Abstract
Background
Amphetamine-type stimulants (ATS) are the most commonly used illicit drugs in Saudi Arabia. Frequency and outcome of ATS-related cardiovascular (CV) complications in the Saudi community have not been previously studied.
Aim
We aimed to determine the incidence and the clinical outcomes of CV complications among individuals with amphetamine-positive urine drug screening (APUDS) tests admitted to a tertiary care facility in Riyadh, Saudi Arabia.
Methods
Retrospective review of consecutive cases with APUDS and concurrently positive cardiac biomarkers admitted to King Abdul-Aziz Medical City in Riyadh, Saudi Arabia, between January 2006 and December 2013. The laboratory database was queried to identify patients with positive APUDS and abnormal cardiac biomarkers. Clinical data were extracted from the electronic medical records.
Results
A total of 7450 urine drug screening tests were performed during the study period, out of which 720 (9.6%) were positive for ATS (APUDS group). Forty-two cases in the APUDS group were documented to have CV complications. All cases were men with a median age of 39 years (range, 21–60 years). Acute coronary syndrome/myocardial infarction was the most frequent clinical presentation (n = 31, 74%), predominantly in the form of ST-elevation myocardial infarction. Other less frequent complications included myopericarditis, cardiomyopathy, and arrhythmia. Coronary procedures were performed in 30 cases. Median hospital stay was 5 days (range, 1–28 days) and in-hospital mortality was 7.2%.
Conclusion
APUDS is frequently encountered in young Saudi men presenting to the emergency department of our institution. Individuals with APUDS are at increased risk of CV complications and in-hospital mortality. The most frequent APUDS-related CV complication is acute coronary syndrome.
Keywords: Amphetamine, Cardiovascular, Complications, Drug abuse, Saudi Arabia, Urine drug screening
Abbreviations
- ACS
Acute Coronary Syndromes
- APUDS
Amphetamine Positive Urine Drug Screening
- CKMB
Creatinine Kinase MB
- ICD
International Classification of Diseases
- MAP
Methamphetamine
- PCI
Percutaneous Coronary Interventions
- STEMI
ST Elevation Myocardial Infarction
- UDS
Urine Drug Screening
- WRS
Water Restraint Stress
Introduction
Drug abuse is a major concern all over the world. It is estimated that 246 million people used illicit drugs in 2013 [1]. Like many other wealthy countries, the Kingdom of Saudi Arabia (SA) is frequently a target of drug smugglers. In its 2014 annual report, the international narcotics control board, clearly indicates that this is an increasingly serious problem in SA and other Gulf countries [2]. Long coastal areas of SA and sharing borders with eight neighboring countries in addition to its sociodemographic characteristics and its strategic geographical location are among the reasons behind targeting SA as a destination country of choice. Drug abuse is most frequently a problem of youth who constitutes the vast majority of the Saudi population, where 27% of them are younger than 25 years and those who are younger than 55 years represent 73% [3]. Health-related consequences of drug abuse are numerous and well documented. A strategic plan and dedicated resources are needed to increase public awareness, implement prevention programs, and initiate early treatment of the users. Published studies about drug-related health issues in Arab countries are limited and may underestimate the magnitude of the problem [4], [5]. There is a great need for more research in this field at national and regional levels.
Amphetamine-type stimulants (ATS) refers to a group of psychostimulant drugs that are related to the parent compound amphetamine and includes amphetamine sulfate, amphetamine hydrochloride, methamphetamine, and phenethylamines [6]. ATS group is currently the most commonly abused substance in SA [7], [8]. In recent years, the frequency of amphetamine use among Saudi people aged ⩽40 years has markedly increased [8]. About 65% of patients in drug rehabilitation programs in SA are reported as addicted to “Captagon” [9], [10]. Captagon is originally a brand name of fenethylline, a synthetic stimulant that has been banned since 1986. Counterfeit versions of Captagon contain amphetamine as the active ingredient and represent the main source of ATS in SA [10]. SA is the main country of destination for Captagon tablets, which accounts for approximately 30% of all global amphetamine seizures and for 80% of the total weight seized in the region [10]. This fact reflects vigilance and collaborative efforts of different government authorities against drug smuggling; however, it may raise a concern that large quantities of drugs may continue to cross borders via unseized smuggling attempts.
