Abstract
Aim
Drug‐induced liver injury is one of the most serious adverse drug reactions and the most frequent reason for restriction of indications or withdrawal of drugs. Some nonsteroidal anti‐inflammatory drugs (NSAIDs) were withdrawn from the market because of serious hepatotoxicity. We estimated the risk of acute and serious liver injury associated with the use of nimesulide and other NSAIDs, with a prevalence of use greater than or equal to 5%.
Methods
This is a multicentre case–control study carried out in nine Italian hospitals from October 2010 to January 2014. Cases were adults, with a diagnosis of acute liver injury. Controls presented acute clinical disorders not related to chronic conditions, not involving the liver. Adjusted odds ratio (ORs) with 95% confidence interval (CI) were calculated initially with a bivariate and then multivariate analysis.
Results
We included 179 cases matched to 1770 controls. Adjusted OR for acute serious liver injury associated with all NSAIDs was 1.69, 95% CI 1.21–2.37. Thirty cases were exposed to nimesulide (adjusted OR 2.10, 95% CI 1.28–3.47); the risk increased according to the length of exposure (OR > 30 days: 12.55, 95% CI 1.73–90.88) and to higher doses (OR 10.69, 95% CI 4.02–28.44). Risk of hepatotoxicity was increased also for ibuprofen, used both at recommended dosages (OR 1.92, 95% CI 1.13–3.26) and at higher doses (OR 3.73, 95% CI 1.11–12.46) and for ketoprofen ≥ 150 mg (OR 4.65, 95% CI 1.33–10.00).
Conclusion
Among all NSAIDs, nimesulide is associated with the higher risk, ibuprofen and high doses of ketoprofen are also associated with a modestly increased risk of hepatotoxicity.
Keywords: case–control study, DILI, liver injury, nimesulide, NSAIDs
What is Already Known about this Subject
Drug‐induced liver injury is one of the most serious adverse drug reactions and the most frequent reason for failure of approval, restriction of indications or withdrawal of drugs.
Some NSAIDs were withdrawn from the market because of serious hepatotoxicity.
Nimesulide is an NSAID suspended from the market in several countries because of high frequency of hepatotoxicity.
What this Study Adds
These findings add new elements to the available data on incidence of drug‐induced hepatotoxicity.
There is an association between the use of some NSAIDs and risk of acute serious liver injury in Italian patients.
Among commonly used anti‐inflammatory drugs, nimesulide is associated with a slight increase of risk, which raises with time exposure and dosage. For ibuprofen and high dosage of ketoprofen, an increased risk was also found.
Introduction
Drug‐induced liver injury (DILI) is a condition that can symptomatically mimic most kinds of acute and chronic liver pathologies and is the most common cause of acute liver failure both in the USA 1, 2 and in Europe 3, 4. DILI is considered among the most serious adverse drug reactions (ADRs) and represents the main cause of discontinuing the development of new drugs at early stages and the most frequent reason for refusal to approve, restriction of indications or withdrawal of drugs by regulatory agencies 5, 6, 7.
Since the diagnosis of this condition is not simple and pre‐marketing studies are unable to detect all possible hepatic ADRs, little is known about the incidence of DILI in the general population and the available data come from spontaneous reports and few epidemiologic studies 8. Björnsson and colleagues defined the incidence of DILI by prospectively examining a population‐based cohort in Iceland: 96 cases of DILI were identified between 2010 and 2011, with an annual incidence of 19.1 cases per 100 000 inhabitants (95% CI, 1.54–23.3) 9. Another French population‐based study reported an incidence of 13.9 cases per 100 000 inhabitants per year, with a 6% mortality rate 10. Two studies from Sweden and the UK, in contrast, found a lower incidence, that is 2.3 and 2.4 per 100 000 inhabitants per year respectively 11, 12. Liver injury can be associated with several drug classes, most commonly antibiotics, antifungal, antituberculosis, antiepileptic and NSAIDs 13.
NSAIDs represent one of the most widely used drug classes in the world and the most commonly used analgesics 14. The major adverse effects of NSAIDs are gastrointestinal, cardiovascular and renal injuries, which are well documented 15, 16; hepatotoxicity is a rare ADR, usually not dose related, but serious and even fatal 15, 17, 18, 19, 20, 21.
Several NSAIDs were withdrawn from the market because of hepatic ADRs (bromfenac, ibufenac, benoxaprofen, droxicam, pirprofen, fenclofenac and, more recently, lumiracoxib); others, such as nimesulide, were never marketed in some countries or withdrawn in others. In 2002 Finland and Spain suspended the marketing of this drug because of the high frequency of hepatotoxicity 22, 23. Consequently, the European Medicines Agency (EMA) started an evaluation procedure on nimesulide safety and in 2004 it concluded that the benefit–risk profile of this drug was favourable. However, the EMA's Committee for Medicinal Products for Human Use (CHMP) recommended restriction of nimesulide indications to the treatment of acute pain, symptomatic treatment of painful osteoarthritis and primary dysmenorrhea, and withdrawal from the market of the 200 mg pharmaceutical formulation, restricting the maximal drug dosage at 100 mg twice a day; nimesulide was also contraindicated in children under 12 years of age 24. In May 2007 the Irish medicines regulatory authority decided to suspend the marketing authorization for systemic nimesulide‐containing medicines owing to new information regarding cases of fulminant hepatic failure requiring liver transplantation, which led the EMA to a further review process 25. Also in this case the risk–benefit balance of nimesulide remained positive, although the EMA restricted the duration of therapy to 15 days, and recommended the drug only as a second‐line treatment. Two years later, in 2009, nimesulide was withdrawn from the market in Argentina as well 26. In 2012 the EMA completed a review of the safety and effectiveness of systemic medicines containing nimesulide and concluded that, compared with other NSAIDs, nimesulide was associated with an increased risk of liver toxicity and its risk–benefit ratio remained positive for its use in acute pain and dysmenorrhea but not for osteoarthritis 27. An important contribution to the debate on the hepatotoxicity of nimesulide was provided by the Italian study published by Traversa and colleagues, which concluded that although the risk of liver injury in patients taking nimesulide and other NSAIDs is small, such risk is higher for nimesulide 28. Another study, conducted in Italy in 2010, showed that NSAIDs were more commonly associated with DILI and 70% of the observed cases were attributable to nimesulide 29. The aim of the Drug‐Induced Liver Injury in Italy (DILI‐IT) study was to estimate the risk of acute and serious liver injury associated with the use of nimesulide and other NSAIDs with a prevalence of use greater than or equal to 5% of the total number of drugs taken by the study population. The hepatotoxic risk associated with paracetamol was assessed as well.
