Abstract
Aims
The aim of this study was to perform an up‐to‐date systematic review and meta‐analysis on the efficacy and safety of prophylactic administration of levetiracetam in brain tumour patients.
Method
A systematic review of studies published until April 2015 was conducted using Scopus/Elsevier, EMBASE and MEDLINE. The search was limited to articles reporting results from adult patients, suffering from brain tumour, undergoing supratentorial craniotomy for tumour resection or biopsy and administered levetiracetam in the perioperative period for seizure prophylaxis. Outcomes included the efficacy and safety of levetiracetam, as well as the tolerability of the specific regimen, defined by the discontinuation of the treatment due to side effects.
Results
The systematic review included 1148 patients from 12 studies comparing levetiracetam with no treatment, phenytoin and valproate, while only 243 patients from three studies, comparing levetiracetam vs phenytoin efficacy and safety, were included in the meta‐analysis. The combined results from the meta‐analysis showed that levetiracetam administration was followed by significantly fewer seizures than treatment with phenytoin (OR = 0.12 [0.03–0.42]: χ2 = 1.76: I2 = 0%). Analysis also showed significantly fewer side effects in patients receiving levetiracetam, compared to other groups (P < 0.05). The combined results showed fewer side effects in the levetiracetam group compared to the phenytoin group (OR = 0.65 [0.14–2.99]: χ2 = 8.79: I2 = 77%).
Conclusions
The efficacy of prophylaxis with levetiracetam seems to be superior to that with phenytoin and valproate administration. Moreover, levetiracetam use demonstrates fewer side effects in brain tumour patients. Nevertheless, high risk of bias and moderate methodological quality must be taken into account when considering these results.
Keywords: brain tumour, levetiracetam, meta‐analysis, prophylaxis, seizures, systematic review
Introduction
Brain tumour surgery might be implicated in the occurrence of early or late seizures 1. Commonly, early seizures appear within the first postoperative week and are attributed to the immediate post‐traumatic effect of the surgical procedure as oedema, inflammation or oxidative stress 2. Conversely, late seizures present beyond the first week after surgical intervention and constitute actual epilepsy 3. The development of postoperative epilepsy, after supratentorial craniotomy for brain tumour biopsy of excision, has an adverse impact on postoperative clinical course and neurological outcome, cost of hospitalization, and rehabilitation 4. Thus, the control of perioperative seizures is of utmost importance for outcome optimization in this population.
Although, the treatment of epilepsy related to brain tumours is indisputable, the prophylactic use of anti‐epileptic drugs (AEDs) for attenuating the risk of postoperative seizures is controversial and the benefits should outweigh the risks associated with the administration 5, 6. Hepatic enzyme induction, mainly that of cytochrome P450 (CYP), is a common side effect of older AEDs, such as phenytoin (PHT), carbamazepine (CBZ) and phenobarbital (PB), while the most prominent adverse effects of valproic acid (VAL) are hepatotoxicity and thrombocytopenia 7, 8, 9, 10. Despite the numerous reports of possible adverse effects, PHT still constitutes the AED of choice for seizure control in most clinical settings 11.
On the other hand, new generation AEDs seem to have an improved safety profile, at least on major complications. For instance, oxcarbazepine (OXC), has minor impact on CYP enzyme induction, but it still can be complicated by hyponatremia and dermatological reactions 12. Levetiracetam (LEV) has gained popularity mainly due to its unique features in terms of mechanism of action, pharmacokinetics and metabolism 13, 14, 15. A growing body of evidence supports the safety and efficacy of LEV compared to other AEDs in various clinical settings 16, 17. Despite the fact that several investigators suggest switching from older AEDs to LEV in brain tumour surgery, the evidence for applying LEV as a single agent for perioperative prophylaxis from seizures in brain tumour patients is limited 18, 19, 20. In order to investigate the efficacy and safety of LEV as first‐line perioperative prophylactic treatment for seizures, a systematic review and meta‐analysis were performed. The study included all published randomized and observational studies of LEV, alone or compared with other AEDs, used in patients with brain tumour undergoing neurosurgical interventions.
Methods
Protocol and registration
A systematic review and meta‐analysis for studies testing the efficacy and safety of LEV in patients that underwent craniotomy for supratentorial brain tumours were conducted. The recommendations of the PRISMA statement for reporting Systematic Reviews and Meta‐analyses were followed throughout the review process. Α protocol was designed before the review started, with registration number PROSPERO 2014:CRD42014013498, and can be accessed at PROSPERO (http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014013498).
