1. Introduction
Strategies to improve cancer care in the field of urothelial carcinoma (UC) in the elderly or in patients unfit to receive cisplatin treatment are urgently needed. This effort requires multidisciplinary collaboration to provide the best care for these patients.
An international multidisciplinary summit was held in Geneva on April 11th 2015 to discuss unmet medical needs in urothelial cancer. Key topics covered in the meeting included defining difficult-to-treat UC patients and the challenges encountered with both surgical and systemic treatment in these patients. The delegates discussed potential therapies, from neoadjuvant treatment to combinations or sequential regimens, maintenance therapy up to second-line management, and future treatment opportunities that could improve on current patient outcomes. Molecular specificities and future perspectives of UC were also discussed. Each individual topic of the meeting is outlined in the chapters below.
2. Defining ‘difficult-to-treat patients’ with UC Maria De Santis, United Kingdom
2.1. Bladder cancer is a disease of the elderly
In the European Union, there were 118,365 new bladder cancer cases in 2012 (>500,000 worldwide), with a prevalence of 389,287 cases, and 39,522 deaths. Bladder cancer incidence peaks around the 7th decade (from the age of 60), and about 20% of patients are aged >80 years. The disease is the 4th most common cancer in males and the 15th in females [1]. Bladder cancer treatment is on a course to becoming an enormous challenge in the context of an increasingly ageing population.
Overall, the fastest growing population segment is that aged 80 years or older, which has increased from 13.8 million in 1950 to 69.2 million in 2000; it is expected to further rise to 379.0 million by 2050 [2]. Due to increased life expectancy and improved diagnostic techniques, cancers are more frequently diagnosed, resulting in higher incidence and prevalence rates. Consequently, more elderly patients will require cancer treatment.
Evidence-based guidelines recommend radical cystectomy (RC) for patients with stage II muscle-invasive bladder cancer (MIBC). However, according to the Surveillance, Epidemiology, and End Results database, only 21% of 3262 patients over the age of 65 underwent RC [3]. Older age at diagnosis and higher comorbidity were associated with decreased odds of receiving cystectomy, even if overall survival (OS) was better for those who underwent cystectomy compared with those who received alternative treatments (chemotherapy and/or radiation) [3]. Similarly, according to the U.S. National Cancer Data Base, out of 28,691 patients with clinical T2–T4a, N0–3 MIBC, no potentially curative therapy was delivered to 47.5% of patients. Use of RC declined dramatically with advancing age [4]. Thus, guideline-recommended RC is underused for patients with MIBC, particularly in elderly patients. The reasons that make bladder cancer unresectable or inoperable are either related to the tumour itself or to the patient. First, extensive local tumours (stage T4) might be technically inoperable; the option of shrinking the tumour with chemotherapy may be considered. Second, inoperability may be due to patient age or comorbidities (renal function impairment, cardiovascular disease, etc.). Other potential factors defining cystectomy candidates include functional (physical) status, adequacy of social support and psychological state, nutritional status, cognitive status, economic and environmental status [5].
In the context of the overall ageing population and the expected increase in incidence of invasive cancer in patients above the age of 65 years [6], comorbidities in elderly patients associated with prognostic implications must be taken into account by treating physicians. In uro-oncology, age-related physiological changes and comorbidities affect treatment choices and outcomes. Comorbidities in the bladder cancer population include renal function impairment, cardiovascular disease, neuropathy and hearing loss. Cardiovascular disease and chronic kidney disease (CKD) are more prevalent in the elderly (>65 years) [7], [8], [9]. In addition, 10–25% of individuals over the age of 65 are characterised as frail [10]. Older and ‘unfit’ bladder cancer patients are frequently underrepresented in clinical trials [11], [12], [13]. Importantly, not all individuals over the age of 65 are ‘elderly.’ A distinction between chronological and functional age must be made. Fit 70-year-olds with adequate renal function tolerate cisplatin-based chemotherapy as well as their younger counterparts and achieve comparable clinical outcomes [14]. Patients should be routinely categorised according to their physiological age in order to gauge whether elderly bladder cancer patients are fit enough to receive cisplatin, which forms the backbone of standard chemotherapeutic treatment.
2.2. Geriatric screening tools
Elderly cancer patients are a heterogeneous group with respect to overall health status, due to differences in comorbidities, functional status, geriatric syndromes and socioeconomic aspects. Although it is not standard, the comprehensive geriatric assessment (CGA) is recommended for routine use in the older patient population with cancer by several societies, including the International Society of Geriatric Oncology [15], [16] and the National Comprehensive Cancer Network [17]. The CGA can distinguish fit patients from vulnerable or frail patients more precisely than physician's evaluations [18], in order to provide an estimate of physiological age and improve anti-cancer treatment. However, because the CGA is a resource-consuming process that is not necessary in all patients, several geriatric screening tools have been developed to identify elderly cancer patients who would benefit from a CGA and multidisciplinary approach (reviewed in [15]). With the Flemish version of the Triage Risk Screening Tool and Vulnerable Elders Survey-13, the G-8 geriatric screening tool is one of the most studied tools, and has the highest sensitivity [15]. The G-8 total score ranges from 0–17, with a cut-off value of 14 (14 and above being favourable, and <14 indicating impairment, which requires CGA). The vast majority of users (98.7%) complete the test in less than 10 min (Table 1) [19].
Table 1.
The G-8 questionnaire [19].
| Items | Possible responses (score) | |
|---|---|---|
| A | Has food intake declined over the past 3 months due to loss of appetite, digestive problems, chewing, or swallowing difficulties? | 0 = severe decrease in food intake |
| 1 = moderate decrease in food intake | ||
| 2 = no decrease in food intake | ||
| B | Weight loss during the last 3 months? | 0 = weight loss >3 kg |
| 1 = does not know | ||
| 2 = weight loss between 1 and 3 kg | ||
| 3 = no weight loss | ||
| C | Mobility? | 0 = bed or chair bound |
| 1 = able to get out of bed/chair but does not go out | ||
| 2 = goes out | ||
| E | Neuropsychological problems? | 0 = severe dementia or depression |
| 1 = mild dementia | ||
| 2 = no psychological problems | ||
| F | BMI? (weight in kg)/(height in m2) | 0 = BMI <19 |
| 1 = BMI 19–<21 | ||
| 2 = BMI 21–<23 | ||
| 3 = BMI ≥23 | ||
| H | Takes more than three prescription drugs per day? | 0 = yes |
| 1 = no | ||
| P | In comparison with other people of the same age, how does the patient consider his/her health status? | 0.0 = not as good |
| 0.5 = does not know | ||
| 1.0 = as good | ||
| 2.0 = better | ||
| Age | 0: >85 | |
| 1: 80–85 | ||
| 2: <80 | ||
| Total score | 0–17 |
BMI, body mass index.
Reproduced from Bellera CA, Rainfray M, Mathoulin-Pélissier S, et al. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol 2012;23:2166–72. With permission from Oxford University Press.
Thus, a treatment algorithm can be derived, based on G-8 screening scores (Fig. 1). ‘Fit’ patients are those with a score of 14 and above and they require no geriatric assessment. ‘Vulnerable’ and ‘frail’ patients are those with a score below 14 and require geriatric assessment. In the case of ‘vulnerable’ patients, geriatric interventions can reverse various comorbidities such as abnormal activities of daily living (ADL) grades I or II, malnutrition, depression, or cumulative illness rating scale for geriatrics (CISR-G) grades I or II. ‘Frail’ patients are those for whom geriatric interventions cannot reverse comorbidities, such as abnormal ADL ≥3, severe malnutrition, cognitive impairment, CISR-G grades III or IV [19].
Fig. 1.
The G-8 geriatric screening tool (based on [19]). ADL, activities of daily living; CISR-G, cumulative illness rating scale for geriatrics; IADL, instrumental activities of daily living.
In cancer patients as a whole, it is becoming increasingly clear that treatment should be adapted to health status. On the one hand, ‘fit’ patients should receive the same standard treatment as younger patients, corresponding to approximately 50% of men aged 70–75 years and about 25% of men aged 80–85 years. ‘Vulnerable’ patients require geriatric intervention followed by standard treatment. On the other hand, ‘frail’ patients should receive geriatric intervention followed by adapted or palliative treatment, and those who are ‘too sick’ should receive palliative treatment only [20].
2.3. Conclusions
Performing a geriatric approach as part of a collaborative multidisciplinary effort is simply equivalent to good medicine. It is important to invest time in the care of elderly patients, in order to ultimately save time, provide better treatment and quality of life for these patients. Strategies to improve cancer care in the elderly are urgently needed.
3. Limitations of local treatment and alternative options in invasive bladder tumours Nicolas Mottet, France
3.1. Local treatment: standard of care
The standard or care in MIBC is neoadjuvant chemotherapy followed by RC and pelvic lymph node dissection (PLND). Neoadjuvant chemotherapy is recommended for T2–T4a, cN0M0 bladder cancer, and should always be cisplatin-based combination therapy. Neoadjuvant chemotherapy is not recommended in patients who are ineligible for cisplatin-based combination chemotherapy. Surgical intervention or multimodality treatments are the preferred curative therapeutic approaches, as they are more effective than radiotherapy (RT) alone. Multimodality treatment could be offered as an alternative in selected, well-informed and compliant patients, especially for whom cystectomy is not an option [21]. Stratifying elderly patients according to their risk–benefit profile using a multidisciplinary approach will help to select patients most likely to benefit from radical surgery and to optimise treatment outcomes. The decision regarding bladder-sparing or RC in elderly/geriatric patients with MIBC should be based on tumour stage and comorbidity best quantified by a validated score [21] and patient's wishes, and not on the patient's age.
3.2. Cystectomy
3.2.1. Undertreatment of MIBC
Evidence-based guidelines recommend RC for patients with MIBC, but many patients receive alternative therapies, such as chemotherapy or radiation. A U.S. National Cancer Database study found that potentially curative aggressive therapy (i.e. radical or partial cystectomy or definitive radiation/chemoradiotherapy [CRT, total dose ≥50 Gy]) was only delivered to 52.5% of patients, and use of aggressive therapy significantly decreased with advancing age (Fig. 2) [4].
Fig. 2.
Distribution of primary therapies received by MIBC patients by age group [4]. Reproduced with permission. © European Association of Urology 2012.
Other studies have shown that cystectomy is underused in senior adults, including analyses stratifying patients by T stage [22], [23]. Further, a study on bladder cancer patterns of care observed that cystectomy for muscle-invasive disease was influenced by patient age and geographic region and not by comorbidities [24].
3.2.2. Survival data
Even though cystectomy is underused in MIBC, it is associated with better survival, independently of age, socio-economic status and serious comorbidity [25], [26], [27]. Tumour stage and number of lymph nodes removed have an impact on specific survival.
Therefore, even if older patients have a higher American Society of Anesthesiologists score, and receive less adjuvant treatment, they should not be denied RC if they are deemed fit to undergo surgery [26], [27]. Studies confirm that a considerable proportion of elderly patients benefit from RC with curative intent. Of note, postoperative outcomes after RC depend on surgeon experience and case load [28].
3.3. Neoadjuvant chemotherapy
A systematic review and meta-analysis assessed the effect of neoadjuvant chemotherapy in the treatment of patients with invasive bladder cancer. Based on 11 trials involving 3005 patients, a significant survival benefit associated with cisplatin-based combination chemotherapy (hazard ratio [HR] = 0.86, 95% confidence interval [CI] 0.77–0.95, p = 0.003) was observed, equivalent to a 5% absolute improvement in survival at 5 years. However, there was not sufficient evidence to reliably determine the effect of single-agent cisplatin on survival [29].
A Medical Research Council/European Organisation for Research and Treatment of Cancer (MRC/EORTC) trial presented the long-term results (median follow-up 8 years) of the international multicentre randomised trial that investigated the use of neoadjuvant cisplatin, methotrexate, and vinblastine (CMV) chemotherapy in patients with MIBC treated by cystectomy and/or RT (of note, 17% were older than 70 years). Results showed a statistically significant 16% reduction in the risk of death (HR, 0.84; 95% CI, 0.72–0.99; p = 0.037, corresponding to an increase in 10-year survival from 30–36%) after CMV. In MIBC, neoadjuvant chemotherapy followed by definitive local therapy should be viewed as state of the art, compared with cystectomy or RT alone [30].
3.4. Adjuvant chemotherapy
The role of postoperative adjuvant cisplatin-based chemotherapy compared with control (surgery alone) in the management of MIBC has been assessed in a meta-analysis involving 945 patients from nine randomised controlled trials (RCTs). For OS, the pooled HR across all nine trials was 0.77 (95% CI, 0.59–0.99; p = 0.049) [31].
After stratification of studies by nodal involvement, the HR for disease-free survival (DFS) associated with adjuvant cisplatin-based chemotherapy in studies where more than 50% of patients had pN+ was 0.39 (95% CI, 0.28–0.54), compared with an HR of 0.89 (95% CI, 0.69–1.15) when less than 50% of patients were pN+. Thus, according to this review, postoperative adjuvant cisplatin-based chemotherapy mainly benefits patients with nodal involvement. Furthermore adjuvant chemotherapy will never be able to compensate for poor surgical procedure, such as omitting the extended lymph node dissection (LND) [32].