Furthermore, the study assessed the risk of acute and serious liver injury induced by amoxicillin and amoxicillin with clavulanic acid, macrolides, antidepressants and statins.
Here, we present the results of the main analysis on the risk of liver injury associated to nimesulide and NSAIDs.
Methods
Study population
We conducted a multicentre case–control study where cases and controls were all recruited among hospital patients in Italy, from October 2010 to January 2014.
Nine hospitals located in four regions representative of the North (Veneto and Emilia Romagna), Central (Tuscany) and South (Campania) of Italy participated in the study; the population covered by the hospitals involved is approximately 4938 700 inhabitants (8.3% of the Italian population). Patients eligible were those visited by hospital physicians participating in the study.
Cases were defined as all patients aged 18 or over admitted with a primary diagnosis of acute liver injury. According to the definition of DILI 13 and in accordance with the hepatologists involved in the study, we used the following criteria for case definition: (1) increase of 2 N (N is the upper limit of normal range and each activity is expressed as a multiple of N) for alanine aminotransferase (ALT), aspartate aminotransferase (AST) serum activity in patients who presented symptoms or not; (2) increase of 1.5 N of alkaline phosphatase (AP) associated with an increase of ALT or AST and/or total bilirubin in patients with or without symptoms.
Ten hospital controls were selected consecutively for each case, matched according to gender and age (+/− 5 years), and to hospital and time from admission (within 2 months).
Controls were patients with a minimum age of 18 years, admitted for acute clinical disorders not related to chronic conditions, not involving the liver (with normal liver enzyme values) and without specific indications or contraindications for NSAID use. The selected admission diagnoses were: non‐alcohol‐related trauma or fracture, acute appendicitis, bowel obstruction, intestinal perforation, acute pancreatitis, pneumonia in patients without risk factors, pneumothorax without previous chronic obstructive bronchitis or chronic obstructive pulmonary disease, renal colic, euthyroid nodule, bite, accidental injuries or burns, foreign bodies, abdominal gestation/fallopian tube rupture or miscarriage, testicular torsion and umbilical hernia.
Both cases and controls were excluded if the patients had a diagnosis of viral hepatitis, biliary abnormality, history of alcohol abuse, autoimmune disease, genetic and metabolic disorders which may determine liver injury, a low alpha‐1‐antitrypsin level and an abnormal phenotype (that may suggest disease associated with a deficiency of this protein), Wilson's disease, HIV/AIDS, hepatic neoplasia or liver metastasis, systemic lupus erythematosus, mushroom poisoning and drug addiction or detoxification treatment in the last 3 months; finally, patients who were not resident in the study areas were also excluded (primary exclusion criteria). Patients who were discharged or died before interview and those refusing the interview or unable to answer were also excluded (secondary exclusion criteria). A panel of experts (External Advisory Board) was established to monitor the appropriateness of inclusion and exclusion criteria for cases and controls; they represented also an important support in the debate about the problems that emerged during patient recruitment.
Exposure to drugs
Exposure to drugs was defined as any use in the 90 days prior to the index day (ID) 30. For each case, the ID was considered as the onset day of the liver damage symptoms or the date corresponding to the first available abnormal value of liver enzyme tests. For controls, the ID was taken as the onset day of any symptom relating to the disease for which the patient was selected as a control.
For NSAIDs with a prevalence of use >5% and for paracetamol, different periods of use/non‐use were compared and cumulative time of exposure was defined as less than or equal to 15 days, between 15 and 30 days and greater than 30 days. Moreover, the average daily dose of each NSAID and paracetamol was calculated for each patient and two dose categories were considered on the basis of the corresponding DDD (defined daily dose) 31. The number of exposed patients according to time and dose categories was calculated.
Information retrieval
Drug exposure was investigated by trained monitors by face‐to‐face interview using a standardized questionnaire (Case Report Form, CRF). Before the interview, the aim of the study was explained to patients and written informed consent was obtained. The CRF covered information regarding demographic data, medical history, coexisting illnesses, lifestyle and dietary habits, alcohol, tobacco and coffee intake and use of herbal products. Data concerning the diagnosis of liver injury and the evolution of the disease were collected from medical records. Clinical records were also used to evaluate the diagnoses of the controls.
To ensure that drug history was as complete as possible and to reduce recall bias, after an open question about previous use of drugs, patients were questioned about a list of common symptoms often prompting use of medicines of interest. Patients were also shown a photographic collection reproducing the packaging of main medicinal products concerned (most used NSAIDs, amoxicillin and amoxicillin with clavulanic acid, macrolides, antidepressants and statins).
Statistical analysis
Data are presented as mean values (SD) and frequencies (%). Odds ratios (OR) and their 95% confidence intervals (95% CI) have been calculated by means of a conditional logistic regression for matched case–control groups, using the Stata Statistical Software version 11.0 (StataCorp, College Station, TX, 2009). OR has been computed initially with a bivariate and then multivariate analysis, to assure that the risk estimates for liver injury and use of drugs were appropriately adjusted for possible confounders and effect modifiers. Covariates included were: smoking, alcohol, body mass index (BMI), liver comorbidities (hepatitis, gallstones, liver cirrhosis, hepatic nodules and other hepatic diseases), heart comorbidities (stroke, angina pectoris, heart failure, arrhythmia and cardiac surgery), and coprescribed drugs (other NSAIDs, paracetamol, amoxicillin, amoxicillin/clavulanic acid, macrolides, antidepressants and statins). There was a negligible amount of missing data (all found on control group) for: BMI (less than 1%; 11 subjects out of 1770), alcohol consumption (less than 1%; 3 subjects out of 1770), and smoking (less than 1%; 2 subjects out of 1770). Therefore no methods to account for missing data have been used and complete case analyses have been performed. The effect of gender and age on risk of liver injury among NSAID users have been evaluated using an unconditional logistic regression.