Eligibility criteria
Types of studies
All type of studies (including randomized‐controlled studies, non‐randomized studies, prospective cohort studies, retrospective studies and case series), were eligible for inclusion. No language or publication date restrictions were imposed.
Types of participants
Patients over 18 years of age, suffering from brain tumour, undergoing supratentorial craniotomy for tumour resection or biopsy and administered LEV in the perioperative period for seizure prophylaxis, were included in this study. Exclusion criteria were patients under the age of 18, pregnancy, breast‐feeding, severe co‐morbidities (including renal and liver failure) and craniotomy for disease other than brain tumour.
Types of interventions
Studies that examined LEV administration as seizure prophylaxis in patients who underwent supratentorial craniotomy for brain tumour were eligible for inclusion. Furthermore, articles comparing LEV administration to no antiepileptic drug, placebo or other drug were also included.
Types of outcome measures
Primary outcome measures were the efficacy and the safety of LEV. Efficacy was defined either by the appearance or not of seizures or the reduction in the incidence of seizures during the study period. Safety was defined by the reports of side effects (severe, moderate and zero), which were directly attributable to LEV. A secondary outcome measure was the tolerability of the specific regimen, defined by the discontinuation of the treatment due to side effects.
Systematic search
The literature search was conducted in MEDLINE, EMBASE, Scopus/Elsevier, The Cochrane Central Register of Controlled Trials (CENTRAL) and The International Web of Science databases up to 20 February 2014. Additional search was conducted on 20 March 2015. Also, the reference lists of the retrieved articles were searched for further relevant studies. The search was limited to articles reporting results from adult patients. The search strategy is presented in Appendix 1. Based on the search strategy, all titles and abstracts retrieved were independently scanned by two authors (CP, GT). Eligibility of each article retrieved was firstly assessed from the title or the abstract. If eligibility could not be ascertained from the title or the abstract, the full text of the study was retrieved and searched. The article was included for review if eligibility criteria were met, as judged by both authors. In case of disagreements between the two reviewers, the discrepancy was resolved by consulting a third author (DK).
Data collection
A data collection sheet was created and included articles were assessed for:
Study design,
Total study duration,
Risk of bias (randomization if any, sequence generation, allocation sequence concealment, blinding, other concerns about bias),
Total number of participants,
Setting where the administration of the drug took place (in‐hospital or outpatient basis),
Diagnostic criteria for seizures (clinical observation or EEG),
Age of participants,
Sex of participants,
Tumour type,
Location,
Co‐administration of corticosteroids,
Number of different intervention groups (LEV, placebo, other AED),
Route of administration,
Dose regimen,
Duration of administration,
Incidence of side effects in the preoperative or postoperative period (somnolence, nausea/vomiting, headache, insomnia or other rare side effect),
Treatment discontinuation due to side effects,
Incidence of seizures preoperatively,
Incidence of seizures postoperatively.
Preoperative period was defined from the commencement of the treatment until the time of the surgical operation. Postoperative period was further divided into three periods and assessed separately: early postoperative period (the first 48 hours after completion of operation), late postoperative period (48 hours postoperatively–4 weeks postoperatively), late observation period (4 weeks postoperatively–completion of the protocol). Values provided as percentages were converted into actual patient numbers for analysis.
Statistical analysis
The effect sizes measured were odds ratio (OR) with 95% confidence interval (CI) for the categorical variable. OR < 1 favoured LEV and OR > 1 favoured PHT. Forest plots were used to graphically display the results of the meta‐analysis. The random effects model described by DerSimonian and Laird was used to combine the results from the studies 21. This model calculates a weighted average by incorporating within‐study and between‐study variations. The Mantel–Haenszel method (fixed effect model) was also used to assess the effect of model assumptions on our conclusions, depending on study heterogeneity 22.
Between‐study heterogeneity was assessed with the Cochrane Q test using a χ2 function (P values < 0.10 were considered significant). In addition, I2 values were calculated to estimate inconsistency across studies. I2 values of 25% or less may represent low heterogeneity, values around 50% may represent moderate heterogeneity, and values of 75% or more may represent high heterogeneity. An I2 value > 25% was considered significant in this meta‐analysis.
Where no significant statistical heterogeneity was identified, the fixed‐effect estimate was used preferentially as the summary measure. Sensitivity analyses were performed to assess the contribution of each study to the pooled estimate by excluding individual trials one at a time and recalculating the pooled OR estimates for the remaining studies. All analyses were conducted using RevMan 5.3.