ATS are known as potent sympathomimetic amines that lead to several central nervous system and cardiovascular (CV) system adverse effects [11]. Acute central nervous system manifestations of ATS abuse include euphoria, talkativeness, anxiety, restlessness, agitation, seizures, and coma. Long-term abuse may lead to loss of weight, pulmonary hypertension, cardiomyopathy, and paranoid psychosis [12]. For social considerations and privacy reasons, a history of drug abuse may be denied by many users at the time of clinical presentation. ATS abuse is commonly suspected in young individuals presenting with acute neuropsychiatric manifestations or cardiac complications. Suspected cases are frequently tested for presence of ATS or any of their metabolites in the urine via urine drug screening (UDS).
The medical literature is deficient in publications on ATS from the Gulf and Arab regions. Most of the available literature is focused on the addiction and psychiatric aspects. Very little scientific work has been published on nonpsychiatric ATS-related health problems, including CV complications [5]. The outcome of our study is expected to enhance the medical staff and public awareness of potential ATS-related CV complications.
This study aimed to determine the incidence and outcomes of ATS-related CV complications in patients admitted to King Abdul-Aziz Medical City in Riyadh, Saudi Arabia over a period of 8 years. As a secondary objective, we aimed also to evaluate the demographic profile of amphetamine-positive UDS (APUDS) population as well as the trends of UDS and its positivity rate for ATS over the study period.
Materials and methods
This hospital-based, retrospective, case-series, study was approved by the King Abdullah International Medical Research Center (KAIMRC) Scientific Committee and was exempted from Institutional Review Board (IRB) approval for obtaining consent, as it did not involve patient contact or human participant intervention. The study included patients screened for ATS between January 2006 and December 2013. Potential candidates were identified through searching the biochemistry laboratory database for all individuals who underwent UDS during the study period. UDS was performed using Enzyme Immunoassay (Multigent, Architect c System; Abbott Laboratories, Chicago, IL, USA) that detects amphetamine and methamphetamine with a cutoff value of 1000 ng/mL for a positive test. Confirmatory test using gas chromatography/mass spectrometry (GC/MS) was not available for study candidates as it is reserved, in our institution, for legal circumstances and not for a routine clinical use. Cardiac biomarkers, i.e., troponin I (TnI) and creatinine kinase MB isoenzyme (CK-MB; Architect STAT Troponin-I & CK-MB, Architect i System; Abbott Laboratories) were also collected from the laboratory database for all individuals who underwent UDS during the study period.
A comprehensive search for the discharge diagnosis of substance abuse, particularly for the ATS abuse, according to the International Classification of Diseases 9 and 10 coding systems was performed through the Health Information Management Department. A list of all individuals with APUDS tests and their respective concurrent cardiac biomarkers were electronically extracted and carefully reviewed for potential study candidates. Records of all individuals with APUDS who were admitted to the hospital or were found to have positive cardiac biomarkers (0.30 ng/mL for TnI and 7.2 ng/mL for CK-MB according to the manufacturer cutoff values and our laboratory reference range) at the time of the index APUDS test were carefully reviewed for potential cardiac complications. Cardiac complications of interest included acute coronary syndrome (ACS), acute myopericarditis, cardiomyopathy, heart failure, and arrhythmia. Relevant demographic and clinical parameters were collected from multiple electronic hospital data management and archiving systems. All data variables were managed and analyzed using Microsoft Excel (Microsoft, Redmond, WA, USA) and IBM-SPSS software, version 20 (SPSS Inc.). Data security and confidentiality were ensured at all times.
