Abstract
Aim
Drug‐induced Raynaud's phenomenon (RP) has long been associated with the use of different drugs, including cancer chemotherapy or β‐adrenoceptor blockers. However, sources report extremely variable prevalence and the level of evidence for each class is heterogeneous. Moreover, new signals are emerging from case reports and small series. Our objective was therefore to review available evidence about this adverse drug effect and to propose a mechanistic approach of drug‐induced RP.
Methods
A systematic review of English and French language articles was performed through Medline (1946–2015) and Embase (1974–2015). Further relevant papers were identified from the reference lists of retrieved articles.
Results
We identified 12 classes of drugs responsible for RP, with a variety of underlying mechanisms such as increased sympathetic activation, endothelial dysfunction, neurotoxicity or decreased red blood cell deformability. Cisplatin and bleomycin were associated with the highest risk, followed by β‐adrenoceptor blockers. Recent data suggest a possible involvement of tyrosine kinase inhibitors (TKI), through an unknown mechanism.
Conclusion
Drug‐induced RP is a probably underestimated adverse drug event, with limited available evidence regarding its prevalence. Although rare, serious complications like critical digital ischaemia have been reported. When these treatments are started in patients with a history of RP, careful monitoring must be made and, if possible, alternative therapies that do not alter peripheral blood flow should be considered.
Keywords: β‐adrenoceptor blockers, peripheral vasoconstriction, Raynaud's phenomenon
Introduction
Raynaud's phenomenon (RP) is characterized by transient ischaemia of the extremities in response to environmental stress or emotions 1. It typically manifests as changes to the fingers, with pallor (vasospasm and decreased blood flow), cyanosis (deoxygenation of the static venous blood) and rubor (reperfusion), often accompanied by pain. RP can be primary (i.e. idiopathic) or secondary to an underlying cause. In both cases, abnormalities of the cutaneous microcirculation are primarily involved in the pathophysiology of RP 2.
The prevalence of RP in the general population varies between 0.5 and 19%, with major geographic variability 3, 4, 5, 6. While primary RP is the most frequent form (80–90%) 7, RP may also be secondary to various auto‐immune diseases (such as systemic sclerosis (SSc), systemic lupus erythematosus, vasculitis, etc.), or other systemic diseases 1. Several drugs with peripheral vascular effects leading to decreased microvascular perfusion may induce or aggravate RP. Drug‐induced RP probably goes unrecognized because of the limited knowledge of this side effect.
Literature reviews and textbooks usually have comprehensively reviewed drugs that have long been known to be responsible for RP 8. However, new signals are emerging from numerous case reports. Yet, to our knowledge, no systematic review has been performed and little is known about the prevalence and the level of evidence of drug‐induced RP. Our objective in the present work was therefore to summarize available evidence and to propose a mechanistic approach of drug‐induced RP.
Methods
The MEDLINE database was searched for English or French language articles published between January 1946 and May 2015 using the following search terms: ‘Raynaud disease/chemically induced’ [MESH] and ‘raynaud’ AND ‘clonidine’, ‘betablocker’, ‘ergot alkaloid’, ‘dopaminergic agonist’, ‘selective serotonin re‐uptake inhibitors’, ‘sympathomimetic drugs’, ‘chemotherapy’, ‘tyrosine kinase inhibitors’, ‘interferon’ and ‘ciclosporin’. Further relevant papers were identified from the reference lists of retrieved articles. We used the Oxford Centre for Evidence‐based Medicine ‐ Levels of Evidence to graduate the strength of the link between RP and drug classes 9. Among 253 records screened, 131 full texts were assessed for eligibility and included is the review (Figure 1).
Figure 1.

Flow diagram of studies included in the review
Results
Drugs enhancing vasoconstriction
β‐adrenoceptor blockers
β‐adrenoceptor blockers have long been known as causing drug‐induced RP, but data about its prevalence are scarce. Analysis of the Framingham Heart study data identified β‐adrenoceptor blocker use as the most common cause of secondary RP (34.2% of secondary RP). A meta‐analysis published in 2012 that included 13 studies (1012 patients) found a prevalence of 14.7% in patients receiving β‐adrenoceptor blockers 4. However, the studies were old (1971 to 1984) and of varying quality. A network meta‐analysis of prospective randomized controlled trials revealed a prevalence of peripheral vasoconstriction among patients treated with β‐adrenoceptor blockers of 7% (1966/28072), whereas 4.6% (555/12060) and 1.7% (305/17492) of patients treated with placebo or active control experienced this adverse effect, respectively (P<0.001) (Khouri et al., submitted).
The pathophysiology of this side effect remains unclear. Studies exploring the effect of β‐adrenoceptor blockers on patients with primary RP failed to show any worsening of their symptoms 10, 11, 12, 13. There is no evident explanation for this discrepancy, but the studies have small sample size.
The influence of the ancillary properties of β‐adrenoceptor blockers (e.g. intrinsic sympathomimetic activity, β1‐selectivity, vasodilator activity) should theoretically influence their propensity to induce peripheral vasoconstriction, although studies report conflicting results 13, 14, 15, 16. The recent network meta‐analysis conducted by our group suggests that β‐adrenoceptor blockers are a heterogeneous class. High affinity for β1‐adrenoceptors does not protect from RP while ancillary properties such as intrinsic sympathomimetic activity and vasodilator properties seem to be protective (Khouri et al., submitted to British Journal of Clinical Pharmacology).
Clonidine
RP induced by clonidine is a well‐known adverse reaction, described since many years although its frequency is not known 17. In patients with RP, cold‐amplified α2c‐adrenoceptors mediated vasoconstriction is increased 18, 19. It has been identified that skin vasoconstriction in response to local cooling is mediated by the translocation of α2c‐adrenoceptors to the vascular smooth muscle cells surface, through a pathway involving RhoA–Rho kinase 20. In cold situations, clonidine direct α2c‐vascular agonism may become pre‐eminent on the usually desired central reduction of the adrenergic tone.
Ergot alkaloids
Ergotamine and its derivatives are used to treat migraine disorders and cluster headache 21. They display affinity for a wide variety of receptors including those for 5‐HT (serotonin), dopamine and norepinephrine 22. They are partial agonists of various serotoninergic receptors and the usual response of blood vessels to 5‐HT is contraction 23. More precisely, they exert a central vasoconstrictor effect through serotoninergic 5‐HT1B/1D receptors, which are mostly in the cranial vessels and at therapeutic dose exert only a weak constricting effect on peripheral blood vessels 24. However 5‐HT2 agonism seems to be the main effector of their peripheral serotoninergic vasoconstrictor effect. Moreover, they are α1‐, α2‐adrenergic and dopaminergic D2‐receptor agonists. Numerous case reports illustrating this effect are found in the literature 25, 26. However, the accountability of ergot alkaloids in RP is difficult to assess because of a significantly higher prevalence of RP in the migraine population 27, 28. Furthermore, the peripheral vasoconstriction caused by ergot alkaloids is sometimes interpreted as RP. ‘Ergotism’ is rarely observed (estimated incidence is 0.1%), but the prolonged vasoconstriction can lead to gangrene.
In contrast, other drugs targeting serotonin receptors such as triptans, selective agonists of 5‐HT1B/1D, do not induce vasoconstriction of extremities and RP.
Dopaminergic agonists
RP cases have been reported following the use of bromocriptine, another ergot alkaloid 29, 30, 31, 32. One report describes severe RP with vascular morphological injury (presence of megacapillary on nailfold capillaroscopy) attributed to 6 years of treatment with bromocriptine 31. Bromocriptine is mainly a dopaminergic agonist. At low doses it has vasodilatative properties resulting from D1‐receptor activation and leading to the well‐identified orthostatic hypotensive state. At high doses it exhibits α1‐adrenoceptor properties 33 and peripheral release of catecholamines both resulting in vasoconstriction. Moreover, direct activation of α2‐adrenoceptors by bromocriptine has been described and could explain increased sensitivity to cold 34, like clonidine. Microvascular injury with long term use of bromocriptine has also been suspected. 31 Nevertheless, a large case–control study (542 cases and 2155 controls) did not support the association between dopamine agonists and an increased risk of ischaemic events requiring hospitalization 35. Unfortunately this study did not provide detailed information on RP.
