Abstract
Aim
The aim of this paper is to investigate 25‐year trends in community use of prescribed opioid analgesics in Australia, and to map these trends against major changes to opioid registration and subsidy.
Methods
We obtained dispensing data from 1990 to 2014 from two sources: dispensing claims processed under Australia's national drug subsidy programme, the Pharmaceutical Benefits Scheme, including under co‐payment records from 2012; and estimates of non‐subsidized medicine use from a survey of Australian pharmacies (until 2011). Utilization was expressed in defined daily doses (DDD)/1000 population/day.
Results
Opioid dispensing increased almost four‐fold between 1990 and 2014, from 4.6 to 17.4 DDD/1000 pop/day. In 1990, weak, short‐acting or orally administered opioids accounted for over 90% of utilization. Use of long‐acting opioids increased over 17‐fold between 1990 and 2000, due primarily to the subsidy of long‐acting morphine and increased use of methadone for pain management. Between 2000 and 2011, oxycodone, fentanyl, buprenorphine, tramadol and hydromorphone use increased markedly. Use of strong opioids, long‐acting and transdermal preparations also increased, largely following the subsidy of various opioids for noncancer pain. In 2011, the most dispensed opioids were codeine (41.1% of total opioid use), oxycodone (19.7%) and tramadol (16.1%); long‐acting formulations comprised approximately half, and strong opioids 40%, of opioid dispensing.
Conclusions
Opioid utilization in Australia is increasing, although these figures remain below levels reported in the US and Canada. The increased use of opioids was largely driven by the subsidy of long‐acting formulations and opioids for the treatment of noncancer pain.
Keywords: analgesics, Australia, dispensing, opioids, pharmacoepidemiology
What is Already Known about this Subject
Increases in prescribed opioid use have been reported globally over the past two decades.
There is no comprehensive and long‐term account of opioid use in Australia to date, and comparisons between available studies are limited by differences in medicine capture, datasets and measures of utilization.
What this Study Adds
Opioid utilization in Australia increased almost four‐fold between 1990 and 2014, with consumption exceeding levels reported in the UK, but lower than those in the US and Canada.
Increased opioid use appeared largely driven by the subsidy of new long‐acting formulations and of opioids for the treatment of noncancer pain.
Introduction
Chronic pain is one of the largest contributors to global disability 1. Affected individuals experience reduced physical and mental health, high levels of healthcare utilization, and reduced work attendance and productivity 2, 3, 4, making the effective treatment of pain of vital importance from an individual, societal and economic perspective. Pain management relies heavily on opioid analgesics, in line with their demonstrated efficacy in acute pain and in the short‐term treatment of chronic cancer and noncancer pain 5, 6, 7. Indeed, opioids comprise 36% of the US pain market, ranking ahead of the nonsteroidal anti‐inflammatories (NSAIDs, 28%), anticonvulsants (13%), antidepressants (11%) and selective cyclooxygenase‐2 (Cox‐2) inhibitors (7%) 8. In Europe, opioids are used by approximately 23% of individuals using prescription medication for chronic pain 3.
Opioid analgesics were initially developed for the treatment of cancer pain, and remain the treatment of choice for the 50% of cancer patients affected by chronic pain 9. In the last two decades, various opioids, including oxycodone, hydromorphone, buprenorphine and fentanyl, have been registered and subsidized for the treatment of chronic noncancer pain (CNCP) in many health care settings around the globe. These changes brought dramatic growth in opioid prescribing and use, despite uncertainty about their efficacy in the long‐term treatment of this indication 10, 11. Indeed, opioid sales quadrupled in the US between 2000 and 2010 12, with approximately 7% of US adults using prescribed opioid analgesics by 2012 13. Similar increases have been observed in the UK; between 2000 and 2012, there was a 5.5‐fold increase in the number of patients prescribed strong opioids (morphine, oxycodone, buprenorphine and fentanyl) in UK primary care 14, although rates of opioid use in the UK are at most one third of those in the US 15, 16. In Australia, total community opioid dispensing increased by 24% between 2002 and 2009 17, with particularly notable growth in the use of oxycodone, fentanyl and buprenorphine in recent years 17, 18, 19, 20. Marked increases in opioid abuse, diversion and related harms have accompanied these upward trends in prescribing 18, 19, 21, 22, sparking concerns of an emerging ‘epidemic’ of opioid use and misuse.
The aim of this study is to investigate trends in the use of prescribed opioid analgesics in Australia over a 25‐year period, from 1990. We will map these trends against major changes to opioid registration and subsidy.
Methods
Setting
Australia has a publically funded universal health care system offering all Australian citizens and permanent residents access to subsidized prescription medicines under the Pharmaceutical Benefits Scheme (PBS), which services the general population, and the Repatriation Pharmaceutical Benefits Scheme (RPBS) for returned servicemen and women and their dependants. A medicine may be listed on the PBS/RPBS subsequent to its registration for supply by Australia's Therapeutic Goods Administration (TGA). PBS listing is conducted on the basis of efficacy, safety and cost‐effectiveness, and may occur for the same or narrower indication as its TGA registration.
PBS‐eligible patients pay a co‐payment according to their beneficiary status. Concessional beneficiaries are patients eligible for government entitlements, such as pensioners, veterans and low‐income earners; these patients have a lower co‐payment threshold. All other patients are general beneficiaries and have a higher co‐payment threshold. PBS medicines priced below the general beneficiary co‐payment threshold and private prescriptions are not subsidized by the PBS 23.
Data
We obtained aggregated dispensing data for the period January 1990 to December 2014 from a database maintained by the Drug Utilization Sub‐Committee (DUSC) of the Pharmaceutical Benefits Advisory Committee (PBAC) of the Australian Government Department of Health. The database includes (a) records of prescriptions subsidized under the PBS/RPBS (and under co‐payment data from 2012), and (b) estimates of nonsubsidized under co‐payment and private prescriptions ascertained from a Pharmacy Guild Survey of a representative sample of approximately 370 community pharmacies (for more detail, see 24). The Survey was initiated in 1989 and ceased on 1 August 2012 25. The DUSC dataset does not capture medicines dispensed over‐the‐counter or inpatient prescriptions in public hospitals, but has captured an increasing number of prescriptions supplied to public hospital outpatients and inpatients upon discharge since 2002 24. The data included monthly figures on number of dispensings and defined daily doses (DDD) per 1000 population per day (DDD/1000 pop/day), including PBS item codes, names, formulations, strengths and type of script (PBS/RPBS, under co‐payment, private). Data were released by DUSC in de‐identified aggregated form; as such, ethical approval was not required.
Medicines of interest (Table 1)
Table 1.