Results
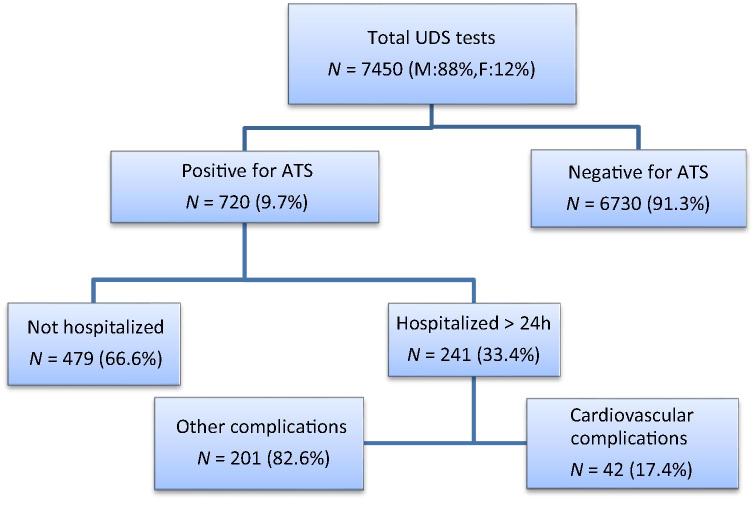
A total of 7450 UDS tests were performed during the study period (88% men and 12% women), out of which 720 (9.6%) were positive for ATS (Fig. 1). Median age of the APUDS population was 29 years with male predominance (98%). Out of the 720 APUDS, 209 (29%) and 134 (18.6%) underwent concurrent TnI and CK-MB testing, respectively. Values of 50 TnI and 60 CK-MB tests were identified to satisfy the definition of acute myocardial injury (>0.3 μg/L and >7.5 μg/L, respectively, according to the manufacturer cutoff values and our laboratory reference range). Forty-two individuals (5.8% of the total APUDS population and 17.4% of the admitted APUDS individuals) were identified to have documented CV complications (CV group). These individual were all men and their baseline characteristics are shown in Table 1 and Fig. 2.
Figure 1.
Flow chart of study population identification, using urine drug screening (UDS) for detection of amphetamine-type stimulants (ATS). F = female; M = male.
Table 1.
Demographic profile and baseline characteristics of amphetamine positive individuals who developed cardiovascular complications (n = 42).
| Parameter | Median (range) or n (%) |
|---|---|
| Age (y) | 39 (21–60) |
| Sex (male) | 42 (100) |
| Nationality (Saudi) | 40 (95%) |
| Body mass index (kg/m2) | 27 (18.5–40.8) |
| Heart rate (beats/min) | 87 (36–130) |
| >100 | 9 (21) |
| <60 | 3 (7) |
| Respiratory rate (breaths/min) | 20 (12–32) |
| Systolic blood pressure (mmHg) | 126 (70–160) |
| >140 | 7 (16.6) |
| <100 | 6 (14) |
| Diastolic blood pressure (mmHg) | 71 (41–114) |
| >90 | 3 (7) |
| <60 | 9 (21) |
| Oxygen saturation (%) | 98 (90–100) |
| Smoking: current/ex-smoker | 25 (60) |
| Nonsmoker | 9 (20) |
| Unknown | 8 (20) |
| Admit amphetamine-type stimulants use | 19 (45) |
| Multidrug use by urine test | 17 (41) |
| Type II diabetes mellitus | 11 (27.5) |
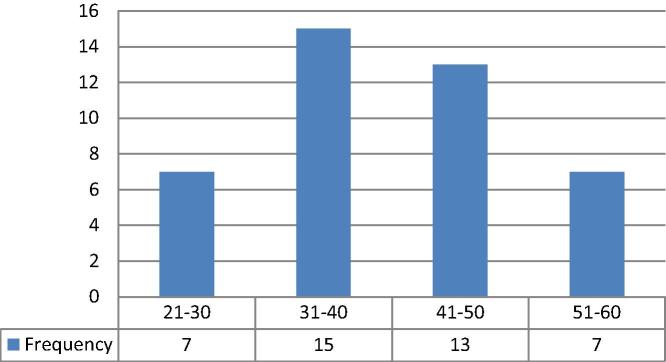
Figure 2.
Age distribution of the study population (total n = 42).