Surprisingly, two cases of erythromelalgia have been described with bromocriptine, in association with calcium channel blockers 36, 37
Selective serotonin re‐uptake inhibitors (SSRIs)
Contradictory effects of SSRIs on peripheral vasoreactivity have been reported. On the one hand, SSRIs have been proposed as a treatment for RP, following the observation of the relief of patients with erythromelalgia or RP with fluoxetine and sertraline 38, or paroxetine and escitalopram 39. Indeed, fluoxetine blocks the uptake of serotonin by platelets and decreases the amount of serotonin that is released during platelet activation/aggregation, which may explain the favourable outcome in patients with primary or secondary RP participating in an open randomized clinical trial 40. On the other hand, other authors have described a deleterious association between RP and the SSRIs, fluoxetine 41, 42, fluvoxamine 43, citalopram 44 and milnacipran 45, together with the relief of erythromelalgia symptoms 46. A case of emerging RP 2 days after beginning tergaserod treatment, a partial 5‐HT4 serotonin receptor agonist, has also been described 47.
Currently this discrepancy between vasoconstriction and vasodilatation remains unexplained. Some authors suggested that endothelial damage is necessary for the development of a vasoconstrictive effect during SSRI treatment 48. In a healthy vascular bed it has been proposed that blocking serotonin re‐uptake could increase free plasma serotonin concentrations and produce, in stasis conditions, a local accumulation of serotonin, exacerbating vasoconstriction through 5‐HT2 receptors that may worsen RP 44. In contrast, SSRIs decrease the amount of serotonin that is released during platelet activation/aggregation. For example fluoxetine is known to deplete platelet serotonin by 95% 49. Individual variability in metabolism or in signalling serotonin pathways could explain this variability in response to SSRIs 40.
Stimulants
Central stimulation of the dopaminergic and noradrenergic system is responsible for the peripheral release of catecholamines leading to vasoconstriction. Cases of RP induced by central nervous system stimulants have been reported 50. A retrospective case–control study investigated whether medications used for the treatment of attention deficit hyperactivity disorder (ADHD) were associated with the development of RP. Sixty‐four children were enrolled in the study (32 cases with RP and 32 age and gender matched control patients) and a significant association between the presence of RP and past or current use of ADHD stimulants (methylphenidate and dextroamphetamine) was found 51. Atomoxetin, a selective norepinephrine re‐uptake inhibitor, was excluded from this study because it was not considered as a central nervous system stimulant. However the case of dose dependent RP following the use of atomoxetin on a girl has recently been described 52. Two cases of RP induced by reboxetin, an inhibitor of norepinephrine re‐uptake, have been described 47. Amphetamine‐like drugs have also been associated with the emergence of RP and vasculopathy, as has phentermine, a weak sympathomimetic agent, used most commonly as an appetite suppressant in the treatment of obesity 53.
Ciclosporin
A study assessing the prevalence of RP in 100 renal transplant patients treated with ciclosporin monotherapy who were then transferred to prednisolone and azathioprine observed the development of de novo symptoms in 39% of patients on the introduction of ciclosporin. After withdrawal of ciclosporin, symptoms improved in 89% 54. Moreover four case studies of RP induced by ciclosporin use have been described. Three of them appeared a few days after ciclosporin introduction and totally disappeared after cessation 55, 56. The second case was dose related but persistent RP symptoms were observed after cessation of ciclosporin 57. The mechanism for ciclosporin induced RP remains unclear. A vasospastic effect of ciclosporin on both the macro and microcirculation has been shown 58 leading to systematic monitoring of hypertension or acute renal failure in the early treatment phase. Furthermore, changes in the viscosity of the blood, a decrease in the deformability of red blood cells and an increase in the aggregation of platelets can also be induced by ciclosporin use and contribute to RP 55.
It is worth noting that drugs increasing blood viscosity such as erythropoietins or intravenous immunoglobulins are not a known cause of RP. Much about the physiopathology of drug‐induced RP remains to be learnt.
Sympathomimetics
Digital necrosis was described following the localized use of lidocaine/epinephrine in a patient with primary RP 59. Data concerning sympathomimetic nasal decongestants (pseudoephedrine, phenylephrine) are scarce. Thus, pharmacologic properties of these drugs and their poor clinical benefit suggest that they should be contraindicated in patients with scleroderma‐related RP 60.
Toxic substances
Among recreational drugs, RP with ischaemic finger necrosis was attributed to cocaine abuse in the case of in a 37‐year‐old man 61. Cocaine has a potent vasoconstrictor effect through its α2‐adrenoceptor activity. In animal studies it has also been shown to alter prostaglandin production with disproportionate increases in thromboxane in rabbit endothelium resulting in vasospasm, platelet activation and thrombus formation 62, 63, although in some cases cocaine vasculopathy is more likely to be related to a Buerger‐like syndrome 64, 65, 66 as described with cannabis use or arsenic exposure 67, 68, 69.
Endothelium damage and/or neurotoxicity
Cancer chemotherapies
The link between RP and chemotherapies has long been clearly identified. First descriptions of chemotherapy‐induced RP were related to treatments for testicular cancer 70, 71, 72. A study in 1995 that included 90 patients treated with cisplatin‐based chemotherapy for more than 1 year after testicular cancer found that 37% of them had developed RP after four cycles of chemotherapy combining cisplatin, bleomycin and vinblastine 73. RP typically appeared 3 to 6 months after the start of chemotherapy and often persisted for several years 74. The risk factors identified for the development of RP were high cumulative doses of bleomycin and a combination of bleomycin with vinblastine rather than etoposide.
Furthermore, a trend towards the increased prevalence of RP was observed in patients who received bleomycin as a bolus compared with continuous infusion. No significant correlation was seen with the cumulative or single doses of cisplatin, etoposide or vinblastine, serum magnesium concentrations during or after chemotherapy or a history of smoking 73.
These results were confirmed by the follow‐up of a cohort study that included 739 patients treated for testicular cancer between 1982 and 1992. Patients were divided between chemotherapy (n = 384) and non‐chemotherapy (n = 355) groups. The prevalence of RP was significantly higher among patients who received chemotherapy (20.7% vs. 1.7%, P<0.001) 75. Once again, a significant relationship between the cumulative dose of bleomycin and the prevalence of RP was found (OR 2.98, 95% CI, 2.286, 3.388, P<0.001); P<0.001). Thirteen percent of patients still suffered from RP 10 years after having received a cumulative bleomycin dose of <180000 IU (corresponding approximately to three cycles of cisplatin‐etoposide‐bleomycin), 24.6% after a cumulative dose of 180 000 IU to 360 000 IU, and 29% after a cumulative dose >360 000 IU. A large observational study 76 including 1409 testicular cancer survivors found a prevalence of RP among the chemotherapy group of 39%. The cancer chemotherapy associated Vinca alkaloids, cisplatin and bleomycin. The odds ratios for Raynaud‐like phenomena in those who received one to four cycles of chemotherapy compared with those who received no chemotherapy were 2.9 [95% CI, 2.2, 3.9] and 8.0 [95% CI, 4.4, 14.7] if they received more than five cycles of chemotherapy. When these drugs have been used to treat Kaposi's sarcoma RP has also been described 77, 78, 79, 80, 81. Nevertheless, emergence of severe RP with digital necrosis after a single cycle of doxorubicin, bleomycin, vincristine and dacarbazine chemotherapy, with a cumulative dose of only 40 000 IU of bleomycin has also been described 82. Cases describing the occurrence of RP after the local injection of bleomycin to treat warts have also been reported 83, 84, 85, 86, 87.
