 71.Tobi M, Darmon CE, Rozen P, Harpaz N, Fink A, Maliakkal B, Halline A, Mobarhan S, Bentwich Z. Urinary organ specific neoantigen. A potentially diagnostic test for colorectal cancer. Dig Dis Sci. 1995;40:1531–1537. doi: 10.1007/BF02285204. [DOI] [PubMed] [Google Scholar]
- 72.Tobi M, Clapp N. Is the Cotton-Top Tamarin an appropriate model for human inflammatory bowel disease? Agents and Actions. 1994;41:C246–C248. [Google Scholar]
- 73.Tobi M, Chintalapani S, Kithier K, Clapp N. Carcinoembryonic antigen family of adhesion molecules in the cotton top tamarin (Saguinus oedipus) Cancer Lett. 2000;157:45–50. doi: 10.1016/s0304-3835(00)00482-1. [DOI] [PubMed] [Google Scholar]
- 74.Tobi M, Maliakkal B, Zitron I, Alousi M, Goo R, Nochomovitz L, Luk G. Adenoma-derived antibody, Adnab-9 recognizes a membrane-bound glycoprotein in colonic tissue and effluent material from patients with colorectal neoplasia. Cancer Lett. 1992;67:61–69. doi: 10.1016/0304-3835(92)90009-k. [DOI] [PubMed] [Google Scholar]
- 75.Tobi M, Yordanova V, Hatfield J, Hallman J, Khoury N, Bajenova O, Clapp NK, Lawson MJ, Sehgal PV, Carville A, et al. Inhibition of Kupffer cell CEA-uptake averts liver metastases in a spontaneous colorectal cancer animal model: Chemoblockade in humans may provide a target for intervention. Cancer Res. 2006;66:820. [Google Scholar]
- 76.Hu Y, Gao H, Vo C, Ke C, Pan F, Yu L, Siegel E, Hess KR, Linskey ME, Zhou YH. Anti-EGFR function of EFEMP1 in glioma cells and patient prognosis. Oncoscience. 2014;1:205–215. doi: 10.18632/oncoscience.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Naghibalhossaini F, Yoder AD, Tobi M, Stanners CP. Evolution of a tumorigenic property conferred by glycophosphatidyl-inositol membrane anchors of carcinoembryonic antigen gene family members during the primate radiation. Mol Biol Cell. 2007;18:1366–1374. doi: 10.1091/mbc.E06-10-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zimmer R, Thomas P. Mutations in the carcinoembryonic antigen gene in colorectal cancer patients: implications on liver metastasis. Cancer Res. 2001;61:2822–2826. [PubMed] [Google Scholar]
- 79.Kithier K, Samal B, Cejka J, Whitcomb MP, Mood DW. Pancreatic oncofetal antigen and carcinoembryonic antigen in breast and colon carcinoma. Tumour Biol. 1988;9:307–314. doi: 10.1159/000217577. [DOI] [PubMed] [Google Scholar]
- 80.Wilson AD, Shooshtari M, Finerty S, Watkins P, Morgan AJ. Selection of monoclonal antibodies for the identification of lymphocyte surface antigens in the New World primate Saguinus oedipus oedipus (cotton top tamarin) J Immunol Methods. 1995;178:195–200. doi: 10.1016/0022-1759(94)00256-v. [DOI] [PubMed] [Google Scholar]
- 81.Kap YS, van Meurs M, van Driel N, Koopman G, Melief MJ, Brok HP, Laman JD, ’t Hart BA. A monoclonal antibody selection for immunohistochemical examination of lymphoid tissues from non-human primates. J Histochem Cytochem. 2009;57:1159–1167. doi: 10.1369/jhc.2009.954123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Podolsky DK, Lobb R, King N, Benjamin CD, Pepinsky B, Sehgal P, deBeaumont M. Attenuation of colitis in the cotton-top tamarin by anti-alpha 4 integrin monoclonal antibody. J Clin Invest. 1993;92:372–380. doi: 10.1172/JCI116575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Adam R. Chemotherapy and surgery: new perspectives on the treatment of unresectable liver metastases. Ann Oncol. 2003;14 Suppl 2:ii13–ii16. doi: 10.1093/annonc/mdg731. [DOI] [PubMed] [Google Scholar]
- 84.Van Cutsem E, Nordlinger B, Adam R, Köhne CH, Pozzo C, Poston G, Ychou M, Rougier P; European Colorectal Metastases Treatment Group. Towards a pan-European consensus on the treatment of patients with colorectal liver metastases. Eur J Cancer. 2006;42:2212–2221. doi: 10.1016/j.ejca.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 85.Nordlinger B, Van Cutsem E, Rougier P, Köhne CH, Ychou M, Sobrero A, Adam R, Arvidsson D, Carrato A, Georgoulias V, et al. Does chemotherapy prior to liver resection increase the potential for cure in patients with metastatic colorectal cancer? A report from the European Colorectal Metastases Treatment Group. Eur J Cancer. 2007;43:2037–2045. doi: 10.1016/j.ejca.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 86.Smedsrød B, Le Couteur D, Ikejima K, Jaeschke H, Kawada N, Naito M, Knolle P, Nagy L, Senoo H, Vidal-Vanaclocha F, et al. Hepatic sinusoidal cells in health and disease: update from the 14th International Symposium. Liver Int. 2009;29:490–501. doi: 10.1111/j.1478-3231.2009.01979.x. [DOI] [PubMed] [Google Scholar]