Anatomical Therapeutic Chemical (ATC) codes and defined daily doses (DDD) for opioid analgesics included in the study
| Medicine | ATC codes | Route of administration | DDD (mg) |
|---|---|---|---|
| Buprenorphine | N02AE01 | P, SL, TD | 1.2 |
| Codeine | R05DA04*, N02AA59† | O | 100 |
| Dextropropoxyphene | N02AC04, N02AC54† | O | 300 |
| Fentanyl | N02AB03 | SL | 0.6 |
| TD | 1.2 | ||
| Hydromorphone | N02AA03 | O | 20 |
| P | 4 | ||
| Methadone | N07BC02‡ | O, P | 25 |
| Morphine | N02AA01, N02AA51† | O | 100 |
| P | 30 | ||
| Oxycodone | N02AA05, N02AA55† | O | 75 |
| P, R | 30 | ||
| Pethidine | N02AB02 | O, P | 400 |
| Tapentadol | N02AX06 | O | 400 |
| Tramadol | N02AX02 | O, P | 300 |
Listings under the R05DA04 code are also used in pain.
Combination product.
Methadone is subsidized under the PBS for several indications: (1) Treatment of opiate dependence, (2) severe disabling pain not responding to non‐narcotic analgesics, (3) chronic severe disabling pain in palliative care patients. All PBS item codes for methadone are under the N07BC02 ATC code (for opiate dependence). As medicines funded by the S100 Opiate Dependence Treatment Program are not captured by the DUSC database, all methadone dispensing data captured under N07BC02 likely reflects the use of methadone in pain.
ATC, Anatomical Therapeutic Chemical; DDD, defined daily dose; N, nasal; O, oral; P, parenteral; R, rectal; SL, sublingual/buccal; TD, transdermal.
We obtained data for the eleven opioid analgesics (including combination products) available in Australia between 1990 and 2014: buprenorphine, codeine, dextropropoxyphene, fentanyl, hydromorphone, methadone, morphine, oxycodone, pethidine, tapentadol and tramadol. Opioid formulations primarily used for indications other than analgesia (e.g. anaesthetics, opiate dependence) were excluded from our analysis. Opioids supplied for opiate dependence (methadone, buprenorphine) are funded by the Government under the Opiate Dependence Treatment Programme and not captured by the DUSC database. We classified codeine, dextropropoxyphene and tramadol as weak opioids; the remainder were strong opioids 7, 26. Long‐acting opioids included methadone (which possesses inherent long‐acting properties), and extended‐release (ER), sustained‐release (SR), controlled‐release (CR) and transdermal (TD) patch formulations. Long‐acting formulations are available for buprenorphine, fentanyl, hydromorphone, morphine, oxycodone, tapentadol and tramadol. These medicines, with the exception of tapentadol, also come in short‐acting forms.
Analysis
DDD/1000 pop/day was our primary measure of drug utilization. The DDD metric, established by the World Health Organization (WHO) Collaborating Centre for Drug Statistics Methodology, corresponds to an estimated mean daily dose of the drug when used for its main indication in adults and allows for simple comparisons of drug use across countries and across different formulations of the drug 27. We present opioid utilization using DDD/1000 pop/day for the period 1990–2014. Specifically, total community use (PBS/RPBS subsidized, under co‐payment and private dispensings) is reported for the period 1990–2011. The remainder of the study period does not include data on private dispensings due to the termination of the Pharmacy Guild Survey in 2012. For combination products, DDD/1000 pop/day was calculated for the opioid component only. Yearly population estimates required for DDD/1000 pop/day calculations were obtained from the Australian Bureau of Statistics (ABS) 28. For analyses of opioid use by formulation strength, we used volume of prescriptions as our outcome measure. Analyses were undertaken using SAS 9.4 and Microsoft Excel 2010; graphs were generated using GraphPad Prism 5.0c.
We mapped utilization trends against major changes in opioid registration and subsidy. These changes were identified using several sources, including historical PBS item listing records obtained by request from DUSC; publically‐available PBS publications, including monthly releases of the Schedule of Pharmaceutical Benefits, Summary of Changes, and Errata 29; and Australian publications on opioid dispensing using PBS data. The latter were identified using the methods outlined in our recent systematic review of published literature using PBS dispensing records 30.
Results
Community opioid dispensing (PBS, RPBS, under co‐payment and private prescriptions)
In 1990 there were seven opioids available in Australia, increasing to 11 by 2011, and to 12 by 2014. Total opioid analgesic dispensing increased 3.8‐fold between 1990 and 2014, from 4.6 to 17.4 DDD/1000 pop/day. Overall, utilization increased steadily over the study period, peaked in 2012, and declined by 0.7 DDD/1000 pop/day between 2012 and 2014. Given that the dispensing of non‐private (PBS/RPBS and under co‐payment) prescriptions was stable at 17.4 DDD/1000 pop/day between 2012 and 2014, this decline reflects the loss of data on private prescriptions from August 2012. As such, we hereafter report utilization in the text primarily for the period 1990 to 2011; figures include data to 2014. Total community dispensing of prescribed opioids increased 3.9‐fold, from 4.6 to 18.0 DDD/1000 pop/day, over this period.
The most dispensed opioids in 1990 were codeine (68.8% of total opioid DDDs/1000 pop/day) and dextropropoxyphene (21.5%), compared to codeine (41.1%), oxycodone (19.7%) and tramadol (16.1%) in 2011 (Figure 1). Codeine was the most popular opioid dispensed in all study years; the majority of codeine utilization was accounted for by codeine with paracetamol combination products (74.0% in 1990 rising to 97.7% in 2011).
Figure 1.