The sample size for the first primary objective (relative risk of nimesulide‐induced liver injury) was estimated assuming a minimal detectable risk (OR) of 2.0 with alpha 0.05 and at a power of 80%, and a prevalence of nimesulide use of 8%. Given these assumptions, 163 cases and 1630 controls (matched case–control ratio 1:10) were required. This sample size was considered adequate even with a slightly different prevalence in the control population (range: 5–10%): this would detect an odds ratio ranging from 2.28 (alpha 0.05; power: 80%) for a 5% prevalence to an odds ratio of 1.91 (alpha 0.05; power: 80%) for a 10% prevalence. The latter proportion has been found (184 nimesulide users among 1770 matched controls) in the present study. The Pass 11 statistical software, version 11.0.7, was used for these calculations.
Ethical considerations
The study was approved by the Independent Ethics Committee (IEC) of Verona Hospital (principal investigator and coordinating centre) and consecutively notified to the IECs of participating centres.
Results
Population
Overall, 2232 patients with diagnosis of acute serious liver injury were recorded; of those, 2028 were not included in the study, according to the primary exclusion criteria. The 204 incident cases resulted in an annual incidence of DILI of 4.1 cases per 100 000 inhabitants. Subsequently, 25 other cases were excluded on the basis of secondary criteria. Therefore, the analysis was performed on 179 cases. In the same period, 3059 patients were selected and interviewed as possible controls. From this sample, 1770 patients were matched to the 179 cases, according to gender, age, centre and time from admission (Figure 1). Controls included in the analysis were enrolled for the following diagnosis: trauma or fracture (46%), acute appendicitis (15%), gastrointestinal disorders (14%), respiratory disorders (13%) and other acute events (12%).
Figure 1.
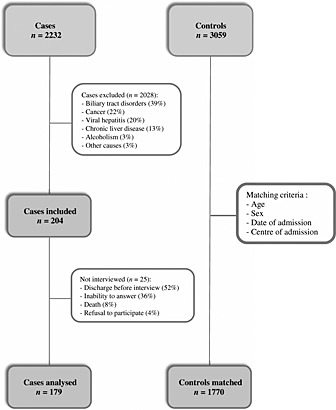
Flow diagram of patients included in the study
Table 1 shows the baseline characteristics of recruited patients. Women were slightly more represented than men (58% vs 42%). Controls were found to be slightly more overweight compared to cases and they consumed alcohol (as current drinkers) and tobacco (as former smokers) more frequently than cases. Again, in a slightly greater number of cases compared to controls liver and heart co‐morbidities were observed. Cases used more drugs than controls (with an average number of drugs of 5.0 and 2.6 respectively). Nevertheless, except for drug consumption, there were no significant differences between cases and controls. The profile of cases and controls utilizing NSAIDs did not differ from that of the whole study population.
Table 1.
Baseline characteristics of patients in study population and in NSAID users
| Study population | NSAID users | |||||
|---|---|---|---|---|---|---|
| Characteristic | Cases n = 179 | Controls n = 1770 | P value | Cases NSAIDs n = 97 | Controls NSAIDs n = 737 | P value NSAIDs |
| Sex, n (%) | — | — | ||||
| Male | 75 (42) | 750 (42) | 43 (44) | 306 (42) | ||
| Female | 104 (58) | 1020 (58) | 54 (56) | 431 (58) | ||
| Age (years), mean (SD) | 52.8 (19.4) | 53.3 (19.3) | 0.74 | 49.4 (18.7) | 51.6 (19.4) | 0.29 |
| Schooling level (years), mean (SD) | 10.5 (4.8) (n = 174) | 10.5 (5.2) (n = 1721) | 0.86 | 10.6 (4.5) (n = 96) | 10.7 (4.7) (n = 714) | 0.84 |
| BMI, mean (SD) | 24.8 (4.6) | 25.3 (4.4) (n = 1759) | 0.15 | 25.0 (5.1) | 25.3 (4.7) | 0.56 |
| Alcohol, n (%) | 0.58 | 0.62 | ||||
| Current drinker | 102 (57) | 1072 (61) | 59 (61) | 481 (65) | ||
| Former drinker | 8 (4) | 67 (4) | 3 (3) | 26 (4) | ||
| Non‐drinker | 69 (39) | 628 (35) | 35 (36) | 227 (31) | ||
| Smoke, n (%) | 0.59 | 0.38 | ||||
| Current smoker | 45 (25) | 445 (25) | 32 (33) | 202 (27) | ||
| Former smoker | 26 (15) | 311 (18) | 14 (14) | 141 (19) | ||
| Non‐smoker | 108 (60) | 1012 (57) | 51 (53) | 392 (53) | ||
| Co‐morbidities, n (%) | ||||||
| Liver diseases | 19 (10) | 146 (8) | 0.26 | 15 (15) | 99 (13) | 0.64 |
| Heart diseases | 30 (17) | 237 (13) | 0.21 | 17 (17) | 140 (19) | 0.78 |
| Drugs, mean (SD) | 5.0 (3.2) | 2.6 (2.4) | <0.001 | 5.1 (3.0) | 3.4 (2.3) | <0.001 |
Risk of acute liver injury related to NSAIDs and nimesulide
Table 2 shows the OR estimates of acute serious liver injury associated with all NSAIDs: during the three months before the index day, 97 out of 179 cases (corresponding to 54%) and 737 out of 1770 controls (42%) were exposed to NSAIDs. The annual incidence of DILI induced by NSAIDs (N‐DILI) was two cases per 100 000 inhabitants and the OR, adjusted for all covariates considered in the analysis, was 1.69 (95% CI, 1.21–2.37).
Table 2.
Odds Ratios (OR) and 95% confidence intervals (95% CI) of acute serious liver injury associated with NSAIDs (sex and age related analysis)
| Drug | No. (%) of cases | No. (%) of controls | OR (95% CI) | OR Adg (95% CI) † |
|---|---|---|---|---|
| NSAIDs * | 97 (54) | 737 (42) | 1.71 (1.23–2.36) | 1.69 (1.21–2.37) |
| Sex | ||||
| Male | 43 (44) | 306 (42) | 1 | 1 |
| Female | 54 (56) | 431 (58) | 0.89 (0.57–1.40) | 0.71 (0.42–1.19) |
| Age | ||||
| <45 | 46 (47) | 293 (40) | 1 | 1 |
| 45–65 | 29 (30) | 225 (30) | 0.82 (0.48–1.38) | 1.21 (0.66–2.20) |
| >65 | 22 (23) | 219 (30) | 0.64 (0.36–1.12) | 0.75 (0.36–1.56) |
NSAIDs with at least one case included nimesulide, acetylsalicylic acid (ASA), ibuprofen, ketoprofen, diclofenac, celecoxib, etoricoxib, naproxen, flurbiprofen, piroxicam, indomethacin, and dexibuprofen.