While RP has been associated with cisplatin‐based chemotherapies the immutability of cisplatin itself remains unclear 73, 88. A recent meta‐analysis of cisplatin‐based chemotherapies included 24 studies (n = 2479 patients) and found a prevalence of RP of 24% (95% CI, 17.5, 31.3) 72. However, cisplatin was almost always associated with bleomycin and Vinca alkaloids making imputability difficult. Agents targeting the VEGF‐VEGFR axis are associated with hypertension, thromboembolic events and induce microvascular rarefaction 89, but their use is not associated with RP and peripheral vasoconstriction.
Among other cancer chemotherapies that could be responsible for RP, there is limited evidence for gemcitabine 90, 91, 92, vincristine 93, 5‐fluorouracil 94, oxaliplatin 95, tegafur and uracil 96 and cyclophosphamide‐methotrexate‐5‐fluorouracil adjuvant therapy 97.
The pathophysiology of RP induced by cancer chemotherapies is not well understood and is probably multifactorial. Some studies showed an exaggerated response to cold not only in patients with RP but also in patients without finger symptoms before testicular chemotherapy 74, 98. An increased central sympathetic vasoconstrictor reflex and an impaired non‐neurogenic vasomuscular, auto‐regulation was highlighted in patients suffering from RP syndrome after chemotherapy when compared with the control group (patients without RP after chemotherapy) 99. Currently, one of the main mechanisms proposed is through the vascular damage induced by chemotherapy, i.e. endothelial dysfunction that persists after chemotherapy 75. Indeed, some authors 100 showed that microalbuminuria, considered to be a sign of endothelial damage, was significantly higher in patients who received testicular cancer chemotherapy 83. Another possible mechanism is the neurotoxicity of chemotherapies toward arteriolar tone regulation, particularly through hypomagnesia related to cisplatin administration leading to dysregulation of vascular smooth muscle tone 101. RP would appear at the same time as the tubular damage. It is interesting to note that bleomycin is used to induce a sclerodermic phenotype in animals 102, scleroderma being the main aetiology of secondary RP.
Occupational and/or environmental exposure
For some time vinyl chloride exposure has been linked to RP 103. The vascular endothelial toxicity of vinyl chloride has been shown by angiographic studies of arteries in the hand and by capillaroscopy 104, 105. The prevalence of RP in vinyl chloride workers ranges from 6 to 33% 106. In 1980 a prospective exposed/non‐exposed cohort study showed a strong association between vinyl chloride exposure and RP (P<0.006) 107.
Drugs increasing blood viscosity and enhancing vasoconstriction
Interferons (IFN)
RP is a known side effect of treatment with interferon supported by numerous cases reports 41, 108, 109, 110, 111, 112, 113, 114, 115, 116. On direct questioning of patients taking IFN 117, symptoms of RP were reported by more than half. Analysis of 24 case reports of RP associated with interferon 118 highlighted that IFNα is the most common substance implicated (n = 14), followed by IFN γ (n = 5) and IFN β (n = 3). The treatment period was variable and lasted from 2 weeks to 49 months (mean 15.5 months). Clinical findings varied from mild and transient vasospasm (1 h after injection) to digital necrosis in 14 cases. Outcomes were known for 15 patients. Spontaneous recovery occurred for 50% of them after withdrawal of the drug. The remaining patients needed specific medication and six amputations were necessary, underlining the severity of this adverse reaction. A recent meta‐analysis 119 with six eligible studies and 183 patients estimated the prevalence of RP in patients taking interferon to be 13.6% (95% CI 0.026, 0.313).
Currently, the pathophysiology of this reaction is not fully understood. However numerous hypotheses have been proposed: a direct vasospastic effect 113, 120, increasing levels of intracellular fibroblast growth factor in endothelial cells leading to proliferation of these cells and increasing angiogenesis 121 and induction or exacerbation of a dormant collagen disease 109. Although some case reports of RP induced by interferons are described without any immune deficiency 122 it is known that interferon therapy can be related to an autoimmune disease 123, 124. Increasing blood viscosity by induction of serum cryoprecipitation 125, deposition of immune complexes 126 and arterial occlusion by thrombi due to the procoagulant activity of interferon 112, 113 have been proposed. A study of 108 patients with SSc found a higher level of IFN‐γ in patients with associated RP and suggested a pathogenic role of INF‐γ in SSc patients with RP, but this role still remains unclear 127.
Unknown mechanisms
Tyrosine kinase inhibitors
The relationship between tyrosine kinase inhibitors (TKI) and RP is complex. Experimental studies have shown that receptors with a tyrosine kinase activity may play a role in the exaggerated vasoconstriction in response to cold 128. On one hand, a pilot study 129 that included three SSc patients treated with 100 mg day–1 of imatinib for 6 months showed improvement of their RP 86. Indeed in each patient, RP was attenuated at around 3 months and had completely disappeared at 6 months. On the other hand, exactly the opposite reaction has been described with other TKIs. Emergence of RP during the first week of treatment with nilotinib has been described in two patients 130. One of them experienced improvement after the treatment was switched to imatinib, with recurrence of RP on the reintroduction of nilotinib. Another patient experienced recurrent RP with nilotinib 131. Erlotinib had also been implicated in the case of a 72‐year‐old patient suffering from scleroderma and secondary RP who experienced digital necrosis 20 days after starting daily oral treatment of 150 mg 132. Erlotinib was promptly discontinued and treatment with calcium channel blockers, nitrates and anti‐platelet drugs was initiated. After 3 weeks of therapy, the digital lesion was completely healed. Erlotininib was scored as producing a probable adverse drug reaction (7/10 on the Naranjo scale).
Other
In the literature sporadic case reports of RP potentially induced by drugs can be found, such as the two cases of fluorescein induced RP 133, 134, sulfasalazine 135, 136, propofol 137 and amphotericin B 138 without being able to determine the pathophysiological mechanism. Some paradoxical reactions following the repeated administration of iloprost 139 or yohimbine 140, a selective α2 adrenergic antagonist, have even been described.
To our knowledge, no pathophysiologic mechanism has been identified yet.
Conclusion
RP is complex, multifactorial and not fully understood yet. This present review summarises the prevalence and level of evidence of the association between drugs and RP (Table 1). Microvascular impairment is a key feature of its pathophysiology. Only symptomatic treatment with vasodilators such as calcium channel blockers or phosphodiesterase‐5 inhibitors has been proposed as a treatment for RP. Logically, vasoconstrictors have long been known to induce or aggravate RP. Increased vascular tone may be related to increased sympathetic activation, but also to endothelial dysfunction or neurotoxicity. Other mechanisms include decreased red blood cell deformability and increased platelet aggregation, both leading to increased blood viscosity (Figure 2). The need for future high quality research including prospective and vascular physiology studies to clarify these mechanisms is obvious. Indeed, this review highlights the lack of available evidence regarding the prevalence of drug‐induced RP, as well as the heterogeneity of its clinical presentation. This probably contributes to the underestimation of drug‐induced RP, as well as the fact that RP is a usually benign condition. However, such an adverse event may rarely lead to serious complications like critical digital ischaemia. Therefore, when these treatments are started in patients with a history of RP, careful monitoring must be made and, if possible, alternative therapies that do not alter peripheral blood flow should be considered.
Table 1.
