Abstract
Objectives
Red cell distribution width (RDW) and neutrophil-to-lymphocyte ratio (NLR) are the two markers used to determine risk of mortality and adverse cardiovascular outcomes in patients with acute myocardial infarction. The relationship between RDW, NLR, and left ventricular (LV) systolic functions has not been reported. In this report, we aimed to investigate the relationship between RDW, NLR, and LV systolic function in anterior ST-segment elevation myocardial infarction (STEMI) patients who underwent primary percutaneous coronary intervention (PCI).
Methods
RDW and NLR were measured on admission in 106 STEMI patients treated with primary PCI. Patients were divided into two groups according to left ventricular ejection fraction (LVEF), as Group I (systolic dysfunction, LVEF <50%) and Group II (preserved global left ventricle systolic function, LVEF ⩾50%). The first group included 47 patients and the second group included 59 patients.
Results
Mean RDW and NLR were significantly higher in Group I compared to Group II [13.7 ± 0.9% vs. 13.4 ± 0.7%, p = 0.03 and 5.86 (range, 0.66–40.50) vs. 2.75 (range, 0.51–39.39), p = 0.013, respectively].
Conclusion
Increased RDW and NLR on admission, in anterior STEMI patients treated with primary PCI are associated with LV systolic dysfunction.
Keywords: Myocardial infarction, Neutrophil-to-lymphocyte ratio, Primary percutaneous coronary intervention, Red cell distribution width, Systolic dysfunction
Abbreviations
- RDW
Red cell distribution width
- NLR
Neutrophil-to-lymphocyte ratio
- LV
Left ventricular
- AMI
Acute myocardial infarction
- WBC
White blood cell
- STEMI
ST-segment elevation myocardial infarction
- LVEF
Left ventricular ejection fraction
- PCI
Primary percutaneous coronary intervention
- LAD
Left anterior descending coronary artery
- TIMI
Thrombolysis in myocardial infarction
- DM
Diabetes mellitus
Introduction
Atherosclerosis is a multifactorial disease and the major cause of cardiovascular disease that still accounts for most of the mortality worldwide [1]. The role of inflammation in the development and progression of atherosclerosis has been clarified and several biological markers of inflammation predict cardiovascular risk [2], [3]. Since it is an inflammatory disease, some inflammatory markers have been proposed for the evaluation of the cardiovascular risk. Red cell distribution width (RDW) and neutrophil-to-lymphocyte ratio (NLR) are the two markers of inflammation that are used to determine risk of mortality and adverse cardiovascular outcomes in patients with acute myocardial infarction (AMI) [4], [5].
RDW is a laboratory measure of the variability in erythrocyte volume and is easily measured during routine complete blood counts [6]. RDW is generally used as an indicator of the differential diagnosis of anemia [5]. However, recent studies have reported that higher RDW is found to be a strong independent predictor of increased risk of mortality and adverse cardiovascular events in patients with heart failure, stable coronary artery disease, acute coronary syndrome, AMI, cardiovascular disease, and also in the general population [5], [7], [8], [9], [10], [11].
White blood cell (WBC) count and its subtypes are also known as classic markers of inflammation in cardiovascular diseases [12]. NLR was introduced as a potential marker to determine inflammation in cardiac and noncardiac disorders, and shown as a predictor of long-term mortality in patients who underwent percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction (STEMI) [13], [14]. As neutrophil and lymphocyte values are readily available in routine blood count analysis, NLR may be used as a cost-effective predictor of inflammation and cardiovascular complications [4].
Left ventricular ejection fraction (LVEF) is a parameter used for assessment of left ventricular systolic function in daily practice and has been shown to be an efficacious predictor of prognosis after AMI [15]. The relationship between RDW and NLR obtained from complete blood count during first admission to hospital and left ventricular systolic functions is unknown. The goal of this study was to evaluate the relationship between RDW and NLR level on admission and left ventricular systolic functions in first anterior STEMI patients who underwent primary percutaneous coronary intervention (PCI).