The EORTC 30994 study compared immediate versus deferred cisplatin-based combination chemotherapy after RC in patients with pT3–pT4 or N+ M0 UC of the bladder. In total, 284 (out of 660 planned) patients were randomly assigned (141 to immediate treatment and 143 to deferred treatment), and after a median follow-up of 7 years, 47% of patients in the immediate treatment group had died compared with 57% in the deferred treatment group. No significant improvement in OS was noted with immediate treatment when compared with deferred treatment [33]. However, these results are underpowered and still inconclusive, while suggesting a survival benefit only in node-negative patients. Therefore, no formal conclusion can be made regarding which patients benefit the most from adjuvant chemotherapy.
3.5. CRT with transurethral resection of the bladder
Radiotherapy is an alternative to cystectomy in MIBC patients. Numerous phase I/II studies have shown that CRT with transurethral resection of the bladder (TURB) is feasible and safe. Three phase III studies have demonstrated a benefit in terms of local control compared to RT alone: (i) RT versus RT + cisplatin [34]; (ii) RT versus RT + nicotinamide/carbogen [35]; (iii) RT versus RT + fluorouracil/mitomycin C [36]. The latter randomly assigned 360 MIBC patients with a median age of 72 years. At 2 years, the rates of locoregional DFS were 67% (95% CI, 59–74) in the CRT group and 54% (95% CI, 46–62) in the RT group. Five-year OS rates were 48% (95% CI, 40–55) in the CRT group and 35% (95% CI, 28–43) in the RT group (HR, 0.82; 95% CI, 0.63–1.09; p = 0.16).
A combined analysis of six prospective Radiation Therapy Oncology Group (RTOG) protocols evaluated bladder-preserving combined-modality therapy (CMT, all of which included cisplatin) in 468 MIBC patients (median age 66 years [range, 34–93 years, 36% were >70 years], clinical T stage T2–T4a). With a median follow-up of 4.3 years among all patients and 7.8 years among survivors (n = 205), the 5- and 10-year OS rates were 57% and 36%, respectively, and the 5- and 10-year disease-specific survival (DSS) rates were 71% and 65%, respectively. This pooled analysis of multicentre, prospective RTOG bladder-preserving CMT protocols suggests long-term DSS comparable to modern immediate cystectomy studies, for patients with similarly staged MIBC [37].
Additional reports from multiple institutional and cooperative group studies have shown that this approach is safe and effective, with OS rates similar to RC in well-selected patients [38], [39]. The best cancers eligible for bladder preservation are those with low-volume T2 disease without hydronephrosis or extensive carcinoma in situ [39] and treated initially with a thorough TURB (as complete as possible). Thus, concomitant CRT is emerging as an attractive alternative for bladder preservation in selected MIBC patients, provided, patients be fit enough to receive the chemotherapy drugs.
In order to address whether cystectomy is better than external-beam radiotherapy, the survival benefit achieved with RC was compared with ERBT in patients with MIBC stratified by age. Those who underwent RC had an OS advantage in all age groups, except for octogenarians (18 versus 15 months). Patients above the age of 80 who receive RC with a limited PLND or RC alone showed little (16 versus 15 months) or no survival benefit. However, DSS was significantly higher in patients who underwent RC, including octogenarians. Even if this comparison is limited by its retrospective nature, it once again highlights that RC is effective, provided it is well done and includes a real lymphadenectomy [40].
In the MRC/EORTC trial that presented the long-term results of CMV chemotherapy in 976 MIBC patients treated by cystectomy and/or RT [30], there was no evidence that neoadjuvant CMV was more or less effective when combined with either RT or cystectomy. For locoregional DFS, there was some evidence of a greater impact with CMV over no CMV given before cystectomy than the same chemotherapy given before RT. However, this comparison is limited, as the patient groups cannot be compared due to differences in patient and tumour characteristics (tumour stage, N0 nodal status, and performance status [PS]).
In summary, CRT with TURB results in optimal outcomes, provided the tumour is a single lesion T2, complete TURB is performed, there is no hydronephrosis or tumour invasion into prostate stroma, the patient has a well functioning bladder, and no carcinoma in situ. A formal efficacy comparison between this bladder-sparing approach and a RC is still lacking.
3.6. TURB alone
A Spanish study analysed the long-term results of an aggressive TURB for MIBC treatment in patients who were biopsied in the deep muscle layer of the tumour bed. A comparison to a control group of 76 patients with invasive pathological stage pT2–3a, N0-3 bladder cancer treated with RC was performed. At 5 and 10 years of follow-up, cause-specific survival rates were 80.5% and 74.5%, respectively. No significant difference was noted in terms of cause-specific survival with respect to the control group. For patients with invasive bladder cancer, radical TURB might be an option when the tumour is clinically limited to the muscular layer and when all biopsies of the periphery and depth of the tumour bed show muscular tissue negative for tumour cells [41].
Another study determined the 10-year outcome of MIBC patients treated with TURB alone. Of 432 patients (tumour stage ≥T2, N0, M0), 151 had a restaging TUR of the primary tumour site showing no (T0) or only non-muscle-invasive (T1) residual tumour. 52 patients opted for immediate RC, while 99 chose a bladder-sparing approach. The 10-year DSS was 76% of 99 patients who accepted to receive TURB as definitive therapy (57% with bladder preserved) compared with 71% of 52 patients who had immediate cystectomy (p = 0.3). The study suggested that radical TURB is a successful bladder-sparing therapeutic strategy in selected patients who have no residual tumour on a repeat extended resection of the primary tumour [42].
3.7. Partial cystectomy
There is very little evidence on partial cystectomy in MIBC patients. The most recently published study was a retrospective analysis that included 101 patients followed-up for a median of 53 months [43]. Multivariate analysis showed that prior history of UC was associated with a decrease of both cancer-specific survival (CSS) and recurrence-free survival (RFS), and was weakly associated with OS; while lymphovascular invasion (LVI) and ureteral reimplantation were associated with a decreased OS, CSS, and RFS.
An earlier retrospective study in 58 patients who had undergone partial cystectomy with LND reported an overall 5-year survival of 69% with a mean follow-up of 33 months. Of these patients, 74% were alive with an intact bladder, and 55% had been continuously disease-free with an intact bladder. On multivariate analysis, concomitant carcinoma in situ (odds ratio [OR] 7.05, p = 0.004) and lymph node involvement (OR 4.38, p = 0.031) were predictors of advanced recurrence [44]. However, unlike reports by Dandekar et al. and Malkowicz et al., this study did not comment on lesion localisation [45], [46].
Recent population-based series and single institution cohorts have found that partial cystectomy did not compromise survival when compared to RC in very selected patients with organ-confined disease, a single limited lesion without carcinoma in situ, N0, and mainly located at the bladder dome. Additional research is needed to clarify patient selection and outcomes [47].
3.8. Palliative radiotherapy: hypofractionation
Reasons for being unfit and for qualifying to receive palliative treatment alone include age, comorbidity, palliation of symptoms, and short life expectancy. In these situations, palliation is the priority. Hypofractionation might represent an attractive modality, avoiding too many journeys to the radiotherapy department and the associated fatigue (Table 2).
Table 2.
Hypofractionation palliative radiotherapy studies.
| Reference | N | Treatment | Approach | Patient characteristics |
|---|---|---|---|---|
| Turgeon, 2014 (retrospective) [48] | 24 | Hypofractionated intensity modulated RT (50 Gy in 20 fractions) with concomitant weekly radiosensitising chemotherapy | Curative, QoL | Elderly (>70 years), T2–T3N0M0 |
| Lacarriere, 2013 (retrospective) [49] | 32 | Haemostatic radiation therapy: 2 schedules:
|
Palliative-care, haematuria related to bladder cancer | ‘Unfit’ for surgery, gross haematuria from advanced bladder cancer (G3) |
| Kouloulias, 2013 (prospective) [50] | 58 | Weekly hypofractionated 3DCRT 36 Gy in 6 weekly fractions | Symptom palliation | Elderly (>75 years), PS <70%, cT1–2N0 |
| Zygogianni, 2013 (prospective) [51] | 43 | Weekly hypofractionated RT (total dose: 36 Gy in 6 fractions) | Safety, symptom palliation | Elderly, poor PS or unfit for surgery, symptomatic (daily pain on urination), T2–T3 |
PS, performance status; QoL, quality of life; RT, radiotherapy.
3.9. Conclusions
The standard treatment approach in MIBC is neoadjuvant chemotherapy followed by RC and PLND. Age is not a limitation for optimal treatment, whereas comorbidity and individual life expectancy are. There are effective alternative treatments in the form of bladder-sparing strategies, of partial surgery (super selection) and combined CRT (with or without cisplatin). Finally, palliative RT is proposed to very unfit patients.
4. Challenging patient profiles and ‘unfit’ patients in advanced UC Maria De Santis, United Kingdom
4.1. Standard chemotherapy
The standard of care for advanced and metastatic UC is cisplatin-based combination chemotherapy. Long-term OS and progression-free survival (PFS) after treatment with gemcitabine plus cisplatin (GC) or methotrexate/vinblastine/doxorubicin/cisplatin (MVAC) have been shown to be similar, as were response rates. Only toxicity was in favour of GC [52], [53] (Table 3).
Table 3.
Long-term follow-up data of cisplatin combination chemotherapy.
| Author Treatment arm | N (ITT) | Median follow-up (years) | Median survival (months) | 5-year (%) |
|---|---|---|---|---|
| Sternberg, 2006 [54] | 263 | 7.3 | 14.9 | |
| MVAC | 129 | 15.1 | 13.5 | |
| HD-MVAC | 134 | 21.8 | ||
| von der Maase, 2005 [53] | 405 | >5 | ||
| MVAC | 203 | 14.0 | 15.3 | |
| GC | 202 | 15.2 | 13.0 | |
| Visceral metastases | 6.8 | |||
| No visceral metastases | 21.9 |
GC, gemcitabine plus cisplatin; HD, high dose intensity; ITT, intention to treat; MVAC, methotrexate, vinblastine, doxorubicin, and cisplatin.
Cisplatin has also been compared with carboplatin in cisplatin-eligible UC patients (Table 4). The carboplatin combinations of all trials resulted in lower complete response (CR) rates and shorter OS. A comparative effectiveness study of 286 patients from four randomised trials confirmed that cisplatin-based chemotherapy was associated with a significantly higher likelihood of achieving a CR (risk ratio [RR] = 3.54; 95% confidence interval [CI] 1.48–8.49, p = 0.005) and overall response rate (ORR) (RR = 1.34; 95% CI 1.04–1.71, p = 0.02) [55]. Thus, the standard first-line chemotherapy for UC should be cisplatin-based, which provides 13–15 months of OS.
Table 4.
Randomised phase II studies of cisplatin versus carboplatin in cisplatin-eligible UC patients.
| Regimens | CR (%) | OS (months) | Reference |
|---|---|---|---|
| MVAC versus MVECa | 25 11 |
13 9.5 |
Petrioli, 1996 [56] |
| MVAC versus Carbo/MV | 13 | 16 | Bellmunt, 1997 [57] |
| 0 | 9 | ||
| GC versus Carbo/gem | 14.5 | 12.8 | Dogliotti, 2007 [58] |
| 1.8 | 9.8 |
Carbo/gem, carboplatin, gemcitabine; Carbo/MV, carboplatin, methotrexate, vinblastine; CR, complete response; GC, gemcitabine plus cisplatin; MVAC, methotrexate, vinblastine, doxorubicin, and cisplatin; MVECa, methotrexate, vinblastine, epirubicin and carboplatin; OS, overall survival; UC, urothelial carcinoma.
The use of cisplatin is limited in daily practice. Only 35.9% of 298 patients presenting with advanced UC to 42 community cancer centres were treated with cisplatin [59]. This percentage was similar for 1077 ‘real world’ patients treated in 23 centres of excellence [60] (Table 5). A more recent published study on patterns of practice in 327 advanced UC patients in Greece reported that 55% did not receive cisplatin-based therapy [61]. Given that the majority of patients may not be eligible to receive cisplatin-based chemotherapy, this population has a significant unmet need.
Table 5.
First-line chemotherapy in advanced/metastatic UC in routine practice.
| Community database [59] N = 298 | RISC group [60] N = 1077 | |
|---|---|---|
| No chemotherapy | 24% | 30% |
| Cisplatin-based | 36% | 36% |
| Carboplatin-based | 27% | 20% |
| Non-platinum regimen | 8% | 14% (1 agent: 11%) |
| Data non-available | 5% | – |
RISC, Retrospective International Study of Invasive/Advanced Cancer of the Urothelium; UC, urothelial carcinoma.
4.2. ‘Unfit’ for cisplatin
There is so far no established standard chemotherapy in patients who are ‘unfit’ to receive cisplatin, even though more than 50% of UC patients are not eligible for standard cisplatin-based chemotherapy [20], [62], [63], [64]. As discussed, comorbidities and age mean that elderly patients are often excluded or under-represented in clinical trials. It is, therefore, not clear whether dosages investigated in clinical trials are realistic or safe in the elderly and comorbid population.
In the first randomised phase III trial for cisplatin-ineligible patients conducted by the EORTC, patients were defined as unfit to receive cisplatin if their creatinine clearance (CrCl) was <60 ml/min and/or the ECOG PS was 2 [62], [65], [66]. In 2011, a consensus conference provided a more uniform description of patients unfit for cisplatin for inclusion in clinical trials [65] (Table 6).
Table 6.
Eligibility criteria for enrolling metastatic UC patients ‘unfit’ for cisplatin-based chemotherapy in clinical trials [65].
| Eligibility criteria (at least one of the following) |
|---|
| 1. WHO or ECOG PS 2 or Karnofsky PS of 60−70% |
| 2. CrCl (calculated or measured) <60 ml/min |
| 3. CTCAE v4 grade ≥II audiometric hearing loss |
| 4. CTCAE v4 grade ≥II peripheral neuropathy |
| 5. NYHA class III heart failure |
CrCl, creatinine clearance; CTCAE, Common Terminology Criteria for Adverse Events; ECOG, Eastern Cooperative Oncology Group; NYHA, New York Heart Association; PS, performance status; WHO, World Health Organization; UC, urothelial carcinoma.