Risk of bias was assessed as described by the Cochrane Handbook for Systematic Reviews of Interventions 23. We assessed the risk of bias in sequence generation, allocation concealment, blinding (including participants and personnel, data collectors, outcome assessors), incomplete data, selective outcome reporting and other sources of bias. Every eligible study was evaluated for any of the above‐mentioned risk of bias domains, as having low, high or uncertain risk of bias. All trials that were classified as having low risk of bias, in all of the previously listed domains, were considered as low risk of bias trials. An assessment of reporting biases (such as publication bias) by constructing a funnel plot and using tests for funnel plot asymmetry was planned if there were at least ten studies included in the meta‐analysis.
Results
Study selection
A flow chart describing the results of the database and other source search is shown in Figure 1 24. Searches returned a total of 1006 records through databases. After removal of duplicates, records were firstly screened by title. Of those, 944 records were excluded as irrelevant and the remaining 62 articles were further screened by abstract. A total of 18 articles were further excluded, based on abstract and the full‐text copies of the remaining 44 articles were evaluated for eligibility. Additional searching up to April 2015 revealed one more study eligible for inclusion 25. Finally, 32 articles were excluded for various reasons, 12 studies met the predetermined inclusion criteria and were subjected to qualitative analysis, and three studies were included in the meta‐analysis 18, 25, 26.
Figure 1.
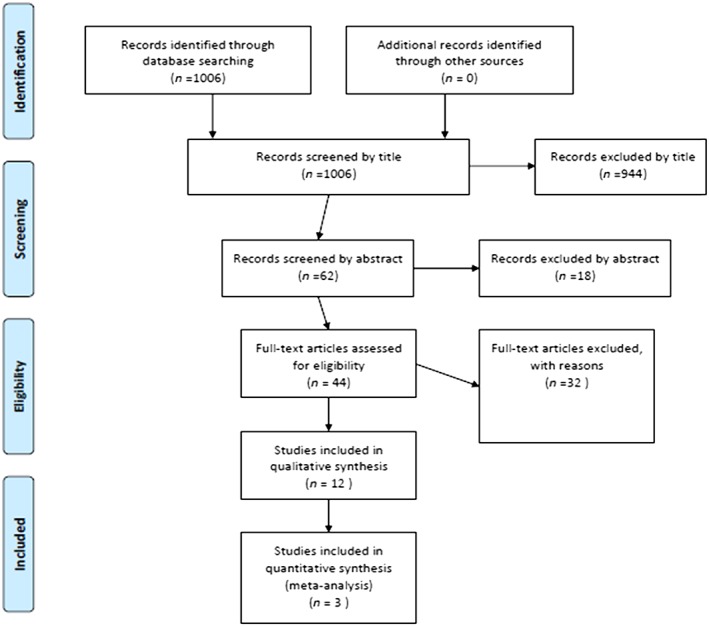
Flow diagram of study selection in the systematic review and meta‐analysis of the efficacy and safety of LEV (levetiracetam) prophylactic administration in brain tumour patients 24
Of the 32 full‐text articles excluded, four concerned patients under LEV, in which it was impossible to retrieve data regarding safety and efficacy 4, 27, 28, 29, three concerned patients under LEV who underwent craniotomy, but data regarding the perioperative period were not described 30, 31, 32, four were excluded because patients receiving LEV did not undergo craniotomy 33, 34, 35, 36, one study was excluded because patients did not received LEV 37, one was excluded because patients received a combination of LEV with PHT 38 and one study was excluded because the authors enrolled patients under the age of 18 39.
Furthermore, 18 articles were also excluded because they were reviews, and data regarding LEV efficacy and safety could not be retrieved 11, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56. However, the reference lists of these articles were manually screened for possible eligible publications, but all relevant articles cited were already screened from the database screening process.
Study characteristics
The characteristics of the 12 included studies are shown in Table 1. Among them only one was a randomized controlled study while four were prospective studies and the remaining seven studies were retrospective. Four of them studied the effect of LEV administration without a control group 20, 56, 57, 58, one compared LEV with no treatment 59, one compared LEV with VAL administration 10, while another compared LEV with VAL administration both as adjuvant therapy to Temozolomide (TMZ) 60 and five compared LEV with PHT administration 18, 25, 26, 61, 62. Ultimately, four of them 18, 25, 26, 62 met the criteria to be included in the meta‐analysis.
Table 1.