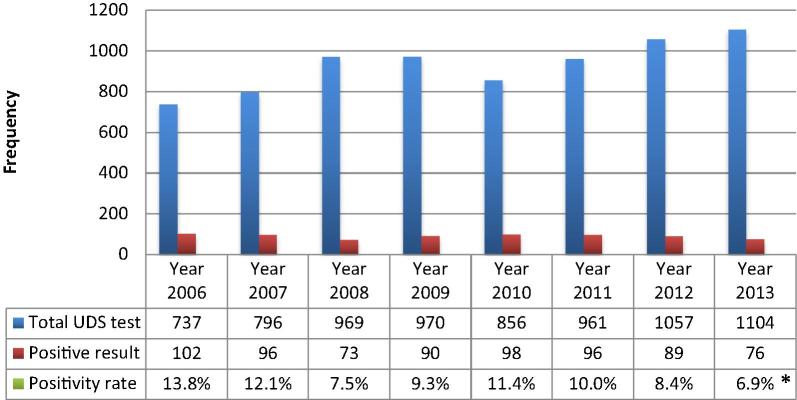
The majority of cases (40 out of 42) were admitted under cardiology services, while one patient was admitted to the general Intensive Care Unit and another one to a general medical ward. Most cases were identified through the positive cardiac biomarkers, while only three cases were coded for substance abuse upon discharge. Details of the cardiac investigations that were performed in the 42 patients are shown in Table 2. Diagnosis of ACS/myocardial infarction (MI) was made in 31 cases (74%), while other complications were less frequent (Fig. 3). Cardiac procedures and other clinical outcomes are shown in (Table 3). Despite the perceived trends of increasing UDS over the study period, the ATS positivity rate has fallen by 50% between 2006 and 2013 (Fig. 4). Individuals older than 40 years were noted to have higher proportion of diabetes mellitus and were more likely to present with ACS, while admitting drug abuse was more frequent in younger population (Table 4).
Table 2.
Cardiac investigations among patients with cardiovascular complications (n = 42).
| Parameter | n (%) or mean (SD) |
|---|---|
| ECG-ST segment: Elevation | 20 (48) |
| Depression | 6 (14) |
| Normal | 16 (38) |
| Site of ST elevation: Anterior | 12 (29) |
| Inferior | 8 (19) |
| ECG-rhythm: Normal sinus | 30 (72) |
| Sinus bradycardia | 3 (7) |
| Sinus tachycardia | 3 (7) |
| Atrial fibrillation | 3 (7) |
| Others | 3 (7) |
| Echo: LV wall: Hypertrophy | 12 (28) |
| Thinning | 2 (5) |
| Normal | 28 (67) |
| Echo: ejection fraction (%) | 39.6 (± 11) |
| ⩾50 | 10 (23.8) |
| 30–50 | 21 (50) |
| <30 | 11(26.2) |
| Troponin I (ng/mL) | 36.8 (± 73) |
ECG = electrocardiogram; LV = left ventricle; SD = standard deviation.
Figure 3.
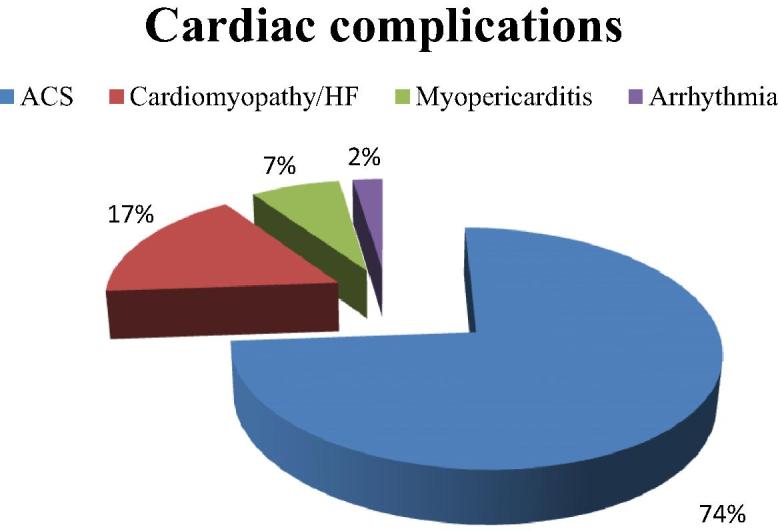
Distribution and frequency of cardiac complications in the study group (n = 42). ACS = acute coronary syndrome; HF = heart failure.
Table 3.