See methods sections for details of adjustment.
Taking into account sex and age, we did not find any statistically significant differences in the risk of DILI. Table 3 reports the acute serious liver injury OR for single NSAIDs with a prevalence of use greater than or equal to 5% and for paracetamol. Apart from ASA, which is also used in cardiovascular disorders, nimesulide was the most used NSAID in the study period (prevalence of use of 11.1%). Paracetamol too was largely utilized in our patients, with a prevalence of use of 16.7%.
Table 3.
Odds ratios (OR) and 95% confidence intervals (95% CI) of acute serious liver injury associated with all NSAIDs and paracetamol
| Drug (prevalence of use) | No. (%) of cases | No. (%) of controls | OR (95% CI) | OR Adg (95% CI) * |
|---|---|---|---|---|
| ASA (13.5) | 31 (17) | 253 (14) | 1.30 (0.85–2.00) | 1.54 (0.95–2.48) |
| Nimesulide (11.1) | 30 (17) | 184 (10) | 1.88 (1.20–2.95) | 2.10 (1.28–3.47) |
| Ketoprofen (10.7) | 19 (11) | 177 (10) | 0.98 (0.57–1.68) | 1.10 (0.60–2.00) |
| Ibuprofen (8.1) | 25 (14) | 170 (10) | 1.59 (0.98–2.57) | 1.92 (1.13–3.26) |
| Diclofenac (5.1) | 12 (7) | 85 (5) | 1.48 (0.77–2.84) | 1.50 (0.74–3.06) |
| Paracetamol (16.7) | 69 (39) | 309 (17) | 3.27 (2.32–4.59) | 2.97 (2.09–4.21) |
See methods sections for details of adjustment.
As shown in Table 3, 30 cases (17%) and 184 controls (10%) were exposed to nimesulide resulting in an adjusted risk of acute and serious liver injury of 2.10 (95% CI, 1.28–3.47). Besides nimesulide, only ibuprofen was associated to a statistically significant increased risk of liver damage, with an adjusted OR equal to 1.92 (95% CI, 1.13–3.26). The well‐known paracetamol‐associated hepatotoxicity is confirmed by three‐fold increase of risk (adjusted OR 2.97, 95% CI 2.09–4.21).
During the study four fatal cases of hepatitis were observed. For three patients the physicians established a possible relation between hepatitis and drug treatment (metolazone, rifaximin and amiodarone); the fourth patient was suffering from many concomitant diseases and he was treated with several drugs, some of which are known to be associated with disorders of the hepatobiliary system (e.g. valproic acid, phenobarbital, lansoprazole, carbamazepine). Both this patient and that associated with metolazone also received low‐dose ASA. No deaths were observed among the controls.
Risk of acute liver injury related to time of exposure and dose
Except for ASA (mainly used as an antiplatelet drug), NSAIDs and paracetamol seem to be used for a short period of time not exceeding 15 days, suggesting, therefore, an occasional consumption.
The analysis showed an increased risk of liver injury related to the length of exposure to nimesulide (OR > 30 days: 12.55; 95% CI, 1.73–90.88) and paracetamol (OR > 30 days: 18.36; 95% CI, 4.60–73.34). Considering exposures shorter than 15 days, nimesulide, ibuprofen and paracetamol are associated with a statistically significant adjusted odd ratio (OR 1.89; 95% CI, 1.12–3.20; OR 1.89; 95% CI, 1.09–3.26; OR 2.66; 95% CI, 1.83–3.88 respectively). Due to the low number of cases, time‐related analysis was not completely possible for ketoprofen (Table 4).
Table 4.
Odds ratios (OR) and 95% confidence intervals (95% CI) of acute serious liver injury associated with different time of exposure of NSAIDs and paracetamol
| DRUG Time of exposure | No. (%) of cases | No. (%) of controls | OR (95% CI) | OR Adg (95% CI) * |
|---|---|---|---|---|
| NIMESULIDE | ||||
| <15 days | 25 (13.97) | 176 (9.94) | 1.66 (1.03–2.68) | 1.89 (1.12–3.20) |
| 15–30 days | 2 (1.12) | 5 (0.28) | 4.82 (0.92–25.34) | 4.89 (0.80–30.00) |
| >30 days | 3 (1.68) | 3 (0.17) | 16.05 (2.60–98.94) | 12.55 (1.73–90.88) |
| ASA | ||||
| <15 days | 7 (3.93) | 76 (4.30) | 0.93 (0.42–2.08) | 1.06 (0.45–2.50) |
| 15–30 days | 1 (0.56) | 1 (0.06) | 10.60 (0.66–169.75) | 6.31 (0.37–106.38) |
| >30 days | 22 (12.36) | 173 (9.79) | 1.40 (0.83–2.35) | 1.66 (0.93–2.96) |
| KETOPROFEN | ||||
| <15 days | 18 (10.06) | 173 (9.77) | 0.94 (0.54–1.64) | 1.05 (0.57–1.93) |
| 15–30 days | 1 (0.56) | 2 (0.11) | 4.97 (0.45–54.87) | 6.55 (0.56–76.66) |
| >30 days | 0 (0) | 2 (0.11) | NA | NA |
| IBUPROFEN | ||||
| <15 days | 23 (12.85) | 162 (9.16) | 1.52 (0.92–2.50) | 1.89 (1.09–3.26) |
| 15–30 days | 1 (0.56) | 4 (0.23) | 2.57 (0.29–23.05) | 2.53 (0.26–24.69) |
| >30 days | 1 (0.56) | 3 (0.17) | 3.46 (0.36–33.29) | 2.58 (0.25–26.95) |
| DICLOFENAC | ||||
| <15 days | 10 (5.59) | 74 (4.19) | 1.42 (0.70–2.87) | 1.35 (0.63–2.92) |
| 15–30 days | 1 (0.56) | 3 (0.17) | 3.47 (0.36–33.40) | 4.23 (0.40–45.07) |
| >30 days | 1 (0.56) | 4 (0.23) | 2.58 (0.29–23.13) | 4.66 (0.49–44.15) |
| PARACETAMOL | ||||
| <15 days | 61 (34.08) | 286 (16.16) | 3.08 (2.14–4.43) | 2.66 (1.83–3.88) |
| 15–30 days | 3 (1.68) | 7 (0.40) | 6.01 (1.51–23.89) | 5.25 (1.21–22.82) |
| >30 days | 5 (2.79) | 6 (0.34) | 14.78 (3.90–56.00) | 18.36 (4.60–73.34) |
See methods sections for details of adjustment
No single NSAID was associated with a significant risk of hepatotoxicity when taken at doses equal to or less than recommended, while for paracetamol this condition is present (Table 5). Exposure to higher doses of nimesulide was associated with a six‐fold increase in risk of acute liver injury (adjusted OR 10.69; 95% CI, 4.02–28.44 vs adjusted OR 1.55; 95% CI, 0.89–2.70 for recommended dose). Risk of hepatotoxicity was increased also for patients receiving higher doses of ketoprofen (adjusted OR 4.65; 95% CI, 1.33–10.00) and ibuprofen (adjusted OR 3.73; 95% CI, 1.11–12.46). The number of cases and controls considered in the time of exposure and dosage analysis may not correspond to the total, due to missing data on duration of therapy and doses.