Characteristics of the included studies
| Time of outcome assessment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Source | Study design | Study period | Intervention/control | No of pts | Age | <4 weeks | 4w‐12mo | Side effects |
| Bahr et al. 56 | Prospective, open‐label | 4 weeks | LEV po or iv 500 × 2/3d po, then 1000 × 2 | LEV 25 | 23–78 yrs | 6/25 (3/25 48 h postop) | 1/25 paresthesia + visual field deficit | |
| In case of seizures up to 1500 × 2 | ||||||||
| Fuller et al. 26 | Prospective, open‐cohort | 3 mo | LEV vs PHT | LEV 36 | LEV 25–88 yrs | LEV 0/36 | 1/25 paresthesia + nystagmus | |
| LEV 250–1000 × 2 po or iv | PHT 38 | PHT 27–89 yrs | PHT 6/38 (1/38 48 h postop) | |||||
| PHT 300 × 3 or 1gr iv (loading)‐ 300 mg/d po or iv | ||||||||
| Gokhale et al. 58 | Retrospective | 1 week | LEV p.o or i.v. 1000–2000 mg/day | LEV 165 | 18–82 yrs | 12/165 (10 generalized and 2 partial seizures | 7/165 somnolence | |
| Garbossa et al. 59 | Retrospective 2‐centre study | 6 mo | LEV vs control (none) | LEV 43 | LEV 59 | LEV 1/43 | LEV 5/43 (3mo) | LEV 1/43 ataxia |
| LEV 500 × 2/3‐5d preop | Control 48 | 47 (SD 12) | Control 0/48 | Control 3/48 | ||||
| LEV 2/41 (6 mo) | ||||||||
| 500 × 2 for 6 mo postop or 1000 × 2 (in case of seizures) | ||||||||
| Control 63.9 (SD 11.8) | Control 6/45 (6 mo) | |||||||
| Iuchi et al. 25 | randomized, prospective, open‐cohort, study | 7 days | LEV 500 × 2 sup → po | 73 LEV | 1/73 LEV | 3/73 LEV (liver dysfunction | 3/73 LEV liver dysfunction | |
| 11/73 PHT | 8/73 PHT (2 liver dysfunction, 2 hyponatriemia, 2 skin rash, 2 atrial fibrillation) | 2/73 PHT liver dysfunction | ||||||
| 73 PHT | ||||||||
| 2/73 PHT hyponatriemia | ||||||||
| PHT 15–18 mg kg−1 iv → 5–7.5 mg kg−1/day iv, then 250 mg po daily | ||||||||
| 2/73 PHT skin eruption | ||||||||
| 2/73 PHT atrial fibrillation | ||||||||
| Kerkhof et al. 60 | Retrospective | 6 mo | LEV vs VAL | LEV 36 | 24–85 yrs | LEV 8/36 | LEV 1/36 severe fatigue + allergic reaction | |
| LEV p.o or i.v. 1000 mg/day (or 2000 mg/day in case of seizures) | ||||||||
| VA L36 | VAL 11/36 | |||||||
| VAL p.o or i.v. 1000 mg/day (or 2000 mg/day in case of seizures) | ||||||||
| Lee et al. 10 | Retrospective | 4 weeks | LEV vs VAL | LEV 51/282 | LEV 50.6 (SD 16.6) | LEV 4/51 | Total LEV 5/51 | |
| VAL 231/282 | ||||||||
| LEV 500 × 2 iv (preop‐1st postop days) | ||||||||
| VAL 50.9 (SD 17.3) | VAL 15/231 | VAL 62/231 | ||||||
| LEV 500 × 2 1500 × 2 (in case of seizures) | ||||||||
| VAL 600 mg for 12 h preop | ||||||||
| VAL 50 mg for 24 h 1st postop | ||||||||
| VAL 600 mg × 2 titration to serum levels | ||||||||
| Lim et al. 18 | RCT | 6 mo | LEV vs PHT (0 postop) | LEV 15 | LEV 20–56 | LEV 2/15 | LEV 0/15 | |
| LEV 1000 × 2 iv + tapering off PHT up to POD3 | PHT 8 | PHT 32–83 | PHT 2/8 | PHT 3/8 difficulty in coordination | ||||
| PHT 300‐400 mg × 1 according serum levels | ||||||||
| 6 mo evaluation | ||||||||
| Merrell et al. 62 | Retrospective | 4 weeks | LEV vs PHT | LEV 51 | LEV 25–77 | LEV 2/51 | LEV:3/51 | |
| NR | PHT 25 | PHT 32–79 | PHT 5/25 | PHT5/25 | ||||
| Milligan et al. 61 | Retrospective | 12 mo | LEV vs PHT | LEV 105 | Primary brain tumour | LEV 1/43 | LEV 5/11 | |
| LEV 500–3000 mg/d (1000 mg) | ||||||||
| PHT 200–800 mg/d (300 mg) | PHT 210 | LEV 43/105 | PHT 2/56 | PHT 24/44 | ||||
| PHT 56/210 | ||||||||
| Usery et al. 20 | Prospective | 4 weeks | LEV 500 × 2 iv → po | LEV 20 | LEV 27–77 | LEV 1/17 | 3/17 somnolence | |
| Titrated 500 mg per day | 1/17 nausea/vomiting | |||||||
| 1/17 headache | ||||||||
| Up to 3000/d | 1/17 insomnia | |||||||