Materials and methods
Patients
Medical records of consecutive patients with acute anterior STEMI who were admitted to the emergency department of our hospital between February 2008 and August 2013 were examined retrospectively. Patients <6 hours from symptom onset and who underwent primary PCI for the proximal or the mid-left anterior descending coronary artery lesions and have postinterventional thrombolysis in myocardial infarction (TIMI) III flow after primary PCI were enrolled in the study. Patients were excluded from the study if they had the following criteria: (1) a history of any heart disease including myocardial infarction, revascularization, angina pectoris, heart failure, valvular heart disease, congenital heart disease, and atrial fibrillation; (2) cardiogenic shock before the procedure, resuscitated arrest and arrest under mechanical ventilation, hemodynamically important atrioventricular block (2nd or 3rd-degree atrioventricular block), thrombolytic administration before primary PCI; or (3) clinical evidence of active infection, cancer, hematological disease, systemic inflammatory conditions, autoimmune disease, end-stage liver disease and renal failure, anemia (hemoglobin levels <13 g/dL in men and 12 g/dL in women), pregnancy, or recent blood transfusion.
Study protocol
A 12-lead electrocardiogram was recorded in each patient immediately after hospital admission. On admission, venous blood was obtained from all the patients. RDW, neutrophils, lymphocytes, and white blood cells were measured as part of the automated complete blood count before starting any medication. The NLR was calculated as the ratio of the neutrophils and lymphocytes, both obtained from the same automated blood sample at admission. All measurements were performed 30 minutes after blood collection by an automatic blood counter. The lipid profile was measured following the first fasting period. A clinical history of age, sex, diabetes mellitus (DM), hypertension, hyperlipidemia, and smoking was determined from medical records.
All patients received 300 mg clopidogrel loading dose and 300 mg aspirin before the primary PCI. The patients’ angiographic data were evaluated from catheter laboratory records. Emergency coronary angiography was performed using the percutaneous femoral approach. Unfractioned heparin (60–100 U/kg) was administered when coronary anatomy was first defined. Primary PCI was performed to infarct related artery. After angioplasty, all patients were admitted to the coronary care unit, where intravenous heparin or subcutaneous low-molecular weight heparin was administered; 100 mg aspirin and 75 mg clopidogrel were continued in all patients. Use of glycoprotein IIb/IIIa inhibitors was left to operators’ preference. TIMI flows were estimated according to Gibson et al.’s [16] method. Patients without TIMI III flow after angioplasty were excluded from the study.
Echocardiographic data were obtained from patients’ record. A two-dimensional transthoracic echocardiography was performed in the left lateral decubitus position with the Vivid 7 system (GE-Vingmed Ultrasound AS, Horten, Norway) ultrasound device and a 3S-RS (3.5 MHz) probe. Echocardiographic examinations were performed according to the guidelines of American Society of Echocardiography [17]. Patients were divided into two groups according to their LVEF. The first group included patients with a depressed left ventricle systolic function (LVEF <50%), and the second group included patients with a preserved left ventricle systolic function (LVEF ⩾50%).
Definitions
Anterior STEMI was defined when a patient had typical chest pain for at least 30 minutes with >2 mm ST elevation in at least two consecutive anterior derivations. Hypertension was considered to be present if the systolic pressure was >140 mmHg and/or diastolic pressure was >90 mmHg for at least two separate measurements, or the previous use of antihypertensive drugs. DM was defined as a fasting blood glucose level >126 mg/dL or current use of a diet or medication to lower blood glucose. Anemia was defined as hemoglobin levels <13 g/dL in men and 12 g/dL in women, in accordance with the World Health Organization criteria [18].
Statistical analysis
Pearson Chi-square and Fisher’s exact tests were used to compare the incidence of categorical variables among groups. Categorical variables were presented as counts and percentages. The Kolmogorov–Smirnov and Shapiro–Wilks tests were used to evaluate whether the distribution of variables was normal. The two independent sample t test or Mann–Whitney U test was used to compare continuous variables between the two groups. Continuous variables were presented as mean (standard deviation) or as median (range). SPSS software 18.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for all statistical analysis. A multivariable logistic regression model was used to evaluate the independent contribution of NLR and RDW to the risk of LV systolic dysfunction. Age, NLR, RDW, and fasting glucose levels were selected for multivariable logistic regression analyses. The RDW and NLR values were implemented as continuous variables for analyses. The adjusted odd ratios and 95% confidence intervals are presented. Calculated p-values were considered statistically significant when they were <0.05.