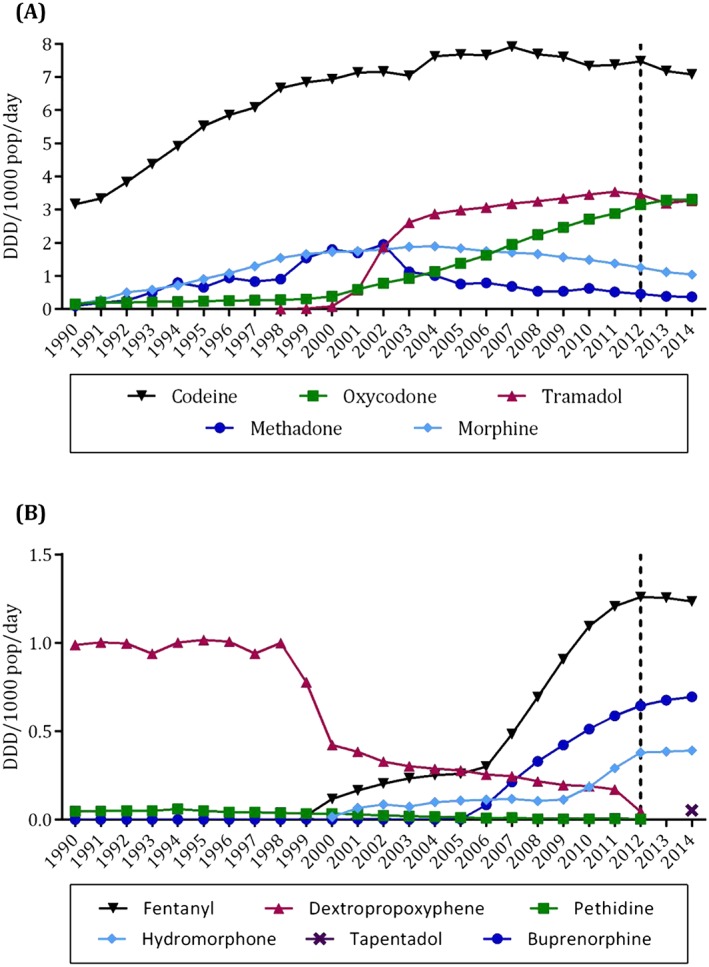
Community use (DDD/1000 pop/day) of (A) the five most dispensed opioids and (B) the remaining six opioids available in Australia between 1990 and 2014. The change in medicines capture in 2012 is indicated with a dotted line. Note the different scales between (A) and (B)
In 1990, the majority of opioid utilization was for weak opioids (90.4% of total opioid DDD/1000 pop/day), short‐acting formulations (95.7%) and oral routes of administration (94.8%). However, the use of strong opioids, long‐acting formulations and transdermal routes of administration increased markedly between 1990 and 2011 (Figure 2). By 2011, strong opioids comprised almost 38.2% and long‐acting formulations 49.9% of total opioid utilization. PBS/RPBS‐subsidized dispensing increased six‐fold over the study period. It comprised the majority of opioid use in all study years, ranging from 52.8% of dispensings in 1990 to 82.2% in 2011. Dispensing of private prescriptions comprised only 6.4% of total opioid use in 2011. Of the currently subsidized opioids, methadone (21.9%) and codeine (7.4%) had the highest levels of private dispensing; private use of buprenorphine and fentanyl (<1%) was low.
Figure 2.

Community use (DDD/1000 pop/day) of opioid analgesics, 1990–2014, by (A) opioid strength, (B) duration of action, (C) route of administration, and (D) prescription type. Data on private prescriptions dispensed in 2012 are omitted from (D) as data were unavailable for the complete year. The change in medicines capture in 2012 is indicated with a dotted line
Trends in the dispensing of individual opioids and policy changes
Figure 3 depicts dispensing trends, from 1990 to 2014, for eight of the nine opioid analgesics currently subsidized in Australia (excluding tapentadol, introduced in 2014), according to key regulatory and subsidy changes. We examined trends across two time periods of equal duration: the 1990s, characterized by the registration and subsidy of the prototypical long‐acting opioid formulations, and the 2000s, during which a number of opioids gained subsidy for the treatment of noncancer pain (Tables 2, 3).
Figure 3.

Trends in the utilization of opioid analgesics in Australia from 1990 to 2014, by medicine formulation: (A) codeine, (B) oxycodone (note: ampoule and oxycodone + paracetamol + aspirin omitted due to low use), (C) tramadol (note: injection and oral drops omitted due to low use), (D) methadone, (E) morphine (note: sachet and combination products with tacrine and aspirin omitted due to low use), (F) fentanyl, (G) buprenorphine, (H) hydromorphone. Note the different scales between graphs. Key regulatory and subsidy changes are indicated numerically and listed in Table 2. A more exhaustive list of changes to opioid approval and subsidy can be found in Supporting Figures S1 and S2 and Table S1
Table 2.
Listing of major changes to opioid regulation and subsidy, 1990–2014
| Medicine and reference number (Figure 3) | Year | Regulatory (TGA) and subsidy (PBS/RPBS) changes |
|---|---|---|
| Oxycodone | ||
| 1 | 1999 | CR tablets TGA‐approved |
| 2 | 2000 | CR tablets PBS‐listed for CNCP |
| 3 | 2001 | Capsules PBS‐listed for severe disabling pain |
| 4 | 2011 | Oxycodone with naloxone PBS‐listed for severe pain |
| Tramadol | ||
| 5 | 1998 | Capsule and injection TGA‐approved |
| 6 | 2000 | Capsule PBS‐listed for moderate–severe pain |
| 7 | 2001 | SR tablet PBS‐listed for moderate–severe pain |
| 8 | 2008 | ER tablets PBS‐listed for pain |
| 9 | 2013 | ER tablets deleted from the PBS |
| Methadone | ||
| 10 | 2003 | Tablets PBS‐listed for chronic severe pain |
| Morphine | ||
| 11 | 1991 | CR tablets TGA‐approved and PBS‐listed |
| 12 | 1994 | SR capsules and oral solution PBS‐listed |
| Fentanyl | ||
| 13 | 1999 | TD patches PBS‐listed for chronic CP |
| 14 | 2002 | Lozenges TGA‐listed for breakthrough CP |
| 15 | 2006 | TD patches PBS‐listed for CNCP |
| 16 | 2008 | Lozenges PBS‐listed for breakthrough CP |
| Buprenorphine | ||
| 17 | 2005 | TD patches TGA‐approved and PBS‐listed for CNCP |
| Hydromorphone | ||
| 18 | 2000 | Injection and oral solution PBS‐listed for severe disabling pain |
| 19 | 2001 | Tablets PBS‐listed for severe disabling pain |
| 20 | 2009 | MR tablets PBS‐listed for CNCP |
Reference numbers correspond to Figure 3. See Supporting Table S1 for a more extensive, chronological list of changes.
CNCP, chronic noncancer pain; CP, cancer pain; CR, controlled‐release; ER, extended‐release; MR, modified‐release; PBS, Pharmaceutical Benefits Scheme; RPBS, Repatriation Pharmaceutical Benefits Scheme; SR, sustained‐release; TD, transdermal; TGA, Therapeutic Goods Administration.
Table 3.