| Zachenhofer et al. 57 | Retrospective | 12 mo | LEV 500 × 2 iv or 500 × 3 | LEV 78 | LEV 27–89 | LEV 2/78 (7 days) | LEV 7 (whole 180 days) | 3/78 somnolence |
| Titrated up to 3000/d | 5/78 (4 weeks) | 2/78 psychosis | ||||||
LEV, levetiracetam; mo, months; PHT, phenytoin; postop, postoperatively; SD, standard deviation; VAL, valproate; yrs, years
Participants
A total of 1148 patients were enrolled in 12 studies included in the systematic review. The study conducted by Milligan et al. included 315 patients with various intracranial pathologies 61. However, only data regarding patients with primary brain tumours have been extracted. Hence, a total of 43 and 56 patients with primary brain tumours were treated with LEV and PHT respectively and were finally included in the present analysis. Also, the study conducted by Kerkhof et al. included 143 patients, only 72 of whom received LEV or VAL as monotherapy and were finally included in the present study. Moreover, among 1148 patients enrolled, 533 took LEV as monotherapy, 200 took PHT, 267 took VAL and 48 took no antiepileptic therapy (Table 1).
Interventions
Regarding time of outcome assessment, the study period varied from 7 days 25, 58 up to 12 months 57, 61. Data from the early postoperative period (during the first 48 hours after surgery) could be extracted from only one article 56, while data from 48 hours to 4 weeks postoperatively could be retrieved from eight studies 10, 20, 26, 56, 57, 59, 61, 62. The late observation period (>4 weeks postoperatively) was terminated at 3 months in one study 26, at 6 months in three studies 18, 59, 60 and at 12 months in two studies 57, 61.
Routes of administration were mentioned in six studies 10, 18, 20, 25, 26, 56. Most used a combination of intravenous plus oral regimen, depending on patient ability to swallow. LEV administration ranged from 500 to 3000 mg daily 20, 61, from 1000 to 3000 mg daily 25, 57, from 500 to 1000 mg 26, from 1000 to 2000 mg 58, 59, 60 and from 2000 to 3000 mg 56. In most studies dose titration was attempted according to effect. Similarly, PHT administration was guided by serum levels in the study by Lim et al. (targeted to serum levels of 10–20 mg dl−1) while Iuchi and colleagues administered PHT starting with a loading dose of 15–18 mg kg−1, followed by an intravenous administration of 5–7.5 mg kg−1/day and then 250 mg daily 18, 25. The trials conducted by Milligan et al. and Merrell et al. did not present data regarding PHT dose regimen 61, 62. Finally, drug administration in the study by Lee et al. was targeted to achieve 50–100 μg ml−1 serum levels of VAL 10.
Evaluation of effectiveness
Data regarding incidence of seizures during the early postoperative period (first 48 hours postoperatively) could be extracted only from the study by Bahr et al., who reported an incidence of 3/25 for patients receiving LEV 56. Among studies evaluating incidence of seizures during the late postoperative period, the combined incidence of seizures was 41/533 for the LEV group (7.69%) 10, 20, 25, 26, 56, 57, 58, 59, 61, 62. Regarding PHT patients, the combined incidence of seizures during the late postoperative period was 22/192 (11.5%). Only two studies included patients under VAL 10. The authors showed an incidence of seizures up to 4 weeks postoperatively in 26/267 patients (9.73%) under VAL therapy. The only study that used no treatment as a control demonstrated no seizures, among 48 patients, during 4 weeks postoperatively (0/48) 59. For the late observation period, 25/168 patients under LEV experienced seizures (14.9%), compared to 26/52 (50%) under PHT. Moreover, among patients receiving no treatment, only 6/48 had seizures (12.5%).