Most relevant prevalence and level of evidence (defined by the Oxford Centre for Evidence‐based Medicine ‐ Levels of Evidence) of the association between each drug and RP
| Mechanism | Drug | Prevalence | Level of evidence |
|---|---|---|---|
| Enhancing vasoconstriction | Clonidine | Unknown | C |
| β‐adrenoceptor blockers | 7% | A | |
| Ergot alkaloids | 0.1% | C | |
| Dopaminergic agonists | Unknown | D | |
| SSRIs | Unknown | D | |
| Sympathomimetic drugs | Unknown | B | |
| Ciclosporin | Unknown | B | |
| Endothelial damage | Chemotherapy | 20.7–37% | A |
| Vinyl chloride | 6–33% | A | |
| Drugs increasing blood viscosity and enhancing vasoconstriction | Interferons | 13.6% | B |
| Unknown mechanism | Tyrosine kinase inhibitors | Unknown | D |
A: systematic review (with homogeneity) of RCTs or individual RCT (with narrow confidence interval); B: cohort or case control studies ;C: Case series; D: Expert opinion or troublingly inconsistent or inconclusive studies of any level; RCT: randomized controlled trial.
Figure 2.
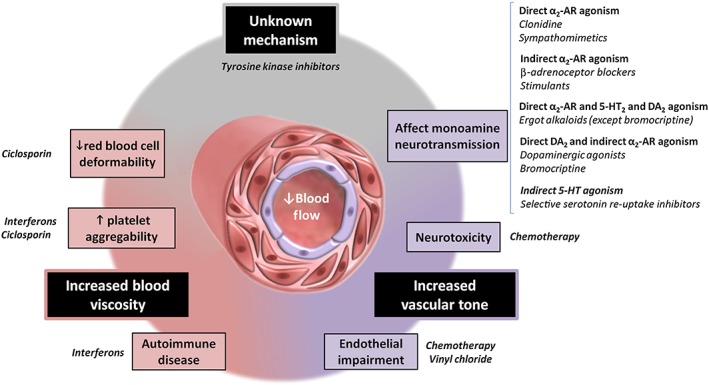
Schematic representation of some of the key mechanisms contributing to the pathogenesis of iatrogenic Raynaud's phenomenon
Competing Interests
They authors do not have any conflict of interest that might bias the present work.
Acknowledgement
We thank Dr Alison Foote (Clinical Research Centre, Grenoble University Hospital) for critically reading and correcting the manuscript.
Khouri, C. , Blaise, S. , Carpentier, P. , Villier, C. , Cracowski, J. ‐L. , and Roustit, M. (2016) Drug‐induced Raynaud's phenomenon: beyond β‐adrenoceptor blockers. Br J Clin Pharmacol, 82: 6–16. doi: 10.1111/bcp.12912.
References
- 1. Herrick AL. The pathogenesis, diagnosis and treatment of Raynaud phenomenon. Nat Rev Rheumatol 2012; 8: 469–79. [DOI] [PubMed] [Google Scholar]
- 2. Herrick AL. Pathogenesis of Raynaud's phenomenon. Rheumatol Oxf 2005; 44: 587–96. [DOI] [PubMed] [Google Scholar]
- 3. Weinrich MC, Maricq HR, Keil JE, McGregor AR, Diat F. Prevalence of Raynaud phenomenon in the adult population of South Carolina. J Clin Epidemiol 1990; 43: 1343–9. [DOI] [PubMed] [Google Scholar]
- 4. De Angelis R, Salaffi F, Grassi W. Raynaud's phenomenon: prevalence in an Italian population sample. Clin Rheumatol 2006; 25: 506–10. [DOI] [PubMed] [Google Scholar]
- 5. Silman A, Holligan S, Brennan P, Maddison P. Prevalence of symptoms of Raynaud's phenomenon in general practice. BMJ 1990; 301: 590–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Suter LG, Murabito JM, Felson DT, Fraenkel L. The incidence and natural history of Raynaud's phenomenon in the community. Arthritis Rheum 2005; 52: 1259–63. [DOI] [PubMed] [Google Scholar]
- 7. Wigley FM, Herrick AL, Flavahan N. (Ed.), Raynaud's Phenomenon: A Guide to Pathogenesis and Treatment. New York, NY: Springer New York; 2015. [Google Scholar]
- 8. Anderson M, Hughes, M . Other Secondary Causes In: Wigley FM, Herrick AL, Flavahan N. (Ed), Raynaud's Phenomenon: A Guide to Pathogenesis and Treatment. New York: Springer, 2015; 141‐162. [Google Scholar]
- 9.Oxford Centre for Evidence‐based Medicine ‐ Levels of Evidence (March 2009) ‐ CEBM
- 10. Coffman JD, Rasmussen HM. Effects of beta‐adrenoreceptor‐blocking drugs in patients with Raynaud's phenomenon. Circulation 1985; 72: 466–70. [DOI] [PubMed] [Google Scholar]
- 11. Heintzen MP, Strauer BE. Peripheral vascular eEffects of beta‐blockers. Eur Heart J 1994; 15 (suppl C): 2–7. [DOI] [PubMed] [Google Scholar]
- 12. Franssen C, Wollersheim H, de Haan A, Thien T. The influence of different beta‐blocking drugs on the peripheral circulation in Raynaud's phenomenon and in hypertension. J Clin Pharmacol 1992; 32: 652–9. [DOI] [PubMed] [Google Scholar]
- 13. Steiner JA, Cooper R, Gear JS, Ledingham JG. Vascular symptoms in patients with primary Raynaud's phenomenon are not exacerbated by propranolol or labetalol. Br J Clin Pharmacol 1979; 7: 401–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feleke E, Lyngstam O, Råstam L, Rydén L. Complaints of cold extremities among patients on antihypertensive treatment. Acta Med Scand 1983; 213: 381–5. [DOI] [PubMed] [Google Scholar]
- 15. VandenBurg MJ, Evans SJW, Cooper WD, Bradshaw F, Currie WJC. Is the feeling of cold extremities experienced by hypertensive patients due to their disease or their treatment? Eur J Clin Pharmacol 1984; 27: 47–9. [PubMed] [Google Scholar]
- 16. Lithell H, Pollare T, Vessby B. Metabolic effects of pindolol and propranolol in a double‐blind cross‐over study in hypertensive patients. Blood Press 1992; 1: 92–101. [DOI] [PubMed] [Google Scholar]
- 17. Winchester JF, Kennedy AC. Iatrogenic Raynaud's phenomenon. Br Med J 1971; 3: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Flavahan NA. A vascular mechanistic approach to understanding Raynaud phenomenon. Nat Rev Rheumatol 2015; 11: 146–58. [DOI] [PubMed] [Google Scholar]
- 19. Freedman RR, Moten M, Migály P, Mayes M. Cold‐induced potentiation of alpha 2‐adrenergic vasoconstriction in primary Raynaud's disease. Arthritis Rheum 1993; 36: 685–90. [DOI] [PubMed] [Google Scholar]
- 20. Roustit M, Cracowski J‐L. Assessment of endothelial and neurovascular function in human skin microcirculation. Trends Pharmacol Sci 2013; 34: 373–84. [DOI] [PubMed] [Google Scholar]
- 21. Tfelt‐Hansen P, Saxena PR, Dahlöf C, Pascual J, Láinez M, Henry P, Diener H‐C, Schoenen J, Ferrari MD, Goadsby. Ergotamine in the acute treatment of migraine A review and European consensus. Brain 2000; 123: 9–18. [DOI] [PubMed] [Google Scholar]
- 22. Dahlöf C, Maassen Van Den Brink A. Dihydroergotamine, ergotamine, methysergide and sumatriptan–basic science in relation to migraine treatment. Headache J Head Face Pain 2012; 52: 707–14. [DOI] [PubMed] [Google Scholar]
- 23. Gilman AG, LS, Rall TW. Goodman and Gilman's the pharmacological basis of thera peutics. Macmillan USA; 7th edition October 1985. [Google Scholar]