Table 5.
Odds ratios (OR) and 95% confidence intervals (95% CI) of acute serious liver injury associated with different doses of NSAIDs and paracetamol
| DRUG Dose | No. (%) of cases | No. (%) of controls | OR (95% CI) | OR Adg (95% CI) † |
|---|---|---|---|---|
| NIMESULIDE | ||||
| <200 mg | 21 (11.73) | 172 (9.72) | 1.41 (0.85–2.35) | 1.55 (0.89–2.70) |
| ≥200 mg | 9 (5.03) | 12 (0.68) | 8.03 (3.36–9.22) | 10.69 (4.02–28.44) |
| ASA | ||||
| <300 mg | 21 (11.73) | 168 (9.49) | 1.35 (0.80–2.27) | 1.46 (0.82–2.58) |
| ≥300 mg | 10 (5.59) | 85 (4.80) | 1.22 (0.62–2.43) | 1.70 (0.81–3.54) |
| KETOPROFEN | ||||
| <150 mg | 10 (5.62) | 142 (8.02) | 0.67 (0.34–1.34) | 0.73 (0.35–1.54) |
| ≥150 mg | 8 (4.49) | 19 (1.07) | 3.29 (1.35–8.04) | 4.65 (1.33–10.00) |
| IBUPROFEN | ||||
| <1200 mg | 20 (11.24) | 155 (8.76) | 1.45 (0.86–2.43) | 1.71 (0.97–3.02) |
| ≥1200 mg | 4 (2.23) | 15 (0.85) | 2.62 (0.82–8.32) | 3.73 (1.11–12.46) |
| DICLOFENAC | ||||
| <100 mg | 5 (2.79) | 30 (1.69) | 1.69 (0.64–4.48) | 2.13 (0.77–5.91) |
| ≥100 mg | 3 (1.68) | 25 (1.41) | 1.19 (0.35–4.09) | 1.21 (0.30–4.85) |
| PARACETAMOL | ||||
| <3000 mg | 42 (23.46) | 265 (14.97) | 2.22 (1.48–3.15) | 2.04 (1.35–3.09) |
| ≥3000 mg | I26 (14.52) | I44 (2.49) | I7.80 (4.53–13.43) | I6.31 (3.56–11.20) |
See methods sections for details of adjustment
Discussion
The annual incidence of DILI observed in this study was 4.1 cases per 100 000 inhabitants and about half of these patients received NSAIDs (giving an annual incidence of N‐DILI of two cases per 100 000 inhabitants). These data are partially in agreement with the evidence available until now: the incidence of DILI reported from Swedish and English studies confirms our results 11, 12; however, these retrospective studies found a six to eight times lower incidence than the population‐based studies from Björnsson and colleagues and Sgro and colleagues 9, 10. Probably, these results reflect some differences between the study methodology that should be taken into account: first, the studies which revealed the lower incidence of DILI used databases as primary sources of information, while the studies by Sgro and Björnsson were based on active participation of physicians and specialists which, in turn, recruited patients in a hospital and/or outpatient setting. Moreover, the first of these two studies considered only DILI in an outpatient setting while the second observed the incidence of DILI in both in‐ and outpatients. Also the period of patient recruitment varied among studies, ranging from 2 to 10 years 9, 10, 11. Our findings about the incidence of N‐DILI corresponds to what is reported in the literature: in most studies these data are rather uniform, ranging from one to nine cases per 100 000 persons exposed to NSAIDs 32.
Considering the association between acute and serious liver injury and NSAID utilization, our results showed that there is a small risk of acute and serious liver injury in NSAID users and that nimesulide and ibuprofen are associated with a higher risk of hepatotoxicity than other NSAIDs.
In general, the available evidence reports that DILI is more likely to occur in females and in elderly people and this is also supported in some NSAID‐associated hepatotoxicity analysis. Traversa and colleagues 28 reported that risk of liver injury was increased among elderly people (age over or equal to 75 years) and a case–control study in primary care in a southwestern area of France showed a significant association between hepatic ADRs and NSAID use only in women 18. At variance with what such studies reported, in our population no significant differences were associated with patients’ gender and age with regard to risk of liver injury.
Although hepatotoxicity is an adverse effect of NSAID class considered worldwide as rare, serious liver damage is the main adverse event which caused some NSAIDs to be withdrawn from the market and the epidemiological data about the individual NSAID risk of liver injury is still divergent 15, 28, 33.
Population‐based studies on liver toxicity induced by NSAIDs indicated a higher risk for diclofenac; however, in these studies the rate of serious hepatic adverse events, hospitalization or death was low 12, 32, 34, 35. In contrast, in a survey on suspected drug‐induced liver fatalities reported to the WHO database (in 88% of the cases the reporting country was the United States), diclofenac was the only NSAID implicated among the top 20 causes and a systematic review reported the prevalence of hospitalization as 22.4 per 100 000 patient‐year of diclofenac exposure 36, 37. These data deviate from our results, which have not revealed any risk of acute and serious liver damage associated with the use of diclofenac. Regulatory actions, restrictions and, in some countries, withdrawal from the market have been applied to nimesulide, another NSAID possibly implicated in hepatotoxicity. The first cases of serious or fatal liver injury in Finland and subsequently in Spain and Ireland led to the withdrawal of the drug in these countries; however, the last EMA evaluation concluded that the risk–benefit profile remains favourable and recommended restrictions in its use [27]. These decisions were supported by epidemiological studies confirming that nimesulide was associated with only a small increase in risk 28, 38. Our study corroborates this conclusion, providing further information on time of exposure and dosage. In most cases nimesulide was administered at recommended time of exposure and daily dosages (time lower than 15 days and dosage lower than 200 mg) and in these conditions the risk seems to be very low; however, an exponential increase of hepatotoxicity risk is observed with increasing duration of treatment and with higher dosages.