In-hospital clinical outcomes of the study group (n = 42).
| Clinical outcome | n (%) or Median (range) |
|---|---|
| Cardiac procedures | |
| Percutaneous coronary intervention | 17 (40) |
| Diagnostic coronary angiogram only | 10 (24) |
| Coronary angiogram followed by CABG | 3 (7) |
| None | 12 (29) |
| Median hospital stay (d) | 5 (1–28) |
| In-hospital mortality | 3 (7.2) |
CABG = coronary artery bypass surgery.
Figure 4.
Annual trends of urine drug screening (UDS) and its respective positivity rate for amphetamine-type stimulants between 2006 and 2013. *p < 0.001.
Table 4.
Comparison of clinical parameters in the study population according to their age category of >40 years or ⩽40 years (n = 42).
| Clinical parameter | ⩽40 years (n = 22) | >40 years (n = 20) | p |
|---|---|---|---|
| Acute coronary syndrome | 13 (59) | 18 (90) | 0.02 |
| ST-elevation myocardial infarction | 10 (46) | 10 (50) | 0.77 |
| Mortality | 1 (5) | 2 (10) | 0.49 |
| Hospital stay | 4.8 (3.0, 6.1) | 7 (3.0, 10.5) | 0.27a |
| Type II diabetes mellitus | 1 (5) | 10 (50) | 0.001⁎ |
| Hypertension | 3 (14) | 6 (30) | 0.27 |
| Admit drug abuse | 14 (64) | 5 (25) | 0.01⁎ |
| Multi drug use | 8 (36) | 9 (45) | 0.57 |
| Smoking | 14 (64) | 11 (55) | 0.57 |
| Troponin I level | 7.5 (0.01, 26.75) | 24.5 (6.0, 47.75) | 0.35a |
Hospital stay and troponin level are presented as median (interquartile range) and all other data are presented as n (%).
Independent samples Mann–Whitney U test.
p < 0.05.
Discussion
Studies have shown that ATS increase heart rate, blood pressure, and myocardial oxygen consumption in a quantity similar to Dobutamine, 20–40 μg/kg/min [13]. The natural history of ATS use indicates that regular use of such agents is likely to cause pathology if taken for many years. Long-term use of ATS has been linked to the development of heart diseases and cerebral hemorrhage [14].
In animal models, combination of methamphetamine with water-restraint stress produced a major rise in the leakage of proteins related to myocardial damage and the levels of cytokines interleukin-6, tumor necrosis factor-α, and interleukin-10 due to cardiac myocytolysis [15]. ATS abuse can lead to cardiovascular toxicity that eventually manifest as myocardial ischemia/infarction, arrhythmia, or heart failure. Pathophysiological mechanisms underlying ATS-related CV toxicity involves; increased heart rate, raised blood pressure, and associated arterial vasospasm, which are largely attributed to a state of persistently elevated catecholamine levels. Long-term use of ATS results in cardiac hypertrophy and destruction of the microtubular and actin structures, which also contributes to the development of CV disease in this population [16]. ATS abuse is considered a potential risk factor for the development of pulmonary arterial hypertension [17]. Worse in-hospital outcome has been observed in khat chewers who were admitted with acute coronary syndrome as compared to nonchewers [18]. Khat (Catha edulis) leaves contain cathinone that is known for its amphetamine-like effects [19], [20].
To our knowledge this is the first study in an Arab country that addresses CV complications in the APUDS population. Total UDS of 7450 test over a period of 8 years is relatively low for a country that is considered as one of the largest targeted markets in the world. In a single-center experience of an outpatient setting in the USA 10,011 UDS tests were performed over a 1.5-year period [21]. Overall positivity rate in that study was 3.6% as compared to 9.6% in our study. Despite the apparently increasing annual trend of UDS tests, the positivity rate has been significantly decreasing over time, by up to 50% between 2006 and 2013 (p < 0.001; Fig. 4). This observation might be explained by the increasing awareness of the medical staff towards substance abuse and its potential clinical consequences on one hand, and by the successful governmental security efforts against drug trafficking and trading on the other [22].