- 24. McCrory DC, Gray RN. Oral sumatriptan for acute migraine. In: Cochrane Database of Systematic Reviews. New York: John Wiley & Sons, Ltd; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hahne T, Balda BR. [Finger tip necroses after dihydroergotamine medication in limited systemic scleroderma]. Hautarzt Z Für Dermatol Venerol Verwandte Geb 1998; 49: 722–4. [DOI] [PubMed] [Google Scholar]
- 26. Robb LG. Severe vasospasm following ergot administration. West J Med 1975; 123: 231–5. [PMC free article] [PubMed] [Google Scholar]
- 27. O'Keeffe ST, Tsapatsaris NP, Beetham WP Jr Association between Raynaud's phenomenon and migraine in a random population of hospital employees. J Rheumatol 1993; 20: 1187–8. [PubMed] [Google Scholar]
- 28. Zahavi I, Chagnac A, Hering R, Davidovich S, Kuritzky A. Prevalence of Raynaud's phenomenon in patients with migraine. Arch Intern Med 1984; 144: 742–4. [PubMed] [Google Scholar]
- 29. Duvoisin RC. Digital vasospasm with bromocriptine. Lancet 1976; 2: 204. [DOI] [PubMed] [Google Scholar]
- 30. Quagliarello J, Barakat R. Raynaud's phenomenon in infertile women treated with bromocriptine. Fertil Steril 1987; 48: 877–9. [DOI] [PubMed] [Google Scholar]
- 31. Zenone T, Durieu I, Nagnoug F, Castell P, Levrat R. [Raynaud phenomenon with organic microangiopathy and prolonged treatment with bromocriptine]. Rev Médecine Interne Fondée Par Société Natl Francaise Médecine Interne 1996; 17: 948–50. [DOI] [PubMed] [Google Scholar]
- 32. Cruaud D, Noël G, Daumont M. [Raynaud's syndrome induced by bromoergocryptine]. Nouv Presse Médicale 1977; 6: 2693. [PubMed] [Google Scholar]
- 33. Newman‐Tancredi A, Cussac D, Audinot V, Nicolas J‐P, Ceuninck FD, Boutin J‐A, Millan MJ. Differential Actions of Antiparkinson Agents at Multiple Classes of Monoaminergic Receptor. II. Agonist and Antagonist Properties at Subtypes of Dopamine D2‐Like Receptor and α1/α2‐Adrenoceptor. J Pharmacol Exp Ther 2002; 303: 805–14. [DOI] [PubMed] [Google Scholar]
- 34. de Leeuw van Weenen JE, Parlevliet ET, Maechler P, Havekes LM, Romijn JA, Ouwens DM, Pijl H, Guigas B. The dopamine receptor D2 agonist bromocriptine inhibits glucose‐stimulated insulin secretion by direct activation of the alpha2‐adrenergic receptors in beta cells. Biochem Pharmacol 2010; 79: 1827–36. [DOI] [PubMed] [Google Scholar]
- 35. Arbouw MEL, Movig KLL, Guchelaar H‐J, Neef C, Egberts TCG. Dopamine agonists and ischemic complications in Parkinson's disease: a nested case–control study. Eur J Clin Pharmacol 2012; 68: 83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cohen JS. Erythromelalgia: New theories and new therapies. J Am Acad Dermatol 2000; 43: 841–7. [DOI] [PubMed] [Google Scholar]
- 37. Skeik N, Rooke TW, Davis MDP, Davis DMR, Kalsi H, Kurth I, Richardson RC. Severe case and literature review of primary erythromelalgia: Novel SCN9A gene mutation. Vasc Med 2012; 17: 44–9. [DOI] [PubMed] [Google Scholar]
- 38. Rey J, Cretel E, Jean R, Pastor M‐J, Durand J‐M. Serotonin reuptake inhibitors, Raynaud's phenomenon and erythromelalgia. Rheumatol Oxf Engl. 2003; 42: 601–2. [DOI] [PubMed] [Google Scholar]
- 39. Buecking A, Rougemont E, Fabio ZD. Treatment of Raynaud's phenomenon with escitalopram. Int J Neuropsychopharmacol Off Sci J Coll Int Neuropsychopharmacol CINP 2005; 8: 307–8. [DOI] [PubMed] [Google Scholar]
- 40. Coleiro B, Marshall SE, Denton CP, Howell K, Blann A, Welsh KI, Black CM. Treatment of Raynaud's phenomenon with the selective serotonin reuptake inhibitor fluoxetine. Rheumatol Oxf Engl 2001; 40: 1038–43. [DOI] [PubMed] [Google Scholar]
- 41. De Broucker T, Lhote F. [Severe Raynaud's phenomenon associated with interferon‐beta 1a and fluoxetine]. Ann Médecine Interne 2000; 151: 424–5. [PubMed] [Google Scholar]
- 42. Rudnick A, Modai I, Zelikovski A. Fluoxetine‐induced Raynaud's phenomenon. Biol Psychiatry 1997; 41: 1218–21. [DOI] [PubMed] [Google Scholar]
- 43. Bell C, Coupland N, Creamer P. Digital infarction in a patient with Raynaud's phenomenon associated with treatment with a specific serotonin reuptake inhibitor. A case report. Angiology 1996; 47: 901–3. [DOI] [PubMed] [Google Scholar]
- 44. Peiró AM, Margarit C, Torra M. Citalopram‐induced Raynaud's phenomenon. Rheumatol Int 2006; 27: 599–601. [DOI] [PubMed] [Google Scholar]
- 45. Bourgade B, Jonville‐Béra AP, Le Garé C, Ferquel D, Autret‐Leca E. Raynaud's syndrome in a patient treated with milnacipran. Ann Pharmacother 1999; 33: 1009–10. [DOI] [PubMed] [Google Scholar]
- 46. Rudikoff D, Jaffe IA. Erythromelalgia: response to serotonin reuptake inhibitors. J Am Acad Dermatol 1997; 37 (2 Pt 1): 281–3. [DOI] [PubMed] [Google Scholar]
- 47. Bertoli R, Girardin F, Russmann S, Lauterburg BH. Raynaud's phenomenon induced by drugs acting on neurotransmission: two cases under reboxetine and one under tegaserod. Eur J Clin Pharmacol 2003; 58: 717–7. [DOI] [PubMed] [Google Scholar]
- 48. Garcia‐Porrua C, Margarinos CC, Gonzalez‐Gay MA. Raynaud's phenomenon and serotonin reuptake inhibitors. J Rheumatol 2004; 31: 2090 author reply 2090–1. [PubMed] [Google Scholar]
- 49. Lemberger L, Bergstrom RF, Wolen RL, Farid NA, Enas GG, Aronoff GR. Fluoxetine: clinical pharmacology and physiologic disposition. J Clin Psychiatry 1985; 46 (3 Pt 2): 14–9. [PubMed] [Google Scholar]
- 50. Syed RH, Moore TL. Methylphenidate and dextroamphetamine‐induced peripheral vasculopathy. J Clin Rheumatol Pract Rep Rheum Musculoskelet Dis 2008; 14: 30–3. [DOI] [PubMed] [Google Scholar]
- 51. Goldman W, Seltzer R, Reuman P. Association between treatment with central nervous system stimulants and Raynaud's syndrome in children: A retrospective case–control study of rheumatology patients. Arthritis Rheum 2008; 58: 563–6. [DOI] [PubMed] [Google Scholar]
- 52. Gökçen C, Kutuk MO, Coşkun S. Dose‐dependent Raynaud's phenomenon developing from use of atomoxetine in a girl. J Child Adolesc Psychopharmacol 2013; 23: 428–30. [DOI] [PubMed] [Google Scholar]
- 53. Jefferson HJ, Jayne DR. Peripheral vasculopathy and nephropathy in association with phentermine. Nephrol Dial Transplant 1999; 14: 1761–3. [DOI] [PubMed] [Google Scholar]