The increased risk of liver injury associated with nimesulide is of particular concern in Italy since the use of this drug is still widespread, despite the restriction in indications (only acute pain and dysmenorrhea) and treatment duration. In fact, according to national drug utilization data, nimesulide was the fourth most prescribed NSAID in 2013, with a consumption of 3.1 DDD/1000 inhabitants/day 39.
Furthermore, our research found a significant risk of serious and acute liver damage associated with ibuprofen and the dose‐related analysis showed that this risk increased with the dose administered. This finding was quite unexpected, as the current literature reports a very low liver toxicity incidence for ibuprofen 28, 32, 33. Some case reports of liver damage associated with ibuprofen were described in the scientific literature 40, 41 and some information emerged from recent observational studies and reviews which investigated DILI and the risk of hepatotoxicity in patients exposed to NSAIDs 38, 42. A case series from Riley and colleagues also suggested that ibuprofen may increase the risk of liver injury in patients with hepatitis C 43, though we were not able to confirm this observation as, people suffering from hepatitis C were excluded from our study. Nevertheless, our result for ibuprofen should be taken into consideration as ibuprofen is the most commonly used NSAID worldwide and among these drugs it is associated with the lower risk of gastrointestinal, cardiovascular and renal serious events.
In the present study, ketoprofen was associated with a significant increased risk of acute and serious liver injury only at dosages higher than recommended. We did not find any additional specific information on ketoprofen hepatotoxicity and our data strengthen the importance of using all NSAIDs within the therapeutic dosages.
Hepatotoxicity of high doses of paracetamol is a well‐documented risk, but the question whether therapeutic use causes acute liver failure is still open, as several individual factors, such as concomitant alcohol use or abuse, concurrent medications, genetic factors and nutritional status, can influence the susceptibility of patients to paracetamol hepatotoxicity [44]. A moderate risk of acute liver injury associated with paracetamol at usual analgesic doses has been reported in some documented cases and in observational studies 45, 46.
A limitation of our study was related to the number of recruited cases, which was lower than expected; in fact, the actual incidence of liver injury events involving hospitalization was lower than reported in the literature, probably due to relevant recent changes in hospital practice (e.g. increased outpatient management of liver injury). The precision of risk estimates of serious liver injury for the time and dosage analysis is affected by the low number of recruited cases and this is reflected in the calculated CI values. Moreover, our analysis did not provide information about the characterization of the liver damage.
The main strength of our case–control analysis is the precision and accuracy in the selection of patients: we excluded other possible causes of hepatic disease to prevent a potential bias of misdiagnosis and we considered all the variables that could influence the risk of developing liver damage. Definition of inclusion and exclusion criteria was made in collaboration with a panel of expert hepatologists. Our findings are also representative of the Italian situation: the study was conducted in four regions distributed in the Northern, Central and Southern Italy and the population covered by this area represents about 10% of the national population. Furthermore, patients were recruited over a long period of time, subsequent to the measures taken by the European and Italian Regulatory Agency, which allowed us to evaluate the effects of these regulatory measures in the clinical practice. Finally, quality control and assurance measures were implemented to ensure that procedures were shared by all centres and data were reasonably valid and coherent as regarding both cases controls. Quality assurance measures were applied to the interview technique, to recruitment and management of the patients, and finally to the quality of data entry in the database. The interview was standardized and the questionnaire tested in a pilot phase to ensure the readability of its content. The monitors were trained before and during the study and appropriate explanations were integrated into the training plan and monitor manuals. Quality control and assurance measures were set for all centres by the Coordinating Centre.
Our findings confirm that hepatotoxicity represents a rare but serious adverse drug reaction and that NSAIDs are associated with an increased risk.
Among NSAIDs, nimesulide is associated with the higher risk, whereas ibuprofen and high doses of ketoprofen are also associated with a modestly increased risk of hepatotoxicity. The results on nimesulide are especially relevant to Italy, where, despite regulatory restrictions by the EMA, the drug is still largely utilized causing a risk of hepatotoxicity that increases with dosage and time of exposure.
Finally, the issue of liver injury induced by NSAIDs at dosage exceeding the DDD should not be underestimated, as extensive evidence is available showing that these drugs are often used inappropriately [47]. In fact, several NSAIDs are currently worldwide available on the market as over‐the‐counter (OTC) drugs, not requiring any medical prescription and with a management of treatment duration and dosage performed only by the patient, without any medical supervision. The consequence of this attitude is the total lack of medical assessment of the possible risks and contraindications, duration and dose of NSAID treatment, which can lead to a greater number of side effects, sometimes serious and unexpected.
Competing Interests
All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: all institutions received financial support from Italian Medicines Agency (AIFA) for the research study; no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
This study was funded by the Italian Medicines Agency (AIFA), through a call for independent research (ID Study. FARM8B2TY7).
Contributors
RL, ACa, DM and AVan conceived the study. RL and UM designed the study. MD, OB, RL, ACo, MCL and RB analysed the data. MD and ACo wrote the manuscript. MD, ACo, MCL, ACa, OB, DM, UM, AVan, CR, AVac, EA, RB, LS and RL contributed to the discussion and reviewed the manuscript.
The lead author affirms that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
No additional data available.
We are grateful for the help and support of the DILI‐IT Study Group, who include the following.
Clinical investigators: Franco Capra, Angelo Tonon, University Hospital of Verona; Marco Zoli, University Hospital S. Orsola‐Malpighi of Bologna and the Head of each Internal Medicine, Geriatric and other participating Units; Enrica Cecchi, Stefano Grifoni, Careggi Hospital of Florence; Fulvio Calise, Cardarelli Hospital of Neaples; Pietro Amoroso, Cotugno Hospital of Neaples; Evangelista Sagnelli, San Sebastiano Hospital of Caserta; Annamaria Frola, Umberto I Hospital Unit of Salerno.
Study monitors: Elena Arzenton, Giovanna Stoppa, Verona; Maria Carmela Lenti, Gemma Benelli, Florence; Carolina Tiani, Bologna; Carla Migliaccio, Andrea Vitale, Nancy Acampa, Neaples.