Results
Baseline characteristics
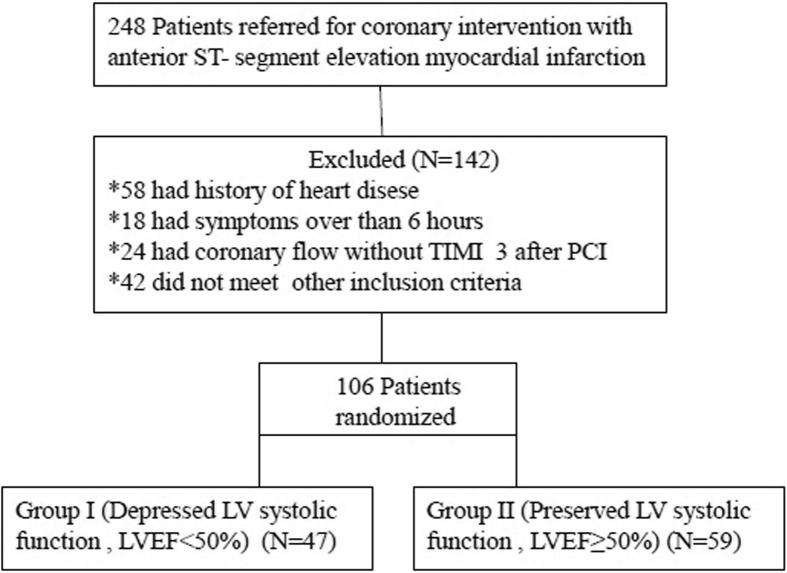
Of 248 patients enrolled with first anterior STEMI, 142 were excluded due to exclusion criteria (Fig. 1). Group I (LVEF <50%) included 47 patients, and Group II (LVEF ⩾50%) included 59 patients. On comparison of the two groups, there were no differences in age, sex, body mass index, hypertension, DM, smoking status, TIMI flow, localization of the culprit lesion, or use of glycoprotein IIb/IIIa inhibitors. Baseline characteristics are shown in Table 1.
Figure 1.
Patient flow.
Table 1.
Baseline characteristics in the study groups.
| Group I (n = 47) |
Group II (n = 59) |
p | |
|---|---|---|---|
| Age (y) | 55 ± 13 | 55 ± 11 | 0.728 |
| Male | 36 (77) | 48 (81) | 0.548 |
| BMI (kg/m2) | 27 ± 4 | 27 ± 3 | 0.314 |
| Creatinine (mg/dL) | 0.89 (0.5–1.7) | 0.90 (0.5–1.7) | 0.187 |
| Troponine (ng/L) | 0.13 (0.003–19.20) | 0.046 (0.001–6.67) | 0.061 |
| LDL cholesterol (mg/dL) | 110 ± 32 | 118 ± 32 | 0.184 |
| HT | 22 (47) | 29 (49) | 0.810 |
| DM | 17 (36) | 14 (24) | 0.162 |
| Current smoker | 22 (47) | 28 (48) | 0.947 |
| Tirofiban | 8 (17) | 17 (29) | 0.155 |
| Culprit lesion | 0.143 | ||
| Proximal | 35 (74.5) | 36 (61.0) | |
| Medial | 12 (25.5) | 23 (39.0) | |
| TIMI flow | 0.712 | ||
| TIMI 0 | 34 (72.3) | 38 (64.4) | |
| TIMI 1 | 8 (17.0) | 12 (20.3) | |
| TIMI 2 | 5 (10.7) | 8 (13.6) | |
| TIMI 3 | 0 | 1 (1.7) |
Data are presented as n (%), mean ± SD, or median (range).
BMI = body mass index; DM = diabetes mellitus; HT = hypertension; LDL = low-density lipoprotein; SD = standard deviation; TIMI = thrombolysis in myocardial infarction.