- 54. Davenport A. The effect of renal transplantation and treatment with cyclosporin A on the prevalence of Raynaud's phenomenon. Clin Transplant 1993; 7 (1 I): 4–8. [Google Scholar]
- 55. Arinsoy T, Derici U, Yuksel A, Reis KA, Sindel S. Cyclosporine–a treatment and a rare complication: Raynaud's phenomenon. Int J Clin Pract 2005; 59: 863–4. [DOI] [PubMed] [Google Scholar]
- 56. Deray G, Le Hoang P, Achour L, Hornych A, Landault C, Caraillon A. Cyclosporin and Raynaud phenomenon. Lancet 1986; 328: 1092–3. [DOI] [PubMed] [Google Scholar]
- 57. Sharma AK, Sunil S, Rustom R, Bone JM, Hammad A, Bakran A, Sells RA. Cyclosporin A‐related Raynaud's phenomenon in a renal transplant recipient. Transpl Int 2002; 15: 517–8. [DOI] [PubMed] [Google Scholar]
- 58. Osman EA, Barrett JJ, Bewick M, Parsons V. Does cyclosporin affect renal blood vessels? Lancet 1984; 1: 1470. [DOI] [PubMed] [Google Scholar]
- 59. Ravindran V, Rajendran S. Digital gangrene in a patient with primary Raynaud's phenomenon. J R Coll Physicians Edinb 2012; 42: 24–6. [DOI] [PubMed] [Google Scholar]
- 60. Francès C, Allanore Y, Cabane J, Carpentier P, Dumontier C, Hachulla É, Hatron P‐Y, Lipsker D, Meaume S, Mouthon L, Senet P, Sibilia J. Prise en charge des ulcères digitaux de la sclérodermie systémique: Recommandations d'un groupe pluridisciplinaire d'experts. Presse Med 2008; 37 (2, Part 2): 271–85. [DOI] [PubMed] [Google Scholar]
- 61. Balbir‐Gurman A, Braun‐Moscovici Y, Nahir AM. Cocaine‐induced Raynaud's phenomenon and ischaemic finger necrosis. Clin Rheumatol 2001; 20: 376–8. [DOI] [PubMed] [Google Scholar]
- 62. Eichhorn EJ, Demian SE, Alvarez LG, Willard JE, Molina S, Bartula LL, Dale Prince M, Inman LR, Grayburn PA, Myers SI. Cocaine‐induced alterations in prostaglandin production in rabbit aorta. J Am Coll Cardiol 1992; 19: 696–703. [DOI] [PubMed] [Google Scholar]
- 63. Rezkalla SH, Mazza JJ, Kloner RA, Tillema V, Chang SH. Effects of cocaine on human platelets in healthy subjects. Am J Cardiol 1993; 72: 243–6. [DOI] [PubMed] [Google Scholar]
- 64. Noel B Cocaine and arsenic‐induced Raynaud's phenomenon. Clin Rheumatol 2002; 21: 343–4. [DOI] [PubMed] [Google Scholar]
- 65. Marder VJ, Mellinghoff IK. Cocaine and Buerger disease: is there a pathogenetic association? Arch Intern Med 2000; 160: 2057–60. [DOI] [PubMed] [Google Scholar]
- 66. Kumar PD, Smith HR. Cocaine‐related vasculitis causing upper‐limb peripheral vascular disease. Ann Intern Med 2000; 133: 923–4. [DOI] [PubMed] [Google Scholar]
- 67. Gröger A, Aslani A, Wolter T, Noah EM, Pallua N. [A rare case of cannabis arteritis]. VASA Z Für Gefässkrankh 2003; 32: 95–7. [DOI] [PubMed] [Google Scholar]
- 68. Schneider F, Abdoucheli‐Baudot N, Tassart M, Boudghène F, Gouny P. [Cannabis and tobacco: cofactors favoring juvenile obliterative arteriopathy]. J Mal Vasc 2000; 25: 388–9. [PubMed] [Google Scholar]
- 69. Lagerkvist B, Linderholm H, Nordberg GF. Vasospastic tendency and Raynaud's phenomenon in smelter workers exposed to arsenic. Environ Res 1986; 39: 465–74. [DOI] [PubMed] [Google Scholar]
- 70. Stefenelli T, Kuzmits R, Ulrich W, Glogar D. Acute vascular toxicity after combination chemotherapy with cisplatin, vinblastine, and bleomycin for testicular cancer. Eur Heart J 1988; 9: 552–6. [DOI] [PubMed] [Google Scholar]
- 71. Scheulen ME, Schmidt CG. [Raynaud's phenomenon following combined cytostatic treatment of malignant testicular tumours]. Dtsch Med Wochenschr 1946 1982; 107: 1640–4. [DOI] [PubMed] [Google Scholar]
- 72. Teutsch C, Lipton A, Harvey HA. Raynaud's phenomenon as a side effect of chemotherapy with vinblastine and bleomycin for testicular carcinoma. Cancer Treat Rep 1977; 61: 925–6. [PubMed] [Google Scholar]
- 73. Berger CC, Bokemeyer C, Schneider M, Kuczyk MA, Schmoll HJ. Secondary Raynaud's phenomenon and other late vascular complications following chemotherapy for testicular cancer. Eur J Cancer Oxf Engl 1990 1995; 31A: 2229–38. [DOI] [PubMed] [Google Scholar]
- 74. Heier MS, Nilsen T, Graver V, Aass N, Fosså SD. Raynaud's phenomenon after combination chemotherapy of testicular cancer, measured by laser Doppler flowmetry. A pilot study. Br J Cancer 1991; 63: 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Glendenning JL, Barbachano Y, Norman AR, Dearnaley DP, Horwich A, Huddart RA. Long‐term neurologic and peripheral vascular toxicity after chemotherapy treatment of testicular cancer. Cancer 2010; 116: 2322–31. [DOI] [PubMed] [Google Scholar]
- 76. Brydoy M, Oldenburg J, Klepp O, Bremnes RM, Wist EA, Wentzel‐Larsen T, Hauge ER, Dahl O, Fossa SD. Observational Study of Prevalence of Long‐term Raynaud‐Like Phenomena and Neurological Side Effects in Testicular Cancer Survivors. JNCI J Natl Cancer Inst 2009; 101: 1682–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Reiser M, Bruns C, Hartmann P, Salzberger B, Diehl V, Fätkenheuer G. Raynaud's phenomenon and acral necrosis after chemotherapy for AIDS‐related Kaposi's sarcoma. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol 1998; 17: 58–60. [DOI] [PubMed] [Google Scholar]
- 78. Hladunewich M, Sawka C, Fam A, Franssen E. Raynaud's phenomenon and digital gangrene as a consequence of treatment for Kaposi's sarcoma. J Rheumatol 1997; 24: 2371–5. [PubMed] [Google Scholar]
- 79. Pechère M, Zulian GB, Vogel JJ, Jeanprêtre M, Hirschel B, Saurat JH. Fingertip necrosis during chemotherapy with bleomycin, vincristine and methotrexate for HIV‐related Kaposi's sarcoma. Br J Dermatol 1996; 134: 378–9. [DOI] [PubMed] [Google Scholar]
- 80. Fertakos RJ, Mintzer DM. Digital gangrene following chemotherapy for AIDS‐related Kaposi's sarcoma. Am J Med 1992; 93: 581–2. [DOI] [PubMed] [Google Scholar]
- 81. von Gunten CF, Roth EL, Von Roenn JH. Raynaud phenomenon in three patients with acquired immune deficiency syndrome‐related Kaposi sarcoma treated with bleomycin. Cancer 1993; 72: 2004–6. [DOI] [PubMed] [Google Scholar]
- 82. McGrath SE, Webb A, Walker‐Bone K. Bleomycin‐induced Raynaud's phenomenon after single‐dose exposure: risk factors and treatment with intravenous iloprost infusion. J Clin Oncol Off J Am Soc Clin Oncol. 2013; 31: e51–2. [DOI] [PubMed] [Google Scholar]