Reproduced with permission. © 2011 American Society of Clinical Oncology. All rights reserved.
Performance status in patients unfit to receive cisplatin must be taken into account with functional status, or with other assessment tools (e.g. CGA). Since no standard chemotherapy has been established for this patient group, the development of well tolerated single-agent therapy or cisplatin-free combination regimens is a priority in view of the substantial number of patients who are not eligible for cisplatin.
4.3. Treatment of bladder cancer in the elderly
The main challenge when treating bladder cancer in the elderly is the use of cisplatin, whose pharmacokinetic properties principally rely on renal elimination [67]. With increasing age, comorbidities increase and renal function decreases [68]. Therefore, serum creatinine will be cleared more slowly in older patients, and will not provide an accurate reflection of renal function. Renal dysfunction is common in oncology patients and many chemotherapy agents, including cisplatin, are nephrotoxic. Renal function should be evaluated before every treatment course in all cancer patients, using either the Cockcroft-Gault or the Modification of the Diet in Renal Disease (MDRD) formulae, including in patients with normal serum creatinine. The use of additional nephrotoxic agents should be avoided whenever possible. Reduced or split-dose cisplatin, or reduced infusion rates, should be administered to avoid excessive toxicity in elderly patients. It is also important to monitor hydration. Moreover, patients above 70 years of age are frequently diagnosed with CKD and have more prevalent comorbidities (hypertension, diabetes, stroke and ischaemic heart disease) than patients without CKD [69].
In addition, the occurrence of neuropathy is associated with cumulative doses of cisplatin and taxanes. Age is also a risk factor for developing peripheral neuropathies induced by chemotherapy [70].
4.4. Bladder preservation after chemotherapy
According to the European Association of Urology guidelines, chemotherapy alone is not recommended for the treatment of the primary tumour [21]. The main reason is the high incidence of relapse in the bladder, with added mortality. The outcomes of patients who did not undergo cystectomy after having received neoadjuvant chemotherapy for MIBC are summarised in Table 7. Clinically complete responders after neoadjuvant chemotherapy for MIBC may have the option to retain the bladder with durable survival, but the added risks and deviation from the guidelines must be openly discussed.
Table 7.
Outcomes of patients who refused cystectomy after receiving neoadjuvant chemotherapy for MIBC.
| Herr, 2008 [71] | Sternberg, 2003 [72] | Meyer, 2014 [73] | |
|---|---|---|---|
| CR after neoadjuvant MVAC (cT0) | N = 63 | N = 37 | N = 25 |
| 5-year survival DSS | 64% | 68% | 88% |
| Intact bladder | 54% | 51% | 72% |
| Relapse in bladder | 64% | 35% | 52% |
| Muscle invasive | 28% | ||
| Non-muscle invasive | 24% | ||
| Relapse metastatic | NR | 24% | – |
| Additional mortality | 30% | 32% | – |
| Alive with bladder intact | NR | 38% | – |
CR, complete response; DSS, disease-specific survival; MVAC, methotrexate, vinblastine, doxorubicin, and cisplatin; NR, not reported; MIBC, muscle-invasive bladder cancer.
4.5. First-line treatments in unfit patients
Various combination regimens in patients unfit for cisplatin-based chemotherapy show an ORR of at best 30–40% and a median OS of 8–10 months [74], [75], [76], [77], [78], [79], [80], [81], [82]. Single arm studies with more positively selected patients, in particular those having a solitary kidney as single inclusion criterion for cisplatin ineligibility, reported longer OS with up to 15 months (Table 8) [82], [83], [84], [85].
Table 8.
Selected first-line treatment studies in unfit patients.
| Author, year | Phase, N | Patient profile | ORR (%) | Median PFS (months) | Median OS (months) |
|---|---|---|---|---|---|
| Doxorubicin–gemcitabine → paclitaxel–carboplatin | |||||
| Galsky, 2007 [83] | II; 25 | CrCl <60 ml/min and/or prior nephrectomy | 56 | NR | 15 |
| Bevacizumab + gemcitabine–carboplatin → bevacizumab | |||||
| Balar, 2013 [84] | II; 51 | CrCl <60 ml/min and/or solitary kidney and/or KPS 60–70% | 43 | 6.5 | 13.9 |
| Gemcitabine | |||||
| Culine, 2011 [82] | R II; 21 | CrCl 30–60 ml/min and/or PS 2 | 43 | 3.8 | 5.4 |
| Sunitinib | |||||
| Bellmunt, 2011 [85] | II; 38 | CrCl 30–60 ml/min (PS 0–1) | 8 | 4.8 | 8.1 |
KPS, Karnofsky performance status; PFS, progression-free survival; ORR, overall response rate; CrCl, creatinine clearance; OS, overall survival; NR, not reported; UC, urothelial carcinoma.
The EORTC 30986 study is the only available phase III trial that compared two carboplatin-containing first-line chemotherapy regimens (methotrexate, carboplatin, and vinblastine [M-CAVI] versus gemcitabine/carboplatin [GCa]) in clearly defined cisplatin-ineligible patients with advanced UC [78] (Table 9).
Table 9.
EORTC 30986 study: first-line therapy in bladder cancer patients unfit for cisplatin-based chemotherapy [78].
| Regimen | ORR (%) confirmed (%) | OS (months) | Severe acute toxicity (%) | Toxic death (%) |
|---|---|---|---|---|
| GCa | 41.2 36.1 |
9.3 | 9.3 | 1.7 |
| M-CAVI | 30.3 21.0 |
8.1 | 21.2 | 3.4 |
GCa, gemcitabine/carboplatin; M-CAVI, methotrexate, carboplatin, and vinblastine; ORR, overall response rate; OS, overall survival.
No significant differences in OS and PFS were observed, but a significant difference for confirmed RR was noted in favour of GCa (36.1% versus 21%, p = 0.01) (Table 9). Fewer adverse events (AEs) were reported with GCa, but there were still grade III/IV AEs (neutropenia 52.5%; thrombocytopenia 48.3%; febrile neutropenia [FN] 4.2%). In patients with both PS 2 and impaired renal function, increased severe acute toxicities (SAT, defined as death, grade IV thrombocytopenia with bleeding, grade III/IV renal toxicity, FN or mucositis), low response rate and a shorter OS were reported. This led to the recommendation against combination chemotherapy in such patients and in favour of single-agent therapy or best supportive care (BSC). The EORTC 30986 study also reported low efficacy and elevated SAT in patients negatively selected according to known prognostic factors [78], [86].
Two additional phase II randomised trials evaluating vinflunine first-line treatment in cisplatin-unfit patients are ongoing. Vinflunine is a novel anti-cancer agent approved for advanced or metastatic UC previously treated with a platinum-based regimen. The Northern Urology Cooperative Oncology Group 1 trial is comparing vinflunine plus gemcitabine versus GCa. The JASINT open-label, multicentre, international randomised phase II study is assessing the combination of gemcitabine or carboplatin with vinflunine [87]. Preliminary safety results showed more haematological grade III/IV AEs in the carboplatin arm [87]. Clinical outcomes, response rates and survival are expected to be published soon.
4.6. Second-line treatment options
Choice of second-line therapy depends on time to progression after first-line treatment, renal function, and PS [21]. Several traditional cytotoxic agents as well as novel targeted agents have been tested in the second-line setting. Response rates of taxanes (weekly paclitaxel, docetaxel, nab-paclitaxel), oxaliplatin, ifosfamide, topotecan, pemetrexed, lapatinib, gefitinib and bortezomib have been modest (up to 28%) in small phase II trials [88], [89]. Gemcitabine has shown very good response rates, but most patients already receive this drug during first-line treatment [90]. Paclitaxel/gemcitabine studies have shown response rates of up to 60%, but randomised phase III trials evaluating this combination in the second-line setting have not included an adequate comparator arm [91], [92], [93].
Several single-agent phase II studies have tested targeted therapies in the second-line setting, but none have shown substantial activity. Trials are also testing targeted agents combined with a cytotoxic drug (paclitaxel + cetuximab or docetaxel + vandetanib), but the combinations exacerbated toxicity, and no synergistic or additive effects were reported [94], [95]. More recent attempts at combining targeted agents with cytotoxic drugs have been more encouraging (docetaxel + ramucirumab versus docetaxel), based on the results of a phase II trial, although toxicity was increased in the combination arm [96]. Another single-arm phase II study which combined pazopanib with paclitaxel reported good ORR values but significant myelosuppression [97].
A phase III trial compared long-term OS of patients with advanced UC treated with vinflunine plus BSC or BSC alone, after failure of platinum-based chemotherapy [98]. The study showed a >2 month survival difference in favour of the vinflunine arm, which was maintained after >3.5 years' follow-up. With vinflunine, the risk of death was reduced by 22%. There were also some long-term survivors in the vinflunine arm (at 40 months follow-up cut off) [98]. For second-line treatment of advanced or metastatic UC, this trial reached the highest level of evidence ever reported. Currently, vinflunine is the only approved second-line treatment [21]. Adverse prognostic factors validated in vinflunine studies for pretreated patients include liver metastases, Hb <10 g/dl, and ECOG PS ≥1 [99], [100]. Available prospective and retrospective data on vinflunine use in routine practice for unselected populations include 422 patients from 87 centres (Table 10).
Table 10.
Vinflunine efficacy in routine practice.
| Germany [101] | France [101] | UK [102] | Spain [103] | Greece [104] | |
|---|---|---|---|---|---|
| Patients | 77 | 134 | 38 | 102 | 71 |
| 1st-line therapy | Platinum-based chemotherapy | ||||
| ECOG PS/Karnofsky | Median Karnofsky: 80 | PS 0: 25% PS 1: 46% PS ≥2: 23% |
PS 0–1: 92% PS 2: 8% |
PS 0: 31% PS 1: 61% PS 2: 8% |
PS 0: 24% PS 1: 53% PS 2: 20% PS 3: 3% |
| Visceral involvement | 60% (visceral) | 57% (lung + liver) | 39% (lung) 29% (liver) | 29% (lung) 17% (liver) | 42% (lung) 30% (liver) |
| Number of cycles | Average: 5 | Median: 5 (1–23) | Median: 3 (1–16) | Median: 4 (1–18) | Median: 4 (1–16) |
| ORR (%) | 23 | 22 | 32 | 25 | 16.3 |
| DCR (%) | 53 | 51 | 53 | 66 | – |
DCR, disease control rate; ECOG, Eastern Cooperative Oncology Group; ORR, overall response rate; PS, performance status.
These recent data from European phase IV studies performed in real life confirm the efficacy of vinflunine, even in patients with adverse prognostic factors [101]. Each of these studies report a median OS that exceeds the 6.9 months reported in the phase III trial [98]. In real life, vinflunine was safe with manageable toxicity (Table 11). Myelosuppression is usually managed with granulocyte colony-stimulating factor in these patients, particularly if dose modifications are insufficient [105].
Table 11.
Vinflunine safety in routine practice.
| Grade III/IV adverse events | Germany (n = 77) [101] | France (n = 134) [101] | UK (n = 38) [102] | Spain (n = 102) [103] | Greece (n = 71) [104] |
|---|---|---|---|---|---|
| Haematological adverse events (% patients) | |||||
| Neutropenic infection or febrile neutropenia (%) | 1 | 3 | 5 | NR | NR |
| Neutropenia (%) | NR | 17 | 3 | 13 | 16.3 |
| Anaemia (%) | 6 | 8 | 5 | NR | 4.1 |
| Non-haematological adverse events (% patients) | |||||
| Constipation (%) | 5 | 8 | 11 | 6 | 12.2 |
| Abdominal pain (%) | NR | 3 | 0 | 5 | NR |
| Asthenia/fatigue (%) | 1 | 21 | 8 | NR | 16.3 |
| Vomiting (%) | 3 | NR | 0 | 2 | NR |
NR, not reported.
Vinflunine is especially interesting in the context of special patient populations, including those with renal or hepatic impairment, and elderly patients. Vinflunine has been shown to be safe in patients with a CrCl as low as 20 ml/min [106]. These conditions are not contraindications to vinflunine use, but require dose adjustments. In the case of renal impairment, the dose needs adjusting according to CrCl values. In patients whose CrCl is ≥60 ml/min, standard vinflunine dosing of 320 mg/m2 every 3 weeks (q3w) is recommended, or 280 mg/m2 q3w in patients who are PS 1 or who have received prior radiotherapy. The same lower dose of 280 mg/m2 q3w is recommended in patients whose CrCl is 40–60 ml/min, and a further reduced dose of 250 mg/m2 q3w in patients whose CrCl is 20–40 ml/min. The large experience in the post-platinum setting, phase II and III study data, and real world experience all demonstrate that vinflunine is safe in patients with CrCl <60 ml/min. Indeed, the ‘tolerance profile of vinflunine in patients with renal dysfunction was similar to that observed in patients with CrCl >60 ml/min’ [106]. Vinflunine can be used in patients with hepatic impairment, provided it is not severe. Doses of 250 mg/m2 or 200 mg/m2 q3w are recommended in patients with mild Child-Pugh Grade A and moderate (Grade B) impairment, respectively.