Laboratory findings
There was no statistically significant difference in blood glucose levels, creatinine, troponin, hemoglobin, hematocrit, leukocyte, mean corpuscular volume, platelet, or low-density lipoprotein level. There was a significant difference in neutrophil and lymphocyte counts [9.39 × 109/L (3.1–19.52 × 109/L) vs. 7.3 × 109/L (3.61–19.70 × 109/L), p = 0.037 and 1700 (400–6930) vs. 2600 (396–8100), p = 0.021, respectively], RDW and NLR levels [13.7 ± 0.9% vs. 13.4 ± 0.7%, p = 0.03 and 5.86 (0.66–40.50) vs. 2.75 (0.51–39.39), p = 0.013, respectively]. Laboratory test results are shown in Table 2. For LV systolic dysfunction; age, NLR, RDW, and fasting glucose level at admission were analyzed using a multivariate logistic regression model. The RDW was the only independent predictor of LV systolic dysfunction (odds ratio 1.797, 95% confidence interval 1.062–3.041, p = 0.029; Table 3).
Table 2.
Hemogram test results in the study groups.
| Group I (n = 47) |
Group II (n = 59) |
p | |
|---|---|---|---|
| Hb (g/dL) | 14.7 ± 1.6 | 14.8 ± 1.3 | 0.857 |
| MCV (fL) | 88 (64–104) | 88 (61–95) | 0.416 |
| WBC (×109/L) | 12.40 (5.31–21.030 | 11.77 (5.51–22.70) | 0.335 |
| PLT (×109/L) | 260 (162–666) | 282 (154–510) | 0.131 |
| RDW (%) | 13.7 ± 0.9 | 13.4 ± 0.7 | 0.030 |
| Neutrophil (×109/L) | 9.39 (3.10–19.52) | 7.30 (3.61–19.70) | 0.037 |
| Lymphocyte (×109/L) | 1.70 (0.40–6.93) | 2.60 (0.40–8.10) | 0.021 |
| NLR | 5.86 (0.66–40.50) | 2.75 (0.51–39.39) | 0.013 |
Data are presented as mean ± SD or median (range).
Hb = hemoglobin; MCV = mean corpuscular volume; NLR = neutrophil lymphocyte ratio; PLT = platelets; RDW = red distribution width; SD = standard deviation; WBC = white blood cells.
Table 3.
Multivariate logistic regression analyses.
| Variables | Odds ratio | CI 95% | p |
|---|---|---|---|
| RDW | 1.797 | 1.062–3.041 | 0.029 |
| NLR | 1.078 | 0.996–1.167 | 0.063 |
| Glucose | 1.005 | 1.000–1.010 | 0.069 |
| Age | 0.995 | 0.960–1.030 | 0.762 |
CI = confidence interval; NLR = neutrophil lymphocyte ratio; RDW = red cell distribution width.
Discussion
RDW and NLR have recently emerged as a potential new biomarkers that single out individuals at risk for future cardiovascular events in STEMI patients. In our study, we investigated the relationship between RDW, NLR and left ventricular systolic functions in first anterior STEMI patients. In our study population patients with depressed left ventricle function had significantly higher RDW and NLR levels than patients with preserved left ventricular function.
RDW reflects the variability in circulating red blood cell size and is increased in conditions such as iron, vitamin B12, and folate deficiency, hemoglobinopathies, and blood transfusion [9]. Also, recent studies showed that RDW was associated with an increased risk of coronary heart disease events, possibly because it reflects the bone marrow’s response to systemic ongoing inflammation [19]. Inflammation may influence erythropoiesis, erythrocyte circulatory half-life, and erythrocyte deformability, promoting anisocytosis and thus increasing RDW level [20]. Increased RDW was found to be an independent predictor of mortality in patients with heart failure, in patients with prior myocardial infarctions without symptomatic heart failure, and in patients presenting with acute myocardial infarction [5], [7]. The underlying mechanism explaining RDW’s association with cardiovascular events is not clear. Possible mechanisms may include oxidative stress, inflammatory, and neurohormonal activation [21], [22]. Oxidative stress has been shown to be associated with RDW. Antioxidants, including serum selenium, total carotenoids were shown to be significantly associated with a decrease in RDW. IL-6 was found to be an attenuating factor for this association [23]. It has been shown that the most important factor in mortality and morbidity after AMI was left ventricular systolic functions. Inflammation, oxidative stress, and neurohormonal activation play major roles in the pathogenesis of atherosclerosis, STEMI, and left ventricular remodeling after AMI [4], [24]. An elevated RDW may reflect underlying inflammation and oxidative stress in STEMI and left ventricular remodeling after AMI and may be proposed as a simple diagnostic marker for monitoring patients with STEMI. Furthermore, neurohumoral activation may influence erythropoiesis. The sympathetic and renin–angiotensin systems may accelerate erythropoiesis by stimulating the release of erythropoietin [25]. Erythropoietin is the hormone responsible for regulation of the production of red cells by bone marrow and increased plasma levels of erythropoietin have been found in STEMI patients independent of hemoglobin levels [26].