Out of the 720 positive UDS tests, 42 individuals (5.8%) were identified to have some sort of CV complication, which represent 17.4% of the total admitted APUDS individuals. The remaining 82.6% of APUDS individuals were admitted under other specialties such as psychiatry, neurosurgery, general surgery, and internal medicine with clearly noncardiac conditions. The CV complications were predominantly due to coronary artery disease and mostly in form of ACS/MI (n = 31, 74%) out of which ST-elevation MI was present in 20 cases (48%). Association between ATS use and ACS/MI has been reported in several studies and case reports [13], [23]. Risk factors for coronary artery disease in this population included being male (100%), diabetes mellitus (27.5%), and smoking (at least 60%), which more or less reflect the usual local population risk factor profile. Individuals with CV complications are 1 decade older than the overall APUDS group (median age, 39 years and 29 years, respectively), a duration that is long enough to contribute to coronary artery disease progression and to expose patients to more ATS-related CV adverse effects [14]. Nonischemic complications in this study cohort included cardiomyopathy (17%), myopericarditis (7%), and arrhythmia (2%). ATS-related cardiomyopathy is a well-known entity that could be attributed to the direct toxic effect of ATS on myocardium or to their long-term adverse hemodynamic consequences or both [15], [24].
Initial clinical presentation of the study population exhibited a wide range of hemodynamic response and heart rate variation (Table 1). These hemodynamic variations are probably multifactorial in nature, with the infarct size and location as well as the degree of left ventricular systolic dysfunction being probably the most important determinants. Acute sympathomimetic effects of ATS use could have been additional contributing factor in some individuals. The in-hospital mortality rate in this cohort was 7.2%, despite being managed in a tertiary care facility and highly specialized cardiac center that provides primary percutaneous coronary interventions and advanced critical care support. This finding is very close to the mortality rate that has been reported in khat chewers who were admitted with acute coronary syndrome [18] and in cases of ATS-related cardiomyopathy [25]. Three patients died before having a coronary angiogram, four patients left against medical advice before coronary angiography could be performed, and five patients refused the procedure. This behavior might reflect the mood instability of such individuals [8], [12].
We believe that the rate of ATS-related CV complications is generally underestimated due to several reasons including but not limited to: underreporting of the drug users to the medical attention; inadequate screening for ATS use and cardiac complications; missing asymptomatic individuals or those with subtle CV disease; and failure to document ATS abuse in the final diagnosis. Only one third (33.4%) of the total APUDS population was admitted to the hospital. Presence of CV complications in some of those individuals with APUDS who were discharged could not be excluded. UDS is a frequently requested emergency room test, particularly for young individuals who present with unusual neuropsychiatric manifestations or unexpected cardiovascular disease [22]. Requesting UDS test depends on the physician’s awareness of drug abuse prevalence in the community and the degree of clinical suspicion for drug abuse. Immunoassay is the most widely used UDS because of its convenience and cost efficiency [26]. Major limitations of UDS include its cross-reactivity with a wide range of commonly prescribed pharmacological agents giving a high rate of false positive result [27] and inability to distinguish different substances of ATS. It is recommended to conduct a confirmatory test for all APUDS samples using GC-MS, which is more expensive and time-consuming, but considered as the gold standard for confirming a positive result on immunoassay [28]. This confirmatory test is not readily available for clinical use in all institutions, including ours. Despite the potential false positive results, UDS remains widely used as an evidence of recent drug use, especially if supported by the appropriate clinical setting. Despite the limited documentation of history of drug abuse in the study population, 45% of cases admitted a history of drug abuse; however, the duration and pattern of drug abuse were not specified. Presence of multidrug use has been reported as almost equivalent to the confirmatory test in supporting positive UDS test [29]. Opioids, cannabis, and benzodiazepines were detected at variable frequencies in the multidrug users.
Our study has the inherent limitations of any retrospective study. Using an unconfirmed UDS test as a marker of active ATS use is another limitation, which could not have been avoided due to lack of the confirmatory test for clinical use in our institution. Being a single center experience with a relatively small study population to detect rare outcomes was an additional limitation.
Conclusion
ATS in the form of Captagon tablets are currently the most frequently abused illicit drugs in SA. APUDS is frequently encountered in young Saudi men presenting to the emergency department of our institution. Individuals with APUDS are at increased risk of CV complications and in-hospital mortality.