In addition, vinflunine is safe in elderly patients; most common AEs do not differ from those seen in younger patients. Similarly to patients with renal impairment, based on pharmacokinetic and safety data, vinflunine should be started at 280 mg/m2 q3w in patients who are aged 75–79 years, and the dose should be lowered to 250 mg/m2 q3w in patients aged ≥80 years who are in good shape [107]. The decision on which dose to administer depends on biological parameters. Upper age thresholds are currently not outlined in clinical trials.
4.7. Conclusions
Urothelial cancer patients are of higher median age and often present with organ impairment and comorbidities. Cisplatin-based chemotherapy is the standard first-line treatment in advanced UC. However, more than 50% of patients are not eligible for cisplatin due to age and/or renal comorbidities, meaning that alternative treatment options are needed for these patients. Medical management should consider nephrotoxicity of drugs and patients' performance status and organ function.
The first randomised phase II/III trial in ‘unfit’ patients showed that M-CAVI and GCa are active, with a toxicity profile in favour of GCa. Thus, in patients ineligible for cisplatin due to a single risk factor (e.g. renal function), the standard regimen is GCa. There may be a role for splitting cisplatin doses in the case of renal function impairment. Patients ineligible for cisplatin are not a uniform group, and successful outcomes of combination therapy depend on renal function, PS and the presence/absence of prognostic factors. In frail patients, recommended treatment options include carboplatin or gemcitabine monotherapy, or GCa if possible. This population is challenging because these patients are difficult to include and accrue in clinical trials.
Novel treatments and combinations with vinflunine are promising in an area of unmet need in the first-line setting in patients who are elderly or unfit to receive cisplatin. Vinflunine has a favourable toxicity profile, including in elderly patients or in patients with impaired renal function, provided that the dose is adjusted. For second-line treatment of advanced or metastatic UC, vinflunine is currently the only approved treatment option, although many trials have evaluated traditional cytotoxic agents and combinations with targeted agents.
5. Management of upper tract UC: surgical aspects Nicolas Mottet, France
5.1. Surgery
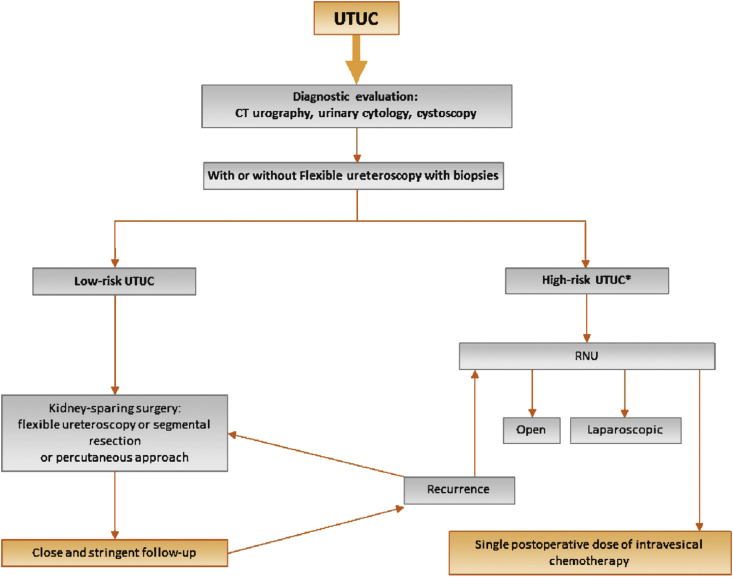
The standard surgical modality in UC of the upper urinary tract (UTUC) is a radical nephroureterectomy (RNU) plus LND followed by single immediate postoperative intravesical instillation (usually with mitomycin C). New data suggest that a systematic conservative approach is standard of care in selected patients (Fig. 3) [108].
Fig. 3.
Proposed flowchart for the management of UTUC [108].*In patients with solitary kidney, consider a more conservative approach. CT, computed tomography; RNU, radical nephroureterectomy; UTUC, upper urinary tract urothelial cell carcinoma.
Low-risk UTUC is defined by the presence of all the following factors: unifocal disease, tumour size <1 cm, low-grade cytology, low-grade ureteroscopic (URS) biopsy, and no invasive aspect on multidetector computed tomography-urography. On the other hand, high-risk UTUC is defined by the presence of either of these factors: hydronephrosis, tumour size >1 cm, high-grade cytology, high-grade URS biopsy, multifocal disease, and previous RC for bladder cancer [108].
In case of lesion suspicion upon imaging and negative or low-grade cytology, it is recommended to perform an ureteroscopy and biopsy, whereas ureteroscopy is optional in the case of positive cytology.
5.1.1. Conservative treatment (low-risk UTUC)
Techniques used in the conservative management of low-risk UTUC are as follows [108]:
-
•
Laser should be used for endoscopic treatment
-
•
Flexible ureteroscopy is preferable to rigid ureteroscopy: especially for renal pelvis, distal, and mid-ureter
-
•
A percutaneous approach remains an option in low-grade tumours unsuitable for URS treatment
Conservative treatment may also be extended to (low-grade only) multifocal lesions (pelvis only) without ureteral locations or tumours <3 cm, provided they are in the pelvis and non-flat. In the future, biological markers associated with low-grade lesions may play a role in patient selection. One unsolved issue is the role of adjuvant (mainly percutaneous) instillations in low-risk UTUC patients.
5.1.2. Lower ureter management
Conservative treatment is performed most of the time in the distal ureter, provided the approach is feasible. Endoscopic removal is recommended in low-grade, small, non-circular tumours. Complete distal ureterectomy and neocystostomy are indicated for non-invasive, low-grade tumours in the distal ureter that cannot be removed completely endoscopically, and for high-grade, locally-invasive (i.e. T2) tumours. Of note, the upper ureter must be normal, and kidneys must be functional [108].
5.1.3. Radical surgery (high-risk UTUC)
RNU plus LND is recommended in high-risk UTUC patients. The procedure involves removal of perinephretic fat. No difference in recurrence or cancer-specific mortality has been reported when comparing open versus laparoscopic RNU in 1249 patients from 13 international centres [109], provided that surgical principles are respected and that the urinary tract is closed.
It is essential to remove the bladder cuff in RNU. The prognostic impact of bladder cuff excision at nephroureterectomy on cancer-specific mortality was quantified in a population-based cohort of 4210 UTUC patients. In univariable and multivariable analyses, omission of bladder cuff excision increased cancer-specific mortality rates in patients with pT3N0/x, pT4N0/x, and pT(any)N1–3 UC of the renal pelvis [110]. Unfortunately, in real practice the standard principle of RNU with systematic bladder cuff removal is not followed in about 50% of patients despite clear guidelines [109].
Intravesical recurrence after RNU is a frequent event requiring intense bladder surveillance with endoscopy. A retrospective analysis in 2681 patients treated with RNU for UTUC at 24 international institutions compared outcomes following RNU using three different methods of bladder cuff management (transvesical, extravesical, and endoscopic) [111]. Of the 2681 patients, 67.5% underwent the transvesical approach; 29.3%, the extravesical approach; and 3.2%, the endoscopic approach. There was no difference in terms of RFS, CSS, and OS among the three distal ureteral management approaches. Patients who underwent the endoscopic approach were at significantly higher risk of intravesical recurrence compared with those who underwent the transvesical (p = 0.02) or extravesical approaches (p = 0.02); the latter two groups did not differ from each other [111].
5.1.4. Nodal dissection
Nodal dissection is not necessary if pTa/1: max 2% pN+, but it is mandatory in the following instances: ≥pT2 (16% pN+); pT2 (8% pN+); pT3 (17% pN+), or pT4 (46% pN+). RNU provides local control and CSS in patients with localised UTUC. Pathologic tumour grade, T stage, LN status, tumour architecture, and LVI are important prognostic variables associated with oncologic outcomes, which could potentially be used to select patients for adjuvant systemic therapy [112].
Having addressed when to perform nodal dissection, clear templates remain to be established to perform the procedure. This depends on lesion location and requires formal clinical validation [108] (Table 12).
Table 12.
Lesion location and nodal dissection.
| Lesion location | Location of nodal dissection |
|---|---|
| Right pyelic lesion | Renal hilum, para, retro cave |
| Left pyelic lesion | Renal hilum, para-aortic |
| Right lumbar ureter (down to iliac vessels) | Renal hilum, para, retro cave, inter-aorticocave |
| Left lumbar ureter (down to iliac vessels) | Renal hilum, para-aortic |
| Right ureter (below iliac vessels) | Primitive external, internal obturator |
| Left ureter (below iliac vessels) | Primitive external, internal obturator |
5.1.5. Postoperative instillation
Guidelines for RNU in UTUC recommend postoperative instillation following surgery to avoid bladder recurrence [108]. Two studies have demonstrated that a single immediate postoperative bladder instillation dose reduces the risk of bladder tumour recurrence [113], [114].
5.2. Conclusions
The main take home message in terms of UTUC surgical management is that a conservative procedure should be applied when feasible. Patients are required to have a closed urinary tract. If RNU is performed, it must include bladder cuff removal and early postoperative instillation at urethral catheter removal; nodal dissection must also be carried out in muscle-invasive lesions.
6. Molecular specificities in UC Joaquim Bellmunt, Spain
6.1. Introduction
New options are needed for treating metastatic bladder cancer, since median survival and response rates with standard therapy (MVAC) have evolved little over the last 30 years. As discussed, superficial disease is treated with TURB plus intravesicular therapy, which includes Bacillus Calmette–Guérin (BCG), mitomycin C, or other investigational agents such as gemcitabine. Localised muscle-invasive disease is managed with cystectomy and neoadjuvant (the standard) or adjuvant chemotherapy; chemoradiation is used if a bladder-sparing approach is opted for. Metastatic disease is treated mainly with chemotherapy, and there are a wide variety of available agents whose role is well established, including cisplatin, carboplatin, or gemcitabine. Immunotherapy and targeted therapies are emerging and highly promising strategies that are awaiting confirmation for being established in this setting.
An improved understanding of molecular specificities of bladder cancer will provide new opportunities for prognostic application and personalised therapy. The sections below present an overview of the latest cancer genomics data in UC, and the potential use of tumour genetics in bladder cancer.
In the future, precision medicine and the analysis of large-scale somatic and germline genomics may impact individual patient treatment in bladder cancer and individualise patient care.
6.2. Recurrence and progression in high-grade ‘superficial’ bladder cancer
Approximately 21% of superficial bladder cancers progress to muscle-invasive or more advanced disease stages, and 50% of localised muscle-invasive disease progresses to metastatic disease. Although risk tables provide a prognostic tool, no molecular biomarkers accurately predict disease recurrence, progression or cancer-specific mortality [115].
A recent meta-analysis in high-grade T1 non-muscle-invasive bladder cancer assessed selection criteria for early cystectomy in these patients. The highest impact risk factor was depth of invasion (T1b/c) into lamina propria (progression: HR = 3.34, p < 0001; CSS: HR = 2.02, p = 0.001). Several other factors also predicted progression and CSS (LVI, associated clinically-isolated syndrome, non-use of BCG, tumour size >3 cm, and older age) [116].
6.3. Chemotherapy in muscle-invasive disease
As discussed, the timing of perioperative chemotherapy in MIBC patients is a subject of debate. Benefit of neoadjuvant chemotherapy is now well established, but survival benefit from adjuvant chemotherapy seems less evidence-based. An updated meta-analysis on the benefit of postoperative adjuvant cisplatin-based chemotherapy versus surgery alone in 945 patients from nine RCTs reported the following efficacy outcomes, providing evidence of a survival benefit in MIBC patients receiving adjuvant cisplatin-based chemotherapy after RC [31] (Table 13).
Table 13.
Efficacy of postoperative adjuvant cisplatin-based chemotherapy versus surgery alone in MIBC patients receiving adjuvant cisplatin-based chemotherapy after RC [31].
| End-point | HR | 95% CI | p Value |
|---|---|---|---|
| OS | 0.77 | 0.59–0.99 | 0.049 |
| OS in LN (+) | 0.66 | 0.45–0.91 | 0.014 |
| DFS | 0.66 | 0.48–0.92 | 0.014 |
CI, confidence interval; DFS, disease-free survival; HR, hazard ratio; LN, lymph node; MIBC, muscle-invasive bladder cancer; OS, overall survival; RC, radical cystectomy.
Larger studies of adjuvant chemotherapy from meta-analyses and retrospective cohorts show some OS benefit, but not with a high level of evidence (Table 14).
Table 14.
Efficacy of adjuvant chemotherapy from meta-analyses and retrospective cohorts.
| Reference | Design | Total N (adjuvant chemo. n) | Level of evidence | OS HR (95% CI) |
|---|---|---|---|---|
| ABC, 2005 [117] | Individual patient data meta-analysis from 6 RCTs | 491 (246) | 2a | 0.75 (0.60–0.96) |
| Leow, 2014 [31] | Literature-based meta-analysis from 9 RCTs | 945 (475) | 2a | 0.77 (0.59–0.99) |
| Svatek, 2010 [118] | Retrospective cohort study from 11 high volume centres | 3947 (932) | 2c | 0.85 (0.72–0.97)* |
| Booth, 2014 | Population-based retrospective cohort | 2809 (541) | 2c | 0.71 (0.62–0.81) |
| Galsky, 2015 | Population-based retrospective cohort | 5653 (1293) | 2c | 0.72 (0.65–0.80) |
Cancer-specific survival. CI, confidence interval; HR, hazard ratio; OS, overall survival; RCT, randomised controlled trial.