Steering Committee: Nicola Montanaro, University of Bologna; Francesco Lapi and Alessandro Mugelli, University of Florence; Francesco Rossi, University of Naples.
Data manager: Giulia Bisoffi, University Hospital of Verona.
We thank the components of External Advisory Board: Maria Grazia Franzosi, Nicola Magrini, Luigi Pagliaro, Giuseppe Traversa and Mauro Venegoni.
Donati, M. , Conforti, A. , Lenti, M. C. , Capuano, A. , Bortolami, O. , Motola, D. , Moretti, U. , Vannacci, A. , Rafaniello, C. , Vaccheri, A. , Arzenton, E. , Bonaiuti, R. , Sportiello, L. , Leone, R. , and on behalf of DILI-IT Study Group (2016) Risk of acute and serious liver injury associated to nimesulide and other NSAIDs: data from drug‐induced liver injury case–control study in Italy. Br J Clin Pharmacol, 82: 238–248. doi: 10.1111/bcp.12938.
References
- 1. Ostapowicz G, Fontana RJ, Schiødt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med 2002; 137: 947–54. [DOI] [PubMed] [Google Scholar]
- 2. Reuben A, Koch DG, Lee WM, Acute Liver Failure Study Group . Drug‐induced acute liver failure: results of a US multicenter, prospective study. Hepatology 2010; 52: 2065–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wei G, Bergquist A, Broomé U, Lindgren S, Wallerstedt S, Almer S, et al. Acute liver failure in Sweden: etiology and outcome. J Intern Med 2007; 262: 393–401. [DOI] [PubMed] [Google Scholar]
- 4. Bechmann LP, Manka P, Best J, Saner FH, Paul A, Canbay A, et al. Drug‐induced liver injury as predominant cause of acute liver failure in a monocenter study. Dtsch Med Wochenschr 2014; 139: 878–82. [DOI] [PubMed] [Google Scholar]
- 5. Watkins PB, Seeff LB. Drug‐induced liver injury: summary of a single topic clinical research conference. Hepatology 2006; 43: 618–31. [DOI] [PubMed] [Google Scholar]
- 6. Navarro VJ, Senior JR. Drug‐related hepatotoxicity. N Engl J Med 2006; 354: 731–9. [DOI] [PubMed] [Google Scholar]
- 7. Abboud G, Kaplowitz N. Drug‐induced liver injury. Drug Saf 2007; 30: 277–94. [DOI] [PubMed] [Google Scholar]
- 8. Leise MD, Poterucha JJ, Talwalkar JA. Drug‐induced liver injury. Mayo Clin Proc 2014; 89: 95–106. [DOI] [PubMed] [Google Scholar]
- 9. Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcome in patients with drug‐induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419–25. [DOI] [PubMed] [Google Scholar]
- 10. Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, Guilleminet C, et al. Incidence of drug‐Induced hepatic injuries: a French population‐based study. Hepatology 2002; 36: 451–5. [DOI] [PubMed] [Google Scholar]
- 11. De Valle MB, Av Klinteberg V, Alem N, Olsson R, Björnsson E. Drug‐induced liver injury in a Swedish University hospital out‐patient hepatology clinic. Aliment Pharmacol Ther 2006; 24: 1187–95. [DOI] [PubMed] [Google Scholar]
- 12. de Abajo FJ, Montero D, Madurga M, García Rodríguez LA. Acute and clinically relevant drug‐induced liver injury: a population based case–control study. Br J Clin Pharmacol 2004; 58: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Björnsson ES. Drug‐induced liver injury: an overview over the most critical compounds. Arch Toxicol 2015; 89: 327–34. [DOI] [PubMed] [Google Scholar]
- 14. Wehling M. Non‐steroidal anti‐inflammatory drug use in chronic pain conditions with special emphasis on the elderly and patients with relevant comorbidities: management and mitigation of risks and adverse effects. Eur J Clin Pharmacol 2014; 70: 1159–72. [DOI] [PubMed] [Google Scholar]
- 15. Day RO, Graham GG. Non‐steroidal anti‐inflammatory drugs (NSAIDs). BMJ 2013; 346: f3195. [DOI] [PubMed] [Google Scholar]
- 16. Ungprasert P, Cheungpasitporn W, Crowson CS, Matteson EL. Individual non‐steroidal anti‐inflammatory drugs and risk of acute kidney injury: a systematic review and meta‐analysis of observational studies. Eur J Intern Med 2015; 26: 285–91. [DOI] [PubMed] [Google Scholar]
- 17. Motola D, Vargiu A, Leone R, Cocci A, Salvo F, Ros B, et al. Hepatic adverse drug reactions: a case/non‐case study in Italy. Eur J Clin Pharmacol 2007; 63: 73–9. [DOI] [PubMed] [Google Scholar]
- 18. Lacroix I, Lapeyre‐Mestre M, Bagheri H, Pathak A, Montastruc JL, Club de Reflexion des cabinets de Groupe de Gastro‐Enterologie (CREGG) , General Practitioner Networks . Nonsteroidal anti‐inflammatory drug‐induced liver injury: a case–control study in primary care. Fundam Clin Pharmacol 2004; 18: 201–6. [DOI] [PubMed] [Google Scholar]
- 19. Pillans PI, Ghiculescu RA, Lampe G, Wilson R, Wong R, Macdonald GA. Severe acute liver injury associated with lumiracoxib. J Gastroenterol Hepatol 2012; 27: 1102–5. [DOI] [PubMed] [Google Scholar]
- 20. Page M, Christin F, Hayi‐Slayman D, Baillon JJ, Ber CE, Delafosse B, et al. Acute liver failure due to a treatment by nimesulide: another case and review. Ann Fr Anesth Reanim 2008; 27: 742–6. [DOI] [PubMed] [Google Scholar]
- 21. Merlani G, Fox M, Oehen HP, Cathomas G, Renner EL, Fattinger K, et al. Fatal hepatoxicity secondary to nimesulide. Eur J Clin Pharmacol 2001; 57: 321–6. [DOI] [PubMed] [Google Scholar]
- 22. FIMEA, Finnish Medicines Agency . The sale of Nimed, an anti‐inflammatory analgesic, is temporarily suspended due to its adverse liver effects, 15 March 2002. Available at http://www.fimea.fi/whatsnew/1/0/thesaleofnimedananti‐inflammatoryanalgesic,istemporarilysuspendedduetoitsadverselivereffects (last accessed 16 April 2015).