A larger, prospective, multicenter study with regional representation using GC-MS confirmed APUDS test is strongly recommended. A national multidisciplinary program for public awareness, early detection, and continuous monitoring of ATS abuse in the Saudi community is required to prevent serious long-term ATS-related adverse effects including CV complications. Hospital-based counseling team, with special training in drug interventions is needed to provide the appropriate psychological and therapeutic support for APUDS individuals.
Acknowledgments
Special thanks to Dr. Waleed Al-Tamimi, head of the Biochemistry Laboratory for providing the biochemical data and their technical specifications, and to Dr. Aamir Omair for his contribution in the statistical analysis. Thanks to Joseph Franke for reviewing and editing the manuscript.
Disclosure: Authors have nothing to disclose with regard to commercial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- 1.UN Office of Drugs and Crime. UN World Drug Report. 2015. Available from: <https://www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf>. [Accessed 8 October 2015].
- 2.International Narcotics Control Board. Report of the International Narcotics Control Board for 2014. 2014. Available from: <https://www.unodc.org/documents/southeastasiaandpacific//Publications/2015/incb/INCB_Annual_Report_2014_EN.pdf>. [Accessed 8 October 2015].
- 3.Saudi Arabia Demographics Profile 2014. Available from: <http://www.indexmundi.com/saudi_arabia/demographics_profile.html>. [Accessed 3 September 2015].
- 4.Sweileh W.M., Zyoud S.H., Al-Jabi S.W., Sawalha A.F. Substance use disorders in Arab countries: research activity and bibliometric analysis. Subst Abuse Treat Prev Policy. 2014;9:33. doi: 10.1186/1747-597X-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghaferi H Al, Osman OT, Matheson C, Bond C, Dhabi A, Emirates UA, et al. Editorial 3 substance misuse in Arabic countries: the need for published research: 7–11. Available from: <http://ijptsud.sljol.info/articles/abstract/10.4038/ijptsud.v1i1.5907/>. [Accessed 8 March 2015].
- 6.National Drug Strategy. National Amphetamine-Type Stimulant Strategy Background Paper 2011. Available from <http://www.nationaldrugstrategy.gov.au/internet/drugstrategy/Publishing.nsf/content/2D1717263A2129CACA2574C50010A827/$File/ats-strategy08.pdf>. [Accessed 10 October 2015].
- 7.Bassiony M. Substance use disorders in Saudi Arabia: review article. J Subst Use. 2015;2013(18):450–466. Available from: < http://www.tandfonline.com/loi/ijsu20>. [Accessed 10 October 2015] [Google Scholar]
- 8.Desoky E.S.E.L., El-Tantawy A.M., Raya Y.M., Al-Yahya A. Amphetamine versus non amphetamine-related first episode psychosis in Saudi Arabian patients. Pharmacol Pharm. 2011;02:101–108. [Google Scholar]
- 9.Murphy C. A Kingdom’s future: Saudi Arabia through the eyes of its twentysomethings. 2012. Available from <https://www.wilsoncenter.org/sites/default/files/kingdoms_future_saudi_arabia_through_the_eyes_twentysomethings_0.pdf>. [Accessed 8 March 2015].
- 10.Greek regional chair of the Dublin Group, Regional Report on the Near East, 4 November 2013. Available from: <http://register.consilium.europa.eu/doc/srv?l=EN&f=ST%2015181%202013%20INIT. [Accessed 8 October 2015].
- 11.Goodman LS, Hardman JG, Limbird LE, Gilman AG. Goodman & Gilman’s. In; The pharmacological basis of therapeutics. 10th ed. New York: McGraw-Hill; 2001. p. 622.