6.4. Molecular determinants of response
Several genetic UC molecular markers that predict response to chemotherapy are emerging. Whole exome sequencing on pretreatment tumour and germline DNA from 50 patients with MIBC who received neoadjuvant cisplatin-based chemotherapy followed by cystectomy identified ERCC2, a nucleotide excision repair gene, as being mutated in cisplatin responders compared with non-responders [119].
Similarly, a pathologic CR to neoadjuvant chemotherapy containing platinum is a strong prognostic determinant for MIBC patients. Erb-b2 receptor tyrosine kinase 2 (ERBB2) mutations characterise a subgroup of MIBC with excellent response to neoadjuvant therapy [120].
A neoadjuvant clinical trial is underway to compare the clinical efficacy of GC versus MVAC and the ability of a gene expression profiling-based algorithm (CoXEN) to predict complete pathological response [121].
6.5. Bladder-preservation as a CMT
Long-term outcomes in MIBC patients after bladder-preserving CMT were assessed in a pooled analysis of five phase II studies and one phase III study that included a total of 468 patients. With a median follow-up of 7.8 years among survivors (n = 205), the 5- and 10-year OS rates were 57% and 36%, respectively, and the 5- and 10-year DSS rates were 71% and 65%, respectively. The study demonstrated long-term outcomes comparable to modern immediate cystectomy studies. Given these long-term outcomes, bladder-preserving CMT can be considered as an alternative to RC, especially in elderly patients not well suited for surgery [37].
Protein expression of DNA damage signalling proteins in tumour samples was measured prior to radical radiotherapy or cystectomy in MIBC patients. In the RT cohort, low tumour MRE11 expression was associated with worse 3-year CSS compared with high expression, highlighting this protein as a predictive factor associated with survival following RT [122].
A report in patients with clinical stage T2–4a MIBC treated with TURB plus cisplatin-based induction and consolidation chemoradiation regimens (and RC for invasive tumour recurrence) showed variations in 3-year OS estimates depending on vascular endothelial growth factor (VEGF) expression patterns [123].
6.6. Precision oncology
The progression towards targeted therapy in UC is underway. Some cancers are still treated with non-specific or non-targeted chemotherapy. ‘Druggable’ tumour-specific genetic targets are increasingly the focus of research. In other cancers, molecular profiling has identified single drug targets such as BCR-ABL in chronic myeloid leukaemia, KRAS in colorectal cancer, or BRAF in melanoma.
So far, no molecularly targeted agents have been approved for bladder cancer treatment. Genomic alterations of anaplastic lymphoma kinase (ALK) have been reported in UC patients, but no association between ALK copy number alteration and OS, ECOG PS, or development of visceral disease was observed [124].
Bladder cancer is a molecularly heterogeneous disease. The Cancer Genome Atlas (TCGA) project reported molecular alterations in several genes involved in cell cycle regulation, chromatin regulation, and kinase signalling pathways, identifying potential therapeutic targets [125]. The TCGA findings have prompted the design of several genomic target driven trials.
6.6.1. Cell cycle regulatory genes, signalling pathways and immunotherapy
An ongoing phase II trial is testing palbociclib (PD-0332991) in patients with metastatic UC with mutated RB and inactivated p16 or overexpressed CCND1 after failure of first-line chemotherapy. The trial will explore the association of molecular markers with outcomes of palbociclib response and the association between UC molecular subtypes (basal-like or luminal) with outcomes of response or resistance to palbociclib (ClinicalTrials.gov Identifier: NCT02334527).
Whole-genome sequencing was used to investigate the genetic basis of a durable remission of metastatic bladder cancer in a patient treated with everolimus, a drug that inhibits the mammalian target of rapamycin (mTOR) signalling pathway. A loss-of-function mutation was identified in tuberous sclerosis complex 1 (TSC1), a regulator of mTOR pathway activation. TSC1 mutation correlated with everolimus sensitivity [126]. As a result, several ongoing phase I/II trials are investigating the impact of PI3K/mTOR pathway genomic alterations in metastatic UC patients treated with various regimens, in order to help identify the best responding patients. In addition, fibroblast growth factor receptor (FGFR) tyrosine kinase inhibitors are an emerging area of interest in cancer therapeutics. Proof-of-concept was recently established by two trials targeting FGFR3 in previously-treated FGFR3-mutant or translocated metastatic UC [127], [128].
Studies are being designed in order to accelerate clinical drug development in UC. The Alliance MATCH-UP study aims to apply molecular findings to clinical trials by allocating drugs based on molecular screening in metastatic UC following platinum-based chemotherapy.
Finally, the programmed death-1 (PD-1) receptor/PD-1 ligand (PD-L1) pathway negatively regulates T-cell-mediated responses. The prognostic impact of PD-L1 expression was defined in UC. Results from 160 tumour samples showed that PD-L1 is widely expressed in tumour cell membrane and tumour-infiltrating mononuclear cells (TIMCs). PD-L1 expression in TIMCs was significantly associated with longer survival in patients who developed metastases [129] and received subsequent chemotherapy. Using PD1 or PD-L1 as a target, several studies have shown encouraging results with anti-PD-L1 antibodies (pembrolizumab and MPDL3280A) in UC patients with PD-L1-positive advanced tumours [130], [131]. Promising long lasting responses have been seen in a selected subgroup of patients. These drugs are now undergoing testing in phase III trials versus chemotherapy, in the second-line setting.
6.7. Intrinsic subtypes in MIBC
In other diseases such as breast cancer, intrinsic subtypes are well characterised (e.g. luminal, HER2-positive, triple-negative) [132], each of which are associated with distinct clinical management approaches. The objective in UC has been to develop similar treatment algorithms. Based on current research, the following intrinsic subtypes and clinical features have been described in MIBC [133], [134]:
-
•
Luminal: enriched with papillary histology and activating FGFR3 mutations and translocations. About half of the tumours are sensitive to neoadjuvant chemotherapy. Therapies include GC/dose-dense MVAC, and possibly FGFR inhibitors.
-
•
p53-like: infiltrated with stromal fibroblasts, bone metastases. Resistant to neoadjuvant chemotherapy. Therapies may include Met-inhibitors or initial surgery.
-
•
Basal: enriched with squamous features, immature signature, angiogenesis. More common in women. Associated with advanced stage, metastatic disease at presentation, shorter DSS and OS in the absence of chemotherapy. About half of the tumours are sensitive to neoadjuvant chemotherapy. Therapies include GC/dose-dense MVAC and possibly CTLA4/PD1/PDL-1, or VEGF inhibitors.
-
•
Claudin-low/mesenchymal: clinical features are not well defined. Probably enriched with sarcomatoid features and associated with high metastatic potential.
6.8. Conclusions
An increasing number of studies are bridging the gap between translational science and novel biomarker-driven clinical trials, establishing molecular-based therapies for bladder cancer. For instance, expression analysis of DNA damage signalling proteins in tumour samples taken before irradiation could be used as a predictive marker of RT response and may ultimately allow patient selection for RT or cystectomy, thus improving overall cure rates.
Studies have demonstrated the feasibility of using whole-genome sequencing in the clinical setting to identify biomarkers of drug sensitivity that can aid in the identification of patients most likely to respond to targeted anti-cancer therapies.
Bladder cancer can no longer be thought of as a single disease; subtyping should be considered, and may have implications on selecting therapy. Application of biomarkers is set to fundamentally change bladder cancer treatment.
7. Perspectives for the future of UC Joaquim Bellmunt, Spain
7.1. First-line therapies
As mentioned earlier, the advances achieved in the last 30 years of randomised trials evaluating systemic chemotherapy in the treatment of advanced bladder cancer are limited. Advanced bladder cancer seems to have reached a plateau with regard to median survival of patients, averaging approximately 15 months.
The standard chemotherapy regimens in 2015 for the first-line treatment or ‘fit’ patients are GC or MVAC or a regimen combining paclitaxel and GC in selected patients. GC and MVAC have level 1 evidence and grade A recommendation [21], [135].
In ‘unfit’ patients, the combination of carboplatin and gemcitabine is now considered the best option based on a randomised phase II/III trial conducted in the EORTC (EORTC 30986) versus the previously used regimen M-CAVI [78].
Knowing that vinflunine is an effective drug with a safe profile in patients with impaired renal function, the open-label, multicentre, international JASINT-1 randomised phase II study assessed the combination of gemcitabine or carboplatin with vinflunine [87]. The initial promising data from JASINT-1 is being further evaluated in the JASINT-2 randomised phase III study comparing vinflunine–gemcitabine versus gemcitabine–carboplatin doublet combinations in 162 patients unfit for cisplatin with advanced or metastatic UC.
7.2. Second-line therapies
The most promising single-agent second-line chemotherapy is vinflunine, a third-generation semi-synthetic vinca alkaloid. In patients progressing after platinum-based combination chemotherapy for metastatic disease, vinflunine should be offered; alternatively, due to the limitations in this setting, treatment within a clinical trial may be offered (grade A* recommendation) [21]. Currently, vinflunine is the only approved second-line drug and the only drug that has proven beneficial both within a phase III study and in real world data [98], [101], [102], [103]. A long list of single-agent second-line chemotherapy trials is available for advanced bladder cancer (reviewed in [89]).
Vinflunine’s mechanism of action is threefold, in that it is a microtubule inhibitor with anti-angiogenic activity that reverses epithelial-to-mesenchymal transition [136], [137]. Moreover, it has anti-angiogenic activity at non-cytotoxic concentrations and can inhibit metastasis and impact on epithelial-to-mesenchymal transition at low doses (in vitro studies) [138], [139], [140].
7.3. Second-line targeted therapies
Many single-agent phase II studies with targeted therapies have been tested in the second-line setting, but no substantial activity has been demonstrated as single agents. These include gefitinib, bortezomib, lapatinib, sorafenib, sunitinib, aflibercept, pazopanib, volasertib, everolimus, vandetanib, temsirolimus, and dovitinib.
Furthermore, targeted agents combined with a cytotoxic drug (paclitaxel + cetuximab or docetaxel + vandetanib) in platinum-pretreated patients did not demonstrate additive activity and resulted in increased toxicities [94], [95].
The VEGF pathway may play an important role in the pathogenesis of bladder cancer. Previous phase II trials failed to demonstrate activity as single agents, but more recent phase II combination studies have led to more promising results. Ramucirumab, a human VEGFR2-targeted monoclonal antibody, has demonstrated clinical activity in several solid tumours with preclinical testing supporting a role for ramucirumab/taxane combinations in UC. In an ongoing phase II study in the second-line setting of advanced or metastatic UC, patients were randomised equally to one of three open-label treatments: docetaxel (n = 44); docetaxel and ramucirumab (n = 46); or docetaxel and icrucumab (n = 49). Randomisation was stratified by the absence/presence of visceral metastases and receipt of prior anti-angiogenic therapies [96]. While docetaxel + icrucumab led to minimal activity (ORR 10%, disease control rate [DCR] 31%) and development of icrucumab was interrupted in bladder cancer, the ORR for docetaxel + ramucirumab was 19.6 versus 4.5% for docetaxel (p = 0.0502) and the DCR was 67.4 versus 43.2% (p = 0.033). Median interim PFS data were also significantly in favour of docetaxel + ramucirumab versus docetaxel: 22 weeks versus 10.4 weeks (p < 0.001), providing a basis for testing ramucirumab in a phase III study now enrolling 524 patients (docetaxel + ramucirumab versus docetaxel + placebo). Nevertheless, toxicities were increased in the docetaxel + ramucirumab arm with a high rate of FN (20% versus 11% with docetaxel only) (Table 15). The most common grade ≥III AEs from the interim analysis are summarised (Table 15) [96].
Table 15.
Planned interim analysis grade ≥III AEs safety results [96].
| Docetaxel (N = 44) | Docetaxel + ramucirumab (N = 46) | |
|---|---|---|
| Haematological toxicities | ||
| Neutropenia | 36 | 28 |
| Febrile neutropenia | 11 | 20 |
| Anaemia | 5 | 9 |
| Thrombocytopenia | 0 | 7 |
| Non-haematological toxicities | ||
| Fatigue | 11 | 33 |
| Pneumonia | 9 | 13 |
| Sepsis | 7 | 9 |
| Stomatitis | 0 | 7 |
| Diarrhoea | 2 | 7 |
AEs, adverse events.
Another recent single-arm phase II study combined an angiogenesis inhibitor (pazopanib) with weekly paclitaxel in 32 pretreated patients with refractory urothelial cancer [97]. Objective responses were observed in 50% of patients, with PFS and OS values of 6 and 8 months, respectively. Seventy-five percent of patients required dose reduction and 44% of patients received growth factors. Despite the impressive ORR value, OS is similar to many other trials, and myelosuppression was significant. The initially planned phase III study was cancelled based on a corporate decision.
7.4. Maintenance therapy
Maintenance therapy has an established role in some diseases such as advanced non-small cell lung cancer, in which patients receive continuation maintenance therapy or switch to another agent (e.g. pemetrexed or erlotinib) based on the benefits observed in randomised trials [141], [142], [143]. The key objectives of maintenance therapy are to delay progressive disease and increase OS. Secondary objectives include the prevention of symptom deterioration and maintenance of PS to allow further therapy.
Which role and which UC patients would best benefit from such a treatment approach remains to be established. Maintenance therapy in UC was investigated with the receptor tyrosine kinase inhibitor sunitinib versus placebo after first-line chemotherapy in a randomised phase II, double-blind study. From 54 randomised patients, the median PFS was 2.9 months with sunitinib versus 2.7 months with placebo. Similarly, no difference in OS was reported (10.5 months with sunitinib versus 10.3 months with placebo). The study was limited by premature closure and a small sample size. Maintenance sunitinib was considered unlikely to confer benefit in this setting [144]. A second biomarker-driven trial with maintenance lapatinib in bladder cancer was presented at ASCO 2015. Despite selecting patients based on her1 and her2 status, the trial was unable to demonstrate any benefit [145].