In previous studies, it has been shown that white blood cell count and its subtypes were indicators of systemic inflammation and had a considerable role in modulating the inflammatory response in the atherosclerotic process [12], [27]. In the acute period, leukocytosis usually accompanies STEMI in proportion to the magnitude of the necrotic process, elevated glucocorticoid levels, and possibly inflammation in the coronary arteries [4], [14]. Neutrophils are the first leukocytes to be found in the damaged myocardial area. Activation of neutrophils produces a large amount of inflammatory mediators that have important microcirculatory effects and regulates the inflammatory response to tissue injury [14]. Additionally, in previous studies, increased neutrophil count has been independently and strongly associated to large infarct size, mechanical complication, and mortality in patients with acute myocardial infarction [28], [29]. In the acute setting of coronary events, lymphocytopenia is a common finding during the stress response secondary to increased corticosteroids levels [30]. Furthermore, in AMI patients, lymphopenia and decreased CD4 counts with inverted CD4/CD8 ratio are related with low ejection fraction, high degree of myocardial necrosis, and mortality [31].
The NLR is a combination of two independent markers of inflammation; neutrophils show the ongoing nonspecific inflammation and lymphocytes show the regulatory pathway [4]. Unlike many other inflammatory markers and bioassays, NLR is an inexpensive and readily available marker that provides an additional level of risk scores in predicting in-hospital and long-term outcomes [4], [14].
In the literature, there are several studies which focused on RDW and NLR and their association with adverse outcomes in patients with AMI. Işık et al. [32] showed that RDW level was associated with mid-term mortality of STEMI patients treated with primary PCI. Moreover, Uyarel et al. [11] demonstrated that a high admission RDW level in patients with STEMI undergoing primary PCI was associated with an increased risk for in hospital and long term cardiovascular events such as advanced heart failure, the time of hospital stay and mortality. Similarly Dabbah et al. [33] concluded that RDW was independently associated with mortality after STEMI. In another study, Baysal et al. [34] examined 102 STEMI patients treated primarily with thrombolytic therapy; they found that RDW and NLR were significantly higher in the failed thrombolysis group than in the successful thrombolysis group and an increased RDW was found to be strongly and independently associated with failed thrombolysis in the setting of acute STEMI. Akpek et al. [35] showed that preprocedural NLR is an independent predictor of no-reflow in STEMI patients. They speculated that neutrophilia may aggravate myocardial ischemia by neutrophil-mediated microvascular plugging and may extend the infarct area [35]. Nuñez et al. [36] evaluated the predictive value of NLR in long-term mortality in STEMI patients and found that an increased NLR is associated with an increased risk of long-term mortality. Moreover Shen et al. [14] concluded that NLR is independently and closely related with long-term mortality in STEMI. In both of these single-center studies; NLR was measured at admission and following days up to 3–4 days. We measured NLR at admission before starting any medication because leukocyte count and its subtypes (neutrophils and lymphocytes) could be affected by infectious disorders, anxiety, and medication.