Use of opioid analgesics, with absolute and percentage changes, over three time periods: 1990–2000, 2001–2011 and 1990–2011. Opioid dispensing and absolute changes are given in DDD/1000 pop/day
| Time period 1: 1990–2000 | Time period 2: 2001–2011 | Total study period: 1990–2011 | ||||||
|---|---|---|---|---|---|---|---|---|
| Dispensing, 1990 | Absolute change | Percent change | Dispensing, 2001 | Absolute change | Percent change | Absolute change | Percent change | |
| All opioids | 4.59 | 6.93 | 150.92 | 12.40 | 5.56 | 44.84 | 13.36 | 291.11 |
| Weak | 4.15 | 3.94 | 79.39 | 8.09 | 2.99 | 36.95 | 6.93 | 167.14 |
| Strong | 0.44 | 3.86 | 821.79 | 4.30 | 2.56 | 59.69 | 6.43 | 1453.81 |
| Short‐acting | 4.39 | 3.68 | 83.68 | 8.51 | 0.48 | 5.67 | 4.60 | 104.68 |
| Long‐acting | 0.20 | 3.25 | 1634.79 | 3.89 | 5.08 | 130.55 | 8.77 | 4406.03 |
| Buprenorphine | <0.01 | <0.01 | * | <0.01 | 0.59 | 607.21† | 0.59 | * |
| Codeine | 3.16 | 3.77 | 119.44 | 7.14 | 0.23 | 3.26 | 4.21 | 133.24 |
| Dextropropoxyphene | 0.99 | −0.57 | −57.29 | 0.38 | −0.21 | −55.54 | −0.82 | −82.78 |
| Fentanyl | — | 0.12 | — | 0.16 | 1.04 | 635.17 | 1.21 | — |
| Hydromorphone | — | 0.01 | — | 0.07 | 0.22 | 342.21 | 0.29 | — |
| Methadone | 0.09 | 1.70 | 1795.68 | 1.70 | −1.18 | −69.38 | 0.42 | 446.99 |
| Morphine | 0.14 | 1.58 | 1116.88 | 1.75 | −0.37 | −21.13 | 1.24 | 873.06 |
| Oxycodone | 0.16 | 0.23 | 142.36 | 0.60 | 2.29 | 381.81 | 2.73 | 1714.47 |
| Pethidine | 0.05 | −0.01 | −25.32 | 0.03 | −0.02 | −82.63 | −0.04 | −89.03 |
| Tramadol | — | 0.08 | — | 0.57 | 2.97 | 519.65 | 3.54 | — |
Percentage change not calculated due to very low use over the period.
Percentage change calculated from 2006–2011 due to very low use prior to 2006.
There was a 2.5‐fold increase in opioid dispensing between 1990 and 2000. This was primarily driven by a large percentage increase in the use of long‐acting opioids (especially methadone and long‐acting morphine formulations), although the absolute change in DDD/1000 pop/day was similar for both long‐ and short‐acting formulations. The 12‐fold increase in morphine dispensing between 1990 and 2000 occurred largely as a result of the subsidy of CR tablets in 1991 and SR capsules in 1993 (see Figure 2D). By 2000, these formulations together comprised almost three quarters of total morphine use. While oxycodone and buprenorphine also increased during this period, their use remained low. Dispensing of dextropropoxyphene remained relatively stable until a dramatic fall in dispensing between 1998 and 2000 (from 1.0 to 0.4 DDD/1000 pop/day); its relative popularity declined from 21.5% of total opioid use in 1990 to 9.6% in 1998, and further to 3.5% by 2000.
The growth in opioid dispensing slowed over the second half of the study period, from an 8.7% annual increase in dispensing during the 1990s to a 3.4% increase from 2001 to 2011. In 2001, the use of weak and short‐acting opioids was roughly double that of strong and long‐acting opioids respectively, and the three most dispensed opioids were codeine (57.6% of total opioid DDD/1000 pop/day), morphine (14.1%) and methadone (13.7%). However, there were marked increases in the use of tramadol, oxycodone, buprenorphine, fentanyl and hydromorphone between 2001 and 2011, as well as a shift towards a greater reliance on strong and long‐acting opioids. Increasing utilization also coincided temporally with the subsidy of various opioids for the treatment of noncancer pain (Figure 2).
Tramadol capsules and SR tablets received subsidy for moderate to severe pain in 2000 and 2001 respectively. Use of SR tablets subsequently grew rapidly (8.6‐fold in 2 years), such that by 2003 this formulation comprised over 80% of tramadol used. Similarly, the subsidy of oxycodone CR tablets for CNCP in 2000 preceded an eight‐fold increase in the use of this formulation between 2001 and 2011, from 0.3 to 2.3 DDD/1000 pop/day (78.8% of oxycodone utilization in 2011). However, use of CR tablets declined following the subsidy of oxycodone with naloxone long‐acting tablets for severe pain in late 2011; the latter increased from <0.1 DDD/1000 pop/day in 2011 to 0.9 DDD/1000 pop/day in 2014.
Hydromorphone was one of the lesser used opioids over the study period: its peak utilization occurred in 2011, when it comprised only 1.6% of total opioid dispensing (0.3 DDD/1000 pop/day). The most pronounced increase in hydromorphone use occurred following the subsidy of a long‐acting formulation for use in CNCP (157.6% increase between 2009 and 2011). Finally, tapentadol was subsidized for chronic severe disabling pain in June 2014; it comprised less than 1% of total 2014 opioid use.
The use of transdermal patch formulations increased approximately 15‐fold between 2001 and 2011. Fentanyl patches were subsidized for use in chronic cancer pain in 1999, precipitating a rise in fentanyl utilization from 0.1 DDD/1000 pop/day in 2000 to 0.3 DDD/1000 pop/day in 2005. In 2006, this listing was expanded to include the treatment of CNCP, leading to a further four‐fold increase in fentanyl patch use by 2011. Similarly, buprenorphine dispensing was negligible prior to the subsidy of the patch formulation for CNCP in 2005; by 2011, buprenorphine was dispensed at a rate of 0.6 DDD/1000 pop/day, 99.7% of which was for transdermal patches. Use of morphine and methadone generally declined over this period. Morphine dispensing peaked in 2004 at 1.9 DDDs/1000 pop/day (12.5% of total opioid utilization), declining to 1.4 DDD/1000 pop/day (7.7% of use) by 2011. Likewise, methadone use was highest in 2002 (1.9 DDD/1000 pop/day; 13.7% total opioid use), but declined to 0.5 DDD/1000 pop/day (2.9% of use) in 2011. Dextropropoxyphene and pethidine dispensing also declined between 2000 and 2011, with very low levels in utilization by the end of the study period (0.2 and <0.1 DDD/1000 pop/day respectively). These opioids are no longer subsidized in Australia (see Supporting Table S1 and Figure S2).
Opioid dispensing (number of prescriptions) by strength (dose)
Considering the most popular formulation of each opioid over the study period, the most dispensed strengths were 10 mg oxycodone CR tablets, 100 mg tramadol SR tablets, 10 mg morphine CR tablets, and 8 mg hydromorphone ER tablets. In all cases, these were the second lowest strengths available. Dispensing was low for the highest dose forms (with the exception of tramadol). The majority of prescriptions for fentanyl and buprenorphine patches were for the lowest strength formulations: the 5 μg h−1 and 10 μg h−1 strength buprenorphine patches each comprised almost 40% of total buprenorphine patch use, while the 12 μg h−1 and 25 μg h−1 fentanyl patches comprised 28.0% and 32.1% of fentanyl patch dispensing, respectively. The relative proportion of use comprised by each strength remained fairly stable over the study years, with no evidence of a shift from low‐dose to high‐dose formulations (Figure 4).
Figure 4.