Some additional ongoing trials are investigating the role of maintenance therapy in advanced UC after first-line chemotherapy. Phase II data in fit patients are expected from two trials. The JASIMA single arm trial is investigating vinflunine up to disease progression after first-line chemotherapy with up to four cycles of GC treatment. Responding patients with at least stable disease were included in the trial. The Spanish MAJA trial is a randomised multicentre study in which up to six cycles of primary treatment with GC were allowed. Fit patients with advanced or metastatic UC not progressing on palliative first-line cisplatin-based chemotherapy were randomised to receive vinflunine maintenance + BSC versus BSC alone. The first preliminary results from the MAJA study presented at ASCO 2015 (66 patients) indicated that maintenance vinflunine post-cisplatin has an acceptable tolerability profile in advanced UC patients. With a median follow-up of 7.2 months, the median PFS was increased by as much as 6 months (10.4 months in the vinflunine arm and 4.6 months in the BSC arm [p = 0.058]), suggesting that more mature data may lead to significant PFS differences between arms [146].
7.5. Conclusions
Molecular understanding of bladder cancer biology has lagged behind other solid cancers, which has represented a major barrier in improving clinical care. To date, vinflunine is the only therapy that has been established and has been approved by the European Medicines Agency for bladder cancer patients who recur or are refractory to platinum-based therapy. Data on vinflunine in first-line ‘unfit’ bladder cancer patients are highly promising and may be confirmed in the ongoing JASINT-2 phase III study. The role of maintenance vinflunine is under study.
Novel targeted therapies in adequately selected, genetically profiled patients might lead to improvement in therapeutic outcomes.
The new emerging immunotherapeutic approach using check-point inhibitors opens a bright future for a selected subgroup of advanced bladder cancer patients.
Conflict of interest statement
Joaquim Bellmunt has served on advisory boards of GSK, Novartis, Pfizer, Bristol-Myers Squibb, Merck and Genentech. Maria De Santis has received honoraria from, and served as a consultant for, Amgen, Astellas, Bayer, Celgene, Dendreon, Ferring, GSK, Janssen Cilag, Novartis, Pfizer, Pierre Fabre, Roche, Sanofi Aventis, Shionogi, Takeda and Teva/OncoGenex, and has received research grants from Pierre Fabre. Nicolas Mottet has received honoraria from, and served as a consultant for, Takeda pharmaceutical/Millennium, Jansen, Astellas, BMS, Bayer, IPSEN, Ferring, Novartis, Nuclétron, Pierre Fabre, Sanofi, AstraZeneca, and has received research grants from Takeda pharmaceutical/Millennium, Astellas, Pierre Fabre, Sanofi Pasteur.
Acknowledgements
Medical writing support was provided by Vanessa Gray-Schopfer at OmniScience SA (Geneva, CH) and funded by Pierre Fabre Médicament.
Footnotes
Proceedings from an international multidisciplinary summit, Geneva, April 2015.
References
- 1.Globocan 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012. Available from: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx (accessed April 2015).
- 2.United Nations Department of Economic and Social Affairs Population Division. World population ageing: 1950–2050. Available from: http://www.un.org/esa/population/publications/worldageing19502050/ (accessed April 2015).
- 3.Gore J.L., Litwin M.S., Lai J. Use of radical cystectomy for patients with invasive bladder cancer. J Natl Cancer Inst. 2010;102:802–811. doi: 10.1093/jnci/djq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray P.J., Fedewa S.A., Shipley W.U. Use of potentially curative therapies for muscle-invasive bladder cancer in the United States: results from the National Cancer Data Base. Eur Urol. 2013;63:823–829. doi: 10.1016/j.eururo.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Balar A., Bajorin D.F., Milowsky M.I. Management of invasive bladder cancer in patients who are not candidates for or decline cystectomy. Ther Adv Urol. 2011;3:107–117. doi: 10.1177/1756287211407543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pal S.K., Hurria A. Impact of age, sex, and comorbidity on cancer therapy and disease progression. J Clin Oncol. 2010;28:4086–4093. doi: 10.1200/JCO.2009.27.0579. [DOI] [PubMed] [Google Scholar]
- 7.Coresh J., Selvin E., Stevens L.A. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 8.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002;39:S1–S266. [PubMed]
- 9.Stern S., Behar S., Gottlieb S. Cardiology patient pages. Aging and diseases of the heart. Circulation. 2003;108:e99–e101. doi: 10.1161/01.CIR.0000086898.96021.B9. [DOI] [PubMed] [Google Scholar]
- 10.Griebling T.L., editor. Geriatric Urology. Springer Science & Business; 1 January 2014. [Google Scholar]
- 11.Crome P., Lally F., Cherubini A. Exclusion of older people from clinical trials: professional views from nine European countries participating in the PREDICT study. Drugs Aging. 2011;28:667–677. doi: 10.2165/11591990-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 12.Pallis A.G., Ring A., Fortpied C. EORTC workshop on clinical trial methodology in older individuals with a diagnosis of solid tumors. Ann Oncol. 2011;22:1922–1926. doi: 10.1093/annonc/mdq687. [DOI] [PubMed] [Google Scholar]
- 13.Hutchins L.F., Unger J.M., Crowley J.J., Coltman C.A., Jr., Albain K.S. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 14.Galsky M.D., Krege S., Lin C.C. Cisplatin-based combination chemotherapy in septuagenarians with metastatic urothelial cancer. Urol Oncol. 2014;32:30.e15–30.e21. doi: 10.1016/j.urolonc.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Decoster L., Van Puyvelde K., Mohile S. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26:288–300. doi: 10.1093/annonc/mdu210. [DOI] [PubMed] [Google Scholar]
- 16.Wildiers H., Heeren P., Puts M. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32:2595–2603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Older Adult Oncology Version 2. 2015. Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp – age (accessed 9 November 2015).
- 18.Wedding U., Kodding D., Pientka L., Steinmetz H.T., Schmitz S. Physicians' judgement and comprehensive geriatric assessment (CGA) select different patients as fit for chemotherapy. Crit Rev Oncol Hematol. 2007;64:1–9. doi: 10.1016/j.critrevonc.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 19.Bellera C.A., Rainfray M., Mathoulin-Pélissier S. Screening older cancer patients: first evaluation of the G-8 geriatric screening tool. Ann Oncol. 2012;23:2166–2172. doi: 10.1093/annonc/mdr587. [DOI] [PubMed] [Google Scholar]
- 20.Balducci L., Extermann M. Management of cancer in the older person: a practical approach. Oncologist. 2000;5:224–237. doi: 10.1634/theoncologist.5-3-224. [DOI] [PubMed] [Google Scholar]
- 21.Guidelines on muscle-invasive and metastatic bladder cancer. European Association of Urology; 2015. [Google Scholar]
- 22.Fleshner N.E., Herr H.W., Stewart A.K., Murphy G.P., Mettlin C., Menck H.R. The National Cancer Data Base report on bladder carcinoma. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1996;78:1505–1513. doi: 10.1002/(sici)1097-0142(19961001)78:7<1505::aid-cncr19>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen M.E., Shariat S.F., Karakiewicz P.I. Advanced age is associated with poorer bladder cancer-specific survival in patients treated with radical cystectomy. Eur Urol. 2007;51:699–706. doi: 10.1016/j.eururo.2006.11.004. discussion-8. [DOI] [PubMed] [Google Scholar]
- 24.Snyder C., Harlan L., Knopf K., Potosky A., Kaplan R. Patterns of care for the treatment of bladder cancer. J Urol. 2003;169:1697–1701. doi: 10.1097/01.ju.0000056727.30546.b7. [DOI] [PubMed] [Google Scholar]
- 25.Goossens-Laan C.A., Leliveld A.M., Verhoeven R.H. Effects of age and comorbidity on treatment and survival of patients with muscle-invasive bladder cancer. Int J Cancer. 2014;135:905–912. doi: 10.1002/ijc.28716. [DOI] [PubMed] [Google Scholar]
- 26.Horovitz D., Turker P., Bostrom P.J. Does patient age affect survival after radical cystectomy? BJU Int. 2012;110:E486–E493. doi: 10.1111/j.1464-410X.2012.11180.x. [DOI] [PubMed] [Google Scholar]
- 27.Chromecki T.F., Mauermann J., Cha E.K. Multicenter validation of the prognostic value of patient age in patients treated with radical cystectomy. World J Urol. 2012;30:753–759. doi: 10.1007/s00345-011-0772-2. [DOI] [PubMed] [Google Scholar]
- 28.Zakaria A.S., Santos F., Dragomir A., Tanguay S., Kassouf W., Aprikian A.G. Postoperative mortality and complications after radical cystectomy for bladder cancer in Quebec: a population-based analysis during the years 2000–2009. Can Urol Assoc J. 2014;8:259–267. doi: 10.5489/cuaj.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol. 2005;48:202–205. doi: 10.1016/j.eururo.2005.04.006. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 30.Griffiths G., Hall R., Sylvester R., Raghavan D., Parmar M.K. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29:2171–2177. doi: 10.1200/JCO.2010.32.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leow J.J., Martin-Doyle W., Rajagopal P.S. Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol. 2014;66:42–54. doi: 10.1016/j.eururo.2013.08.033. [DOI] [PubMed] [Google Scholar]
- 32.Abdollah F., Sun M., Shariat S.F. The importance of pelvic lymph node dissection in the elderly population: implications for interpreting the 2010 National Comprehensive Cancer Network practice guidelines for bladder cancer treatment. J Urol. 2011;185:2078–2084. doi: 10.1016/j.juro.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 33.Sternberg C.N., Skoneczna I., Kerst J.M. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol. 2015;16:76–86. doi: 10.1016/S1470-2045(14)71160-X. [DOI] [PubMed] [Google Scholar]
- 34.Coppin C.M., Gospodarowicz M.K., James K. Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1996;14:2901–2907. doi: 10.1200/JCO.1996.14.11.2901. [DOI] [PubMed] [Google Scholar]
- 35.Hoskin P.J., Rojas A.M., Bentzen S.M., Saunders M.I. Radiotherapy with concurrent carbogen and nicotinamide in bladder carcinoma. J Clin Oncol. 2010;28:4912–4918. doi: 10.1200/JCO.2010.28.4950. [DOI] [PubMed] [Google Scholar]
- 36.James N.D., Hussain S.A., Hall E. Radiotherapy with or without chemotherapy in muscle-invasive bladder cancer. N Engl J Med. 2012;366:1477–1488. doi: 10.1056/NEJMoa1106106. [DOI] [PubMed] [Google Scholar]
- 37.Mak R.H., Hunt D., Shipley W.U. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: a pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol. 2014;32:3801–3809. doi: 10.1200/JCO.2014.57.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rene N.J., Cury F.B., Souhami L. Conservative treatment of invasive bladder cancer. Curr Oncol. 2009;16:36–47. doi: 10.3747/co.v16i4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ploussard G., Daneshmand S., Efstathiou J.A. Critical analysis of bladder sparing with trimodal therapy in muscle-invasive bladder cancer: a systematic review. Eur Urol. 2014;66:120–137. doi: 10.1016/j.eururo.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 40.Chamie K., Hu B., Devere White R.W., Ellison L.M. Cystectomy in the elderly: does the survival benefit in younger patients translate to the octogenarians? BJU Int. 2008;102:284–290. doi: 10.1111/j.1464-410X.2008.07636.x. [DOI] [PubMed] [Google Scholar]
- 41.Solsona E., Iborra I., Ricos J.V., Monros J.L., Casanova J., Calabuig C. Feasibility of transurethral resection for muscle infiltrating carcinoma of the bladder: long-term followup of a prospective study. J Urol. 1998;159:95–98. doi: 10.1016/s0022-5347(01)64022-9. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 42.Herr H.W. Transurethral resection of muscle-invasive bladder cancer: 10-year outcome. J Clin Oncol. 2001;19:89–93. doi: 10.1200/JCO.2001.19.1.89. [DOI] [PubMed] [Google Scholar]
- 43.Ma B., Li H., Zhang C. Lymphovascular invasion, ureteral reimplantation and prior history of urothelial carcinoma are associated with poor prognosis after partial cystectomy for muscle-invasive bladder cancer with negative pelvic lymph nodes. Eur J Surg Oncol. 2013;39:1150–1156. doi: 10.1016/j.ejso.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 44.Holzbeierlein J.M., Lopez-Corona E., Bochner B.H. Partial cystectomy: a contemporary review of the Memorial Sloan-Kettering Cancer Center experience and recommendations for patient selection. J Urol. 2004;172:878–881. doi: 10.1097/01.ju.0000135530.59860.7d. [DOI] [PubMed] [Google Scholar]
- 45.Malkowicz S.B., van Poppel H., Mickisch G. Muscle-invasive urothelial carcinoma of the bladder. Urology. 2007;69:3–16. doi: 10.1016/j.urology.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 46.Dandekar N.P., Tongaonkar H.B., Dalal A.V., Kulkarni J.N., Kamat M.R. Partial cystectomy for invasive bladder cancer. J Surg Oncol. 1995;60:24–29. doi: 10.1002/jso.2930600106. [DOI] [PubMed] [Google Scholar]