Similar to previous studies, our current study has confirmed the relationship between NLR, RDW, and STEMI. However, there is no study evaluating the association between NLR, RDW levels, and left ventricular systolic functions after anterior STEMI patients who underwent primary PCI. In the present study, we investigated whether the relationship between LVEF, NLR, and RDW and we found that patients with depressed left ventricular systolic function (LVEF <50%) had higher admission NLR and RDW levels than patients with preserved left ventricular systolic function (LVEF ⩾50%). We showed that RDW was an independent predictor of depressed left ventricular systolic function in anterior STEMI patients. However, the NLR did not reach a statistically significant level in multivariate analyses. The lack of a statistically significant level in the NLR values was thought to have resulted from the low number of study patients. Also, it was considered that an increased number of patients would produce a statistically significant level in the NLR values. Therefore, we propose that the increased NLR and RDW level may have caused an inflammatory response in the coronary arteries and worsen microvascular perfusion, and thereby affected left ventricular functions.
Study limitations
The limitations of the present study are as follows. (1) This was a retrospective and single-center study that included a relatively small number of patients. Further studies with a larger sample size may be needed. (2) Only one measurement of admission full blood count and calculation of RDW was included in the analysis. (3) We could not compare RDW with other inflammatory markers, such as C-reactive protein, fibrinogen, or myeloperoxidase, because they were not routinely measured in our study population. (4) Elevated RDW levels are affected by many clinical settings such as iron deficiency, pregnancy, vitamin B12 and folate deficiency, and blood transfusions. Only hemoglobin levels were measured in our study and other factors including, iron, vitamin B12, and folate levels were not measured in our study. Also, we did not include patients with anemia in our study. We believe that this makes it highly unlikely that the RDW values were not related to iron deficiency. Furthermore, the incidence of clinically significant vitamin B12 and folate deficiency is low in a modern population. In another study it was found that RDW was an independent predictor of mortality and the investigators concluded that the relationship between RDW and mortality was not confounded by anemia-related deficiencies such as iron, folate, and vitamin B12 [37].
Conclusion
Our findings, although they do not prove a direct relationship, suggest that higher NLR and RDW levels, in patients admitting to the emergency department with acute anterior STEMI, are associated with left ventricular systolic dysfunction and thus, may show potential for use as a prognostic marker. We think that these significant findings can guide further clinical practice. However, these findings must be confirmed on a study with more patients.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.Lopez A.D., Mathers C.D., Ezzati M., Jamison D.T., Murray C.J. Global and regional burden of disease and risk factors, 2001: systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 3.Taşoğlu I., Turak O., Nazli Y., Ozcan F., Colak N., Sahin S. Preoperative neutrophil-lymphocyte ratio and saphenous vein graft patency after coronary artery bypass grafting. Clin Appl Thromb Hemost. 2014;20:819–824. doi: 10.1177/1076029613484086. [DOI] [PubMed] [Google Scholar]
- 4.Oncel R.C., Ucar M., Karakas M.S., Akdemir B., Yanikoglu A., Gulcan A.R. Relation of neutrophil-to-lymphocyte ratio with GRACE risk score to in-hospital cardiac events in patients with ST-segment elevated myocardial infarction. Clin Appl Thromb Hemost. 2015;21:383–388. doi: 10.1177/1076029613505763. [DOI] [PubMed] [Google Scholar]
- 5.Sangoi M.B., da Silva S.H., da Silva J.E., Moresco R.N. Relation between red blood cell distribution width and mortality after acute myocardial infarction. Int J Cardiol. 2011;146:278–280. doi: 10.1016/j.ijcard.2010.10.084. [DOI] [PubMed] [Google Scholar]