- 83. Epstein E. Intralesional bleomycin and Raynaud's phenomenon. J Am Acad Dermatol 1991; 24 (5 Pt 1): 785–6. [DOI] [PubMed] [Google Scholar]
- 84. Epstein E. Persisting Raynaud's phenomenon following intralesional bleomycin treatment of finger warts. J Am Acad Dermatol 1985; 13: 468–71. [DOI] [PubMed] [Google Scholar]
- 85. Gregg LJ. Intralesional bleomycin and Raynaud's phenomenon. J Am Acad Dermatol 1992; 26 (2 Pt 1): 279–80. [DOI] [PubMed] [Google Scholar]
- 86. Vanhooteghem O, Richert B, de la Brassinne M. Raynaud phenomenon after treatment of verruca vulgaris of the sole with intralesional injection of bleomycin. Pediatr Dermatol 2001; 18: 249–51. [DOI] [PubMed] [Google Scholar]
- 87. de Pablo P, Aguillar A, Gallego MA. Raynaud's phenomenon and intralesional bleomycin. Acta Derm Venereol 1992; 72: 465. [PubMed] [Google Scholar]
- 88. Fosså SD, Lehne G, Heimdal K, Theodorsen L. Clinical and biochemical long‐term toxicity after postoperative cisplatin‐based chemotherapy in patients with low‐stage testicular cancer. Oncology 1995; 52: 300–5. [DOI] [PubMed] [Google Scholar]
- 89. Vaklavas C, Lenihan D, Kurzrock R, Tsimberidou AM. Anti‐vascular endothelial growth factor therapies and cardiovascular toxicity: what are the important clinical markers to target? Oncologist 2010; 15: 130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kuhar CG, Mesti T, Zakotnik B. Digital ischemic events related to gemcitabine: Report of two cases and a systematic review. Radiol Oncol 2010; 44: 257–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zaima C, Kanai M, Ishikawa S, Kawaguchi Y, Masui T, Mori Y, Nishimura T, Matsumoto S, Yanagihara K, Chiba T, Mimori T. A case of progressive digital ischemia after early withdrawal of gemcitabine and S‐1 in a patient with systemic sclerosis. Jpn J Clin Oncol 2011; 41: 803–6. [DOI] [PubMed] [Google Scholar]
- 92. D'Alessandro V, Errico M, Varriale A, Greco A, De Cata A, Carnevale V, et al. [Case report: Acro‐necrosis of the upper limbs caused by gemcitabine therapy]. Clin Ter 2003; 154: 207–10. [PubMed] [Google Scholar]
- 93. Gottschling S, Meyer S, Reinhard H, Krenn T, Graf N. First report of a vincristine dose‐related Raynaud's phenomenon in an adolescent with malignant brain tumor. J Pediatr Hematol Oncol 2004; 26: 768–9. [DOI] [PubMed] [Google Scholar]
- 94. Papamichael D, Amft N, Slevin ML, D'Cruz D. 5‐Fluorouracil‐induced Raynaud's phenomenon. Eur J Cancer Oxf Engl 1990 1998; 34: 1983. [DOI] [PubMed] [Google Scholar]
- 95. Karabacak K, Kadan M, Kaya E, Durgun B, Arslan G, Doganci S, Bolcal C, Demirkilic U. Oxaliplatin induced digital ischemia and necrosis. Case Rep Vasc Med 2015; 2015: e248748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Seishima M, Izumi T, Kanoh H. Raynaud's phenomenon possibly induced by a compund drug of tegafur and uracil. Eur J Dermatol EJD 2000; 10: 55–8. [PubMed] [Google Scholar]
- 97. De Angelis R, Silveri F, Bugatti L, Filosa G. Raynaud's phenomenon after combined adjuvant chemotherapy for breast cancer. Chemotherapy 2003; 49: 267–8. [DOI] [PubMed] [Google Scholar]
- 98. Hansen SW, Olsen N. Raynaud's phenomenon in patients treated with cisplatin, vinblastine, and bleomycin for germ cell cancer: measurement of vasoconstrictor response to cold. J Clin Oncol 1989; 7: 940–2. [DOI] [PubMed] [Google Scholar]
- 99. Hansen SW, Olsen N, Rossing N, Rørth M. Vascular toxicity and the mechanism underlying Raynaud's phenomenon in patients treated with cisplatin, vinblastine and bleomycin. Ann Oncol 1990; 1: 289–92. [DOI] [PubMed] [Google Scholar]
- 100. Meinardi MT, Gietema JA, van der Graaf WTA, van Veldhuisen DJ, Runne MA, Sluiter WJ, de Vries EGE, Willemse PBH, Mulder NH, van den Berg MP, Koops HS, Sleijfer DT. Cardiovascular morbidity in long‐term survivors of metastatic testicular cancer. J Clin Oncol 2000; 18: 1725–32. [DOI] [PubMed] [Google Scholar]
- 101. Mohokum M, Hartmann P, Schlattmann P. The association of Raynaud's syndrome with cisplatin‐based chemotherapy — A meta‐analysis. Eur J Intern Med 2012; 23: 594–8. [DOI] [PubMed] [Google Scholar]
- 102. Batteux F, Kavian N, Servettaz A. New insights on chemically induced animal models of systemic sclerosis. Curr Opin Rheumatol 2011; 23: 511–8. [DOI] [PubMed] [Google Scholar]
- 103. Freudiger H, Bounameaux H, Garcia J. Acroosteolysis and Raynaud's phenomenon after vinyl chloride exposure. VASA Z Für Gefässkrankh 1988; 17: 216–8. [PubMed] [Google Scholar]
- 104. Falappa P, Magnavita N, Bergamaschi A, Colavita N. Angiographic study of digital arteries in workers exposed to vinyl chloride. Br J Ind Med 1982; 39: 169–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Maricq HR, Johnson MN, Whetstone CL, LeRoy EC. Capillary abnormalities in polyvinyl chloride production workers. Examination by in vivo microscopy. JAMA 1976; 236: 1368–71. [PubMed] [Google Scholar]
- 106. Fontana L, Marion M‐J, Ughetto S, Catilina P. Glutathione S‐transferase M1 and GST T1 genetic polymorphisms and Raynaud's phenomenon in French vinyl chloride monomer‐exposed workers. J Hum Genet 2006; 51: 879–86. [DOI] [PubMed] [Google Scholar]
- 107. Laplanche A, Clavel F, Contassot JC, Lanouziere C. Exposure to vinyl chloride monomer: report on a cohort study. Br J Ind Med 1987; 44: 711–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Al‐Zahrani H, Gupta V, Minden MD, Messner HA, Lipton JH. Vascular events associated with alpha interferon therapy. Leuk Lymphoma 2003; 44: 471–5. [DOI] [PubMed] [Google Scholar]
- 109. Kruit WHJ, Eggermont AMM, Stoter G. Interferon‐α induced Raynaud's syndrome. Ann Oncol 2000; 11: 1501–2. [DOI] [PubMed] [Google Scholar]
- 110. Cruz BA, Queiroz ED, Nunes SV, Cruz Filho A, Campos GB, Monteiro EL, Crivellari H. [Severe Raynaud's phenomenon associated with interferon‐beta therapy for multiple sclerosis: case report]. Arq Neuropsiquiatr 2000; 58 (2B): 556–9. [DOI] [PubMed] [Google Scholar]
- 111. Campo‐Voegeli A, Estrach T, Marti RM, Corominas N, Tuset M, Mascaró JM. Acrocyanosis induced by interferon alpha(2a). Dermatol Basel Switz 1998; 196: 361–3. [DOI] [PubMed] [Google Scholar]