Opioid dispensing (number of prescriptions) by prescription strength: (A) oxycodone controlled‐release (CR) tablet, (B) tramadol sustained‐release (SR) tablet, (C) morphine CR tablets, (D) buprenorphine transdermal (TD) patches, (E) fentanyl TD patches, (F) hydromorphone modified‐release (MR) tablets. Note the different scales between graphs
Discussion
This paper provides a comprehensive account of Australian trends in prescribed opioid analgesic dispensing. We report an almost four‐fold increase in opioid utilization over the study period, with particular growth in the use of strong and long‐acting opioids. Of note was the rise in morphine and methadone throughout the 1990s, and that of tramadol, oxycodone, buprenorphine, fentanyl and hydromorphone since the turn of the century. Codeine remained the dominant opioid for the entirety of the study period, in line with its established position as the first‐line opioid for pain in general, and as a continuing treatment for mild–moderate pain 31, 32. There was a profound shift in the use of other opioids, with prominence moving from dextropropoxyphene and morphine in 1990, to oxycodone, tramadol and fentanyl in 2014. The use of strong opioids increased at a far greater rate than weak opioids (although the absolute change was similar), reflecting findings from Scotland 33 and Canada 34. While morphine is still considered the strong opioid of choice in Australia due to its familiarity, cost and range of formulations 35, 36, its use declined over the last decade. Similar trends have been observed in the US 37, 38, although morphine remains popular and its use is increasing in Europe 14, 33.
In 2011, we report dispensing of opioid analgesics at a rate of 18 DDD/1000 pop/day (17.4 DDD/1000 pop/day in 2014); this is somewhat lower than rates in Canada (22 DDD/1000 pop/day in 2010) 34 and Scandinavia (approximately 20 DDD/1000 pop/day in 2006) 39, who are among the leading consumers of opioid analgesics globally. A recent report by the International Narcotics Control Board (INCB) ranked Australia eighth out of 179 countries on 2011–2013 opioid consumption measured by DDDs for statistical purposes (S‐DDD), substantially behind the US, Canada and Germany 15. However, levels of consumption in Australia were almost twice those in the UK, despite similar levels of consumption between the two nations in 2009–2011 15, 40.
Clearly, the increasing trends in opioid consumption in Australia have been driven, in the most part, by changes in the subsidy of individual opioid analgesics. The subsidy of long‐acting formulations of morphine (1991/1992), oxycodone (1999) and hydromorphone (2003) coincided with marked increases in the use of these medicines and likely contributed to the decline in the dispensing of methadone (also long‐acting) over the second half of the study period. Long‐acting formulations are now endorsed by most European and Australian guidelines as the preferred treatment for chronic pain (e.g. 36, 41, 42) as they are thought to provide better pain control and fewer side effects through the maintenance of stable blood concentrations 26. However, this claim of superiority is contested, and the US Food and Drug Administration now indicates the use of long‐acting opioids only for severe pain requiring daily, round‐the‐clock, long‐term treatment where non‐opioid analgesics or immediate‐release options are inadequate 43, 44.
The approval and subsidy of various opioids for the treatment of noncancer pain also brought significant growth, particularly in the case of buprenorphine (PBS‐listed for CNCP in 2005) and fentanyl (2006), but also oxycodone (from 2000) and hydromorphone (from 2003). However, the use of opioids in the treatment of CNCP is controversial. While randomized‐controlled trials have demonstrated efficacy of opioids for the short‐term treatment of chronic pain 44, 45, there is no high‐quality evidence for their long‐term efficacy 10, 46, 47. Despite this, treatment of CNCP accounts for almost 50% of opioid prescriptions written by Australian general practitioners 48, and 88% of strong opioids prescribed in UK primary care 14. The prevalence of pain has increased in Australia and globally over the last two decades 49, 50, 51, with a likely impact on opioid utilization. Between 1995 and 2008, the self‐reported prevalence of pain in Australia increased from 57% to 68%, and the prevalence of severe to very severe pain increased from 7% to 10% 52. However, our data show a doubling of opioid use in Australia during this period, which far exceeds the increase in pain prevalence. This may indicate overuse of opioids in the treatment of pain, although concerns about undertreatment have also been raised, particularly in cancer patients and the elderly 53, 54. Other contributors to the increase in opioid utilization may include the ageing population 55; improvements in pain management and physician education 8; recognition of the serious side effects of NSAIDs and Cox‐2 inhibitors 56; and growth in opioid misuse and diversion 22.
Strengths and limitations
This study has several advantages over previous studies of opioid use in Australia. The most extensive existing analyses (spanning from 1992–2012 and 1992–2007, respectively 20, 21) only include data on PBS‐subsidized medicine use; our analyses show that this accounts for less than 60% of total prescription opioid use in 1992, and approximately 82% of use in 2011. Additionally, previous studies including both subsidized and unsubsidized prescriptions cover restricted time periods and/or fewer medicines 17, 18, 19, 57, 58. Finally, variation between studies on measures of utilization (e.g. DDD/1000 pop/day, number of dispensings, sales) limits comparisons between analyses. Therefore, our study considers trends: over a 25‐year period, from 1990 to 2014; in both subsidized and unsubsidized dispensings (to 2011); in all prescription opioid analgesics over the study period; and by formulation, medicine strength and prescription type.
It should be noted that, at the time of writing, a study similar to our own is available online in accepted, unedited format 59. This paper examines trends in opioid use in Australia between 1992 and 2011 by DDD/1000 pop/day and prescriptions dispensed. We note significant departures from the results presented in this paper and other opioid data in the public domain 60. Most notably, the results based on DDD/1000 pop/day are overestimated by between two‐ and seven‐fold, depending on the opioid examined, and some results overestimate the number of dispensings by a factor of ten.
However, this study also has several limitations. The datasets employed provide measures of opioid utilization using dispensing claims, and therefore do not provide a direct measure of medicine consumption, nor an account of over‐the‐counter opioid use. Codeine is the only opioid available over‐the‐counter in Australia (restricted to doses <12 mg and ≤5 days’ supply) 61, thus our figures significantly underestimated total codeine use. The DUSC combined dataset, while providing near‐complete coverage of prescription medicine dispensing in Australia, also contains limited data on public hospital dispensings, and no data on private prescriptions from 2012. Our data show that private use accounts for 6.4% of prescription opioid utilization in 2011.
We used the DDD/1000 pop/day metric in this study due to its established position as the gold standard for measuring drug utilization in pharmacoepidemiological studies. Developed in the 1970s for drug utilization research, DDD allocation does not vary across countries or regions, thereby permitting national and international comparisons of medicine use 31. However, this metric has several limitations, particularly when used to quantify opioid use. The DDDs of many strong opioids were established when the drugs were initially listed for use in cancer pain. These doses are considerably higher than those used for the treatment of noncancer pain. Therefore DDD/1000 pop/day may not optimally represent the clinical dosing of opioids and underestimate the true utilization of strong opioids, particularly morphine, buprenorphine and fentanyl 60. Moreover, the observed increase in opioid use for CNCP between 1990 and 2014 means that the DDD/1000 pop/day metric will underestimate strong opioid use, relative to the use of weak opioids, to a greater degree as the study period progresses. In light of these limitations, recent studies from Australia 60, 62, the US 63, and the United Nations 64 have advocated the use of oral morphine equivalents (MEQ) as a secondary measure of opioid consumption as it accounts for medicine potency. However, use of MEQs in drug utilization research is relatively new, and comparisons between studies using this metric are currently limited due to the absence of universally accepted MEQ conversion factors.