Three studies, enrolling 243 patients in total and comparing LEV with PHT effectiveness, were included in the meta‐analysis 18, 25, 26. Due to the small number of trials included, it was not possible to extract data regarding the incidence of seizures through different study periods (first 48 hours, late postoperative period, late observation period). The combined results from these three studies showed that LEV administration was followed by significantly fewer seizures than with PHT (OR = 0.12 [0.03–0.42]: χ2 = 1.76: I2 = 0%, see Figure 2).
Figure 2.
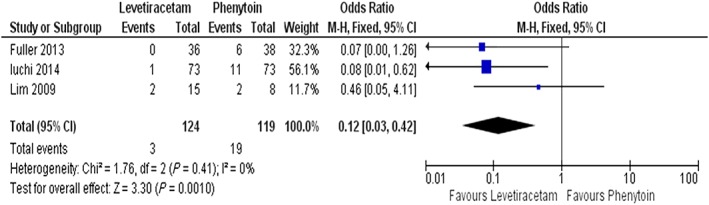
Forest plots and pooled odds ratio (OR) measures with 95% confidence interval (CI) for categorical variable of LEV (levetiracetam) and PHT (phenytoin) postoperative seizures. M‐H: Mantel–Haenszel method (fixed effect model)
Side effects
Considering the combined incidences of side effects, a total of 55/533 (10.3%) patients under LEV were recorded to have had at least one side effect, while the combined incidences for PHT and VAL were 45/200 (22.5%) and 62/267 (23.2%) respectively (Table 1). Analysis showed significant fewer side effects in patients receiving LEV, compared to other groups (P < 0.05). Three studies comparing LEV with PHT were included in the meta‐analysis. The combined results showed fewer (but not statistically significant) side effects in the LEV group, compared with the PHT group (OR = 0.65 [0.14–2.99]: χ2 = 8.79: I2 = 77%, Figure 3). A sensitivity analysis was conducted, and the pooled OR was not significantly changed when individual studies were removed each in turn (Figure 3).
Figure 3.
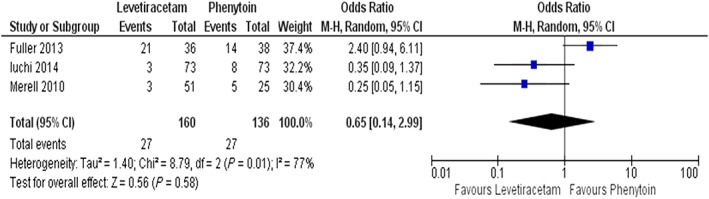
Forest plots and pooled odds ratio (OR) measures with 95% confidence interval (CI) for categorical variable of LEV and PHT side effects. M‐H: Mantel–Haenszel method (fixed effect model)
Risk of bias estimation
We assessed the risk of bias in sequence generation, allocation concealment, blinding (including participants and personnel, data collectors, outcome assessors), incomplete data and selective outcome reporting and other sources of bias (Table 2). Most of the studies enrolled are characterized by high risk of bias, due to the absence of data regarding randomization technique or blinding. Only the study conducted by Fuller et al. is characterized by proper randomization and blinding 26. However, data regarding patient blinding are missing. Publication bias analyses were not pursued because the number of studies included in the meta‐analysis was insufficient and when there are fewer than ten studies, the power of the tests is too low to distinguish chance from real asymmetry.
Table 2.