- 112. Liozon E, Delaire L, Lacroix P, Labrousse F, Ly K, Fauchais AL, Loustaud‐Ratti V, Vidal J, Liozon F, Vidal E. [Raynaud syndrome complicated by digital gangrene during treatment with interferon‐alpha]. Rev Médecine Interne Fondée Par Société Natl Francaise Médecine Interne 1997; 18: 316–9. [DOI] [PubMed] [Google Scholar]
- 113. Rot U, Ledinek AH. Interferons beta have vasoconstrictive and procoagulant effects: A woman who developed livedo reticularis and Raynaud phenomenon in association with interferon beta treatment for multiple sclerosis. Clin Neurol Neurosurg 2013; 115 (Supplement 1): S79–81. [DOI] [PubMed] [Google Scholar]
- 114. Husein–ElAhmed H, Callejas–Rubio JL, Olmo ROD, Ríos–Fernandez R, Ortego–Centeno N. Severe Raynaud syndrome induced by adjuvant interferon alfa in metastatic melanoma. Curr Oncol 2010; 17: 122–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Bachmeyer C, Farge D, Gluckman E, Miclea JM, Aractingi S. Raynaud's phenomenon and digital necrosis induced by interferon‐alpha. Br J Dermatol 1996; 135: 481–3. [PubMed] [Google Scholar]
- 116. Arslan M, Ozyilkan E, Kayhan B, Telatar H. Raynaud's phenomenon associated with alpha‐interferon therapy. J Intern Med 1994; 235: 503. [DOI] [PubMed] [Google Scholar]
- 117. Creutzig A, Caspary L, Freund M. The Raynaud phenomenon and interferon therapy. Ann Intern Med 1996; 125: 423. [DOI] [PubMed] [Google Scholar]
- 118. Schapira D, Nahir AM, Hadad N. Interferon‐induced Raynaud's syndrome. Semin Arthritis Rheum 2002; 32: 157–62. [DOI] [PubMed] [Google Scholar]
- 119. Mohokum M, Hartmann P, Schlattmann P. Association of Raynaud's syndrome with interferons. A meta‐analysis. Int Angiol J Int Union Angiol 2012; 31: 408–13. [PubMed] [Google Scholar]
- 120. Zeidman A, Dicker D, Mittelman M. Interferon‐induced vasospasm in chronic myeloid leukaemia. Acta Haematol 1998; 100: 94–6. [DOI] [PubMed] [Google Scholar]
- 121. Cozzolino F, Torcia M, Lucibello M, Morbidelli L, Ziche M, Platt J, Fabiani S, Brett J, Stern D. Interferon‐alpha and interleukin 2 synergistically enhance basic fibroblast growth factor synthesis and induce release, promoting endothelial cell growth. J Clin Invest 1993; 91: 2504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Iorio R, Spagnuolo MI, Sepe A, Zoccali S, Alessio M, Vegnente A. Severe Raynaud's phenomenon with chronic hepatis C disease treated with interferon. Pediatr Infect Dis J 2003; 22: 195–7. [PubMed] [Google Scholar]
- 123. Vial T, Descotes J. Clinical toxicity of the interferons. Drug Saf Int J Med Toxicol Drug Exp 1994; 10: 115–50. [DOI] [PubMed] [Google Scholar]
- 124. Tóthová E, Kafková A, Stecová N, Fricová M, Guman T, Svorcová E. Immune‐mediated complications during interferon alpha therapy in chronic myelogenous leukemia. Neoplasma 2002; 49: 91–4. [PubMed] [Google Scholar]
- 125. Roy V, Newland AC. Raynaud's phenomenon and cryoglobulinaemia associated with the use of recombinant human alpha‐interferon. Lancet 1988; 1: 944–5. [DOI] [PubMed] [Google Scholar]
- 126. Raza A, Mittal S, Sood GK. Interferon‐associated retinopathy during the treatment of chronic hepatitis C: a systematic review. J Viral Hepat 2013; 20: 593–9. [DOI] [PubMed] [Google Scholar]
- 127. Willeke P, Schlüter B, Schotte H, Domschke W, Gaubitz M, Becker H. Interferon‐gamma is increased in patients with primary Sjogren's syndrome and Raynaud's phenomenon. Semin Arthritis Rheum 2009; 39: 197–202. [DOI] [PubMed] [Google Scholar]
- 128. Furspan PB, Chatterjee S, Freedman RR. Increased tyrosine phosphorylation mediates the cooling‐induced contraction and increased vascular reactivity of Raynaud's disease. Arthritis Rheum 2004; 50: 1578–85. [DOI] [PubMed] [Google Scholar]
- 129. Tamaki Z, Asano Y, Hatano M, Yao A, Kawashima T, Tomita M, Kinugawa K, Nagai R, Sato S. Efficacy of low‐dose imatinib mesylate for cutaneous involvement in systemic sclerosis: a preliminary report of three cases. Mod Rheumatol Jpn Rheum Assoc 2012; 22: 94–9. [DOI] [PubMed] [Google Scholar]
- 130. Hazenberg CLE, Ossenkoppele GJ, Smit WM. Raynaud‐like phenomenon in two patients on nilotinib. Br J Haematol 2012; 158: 431. [DOI] [PubMed] [Google Scholar]
- 131. Quintás‐Cardama A, Kantarjian H, Cortes J. Nilotinib‐associated vascular events. Clin Lymphoma Myeloma Leuk 2012; 12: 337–40. [DOI] [PubMed] [Google Scholar]
- 132. Ballardini P, Margutti G, Manfredini R. Digital necrosis induced by erlotinib treatment in metastatic adenocarcinoma of the lung. Curr Oncol 2011; 18: 109–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Schmutz J‐L, Barbaud A, Tréchot P. [Intravenous fluorescein and Raynaud's phenomenon]. Ann Dermatol Vénéréologie 2009; 136: 96. [DOI] [PubMed] [Google Scholar]
- 134. Blaise P, Ribbens C, Rakic J‐M. Fluorescein‐induced Raynaud's phenomenon. Acta Ophthalmol Scand 2007; 85: 910–1. [DOI] [PubMed] [Google Scholar]
- 135. Ahmad J, Siddiqui MA, Khan AS, Afzall S. Raynaud's phenomenon induced by sulphasalazine in a case of chronic ulcerative colitis. J Assoc Physicians India 1984; 32: 370. [PubMed] [Google Scholar]
- 136. Reid J, Holt S, Housley E, Sneddon DJ. Raynaud's phenomenon induced by sulphasalazine. Postgrad Med J 1980; 56: 106–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Dodd PH, Biswas G. Raynaud's phenomenon and propofol. Anaesthesia 1999; 54: 918. [DOI] [PubMed] [Google Scholar]
- 138. Zernikow B, Fleischhack G, Hasan C, Bode U. Cyanotic Raynaud's phenomenon with conventional but not with liposomal amphotericin B: three case reports. Mycoses 1997; 40: 359–61. [DOI] [PubMed] [Google Scholar]
- 139. Barreira RI, García BB, López MG, Legazpi IR, Díaz HÁ, Penín IR. Paradoxical reaction of Raynaud phenomenon following the repeated administration of iloprost in a patient with diffuse cutaneous systemic sclerosis. Ann Pharmacother 2012; 46: e28–8. [DOI] [PubMed] [Google Scholar]
- 140. Johnson S, Iazzetta J, Dewar C. Severe Raynaud's phenomenon with yohimbine therapy for erectile dysfunction. J Rheumatol 2003; 30: 2503–5. [PubMed] [Google Scholar]