Conclusions
Prescribed opioid analgesic dispensing increased markedly in Australia over the past 25 years, in line with international trends. These trends were driven primarily by the subsidy of long‐acting formulations and extension of subsidy to include the treatment of noncancer pain, demonstrating a profound impact of such policy changes on utilization patterns. Indeed, increasing use has occurred despite uncertainty surrounding the efficacy of opioids in CNCP and concerns about corresponding increases in opioid abuse and diversion. As a result of these concerns, a number of policy and systems‐level changes aimed at reducing opioid misuse and abuse have been employed in Australia and internationally. These diverse interventions include prescription drug monitoring programmes, clinician and patient education, the introduction of abuse‐deterrent formulations, changes to product labelling and treatment guidelines, and pain clinic and opioid disposal legislation 34, 61, 65, 66, 67, 68. However, the impact of these interventions on opioid utilization, abuse, and patient and clinician behaviours is uncertain 65, 69. From a clinical and policy perspective, there is a need for further research into patterns of opioid use and misuse, both at the level of the population and the individual. Indeed, few Australian studies have used person‐level data to explore opioid utilization patterns and outcomes at the level of the individual 70, 71, 72. Knowledge of rates of initiation and prevalence of opioid treatment, duration of therapy, prescribed daily doses and patterns of extra‐medical opioid use (such as excess dosing, and pharmacy/doctor shopping) is much needed and could facilitate targeted future interventions aimed at enhancing the quality use of opioids in the treatment of pain.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships that could appear to have influenced the submitted work. SP is a member of the Drug Utilisation Sub‐Committee (DUSC) of the Pharmaceutical Benefits Advisory Committee (PBAC). The views presented are those of the authors and do not reflect those of the PBAC.
We gratefully acknowledge Alicia Segrave, Chris Raymond and DUSC for access to the data and related support; Melisa Litchfield for data analysis and technical assistance; and Jonathan Brett for his helpful comments.
This study was funded by the National Health and Medical Research Council (NHMRC) Centre of Research Excellence in Medicines and Ageing (CREMA; ID: 1060407); SP is a Cancer Institute New South Wales Career Development Fellow (ID:12/CDF/2‐25); BB is funded by a University of Sydney Postgraduate Scholarship and CREMA Scholarship Top‐Up.
Contributors
SP, BB and EK conceived and designed the study; BB and EK acquired the data; EK supervised and assisted in the analysis; EK drafted the manuscript; EK, BB, NB, and SP contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content; all authors commented on drafts, approved the final manuscript, and can take responsibility for the integrity of the data and the accuracy of the data analysis; SP and EK are study guarantors.
Supporting information
Figure S1 Trends in the utilization of opioid analgesics in Australia from 1990 to 2014, by medicine formulation: (A) codeine, (B) oxycodone (note: ampoule and oxycodone + paracetamol + aspirin omitted due to low use), (C) tramadol (note: injection and oral drops omitted due to low use), (D) methadone, (E) morphine (note: sachet and combination products with tacrine and aspirin omitted due to low use), (F) fentanyl, (G) buprenorphine, (H) hydromorphone. Note the different scales between figures
Figure S2 Trends in the utilization of (A) dextropropoxyphene and (B) pethidine in Australia from 1990 to 2014. Note the different scales for (A) and (B). Key subsidy and regulatory changes are indicated numerically and in Table S1
Table S1 Chronological listing of changes to opioid regulation and subsidy, 1990–2014. Reference numbers refer to events marked in Figures [Link], [Link]
Supporting info item
Supporting info item
Supporting info item
Karanges, E. A. , Blanch, B. , Buckley, N. A. , and Pearson, S. ‐A. (2016) Twenty‐five years of prescription opioid use in Australia: a whole‐of‐population analysis using pharmaceutical claims. Br J Clin Pharmacol, 82: 255–267. doi: 10.1111/bcp.12937.
References
- 1. Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2013; 380: 2163–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lalonde L, Choinière M, Martin É, Berbiche D, Perreault S, Lussier D. Costs of moderate to severe chronic pain in primary care patients – a study of the ACCORD Program. J Pain Res 2014; 7: 389–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reid KJ, Harker J, Bala MM, Truyers C, Kellen E, Bekkering GE, et al. Epidemiology of chronic non‐cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin 2011; 27: 449–62. [DOI] [PubMed] [Google Scholar]
- 4. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006; 10: 287–333. [DOI] [PubMed] [Google Scholar]
- 5. Ballantyne JC, Shin NS. Efficacy of opioids for chronic pain: a review of the evidence. Clin J Pain 2008; 24: 469–78. [DOI] [PubMed] [Google Scholar]
- 6. Michna E, Cheng WY, Korves C, Birnbaum H, Andrews R, Zhou Z, et al. Systematic literature review and meta‐analysis of the efficacy and safety of prescription opioids, including abuse‐deterrent formulations, in non‐cancer pain management. Pain Med 2014; 15: 79–92. [DOI] [PubMed] [Google Scholar]
- 7. Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, Cherny N, et al. Use of opioid analgesics in the treatment of cancer pain: evidence‐based recommendations from the EAPC. Lancet Oncol 2012; 13: e58–68. [DOI] [PubMed] [Google Scholar]
- 8. Melnikova I. Pain market. Nat Rev Drug Discov 2010; 9: 589–90. [DOI] [PubMed] [Google Scholar]
- 9. Marcus DA. Epidemiology of cancer pain. Curr Pain Headache Rep 2011; 15: 231–4. [DOI] [PubMed] [Google Scholar]
- 10. Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, et al. The effectiveness and risks of long‐term opioid therapy for chronic pain: a systematic review for a National Institutes of Health pathways to prevention workshop. Ann Intern Med 2015; 162: 276–86. [DOI] [PubMed] [Google Scholar]
- 11. Breivik H. Opioids in chronic non‐cancer pain, indications and controversies. Eur J Pain 2005; 9: 127–30. [DOI] [PubMed] [Google Scholar]
- 12. Sullivan MD, Howe CQ. Opioid therapy for chronic pain in the United States: promises and perils. Pain 2013; 154: S94–S100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frenk SM, Porter KS, Paulozzi LL. Prescription opioid analgesic use among adults: United States, 1999–2012. NCHS data brief, no. 189. Hyattsville, MD: National Centre for Health Statistics, 2015. [PubMed] [Google Scholar]
- 14. Zin C, Chen L‐C, Knaggs R. Changes in trends and pattern of strong opioid prescribing in primary care. Eur J Pain 2014; 18: 1343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. International Narcotics Control Board (INCB) . Report 2014 – Narcotic drugs: estimated world requirements for 2015; statistics for 2013. New York: United Nations, 2015.