- 47.Knoedler J., Frank I. Organ-sparing surgery in urology: partial cystectomy. Curr Opin Urol. 2015;25:111–115. doi: 10.1097/MOU.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 48.Turgeon G.A., Souhami L., Cury F.L. Hypofractionated intensity modulated radiation therapy in combined modality treatment for bladder preservation in elderly patients with invasive bladder cancer. Int J Radiat Oncol Biol Phys. 2014;88:326–331. doi: 10.1016/j.ijrobp.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Lacarriere E., Smaali C., Benyoucef A., Pfister C., Grise P. The efficacy of hemostatic radiotherapy for bladder cancer-related hematuria in patients unfit for surgery. Int Braz J Urol. 2013;39:808–816. doi: 10.1590/S1677-5538.IBJU.2013.06.06. [DOI] [PubMed] [Google Scholar]
- 50.Kouloulias V., Tolia M., Kolliarakis N., Siatelis A., Kelekis N. Evaluation of acute toxicity and symptoms palliation in a hypofractionated weekly schedule of external radiotherapy for elderly patients with muscular invasive bladder cancer. Int Braz J Urol. 2013;39:77–82. doi: 10.1590/S1677-5538.IBJU.2013.01.10. [DOI] [PubMed] [Google Scholar]
- 51.Zygogianni A., Kouloulias V., Armpilia C. A weekly hypofractionated radiotherapeutic schedule for bladder carcinoma in elderly patients: local response, acute and late toxicity, dosimetric parameters and pain relief. J BUON. 2013;18:407–412. [PubMed] [Google Scholar]
- 52.von der Maase H., Hansen S.W., Roberts J.T. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–3077. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 53.von der Maase H., Sengelov L., Roberts J.T. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. doi: 10.1200/JCO.2005.07.757. [DOI] [PubMed] [Google Scholar]
- 54.Sternberg C.N., de Mulder P., Schornagel J.H. Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer. 2006;42:50–54. doi: 10.1016/j.ejca.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 55.Galsky M.D., Chen G.J., Oh W.K. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann Oncol. 2012;23:406–410. doi: 10.1093/annonc/mdr156. [DOI] [PubMed] [Google Scholar]
- 56.Petrioli R., Frediani B., Manganelli A. Comparison between a cisplatin-containing regimen and a carboplatin-containing regimen for recurrent or metastatic bladder cancer patients. A randomized phase II study. Cancer. 1996;77:344–351. doi: 10.1002/(SICI)1097-0142(19960115)77:2<344::AID-CNCR18>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 57.Bellmunt J., Ribas A., Eres N. Carboplatin-based versus cisplatin-based chemotherapy in the treatment of surgically incurable advanced bladder carcinoma. Cancer. 1997;80:1966–1972. doi: 10.1002/(sici)1097-0142(19971115)80:10<1966::aid-cncr14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 58.Dogliotti L., Carteni G., Siena S. Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: results of a randomized phase 2 trial. Eur Urol. 2007;52:134–141. doi: 10.1016/j.eururo.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 59.Sonpavde G., Watson D., Tourtellott M. Frequency of cisplatin administration in patients presenting with advanced urothelial carcinoma in the community. J Clin Oncol. 2012;30 doi: 10.1016/j.clgc.2011.11.005. abstr 285. [DOI] [PubMed] [Google Scholar]
- 60.Galsky M.D., Chowdhury S., Bellmunt J. Treatment patterns and outcomes in “real world” patients (pts) with metastatic urothelial cancer (UC) J Clin Oncol. 2013;31 abstr 4525. [Google Scholar]
- 61.Bamias A., Peroukidis S., Stamatopoulou S. Utilization of systemic chemotherapy in advanced urothelial cancer: a retrospective collaborative study by the Hellenic Genitourinary Cancer Group (HGUCG) Clin Genitourin Cancer. 2015 doi: 10.1016/j.clgc.2015.09.009. (in press) [DOI] [PubMed] [Google Scholar]
- 62.Dash A., Galsky M.D., Vickers A.J. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107:506–513. doi: 10.1002/cncr.22031. [DOI] [PubMed] [Google Scholar]
- 63.Nogue-Aliguer M., Carles J., Arrivi A. Gemcitabine and carboplatin in advanced transitional cell carcinoma of the urinary tract: an alternative therapy. Cancer. 2003;97:2180–2186. doi: 10.1002/cncr.10990. [DOI] [PubMed] [Google Scholar]
- 64.De Santis M., Bachner M. New developments in first- and second-line chemotherapy for transitional cell, squamous cell and adenocarcinoma of the bladder. Curr Opin Urol. 2007;17:363–368. doi: 10.1097/MOU.0b013e3282c4b0cb. [DOI] [PubMed] [Google Scholar]
- 65.Galsky M.D., Hahn N.M., Rosenberg J. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J Clin Oncol. 2011;29:2432–2438. doi: 10.1200/JCO.2011.34.8433. [DOI] [PubMed] [Google Scholar]
- 66.Witjes J.A., Comperat E., Cowan N.C. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014;65:778–792. doi: 10.1016/j.eururo.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 67.Yamamoto N., Tamura T., Maeda M. The influence of ageing on cisplatin pharmacokinetics in lung cancer patients with normal organ function. Cancer Chemother Pharmacol. 1995;36:102–106. doi: 10.1007/BF00689192. [DOI] [PubMed] [Google Scholar]
- 68.Raj G.V., Iasonos A., Herr H., Donat S.M. Formulas calculating creatinine clearance are inadequate for determining eligibility for cisplatin-based chemotherapy in bladder cancer. J Clin Oncol. 2006;24:3095–3100. doi: 10.1200/JCO.2005.04.3091. [DOI] [PubMed] [Google Scholar]
- 69.de Lusignan S., Chan T., Gallagher H. Chronic kidney disease (CKD) management in southeast England: a preliminary cross-sectional report from the QICKD – Quality Improvement in Chronic Kidney Disease study. Prim Care Cardiovasc J. 2009;2:33–39. [Google Scholar]
- 70.Grisold W., Cavaletti G., Windebank A.J. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro Oncol. 2012;14(Suppl. 4):iv45–iv54. doi: 10.1093/neuonc/nos203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Herr H.W. Outcome of patients who refuse cystectomy after receiving neoadjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2008;54:126–132. doi: 10.1016/j.eururo.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 72.Sternberg C.N., Pansadoro V., Calabro F. Can patient selection for bladder preservation be based on response to chemotherapy? Cancer. 2003;97:1644–1652. doi: 10.1002/cncr.11232. [DOI] [PubMed] [Google Scholar]
- 73.Meyer A., Ghandour R., Bergman A. The natural history of clinically complete responders to neoadjuvant chemotherapy for urothelial carcinoma of the bladder. J Urol. 2014;192:696–701. doi: 10.1016/j.juro.2014.03.078. [DOI] [PubMed] [Google Scholar]
- 74.Carles J., Nogue M., Domenech M. Carboplatin-gemcitabine treatment of patients with transitional cell carcinoma of the bladder and impaired renal function. Oncology. 2000;59:24–27. doi: 10.1159/000012132. [DOI] [PubMed] [Google Scholar]
- 75.Bellmunt J., de Wit R., Albanell J., Baselga J. A feasibility study of carboplatin with fixed dose of gemcitabine in “unfit” patients with advanced bladder cancer. Eur J Cancer. 2001;37:2212–2215. doi: 10.1016/s0959-8049(01)00295-7. [DOI] [PubMed] [Google Scholar]
- 76.Linardou H., Aravantinos G., Efstathiou E. Gemcitabine and carboplatin combination as first-line treatment in elderly patients and those unfit for cisplatin-based chemotherapy with advanced bladder carcinoma: phase II study of the Hellenic Co-operative Oncology Group. Urology. 2004;64:479–484. doi: 10.1016/j.urology.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 77.Bamias A., Lainakis G., Kastritis E. Biweekly carboplatin/gemcitabine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: report of efficacy, quality of life and geriatric assessment. Oncology. 2007;73:290–297. doi: 10.1159/000132394. [DOI] [PubMed] [Google Scholar]
- 78.De Santis M., Bellmunt J., Mead G. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30:191–199. doi: 10.1200/JCO.2011.37.3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vaughn D.J., Manola J., Dreicer R., See W., Levitt R., Wilding G. Phase II study of paclitaxel plus carboplatin in patients with advanced carcinoma of the urothelium and renal dysfunction (E2896): a trial of the Eastern Cooperative Oncology Group. Cancer. 2002;95:1022–1027. doi: 10.1002/cncr.10782. [DOI] [PubMed] [Google Scholar]
- 80.Calabro F., Lorusso V., Rosati G. Gemcitabine and paclitaxel every 2 weeks in patients with previously untreated urothelial carcinoma. Cancer. 2009;115:2652–2659. doi: 10.1002/cncr.24313. [DOI] [PubMed] [Google Scholar]
- 81.Ricci S., Galli L., Chioni A. Gemcitabine plus epirubicin in patients with advanced urothelial carcinoma who are not eligible for platinum-based regimens. Cancer. 2002;95:1444–1450. doi: 10.1002/cncr.10860. [DOI] [PubMed] [Google Scholar]
- 82.Culine S., Flechon A., Guillot A. Gemcitabine or gemcitabine plus oxaliplatin in the first-line treatment of patients with advanced transitional cell carcinoma of the urothelium unfit for cisplatin-based chemotherapy: a randomized phase 2 study of the French Genitourinary Tumor Group (GETUG V01) Eur Urol. 2011;60:1251–1257. doi: 10.1016/j.eururo.2011.08.072. [DOI] [PubMed] [Google Scholar]
- 83.Galsky M.D., Iasonos A., Mironov S., Scattergood J., Boyle M.G., Bajorin D.F. Phase II trial of dose-dense doxorubicin plus gemcitabine followed by paclitaxel plus carboplatin in patients with advanced urothelial carcinoma and impaired renal function. Cancer. 2007;109:549–555. doi: 10.1002/cncr.22454. [DOI] [PubMed] [Google Scholar]
- 84.Balar A.V., Apolo A.B., Ostrovnaya I. Phase II study of gemcitabine, carboplatin, and bevacizumab in patients with advanced unresectable or metastatic urothelial cancer. J Clin Oncol. 2013;31:724–730. doi: 10.1200/JCO.2012.42.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bellmunt J., Gonzalez-Larriba J.L., Prior C. Phase II study of sunitinib as first-line treatment of urothelial cancer patients ineligible to receive cisplatin-based chemotherapy: baseline interleukin-8 and tumor contrast enhancement as potential predictive factors of activity. Ann Oncol. 2011;22:2646–2653. doi: 10.1093/annonc/mdr023. [DOI] [PubMed] [Google Scholar]
- 86.De Santis M., Bellmunt J., Mead G. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer “unfit” for cisplatin-based chemotherapy: phase II–results of EORTC study 30986. J Clin Oncol. 2009;27:5634–5639. doi: 10.1200/JCO.2008.21.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.De Santis M., Wiechno P.J., Lucas C. Mature survival (OS) data of a randomised international phase II trial (JASINT1): vinflunine (VFL)-gemcitabine (GEM) vs. VFL-CBDCA in CDDP-unfit patients (Pts) with advanced urothelial carcinoma. Ann Oncol. 2014;25:iv280–iv304. doi: 10.1093/annonc/mdv609. (812PD) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ko Y.J., Canil C.M., Mukherjee S.D. Nanoparticle albumin-bound paclitaxel for second-line treatment of metastatic urothelial carcinoma: a single group, multicentre, phase 2 study. Lancet Oncol. 2013;14:769–776. doi: 10.1016/S1470-2045(13)70162-1. [DOI] [PubMed] [Google Scholar]
- 89.Yafi F.A., North S., Kassouf W. First- and second-line therapy for metastatic urothelial carcinoma of the bladder. Curr Oncol. 2011;18:e25–e34. doi: 10.3747/co.v18i1.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.von der Maase H. Gemcitabine in transitional cell carcinoma of the urothelium. Expert Rev Anticancer Ther. 2003;3:11–19. doi: 10.1586/14737140.3.1.11. [DOI] [PubMed] [Google Scholar]
- 91.Albers P., Park S.I., Niegisch G. Randomized phase III trial of 2nd line gemcitabine and paclitaxel chemotherapy in patients with advanced bladder cancer: short-term versus prolonged treatment [German Association of Urological Oncology (AUO) trial AB 20/99] Ann Oncol. 2011;22:288–294. doi: 10.1093/annonc/mdq398. [DOI] [PubMed] [Google Scholar]
- 92.Sternberg C.N., Vogelzang N.J. Gemcitabine, paclitaxel, pemetrexed and other newer agents in urothelial and kidney cancers. Crit Rev Oncol Hematol. 2003;46(Suppl):S105–S115. doi: 10.1016/s1040-8428(03)00068-4. [DOI] [PubMed] [Google Scholar]
- 93.Kaufmann D.S., Carducci M.A., Kuzel T. Gemcitabine (G) and paclitaxel (P) every two weeks (GP2w): a completed multicenter phase II trial in locally advanced or metastatic urothelial cancer (UC) Proc Am Soc Clin Oncol. 2002;21(1):192a. Abstr 767. [Google Scholar]
- 94.Wong Y., Litwin S., Plimack E.R. Effect of EGFR inhibition with cetuximab on the efficacy of paclitaxel in previously treated metastatic urothelial cancer. J Clin Oncol. 2011;29 abstr 243. [Google Scholar]