- 6.Simel D.L., DeLong E.R., Feussner J.R., Weinberg J.B., Crawford J. Erythrocyte anisocytosis. Visual inspection of blood films vs automated analysis of red blood cell distribution width. Arch Intern Med. 1988;148:822–824. doi: 10.1001/archinte.148.4.822. [DOI] [PubMed] [Google Scholar]
- 7.Felker G.M., Allen L.A., Pocock S.J., Shaw L.K., McMurray J.J., Pfeffer M.A. CHARM Investigators. Red cell distribution width as a novel prognostic marker in heart failure: data from the CHARM Program and the Duke Databank. J Am Coll Cardiol. 2007;50:40–47. doi: 10.1016/j.jacc.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 8.Osadnik T., Strzelczyk J., Hawranek M., Lekston A., Wasilewski J., Kurek A. Red cell distribution width is associated with long-term prognosis in patients with stable coronary artery disease. BMC Cardiovasc Disord. 2013;13:113. doi: 10.1186/1471-2261-13-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y.L., Hua Q., Bai C.R., Tang Q. Relationship between red cell distribution width and short-term outcomes in acute coronary syndrome in a Chinese population. Intern Med. 2011;50:2941–2945. doi: 10.2169/internalmedicine.50.6407. [DOI] [PubMed] [Google Scholar]
- 10.Tonelli M., Sacks F., Arnold M., Moye L., Davis B., Pfeffer M. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. 2008;117:163–168. doi: 10.1161/CIRCULATIONAHA.107.727545. [DOI] [PubMed] [Google Scholar]
- 11.Uyarel H., Ergelen M., Cicek G., Kaya M.G., Ayhan E., Turkkan C. Red cell distribution width as a novel prognostic marker in patients undergoing primary angioplasty for acute myocardial infarction. Coron Artery Dis. 2011;22:138–144. doi: 10.1097/MCA.0b013e328342c77b. [DOI] [PubMed] [Google Scholar]
- 12.Horne B.D., Anderson J.L., John J.M., Weaver A., Bair T.L., Jensen K.R. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol. 2005;45:1638–1643. doi: 10.1016/j.jacc.2005.02.054. [DOI] [PubMed] [Google Scholar]
- 13.Walsh S.R., Cook E.J., Goulder F., Justin T.A., Keeling N.J. Neutrophil-lymphocyte ratio as a prognostic factor in colorectal cancer. J Surg Oncol. 2005;91:181–184. doi: 10.1002/jso.20329. [DOI] [PubMed] [Google Scholar]
- 14.Shen X.H., Chen Q., Shi Y., Li H.W. Association of neutrophil/lymphocyte ratio with long-term mortality after ST elevation myocardial infarction treated with primary percutaneous coronary intervention. Chin Med J. 2010;123:3438–3443. [PubMed] [Google Scholar]
- 15.Bigger J.T., Jr, Fleiss J.L., Kleiger R., Miller J.P., Rolnitzky L.M. The relationships among ventricular arrhythmias, left ventricular dysfunction, and mortality in the 2 years after myocardial infarction. Circulation. 1984;69:250–258. doi: 10.1161/01.cir.69.2.250. [DOI] [PubMed] [Google Scholar]
- 16.Gibson C.M., Cannon C.P., Daley W.L., Dodge J.T., Jr, Alexander B., Jr, Marble S.J. TIMI frame count: a quantitative method of assessing coronary artery flow. Circulation. 1996;93:879–888. doi: 10.1161/01.cir.93.5.879. [DOI] [PubMed] [Google Scholar]
- 17.Lang R.M., Bierig M., Devereux R.B., Flachskampf F.A., Foster E., Pellikka P.A. Chamber Quantification Writing Group; American Society of Echocardiography’s Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Anon Nutritional anemias. Report of a WHO scientific group. World Health Organ Tech Rep Ser. 1968;405:3–37. [PubMed] [Google Scholar]
- 19.Zalawadiya S.K., Veeranna V., Niraj A., Pradhan J., Afonso L. Red cell distribution width and risk of coronary heart disease events. Am J Cardiol. 2010;106:988–993. doi: 10.1016/j.amjcard.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Weiss G., Goodnough L.T. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 21.Libby P., Ridker P.M., Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 22.Tsimikas S., Willerson J.T., Ridker P.M. C-reactive protein and other emerging blood biomarkers to optimize risk stratification of vulnerable patients. J Am Coll Cardiol. 2006;47(Suppl. 8):C19–C31. doi: 10.1016/j.jacc.