Risk of bias of the included studies
| Source | Sequence generation | Allocation concealment | Patients blinded | Personnel blinded | Data collectors blinded | Outcome assessors blinded | Incomplete outcome data | Selective reporting | Summary |
|---|---|---|---|---|---|---|---|---|---|
| Bahr et al. 56 | No | No | No | No | No | No | Low | Low | High |
| Fuller et al. 26 | No | No | No | No | No | No | Low | Low | High |
| Gokhale et al. 58 | No | No | No | No | No | No | Low | Low | High |
| Garbossa et al. 59 | Low | Low | Unclear | Low | Low | Low | Low | Low | Unclear |
| Iuchi et al. 25 | Low | Low | No | No | No | No | Unclear | Unclear | High |
| Kerkhof et al. 60 | No | No | No | No | No | No | Low | Low | High |
| Lee et al. 10 | No | No | No | No | No | No | Low | Low | High |
| Lim et al. 18 | Unclear | Unclear | No | No | No | No | Low | Low | High |
| Merrell et al. 62 | No | No | No | No | No | No | Low | Low | High |
| Milligan et al. 61 | No | No | No | No | No | No | Low | Low | High |
| Usery et al. 20 | No | No | No | No | No | No | Unclear | Unclear | High |
| Zachenhofer et al. 57 | No | No | No | No | No | No | Unclear | Unclear | High |
Discussion
In the systematic review, the efficacy and safety of LEV in patients who underwent supratentorial craniotomy for brain tumour resection was examined. Studies investigating LEV administration versus other AEDs and LEV versus no therapy were included, as well as studies investigating LEV treatment without a control group. Due to the very limited number of randomized controlled trials (RCTs) evaluating LEV administration, it was decided to also include non‐randomized prospective and retrospective studies in the eligibility criteria. A recently published systematic review by the Cochrane Collaboration evaluated different antiepileptic drugs administered pre‐ or postoperatively as prophylaxis for post‐craniotomy seizures in patients being operated for various central nervous system pathologies and not exclusively brain tumour patients 63. Although the study design of this systematic review included only randomized controlled studies, the notable heterogeneity among them ruled out the possibility of a further meta‐analysis. Moreover, Yuan et al. published a meta‐analysis in order to evaluate only efficacy and not safety of LEV in patients with brain tumours 64. They included studies with significant heterogeneity in study design as well as in intervention groups, leading to results requiring caution in clinical interpretation. To our knowledge the present study is the only systematic review combined with meta‐analysis that examines both efficacy and safety of prophylactic use of LEV in patients with brain tumours.
Based on our eligibility criteria, we included four studies examining LEV administration without comprising a control group in their study design 20, 56, 57, 58. The reported incidence of seizures during the study period was relatively low, ranging from 5.9% 20 to 25% 56. Similarly, a rather limited risk of side effects was identified, ranging from 3.8% 57 to 35.3% 20. However, these can be considered as low quality studies, as two of them are retrospective studies, and the other two, although being prospective, involve a rather limited number of participants, raising questions about the efficacy and safety of prophylactic use of LEV.
Only one study compared LEV administration with no treatment as a control group, in patients undergoing craniotomy for brain tumours 59. In this retrospective, two‐centre study, the authors evaluated the effect of perioperative LEV administration (starting 3–5 days preoperatively and continued up to 6 months after surgery), compared to a control group, in which no perioperative antiepileptic treatment was administered. The authors found that LEV prophylaxis was not a significant predictor of seizure occurrence, although the regression analysis indicated a slight reduction in seizure risk following LEV administration. Furthermore, the 30‐day incidence of seizures after surgery was extremely low (2.4% for the LEV group versus 0% for the no treatment group), while, regarding side effects, only one case of ataxia was recorded among patients.
One retrospective study compared LEV with VAL administration in craniotomy patients 10. The authors examined antiepileptic therapy starting 24 hours before operation, at doses 500 mg twice a day for LEV and 600 mg for VAL and continuing for 4 weeks postoperatively at doses 500 mg twice a day for LEV (titrated to 1500 twice a day according to seizure activity) and 600 mg twice a day for VAL (titrated to serum levels). As far as efficacy was concerned, they found that the postoperative seizure control rates of LEV and VAL acid were not statistically significantly different. Nevertheless, despite comparable incidence of seizures between groups, the authors concluded that the side effects indicated that the long‐term complication rate of the VAL group was significantly higher than that of the LEV group. In the VAL group, 10 cases with hepatic toxicity, 20 cases with hyperammonemia and 10 cases with hematologic disorders were recorded. Switching to other and/or additional anticonvulsants, because of either side effects or uncontrolled seizures, was necessary in 38.5% of the cases receiving VAL, whereas only nine patients (17.6%) in the LEV group changed treatment.
Among the included studies, five compared LEV with PHT administration 18, 25, 26, 61, 62, but only three of them were eligible, regarding LEV efficacy, for quantitative analysis, so a meta‐analysis was conducted 18, 25, 26. The meta‐analysis outlined that LEV was superior to PHT regarding the occurrence of seizures postoperatively. The included studies comprised one RCT 18 and two prospective studies 25, 26, suggesting a moderate quality of evidence. Risk of bias was also considered unclear or high for the included studies.