- 12.Carvalho M., Carmo H., Costa V.M., Capela J.P., Pontes H., Remião F. Toxicity of amphetamine-type stimulants: an update. Arch Toxicol. 2012;86:1167–1231. doi: 10.1007/s00204-012-0815-5. [DOI] [PubMed] [Google Scholar]
- 13.Lester S.J., Baggott M., Welm S., Schiller N.B., Jones R.T., Foster E. Cardiovascular effects of 3,4-methylenedioxymethamphetamine-type stimulants: a double-blind, placebo-controlled trial. Ann Intern Med. 2000;133:969–973. doi: 10.7326/0003-4819-133-12-200012190-00012. [DOI] [PubMed] [Google Scholar]
- 14.Pilgrim J.L., Gerostamoulos D., Drummer O.H., Bollmann M. Involvement of amphetamine-type stimulantss in sudden and unexpected death. J Forensic Sci. 2009;54:478–485. doi: 10.1111/j.1556-4029.2008.00949.x. [DOI] [PubMed] [Google Scholar]
- 15.Tomita M., Katsuyama H., Watanabe Y., Hidaka K., Yoshitome K., Miyaishi S. Water-restraint stress enhances methamphetamine-type stimulants-induced cardiotoxicity. ChemBiol Interact. 2011;190:54–61. doi: 10.1016/j.cbi.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 16.Yu Q., Larson D.F., Watson R.R. Heart disease, methamphetamine-type stimulants and AIDS. Life Sci. 2003;73:129–140. doi: 10.1016/s0024-3205(03)00260-1. [DOI] [PubMed] [Google Scholar]
- 17.Seferian A., Chaumais M.C., Savale L., Günther S., Tubert-bitter P., Humbert M. Drugs induced pulmonary arterial hypertension. Presse Med. 2013;42:e303–e310. doi: 10.1016/j.lpm.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Ali W.M., Al Habib K.F., Al-Motarreb A., Singh R., Hersi A., Al Faleh H. Acute coronary syndrome and khat herbal amphetamine-type stimulants use: an observational report. Circulation. 2011;124:2681–2689. doi: 10.1161/CIRCULATIONAHA.111.039768. [DOI] [PubMed] [Google Scholar]
- 19.Wabe N.T. Chemistry, Pharmacology, and Toxicology of Khat (Catha edulis Forsk): a review. Addict Health. 2011;3:137–149. [PMC free article] [PubMed] [Google Scholar]
- 20.Al-Motarreb A., Baker K., Broadley K.J. Khat: pharmacological and medical aspects and its social use in Yemen. Phyther Res. 2002;16:403–413. doi: 10.1002/ptr.1106. [DOI] [PubMed] [Google Scholar]
- 21.Casey E.R., Scott M.G., Tang S., Mullins M.E. Frequency of false positive amphetamine-type stimulants screens due to bupropion using the Syva Emit II immunoassay. J Med Toxicol. 2011;7:105–108. doi: 10.1007/s13181-010-0131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saudi Arabia 2013 Crime and Safety Report. Washington, DC: The Overseas Security Advisory Council (OSAC). 2013. Available from: <https://www.osac.gov/pages/ContentReportDetails.aspx?cid=13573>. [Accessed 10 April 2015].
- 23.Khattab E., Shujaa A. Amphetamine abuse and acute thrombosis of left circumflex coronary artery. Int J Case Reports Images. 2013;4:698. [Google Scholar]
- 24.Dawson P., Moffatt J.D. Cardiovascular toxicity of novel psychoactive drugs: lessons from the past. Prog Neuro-Psychopharmacol Biol Psychiatry. 2012;39:244–252. doi: 10.1016/j.pnpbp.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Elasfar A., Ahmad K.E., AlShaghaa W. Clinical characteristics and outcome of heart failure and Captagon amphetamine use: an observational prospective study. Egypt Heart J. 2014;66:5. [Google Scholar]
- 26.Riahi-Zanjani B. False positive and false negative results in urine drug screening tests: tampering methods and specimen integrity tests. Pharmacol Online. 2014;1:102–108. [Google Scholar]
- 27.Moeller K.E., Lee K.C., Kissack J.C. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83:66–76. doi: 10.4065/83.1.66. [DOI] [PubMed] [Google Scholar]
- 28.Vincent E.C. What common substances can cause false positives on urine screens for drugs of abuse? J Fam Pract. 2006;55:893–897. [PubMed] [Google Scholar]
- 29.Napper L.E., Fisher D.G., Johnson M.E., Wood M.M. The reliability and validity of drug users’ self reports of amphetamine-type stimulants use among primarily heroin and cocaine users. Addict Behav. 2010;35:350–354. doi: 10.1016/j.addbeh.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]