- 16. Pain and Policy Studies Group . Opioid consumption motion chart. Madison: University of Wisconsin‐Madison, 2015. Available at https://ppsg.medicine.wisc.edu/chart (last accessed 4 September 2015). [Google Scholar]
- 17. Hollingworth SA, Gray PD, Hall WD, Najman JM. Opioid analgesic prescribing in Australia: a focus on gender and age. Pharmacoepidemiol Drug Saf 2015; 24: 628–36. [DOI] [PubMed] [Google Scholar]
- 18. Roxburgh A, Burns L, Drummer OH, Pilgrim J, Farrell M, Degenhardt L. Trends in fentanyl prescriptions and fentanyl‐related mortality in Australia. Drug Alcohol Rev 2013; 32: 269–75. [DOI] [PubMed] [Google Scholar]
- 19. Roxburgh A, Bruno R, Larance B, Burns L. Prescription of opioid analgesics and related harms in Australia. Med J Aust 2011; 195: 280–4. [DOI] [PubMed] [Google Scholar]
- 20. Leong M, Murnion B, Haber P. Examination of opioid prescribing in Australia from 1992 to 2007. Intern Med J 2009; 39: 676–81. [DOI] [PubMed] [Google Scholar]
- 21. Blanch B, Pearson S‐A, Haber PS. An overview of the patterns of prescription opioid use, costs and related harms in Australia. Br J Clin Pharmacol 2014; 78: 1159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Atluri S, Sudarshan G, Manchikanti L. Assessment of the trends in medical use and misuse of opioid analgesics from 2004 to 2011. Pain Physician 2014; 17: e119–28. [PubMed] [Google Scholar]
- 23. Mellish L, Karanges EA, Litchfield MJ, Schaffer AL, Blanch B, Daniels BJ, et al. The Australian Pharmaceutical Benefits Scheme data collection: a practical guide for researchers. BMC Res Notes 2015; 8: 634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Australian Government Department of Health . Australian Statistics on Medicines 2011. Canberra: Commonwealth of Australia, 2013. [Google Scholar]
- 25. Australian Government Department of Health . Report on the collection of under co‐payment data 2013–2014. Canberra, 2014. Available at http://www.pbs.gov.au/info/statistics/under‐co‐payment/ucp‐data‐report (last accessed 5 May 2015).
- 26. Royal Australasian College of Physicians . Prescription opioid policy: improving the management of chronic non‐malignant pain and prevention of problems associated with prescription opioid use. Sydney: RACP, 2008. [Google Scholar]
- 27. WHO Collaborating Centre for Drug Statistics Methodology . Guidelines for ATC classification and DDD assignment, 2014. Oslo: World Health Organization, 2015. [Google Scholar]
- 28. Australian Bureau of Statistics . Australian Historical Population Statistics, 2014; table, Cat. no. 3105.0.65.001. Canberra: Australian Bureau of Statistics, 2014.
- 29. Australian Government Department of Health . PBS Publications archive. Canberra, 2015. Available at http://www.pbs.gov.au/info/publication/schedule/archive (last accessed 16 September 2015).
- 30. Pearson SA, Pesa N, Langton JM, Drew A, Faedo M, Robertson J. Studies using Australia's Pharmaceutical Benefits Scheme data for pharmacoepidemiological research: a systematic review of the published literature (1987–2013). Pharmacoepidemiol Drug Saf 2015; 24: 447–55. [DOI] [PubMed] [Google Scholar]
- 31. Manchikanti L, Abdi S, Atluri S, Balog CC, Benyamin RM, Boswell MV, et al. American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non‐cancer pain: Part 2 – guidance. Pain Physician 2012; 15: S67–116. [PubMed] [Google Scholar]
- 32. Cheung CW, Qiu Q, Choi S‐W, Moore B, Goucke R, Irwin M. Chronic opioid therapy for chronic non‐cancer pain: a review and comparison of treatment guidelines. Pain Physician 2014; 17: 401–14. [PubMed] [Google Scholar]
- 33. Ruscitto A, Smith B, Guthrie B. Changes in opioid and other analgesic use 1995–2010: Repeated cross‐sectional analysis of dispensed prescribing for a large geographical population in Scotland. Eur J Pain 2015; 19: 59–66. [DOI] [PubMed] [Google Scholar]
- 34. Fischer B, Jones W, Rehm J. Trends and changes in prescription opioid analgesic dispensing in Canada 2005–2012: an update with a focus on recent interventions. BMC Health Serv Res 2014; 14: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. National Prescribing Service . Analgesic options for pain relief. MedicineWise News 2006; 47.
- 36. Australian Medicines Handbook 2015. Adelaide: Australian Medicines Handbook Pty Ltd, 2015. [Google Scholar]
- 37. Kenan K, Mack K, Paulozzi L. Trends in prescriptions for oxycodone and other commonly used opioids in the United States, 2000–2010. Open Med 2012; 6: e41. [PMC free article] [PubMed] [Google Scholar]
- 38. Steinman MA, Komaiko KD, Fung KZ, Ritchie CS. Use of opioids and other analgesics by older adults in the United States, 1999–2010. Pain Med 2014; 16: 319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hamunen K, Paakkari P, Kalso E. Trends in opioid consumption in the Nordic countries 2002–2006. Eur J Pain 2009; 13: 954–62. [DOI] [PubMed] [Google Scholar]
- 40. International Narcotics Control Board (INCB) . Report 2013 – Narcotic drugs: estimated world requirements for 2014; statistics for 2012. New York: United Nations, 2014.
- 41. British Pain Society, Faculty of Pain Medicine of the Royal College of Anaesthetists, Royal College of General Practitioners, Faculty of Addictions of the Royal College of Psychiatrists . Opioids for persistent pain. Summary on guidance on good practice from the British Pain Society. London: British Pain Society, 2010. [Google Scholar]
- 42. Analgesic Expert Group . Therapeutic Guidelines: Analgesic. Version 6. Melbourne: Therapeutic Guidelines Ltd, 2012. [Google Scholar]
- 43. Sullivan M. Will data destroy our faith in long‐acting opioids? Pain 2014; 155: 843–4. [DOI] [PubMed] [Google Scholar]
- 44. US Food and Drug Administration . FDA announces safety labeling changes and postmarket study requirements for extended‐release and long‐acting opioid analgesics. Press release, 2013. Available at http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm367726.htm (last accessed 16 September 2015).