- 87.Rozier A, Mazuel C, Grove J, Plazonnet B. Functionality testing of gellan gum, a polymeric excipient material for ophthalmic dosage forms. Int J Pharm. 1997;153:191–198. [Google Scholar]
- 88.Kanari B, Banik RR, Upadhyay SN. Effect of environmental factors and carbohydrate on gellan gum production. Appl Biochem Biotechnol. 2002;102-103:129–140. doi: 10.1385/abab:102-103:1-6:129. [DOI] [PubMed] [Google Scholar]
- 89.Banik RM, Santhiagu A. Improvement in production and quality of gellan gum by Sphingomonas paucimobilis under high dissolved oxygen tension levels. Biotechnol Lett. 2006;28:1347–1350. doi: 10.1007/s10529-006-9098-3. [DOI] [PubMed] [Google Scholar]
- 90.Antony PJ, Sanghavi NM. A New Binder for Pharmaceutic al Dosage Forms. Drug Dev Ind Pharm. 1997;23:417–418. [Google Scholar]
- 91.Devineni D, Ezekwudo D, Palaniappan R. Formulation of maltodextrin entrapped in polycaprolactone microparticles for protein and vaccine delivery: effect of size determining formulation process variables of microparticles on the hydrodynamic diameter of BSA. J Microencapsul. 2007;24:358–370. doi: 10.1080/02652040701279104. [DOI] [PubMed] [Google Scholar]
- 92.Meng H, Xing G, Blanco E, Song Y, Zhao L, Sun B, Li X, Wang PC, Korotcov A, Li W, et al. Gadolinium metallofullerenol nanoparticles inhibit cancer metastasis through matrix metalloproteinase inhibition: imprisoning instead of poisoning cancer cells. Nanomedicine. 2012;8:136–146. doi: 10.1016/j.nano.2011.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tu K, Dou C, Zheng X, Li C, Yang W, Yao Y, Liu Q. Fibulin-5 inhibits hepatocellular carcinoma cell migration and invasion by down-regulating matrix metalloproteinase-7 expression. BMC Cancer. 2014;14:938. doi: 10.1186/1471-2407-14-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Møller HD, Ralfkjær U, Cremers N, Frankel M, Pedersen RT, Klingelhöfer J, Yanagisawa H, Grigorian M, Guldberg P, Sleeman J, et al. Role of fibulin-5 in metastatic organ colonization. Mol Cancer Res. 2011;9:553–563. doi: 10.1158/1541-7786.MCR-11-0093. [DOI] [PubMed] [Google Scholar]
- 95.Hwang CF, Shiu LY, Su LJ, Yu-Fang Yin WS, Huang SC, Chiu TJ, Huang CC, Zhen YY, Tsai HT, Fang FM, et al. Oncogenic fibulin-5 promotes nasopharyngeal carcinoma cell metastasis through the FLJ10540/AKT pathway and correlates with poor prognosis. PLoS One. 2013;8:e84218. doi: 10.1371/journal.pone.0084218. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.Kaur G, Roy I. Therapeutic applications of aptamers. Expert Opin Investig Drugs. 2008;17:43–60. doi: 10.1517/13543784.17.1.43. [DOI] [PubMed] [Google Scholar]
- 97.Wang L, Liu B, Yin H, Wei J, Qian X, Yu L. Selection of DNA aptamer that specific binding human carcinoembryonic antigen in vitro. Nanjing Yike Daxue Zazhi. 2007;21:277–278. [Google Scholar]
- 98.Lee YJ, Han SR, Kim NY, Lee SH, Jeong JS, Lee SW. An RNA aptamer that binds carcinoembryonic antigen inhibits hepatic metastasis of colon cancer cells in mice. Gastroenterology. 2012;143:155–65.e8. doi: 10.1053/j.gastro.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 99.Wagner HE, Toth CA, Steele GD, Thomas P. Metastatic potential of human colon cancer cell lines: relationship to cellular differentiation and carcinoembryonic antigen production. Clin Exp Metastasis. 1992;10:25–31. doi: 10.1007/BF00163573. [DOI] [PubMed] [Google Scholar]
- 100.Orava EW, Abdul-Wahid A, Huang EH, Mallick AI, Gariépy J. Blocking the attachment of cancer cells in vivo with DNA aptamers displaying anti-adhesive properties against the carcinoembryonic antigen. Mol Oncol. 2013;7:799–811. doi: 10.1016/j.molonc.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang LF, Wei J, Yin HT, Qian XP, Yu LX, Liu B. In vitro selection of specific binding DNA aptamer to human carcinoembryonic antigen. Yixue Yanjiusheng Xuebao. 2007;9:2004–2005. [Google Scholar]
- 102.Correa CR, de Barros AL, Ferreira Cde A, de Goes AM, Cardoso VN, de Andrade AS. Aptamers directly radiolabeled with technetium-99m as a potential agent capable of identifying carcinoembryonic antigen (CEA) in tumor cells T84. Bioorg Med Chem Lett. 2014;24:1998–2001. doi: 10.1016/j.bmcl.2014.02.048. [DOI] [PubMed] [Google Scholar]