- 95.Choueiri T.K., Ross R.W., Jacobus S. Double-blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum-pretreated metastatic urothelial cancer. J Clin Oncol. 2012;30:507–512. doi: 10.1200/JCO.2011.37.7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Petrylak D.P., Tagawa S.T., Kohli M. Interim results of a randomized phase 2 study of docetaxel with ramucirumab versus docetaxel in second-line advanced or metastatic urothelial carcinoma. J Clin Oncol. 2015;33 doi: 10.1200/JCO.2015.65.0218. abstr 295. [DOI] [PubMed] [Google Scholar]
- 97.Srinivas S., Narayanan S., Harshman L.C. Phase II study of pazopanib with weekly paclitaxel in refractory urothelial cancer. J Clin Oncol. 2015;33 doi: 10.1016/j.clgc.2016.03.011. abstr 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bellmunt J., Fougeray R., Rosenberg J.E. Long-term survival results of a randomized phase III trial of vinflunine plus best supportive care versus best supportive care alone in advanced urothelial carcinoma patients after failure of platinum-based chemotherapy. Ann Oncol. 2013;24:1466–1472. doi: 10.1093/annonc/mdt007. [DOI] [PubMed] [Google Scholar]
- 99.Bellmunt J., Choueiri T.K., Fougeray R. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28:1850–1855. doi: 10.1200/JCO.2009.25.4599. [DOI] [PubMed] [Google Scholar]
- 100.Sonpavde G., Pond G.R., Fougeray R. Time from prior chemotherapy enhances prognostic risk grouping in the second-line setting of advanced urothelial carcinoma: a retrospective analysis of pooled, prospective phase 2 trials. Eur Urol. 2013;63:717–723. doi: 10.1016/j.eururo.2012.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Serrate C., Pouessel D., Gauthier H., le Maignan C., Teixeira L., Culine S. Vinflunine for the treatment of metastatic transitional cell carcinoma: recent evidence from clinical trials and observational studies. Clinic Investig. 2014;4:305–311. [Google Scholar]
- 102.Hussain S.A., Ansari J., Huddart R.A. VICTOR: vinflunine (Vin) in advanced metastatic transitional cell carcinoma of the urothelium (TCCU)—a retrospective analysis of use of Vin in multicenter, real life setting as second-line chemotherapy (ChT) through free-of charge-programme (FOCP) for patients (pts) in the UK. J Clin Oncol. 2015;33 doi: 10.3892/ijo.2017.3847. abstr 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Castellano D., Puente J., de Velasco G. Safety and effectiveness of vinflunine in patients with metastatic transitional cell carcinoma of the urothelial tract after failure of one platinum-based systemic therapy in clinical practice. BMC Cancer. 2014;14:779. doi: 10.1186/1471-2407-14-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pistamaltzian N., Tzannis K., Pissanidou V. Treatment of relapsed urothelial bladder cancer with vinflunine: real-world evidence by the Hellenic Genitourinary Cancer Group. Anticancer Drugs. 2016;27:48–53. doi: 10.1097/CAD.0000000000000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aapro M.S., Bohlius J., Cameron D.A. 2010 update of EORTC guidelines for the use of granulocyte-colony stimulating factor to reduce the incidence of chemotherapy-induced febrile neutropenia in adult patients with lymphoproliferative disorders and solid tumours. Eur J Cancer. 2011;47:8–32. doi: 10.1016/j.ejca.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 106.Isambert N., Delord J.P., Tourani J.M. How to manage intravenous vinflunine in cancer patients with renal impairment: results of a pharmacokinetic and tolerability phase I study. Br J Clin Pharmacol. 2014;77:498–508. doi: 10.1111/bcp.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tourani J.M., Mourey L., Servent V. Influence of age on the pharmacokinetics of i.v. vinflunine: results of a phase I trial in elderly cancer patients. Journal of Geriatric Oncology. 2012;3:41–48. [Google Scholar]
- 108.Guidelines on urothelial carcinomas of the upper urinary tract. European Association of Urology; 2015. [Google Scholar]
- 109.Capitanio U., Shariat S.F., Isbarn H. Comparison of oncologic outcomes for open and laparoscopic nephroureterectomy: a multi-institutional analysis of 1249 cases. Eur Urol. 2009;56:1–9. doi: 10.1016/j.eururo.2009.03.072. [DOI] [PubMed] [Google Scholar]
- 110.Lughezzani G., Sun M., Perrotte P. Should bladder cuff excision remain the standard of care at nephroureterectomy in patients with urothelial carcinoma of the renal pelvis? A population-based study. Eur Urol. 2010;57:956–962. doi: 10.1016/j.eururo.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 111.Xylinas E., Rink M., Cha E.K. Impact of distal ureter management on oncologic outcomes following radical nephroureterectomy for upper tract urothelial carcinoma. Eur Urol. 2014;65:210–217. doi: 10.1016/j.eururo.2012.04.052. [DOI] [PubMed] [Google Scholar]
- 112.Margulis V., Shariat S.F., Matin S.F. Outcomes of radical nephroureterectomy: a series from the Upper Tract Urothelial Carcinoma Collaboration. Cancer. 2009;115:1224–1233. doi: 10.1002/cncr.24135. [DOI] [PubMed] [Google Scholar]
- 113.Ito A., Shintaku I., Satoh M. Prospective randomized phase II trial of a single early intravesical instillation of pirarubicin (THP) in the prevention of bladder recurrence after nephroureterectomy for upper urinary tract urothelial carcinoma: the THP Monotherapy Study Group Trial. J Clin Oncol. 2013;31:1422–1427. doi: 10.1200/JCO.2012.45.2128. [DOI] [PubMed] [Google Scholar]
- 114.O'Brien T., Ray E., Singh R., Coker B., Beard R. Prevention of bladder tumours after nephroureterectomy for primary upper urinary tract urothelial carcinoma: a prospective, multicentre, randomised clinical trial of a single postoperative intravesical dose of mitomycin C (the ODMIT-C Trial) Eur Urol. 2011;60:703–710. doi: 10.1016/j.eururo.2011.05.064. [DOI] [PubMed] [Google Scholar]
- 115.Knowles M.A., Hurst C.D. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat Rev Cancer. 2015;15:25–41. doi: 10.1038/nrc3817. [DOI] [PubMed] [Google Scholar]
- 116.Martin-Doyle W., Leow J.J., Orsola A., Chang S.L., Bellmunt J. Improving selection criteria for early cystectomy in high-grade t1 bladder cancer: a meta-analysis of 15,215 patients. J Clin Oncol. 2015;33:643–650. doi: 10.1200/JCO.2014.57.6967. [DOI] [PubMed] [Google Scholar]
- 117.Adjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis of individual patient data Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. Eur Urol 2005;48:189–199; discussion 99–201. [DOI] [PubMed]
- 118.Svatek R.S., Shariat S.F., Lasky R.E. The effectiveness of off-protocol adjuvant chemotherapy for patients with urothelial carcinoma of the urinary bladder. Clin Cancer Res. 2010;16:4461–4467. doi: 10.1158/1078-0432.CCR-10-0457. [DOI] [PubMed] [Google Scholar]
- 119.Van Allen E.M., Mouw K.W., Kim P. Somatic ERCC2 mutations correlate with cisplatin sensitivity in muscle-invasive urothelial carcinoma. Cancer Discov. 2014;4:1140–1153. doi: 10.1158/2159-8290.CD-14-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Groenendijk F.H., de Jong J., Fransen van de Putte E.E. ERBB2 mutations characterize a subgroup of muscle-invasive bladder cancers with excellent response to neoadjuvant chemotherapy. Eur Urol. 2015;69:384–388. doi: 10.1016/j.eururo.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 121.Dinney C.P., Hansel D., McConkey D. Novel neoadjuvant therapy paradigms for bladder cancer: results from the National Cancer Center Institute Forum. Urol Oncol. 2014;32:1108–1115. doi: 10.1016/j.urolonc.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Choudhury A., Nelson L.D., Teo M.T. MRE11 expression is predictive of cause-specific survival following radical radiotherapy for muscle-invasive bladder cancer. Cancer Res. 2010;70:7017–7026. doi: 10.1158/0008-5472.CAN-10-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lautenschlaeger T., George A., Klimowicz A.C. Bladder preservation therapy for muscle-invading bladder cancers on Radiation Therapy Oncology Group trials 8802, 8903, 9506, and 9706: vascular endothelial growth factor B overexpression predicts for increased distant metastasis and shorter survival. Oncologist. 2013;18:685–686. doi: 10.1634/theoncologist.2012-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bellmunt J., Selvarajah S., Rodig S. Identification of ALK gene alterations in urothelial carcinoma. PLoS One. 2014;9:e103325. doi: 10.1371/journal.pone.0103325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Iyer G., Hanrahan A.J., Milowsky M.I. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338:221. doi: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sequist L.V., Cassier P., Varga A. American Association for Cancer Research; Philadelphia: 2014. Phase I study of BGJ398, a selective pan-FGFR inhibitor in genetically preselected advanced solid tumors. abstr. CT326. [Google Scholar]
- 128.Bahleda R., Dienstmann R., Adamo B. Phase 1 study of JNJ-42756493, a pan-fibroblast growth factor receptor (FGFR) inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2014;32 doi: 10.1200/JCO.2014.60.7341. abstr 2501. [DOI] [PubMed] [Google Scholar]
- 129.Bellmunt J., Mullane S.A., Werner L. Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol. 2015;26:812–817. doi: 10.1093/annonc/mdv009. [DOI] [PubMed] [Google Scholar]
- 130.Powles T., Eder J.P., Fine G.D. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 131.Kim J.W., Bellmunt J., Powles T. Clinical activity, safety, and biomarkers of MPDL3280A in metastatic urothelial bladder cancer: additional analysis from phase IA study. J Clin Oncol. 2015;33 abstr 297. [Google Scholar]
- 132.Perou C.M., Sorlie T., Eisen M.B. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 133.McConkey D.J., Choi W., Dinney C.P. New insights into subtypes of invasive bladder cancer: considerations of the clinician. Eur Urol. 2014;66:609–610. doi: 10.1016/j.eururo.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 134.Siefker-Radtke A.O., Choi W., Porten S.P. The basal subtype to predict clinical benefit from neoadjuvant chemotherapy: final results from a phase II clinical trial of DDMVAC plus bevacizumab. J Clin Oncol. 2015;33 abstr 291. [Google Scholar]
- 135.Bellmunt J., Orsola A., Leow J.J., Wiegel T., De Santis M., Horwich A. Bladder cancer: ESMO Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl. 3):iii40–iii48. doi: 10.1093/annonc/mdu223. [DOI] [PubMed] [Google Scholar]
- 136.Bennouna J., Delord J.P., Campone M., Nguyen L. Vinflunine: a new microtubule inhibitor agent. Clin Cancer Res. 2008;14:1625–1632. doi: 10.1158/1078-0432.CCR-07-2219. [DOI] [PubMed] [Google Scholar]
- 137.Aparicio L.A., Castosa R., Haz-Conde M. Role of the microtubule-targeting drug vinflunine on cell-cell adhesions in bladder epithelial tumour cells. BMC Cancer. 2014;14:507. doi: 10.1186/1471-2407-14-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pourroy B., Honore S., Pasquier E. Antiangiogenic concentrations of vinflunine increase the interphase microtubule dynamics and decrease the motility of endothelial cells. Cancer Res. 2006;66:3256–3263. doi: 10.1158/0008-5472.CAN-05-3885. [DOI] [PubMed] [Google Scholar]
- 139.Kruczynski A., Poli M., Dossi R. Anti-angiogenic, vascular-disrupting and anti-metastatic activities of vinflunine, the latest vinca alkaloid in clinical development. Eur J Cancer. 2006;42:2821–2832. doi: 10.1016/j.ejca.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 140.Aparicio L.M., Pulido E.G., Gallego G.A. Vinflunine: a new vision that may translate into antiangiogenic and antimetastatic activity. Anticancer Drugs. 2012;23:1–11. doi: 10.1097/CAD.0b013e32834d237b. [DOI] [PubMed] [Google Scholar]
- 141.Ciuleanu T., Brodowicz T., Zielinski C. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 142.Cappuzzo F., Ciuleanu T., Stelmakh L. Erlotinib as maintenance treatment in advanced non-small-cell lung cancer: a multicentre, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2010;11:521–529. doi: 10.1016/S1470-2045(10)70112-1. [DOI] [PubMed] [Google Scholar]
- 143.Paz-Ares L.G., de Marinis F., Dediu M. PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31:2895–2902. doi: 10.1200/JCO.2012.47.1102. [DOI] [PubMed] [Google Scholar]
- 144.Grivas P.D., Daignault S., Tagawa S.T. Double-blind, randomized, phase 2 trial of maintenance sunitinib versus placebo after response to chemotherapy in patients with advanced urothelial carcinoma. Cancer. 2014;120:692–701. doi: 10.1002/cncr.28477. [DOI] [PubMed] [Google Scholar]
- 145.Powles T., Huddart R.A., Elliott T. A phase II/III, double-blind, randomized trial comparing maintenance lapatinib versus placebo after first line chemotherapy in HER1/2 positive metastatic bladder cancer patients. J Clin Oncol. 2015;33 doi: 10.1200/JCO.2015.66.3468. abstr 4505. [DOI] [PubMed] [Google Scholar]
- 146.Bellmunt J., Perez Valderrama B., Font A. Maintenance vinflunine post cisplatin chemotherapy (CT) in patients with advanced urothelial carcinoma (UC): preliminary analysis of a randomized placebo controlled phase II trial (MAJA trial)—SOGUG 2011-02. J Clin Oncol. 2015;33 Abstract 4529. [Google Scholar]