- 45. Furlan AD, Sandoval JA, Mailis‐Gagnon A, Tunks E. Opioids for chronic noncancer pain: a meta‐analysis of effectiveness and side effects. Can Med Assoc J 2006; 174: 1589–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Noble M, Treadwell JR, Tregear SJ, Coates VH, Wiffen PJ, Akafomo C, et al. Long‐term opioid management for chronic noncancer pain. Cochrane Database Syst Rev 2010; 1: CD006605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chaparro LE, Furlan AD, Deshpande A, Mailis‐Gagnon A, Atlas S, Turk DC. Opioids compared to placebo or other treatments for chronic low‐back pain. Cochrane Database Syst Rev 2013; 8: CD004959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Harrison CM, Charles J, Henderson J, Britt H. Opioid prescribing in Australian general practice. Med J Aust 2012; 196: 380–1. [DOI] [PubMed] [Google Scholar]
- 49. Hoy D, Bain C, Williams G, March L, Brooks P, Blyth F, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum 2012; 64: 2028–37. [DOI] [PubMed] [Google Scholar]
- 50. Britt H, Miller G, Henderson J, Bayram C, Valenti L, Harrison C, et al. A decade of Australian general practice activity 2004–05 to 2013–14. Sydney: Sydney University Press, 2014. [Google Scholar]
- 51. Croft P, Blyth FM, van der Windt D. Chronic pain epidemiology: from aetiology to public health. New York: Oxford University Press, 2010. [Google Scholar]
- 52. Australian Bureau of Statistics . Characteristics of bodily pain. Canberra, 2012. Available at http://www.abs.gov.au/ausstats/abs@.nsf/Lookup/4841.0Chapter12011 (last accessed 8 September 2015).
- 53. Deandrea S, Montanari M, Moja L, Apolone G. Prevalence of undertreatment in cancer pain: a review of published literature. Ann Oncol 2008; 19: 1985–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Auret K, Schug SA. Underutilisation of opioids in elderly patients with chronic pain. Drugs Aging 2005; 22: 641–54. [DOI] [PubMed] [Google Scholar]
- 55. Campbell CI, Weisner C, LeResche L, Ray GT, Saunders K, Sullivan MD, et al. Age and gender trends in long‐term opioid analgesic use for noncancer pain. Am J Public Health 2010; 100: 2541–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Makris UE, Abrams RC, Gurland B, Reid MC. Management of persistent pain in the older patient: a clinical review. JAMA 2014; 312: 825–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hollingworth SA, Symons M, Khatun M, Loveday B, Ballantyne S, Hall WD, et al. Prescribing databases can be used to monitor trends in opioid analgesic prescribing in Australia. Aust N Z J Public Health 2013; 37: 132–8. [DOI] [PubMed] [Google Scholar]
- 58. National Drug and Alcohol Research Centre . A review of opioid prescribing in Tasmania: a blueprint for the future. Sydney: University of New South Wales, 2012. [Google Scholar]
- 59. Islam MM, McRae IS, Mazumdar S, Taplin S, McKetin R. Prescription opioid analgesics for pain management in Australia: twenty years of dispensing. Intern Med J 2015. doi:10.1111/imj.12966. [DOI] [PubMed] [Google Scholar]
- 60. Drug Utilisation Sub‐Committee (DUSC) . Opioid analgesics: overview. Canberra: Australian Government Department of Health, 2014. [Google Scholar]
- 61. Tobin CL, Dobbin M, McAvoy B. Regulatory responses to over‐the‐counter codeine analgesic misuse in Australia, New Zealand and the United Kingdom. Aust N Z J Public Health 2013; 37: 483–8. [DOI] [PubMed] [Google Scholar]
- 62. Nielsen S, Degenhardt L, Hoban B, Gisev N. Comparing opioids: a guide to estimating oral morphine equivalents (OME) in research. Sydney: National Drug and Alcohol Research Centre, 2014. [Google Scholar]
- 63. Hastie BA, Gilson AM, Maurer MA, Cleary JF. An examination of global and regional opioid consumption trends 1980–2011. J Pain Palliat Care Pharmacother 2014; 28: 259–75. [DOI] [PubMed] [Google Scholar]
- 64. Svendsen K, Borchgrevink P, Fredheim O, Hamunen K, Mellbye A, Dale O. Choosing the unit of measurement counts: the use of oral morphine equivalents in studies of opioid consumption is a useful addition to defined daily doses. Palliat Med 2011; 25: 725–32. [DOI] [PubMed] [Google Scholar]
- 65. Haegerich TM, Paulozzi LJ, Manns BJ, Jones CM. What we know, and don't know, about the impact of state policy and systems‐level interventions on prescription drug overdose. Drug Alcohol Depend 2014; 145: 34–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Weisberg DF, Becker WC, Fiellin DA, Stannard C. Prescription opioid misuse in the United States and the United Kingdom: cautionary lessons. Int J Drug Policy 2014; 25: 1124–30. [DOI] [PubMed] [Google Scholar]
- 67. Pharmacy Guild of Australia . Oxycontin reformulation – effective 1 April 2014. Canberra, 2014. Available at https://www.guild.org.au/news‐page/2014/03/20/oxycontin‐reformulation–effective‐1‐april‐2014 (last accessed 5 August 2015).
- 68. Shand FL, Campbell G, Hall W, Lintzeris N, Cohen M, Degenhardt L. Real‐time monitoring of Schedule 8 medicines in Australia: evaluation is essential. Med J Aust 2013; 198: 80–1. [DOI] [PubMed] [Google Scholar]
- 69. Schatman ME, Webster LR. The health insurance industry: perpetuating the opioid crisis through policies of cost‐containment and profitability. J Pain Res 2015; 8: 153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Campbell G, Nielsen S, Bruno R, Lintzeris N, Cohen M, Hall W, et al. The Pain and Opioids IN Treatment study: characteristics of a cohort using opioids to manage chronic non‐cancer pain. Pain 2015; 156: 231–42. [DOI] [PubMed] [Google Scholar]
- 71. Rogers KD, Kemp A, McLachlan AJ, Blyth F. Adverse selection? A multi‐dimensional profile of people dispensed opioid analgesics for persistent non‐cancer pain. PLoS One 2013; 8: e80095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gadzhanova S, Bell JS, Roughead EE. What analgesics do older people use prior to initiating oxycodone for non‐cancer pain? A retrospective database study. Drugs Aging 2013; 30: 921–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Trends in the utilization of opioid analgesics in Australia from 1990 to 2014, by medicine formulation: (A) codeine, (B) oxycodone (note: ampoule and oxycodone + paracetamol + aspirin omitted due to low use), (C) tramadol (note: injection and oral drops omitted due to low use), (D) methadone, (E) morphine (note: sachet and combination products with tacrine and aspirin omitted due to low use), (F) fentanyl, (G) buprenorphine, (H) hydromorphone. Note the different scales between figures
Figure S2 Trends in the utilization of (A) dextropropoxyphene and (B) pethidine in Australia from 1990 to 2014. Note the different scales for (A) and (B). Key subsidy and regulatory changes are indicated numerically and in Table S1
Table S1 Chronological listing of changes to opioid regulation and subsidy, 1990–2014. Reference numbers refer to events marked in Figures [Link], [Link]
Supporting info item
Supporting info item
Supporting info item