- 23. AEMPS, Agencia Española de Medicamentos y Productos Sanitarios . Comunicación sobre riesgos de Medicamentos. Ref: 2002/03. Nota Informativa Nimesulida (Guaxan®, Antifloxil®): suspensión cautelar de comercialización, 6 May 2002. Available at http://www.aemps.gob.es/informa/notasInformativaUsoHumano/seguridad/2002/NI_2002‐03_nimesulida.htm (last accessed 16 April 2015).
- 24. EMEA, European Agency for the Evaluation of Medicinal Products . Committee for Proprietary Medicinal Products (CPMP) opinion following an article 31 referral. Nimesulide containing medicinal products, 7 May 2004. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Nimesulide_31/WC500013818.pdf (last accessed 16 April 2015).
- 25. HPRA, Health Products Regulatory Authority . Nimesulide suspension, 15 May 2007. Available at https://www.hpra.ie/homepage/medicines/safety‐notices/item?t=/nimesulide‐suspension&id=7d69f825‐9782‐6eee‐9b55‐ff00008c97d0 (last accessed 16 April 2015).
- 26. ANMAT, Administración Nacional de Medicamentos, Alimentos y Tecnología Médica . ANMAT suspende la comercializacion de la droga nimesulide. Boletin para profesionales 2009; XVII (3): 17–24. [Google Scholar]
- 27. EMEA, European Agency for the Evaluation of Medicinal Products . Assessment report for Nimesulide containing medicinal products for systemic use, 20 January 2012. Available at http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Nimesulide_31/WC500125574.pdf (last accessed 16 April 2015).
- 28. Traversa G, Bianchi C, Da Cas R, Abraha I, Menniti‐Ippolito F, Venegoni M. Cohort study of hepatotoxicity associated with nimesulide and other nonsteroidal anti‐inflammatory drugs. BMJ 2003; 327: 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Licata A, Calvaruso V, Cappello M, Craxì A, Almasio PL. Clinical course and outcomes of drug‐induced liver injury: nimesulide as the first implicated medication. Dig Liver Dis 2010; 42: 143–8. [DOI] [PubMed] [Google Scholar]
- 30. Bénichou C. Criteria of drug‐induced liver disorders. Report of an international consensus meeting. J Hepatol 1990; 1: 272–6. [DOI] [PubMed] [Google Scholar]
- 31. WHO Collaborating Centre for Drug Statistics Methodology . Guidelines for ATC classification and DDD assignment, 2015. Oslo, 2014. Available at http://www.whocc.no/atc_ddd_index/ (last accessed 21 July 2015).
- 32. Bessone F. Non‐steroidal anti‐inflammatory drugs: What is the actual risk of liver damage? World J Gastroenterol 2010; 16: 5651–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Unzueta A, Vargas HE. Nonsteroidal anti‐inflammatory drug‐induced hepatoxicity. Clin Liver Dis 2013; 17: 643–56. [DOI] [PubMed] [Google Scholar]
- 34. Laine L, Goldkind L, Curtis SP, Connors LG, Yanqiong Z, Cannon CP. How common is diclofenac‐associated liver injury? Analysis of 17,289 arthritis patients in a long‐term prospective clinical trial. Am J Gastroenterol 2009; 104: 356–62. [DOI] [PubMed] [Google Scholar]
- 35. Rostom A, Goldkind L, Laine L. Nonsteroidal anti‐inflammatory drugs and hepatic toxicity: a systematic review of randomized controlled trials in arthritis patients. Clin Gastroenterol Hepatol 2005; 3: 489–98. [DOI] [PubMed] [Google Scholar]
- 36. Björnsson E, Olsson R. Suspected drug‐induced liver fatalities reported to the WHO database. Dig Liver Dis 2006; 38: 33–8. [DOI] [PubMed] [Google Scholar]
- 37. Rubenstein JH, Laine L. Systematic review: the hepatotoxicity of non‐steroidal anti‐inflammatory drugs. Aliment Pharmacol Ther 2004; 20: 373–80. [DOI] [PubMed] [Google Scholar]
- 38. Lee CH, Wang JD, Chen PC. Increased risk of hospitalization for acute hepatitis in patients with previous exposure to NSAIDs. Pharmacoepidemiol Drug Saf 2010; 19: 708–14. [DOI] [PubMed] [Google Scholar]
- 39. The Medicines Utilisation Monitoring Centre . National Report on Medicines use in Italy, 2013 (English edition). Available at http://www.agenziafarmaco.gov.it/sites/default/files/OsMed_Report13_Eng.pdf (last accessed 15 July 2015).
- 40. Borel I, Hedelius F, Baumgartner C, Vial T, Scoazec JY, Dumortier J. Severe acute hepatitis associated with ibuprofen treatment. Gastroenterol Clin Biol 2001; 25: 430–2. [PubMed] [Google Scholar]
- 41. Leoz MK, Concejo FB, Fernández JM, Urmeneta JM, Peñuela AM. Ibuprofen‐induced cholestatic hepatitis. Gastroenterol Hepatol 2011; 34: 660–1. [DOI] [PubMed] [Google Scholar]
- 42. Douros A, Bronder E, Andersohn F, Klimpel A, Thomae M, Sarganas G, et al. Drug‐induced liver injury: results from the hospital‐based Berlin Case–Control Surveillance Study. Br J Clin Pharmacol 2015; 79: 988–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Riley TR, Smith JP. Ibuprofen‐induced hepatotoxicity in patients with chronic hepatitis C: a case series. Am J Gastroenterol 1998; 93: 1563–5. [DOI] [PubMed] [Google Scholar]
- 44. Bunchorntavakul C, Reddy KR. Acetaminophen‐related hepatotoxicity. Clin Liver Dis 2013; 17: 587–607. [DOI] [PubMed] [Google Scholar]
- 45. Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, et al. Paracetamol in therapeutic dosages and acute liver injury: causality assessment in a prospective case series. BMC Gastroenterol 2011; 11: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, et al. Risk of acute liver injury associated with the use of drugs: a multicentre population survey. Aliment Pharmacol Ther 2007; 25: 1401–9. [DOI] [PubMed] [Google Scholar]
- 47. Koffeman AR, Valkhoff VE, Celik S, W't Jong G, Sturkenboom MC, Bindels PJ, et al. High‐risk use of over‐the‐counter non‐steroidal anti‐inflammatory drugs: a population‐based cross‐sectional study. Br J Gen Pract 2014; 64: e191–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