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 23.Semba R.D., Patel K.V., Ferrucci L., Sun K., Roy C.N., Guralnik J.M. Serum antioxidants and inflammation predict red cell distribution width in older women: the Women’s Health and Aging Study I. Clin Nutr. 2010;29:600–604. doi: 10.1016/j.clnu.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parodi G., Antoniucci D. Left ventricular remodeling after primary percutaneous coronary intervention. Am Heart J. 2010;160(6 Suppl.):S11–S15. doi: 10.1016/j.ahj.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Akin F., Köse N., Ayça B., Katkat F., Duran M., Uysal O.K. Relation between red cell distribution width and severity of coronary artery disease in patients with acute myocardial infarction. Angiology. 2013;64:592–596. doi: 10.1177/0003319712461931. [DOI] [PubMed] [Google Scholar]
- 26.Ferrario M., Massa M., Rosti V., Campanelli R., Ferlini M., Marinoni B. Early haemoglobin-independent increase of plasma erythropoietin levels in patients with acute myocardial infarction. Eur Heart J. 2007;28:1805–1813. doi: 10.1093/eurheartj/ehm065. [DOI] [PubMed] [Google Scholar]
- 27.Gurm H.S., Bhatt D.L., Lincoff A.M., Tcheng J.E., Kereiakes D.J., Kleiman N.S. Impact of preprocedural white blood cell count on long term mortality after percutaneous coronary intervention: insights from the EPIC, EPILOG, and EPISTENT trials. Heart. 2003;89:1200–1204. doi: 10.1136/heart.89.10.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Donoghue M., Morrow D.A., Cannon C.P., Guo W., Murphy S.A., Gibson C.M. Association between baseline neutrophil count, clopidogrel therapy, and clinical and angiographic outcomes in patients with ST-elevation myocardial infarction receiving fibrinolytic therapy. Eur Heart J. 2008;29:984–991. doi: 10.1093/eurheartj/ehn112. [DOI] [PubMed] [Google Scholar]
- 29.Kirtane A.J., Bui A., Murphy S.A., Barron H.V., Gibson C.M. Association of peripheral neutrophilia with adverse angiographic outcomes in ST elevation myocardial infarction. Am J Cardiol. 2004;93:532–536. doi: 10.1016/j.amjcard.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Onsrud M., Thorsby E. Influence of in vivo hydrocortisone on some human blood lymphocyte subpopulations. I. Effect on natural killer cell activity. Scand J Immunol. 1981;13:573–579. doi: 10.1111/j.1365-3083.1981.tb00171.x. [DOI] [PubMed] [Google Scholar]
- 31.Blum A., Sclarovsky S., Rehavia E., Shohat B. Levels of T-lymphocyte subpopulations, interleukin-1 beta, and soluble interleukin-2 receptor in acute myocardial infarction. Am Heart J. 1994;127:1226–1230. doi: 10.1016/0002-8703(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 32.Işık T., Kurt M., Ayhan E., Tanboga I.H., Ergelen M., Uyarel H. The impact of admission red cell distribution width on the development of poor myocardial perfusion after primary percutaneous intervention. Atherosclerosis. 2012;224:143–149. doi: 10.1016/j.atherosclerosis.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Dabbah S., Hammerman H., Markiewicz W., Aronson D. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. Am J Cardiol. 2010;105:312–317. doi: 10.1016/j.amjcard.2009.09.027. [DOI] [PubMed] [Google Scholar]
- 34.Baysal E., Çetin M., Yaylak B., Altntaş B., Altndağ R., Adyaman Ş. Roles of the red cell distribution width and neutrophil/lymphocyte ratio in predicting thrombolysis failure in patients with an ST-segment elevation myocardial infarction. Blood Coagul Fibrinolysis. 2015;26:274–278. doi: 10.1097/MBC.0000000000000227. [DOI] [PubMed] [Google Scholar]
- 35.Akpek M., Kaya M.G., Lam Y.Y., Sahin O., Elcik D., Celik T. Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol. 2012;110:621–627. doi: 10.1016/j.amjcard.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 36.Nuñez J., Nuñez E., Bodi V., Sanchis J., Miñana G., Mainar L. Usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST segment elevation myocardial infarction. Am J Cardiol. 2008;101:747–752. doi: 10.1016/j.amjcard.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Perlstein T.S., Weuve J., Pfeffer M.A., Beckman J.A. Red blood distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med. 2009;169:588–594. doi: 10.1001/archinternmed.2009.55. [DOI] [PMC free article] [PubMed] [Google Scholar]