Another critical issue was the variability of the dose regimens used in the included studies. Despite the low heterogeneity considering efficacy, there was a significant variability of the dose regimens used among the selected studies. Fuller et al. used LEV doses ranging from 500 to 2000 mg daily, Lim et al. used a standard regimen of 2000 mg and Iuchi et al. administered 1000 mg daily 18, 25, 26. Similarly, PHT administration varied from 300 mg daily in Fuller et al. to 300–400 mg daily and 5–7.5 mg kg−1/day iv then 250 mg daily in Lim et al. and Iuchi et al. respectively 18, 25, 26. Furthermore, investigators titrated dose regimen according to effect. Consequently it is difficult to conclude about doses in which LEV demonstrates its beneficial effect compared to PHT.
Among studies comparing LEV with PHT administration which were not included in the meta‐analysis, Milligan et al. found a similar risk for early and late seizures between the two anticonvulsants, but LEV administration was superior to PHT in terms of the occurrence of side effects 61. Similarly, the study conducted by Merrell et al. (not included in meta‐analysis), demonstrated comparable occurrence of seizures and side effects between LEV and PHT patients 62. Both studies had a retrospective design while data regarding dose regimens could not be retrieved from the Merrell et al. study 62.
Considering side effects, three studies comparing LEV with PHT administration were subjected to quantitative analysis 25, 26, 62. The meta‐analysis conducted suggested that the rate of side effects was rarer in LEV patients compared to PHT patients (OR = 0.65 [0.14–2.99]: χ2 = 8.79, Figure 3). However, the included studies are characterized by high heterogeneity regarding side effects: I2 = 77%. Additionally, among studies comparing LEV with PHT administration which were not included in the meta‐analysis, Milligan et al. reported 1/43 patients under LEV treatment versus 14/56 under PHT with side effects, while Lim et al. reported 0/15 LEV patients compared to 4/8 PHT patients presenting with side effects 18, 61.
Conclusions
In conclusion, few studies examined efficacy and safety of LEV administration in the perioperative period for controlling seizures. Only three studies (one RCT and two prospective studies) that compared LEV with PHT administration could be subjected to quantitative analysis regarding the occurrence of seizures. According to the analysis, LEV administration seems to be more effective in controlling postoperative seizures. Similarly, only three studies comparing LEV with PHT administration could be included in the meta‐analysis regarding side effects, demonstrating fewer not statistically significant side effects in LEV patients. Nevertheless, a high risk of bias and moderate methodological quality must be taken into account when considering these results. Consequently, further well‐designed studies are necessary in order to confirm the superiority of LEV as prophylaxis for controlling seizures in the perioperative period.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: all authors report no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Appendix 1.
Searching strategy, combining free text and medical subject headings (MeSH terms) was set up for PUBMED as follows:
(“keppra”[All Fields] OR “levetiracetam”[All Fields]) AND ((“surgery”[Subheading] OR “surgery”[All Fields] OR “surgical procedures, operative”[MeSH Terms] OR (“surgical”[All Fields] AND “procedures”[All Fields] AND “operative”[All Fields]) OR “operative surgical procedures”[All Fields] OR “surgery”[All Fields] OR “general surgery”[MeSH Terms] OR (“general”[All Fields] AND “surgery”[All Fields]) OR “general surgery”[All Fields]) OR (“craniotomy”[MeSH Terms] OR “craniotomy”[All Fields]) OR (“surgical procedures, operative”[MeSH Terms] OR (“surgical”[All Fields] AND “procedures”[All Fields] AND “operative”[All Fields]) OR “operative surgical procedures”[All Fields] OR “operation”[All Fields]) OR (“brain tumour”[All Fields] OR “brain neoplasms”[MeSH Terms] OR (“brain”[All Fields] AND “neoplasms”[All Fields]) OR “brain neoplasms”[All Fields] OR (“brain”[All Fields] AND “tumour”[All Fields]) OR “brain tumour”[All Fields]) OR (“supratentorial tumour”[All Fields] OR “supratentorial neoplasms”[MeSH Terms] OR (“supratentorial”[All Fields] AND “neoplasms”[All Fields]) OR “supratentorial neoplasms”[All Fields] OR (“supratentorial”[All Fields] AND “tumour”[All Fields]) OR “supratentorial tumour”[All Fields]) OR ((“seizures”[MeSH Terms] OR “seizures”[All Fields] OR “seizure”[All Fields]) AND (“prevention and control”[Subheading] OR (“prevention”[All Fields] AND “control”[All Fields]) OR “prevention and control”[All Fields] OR “prophylaxis”[All Fields]))).
Searching strategy, using combination of terms was set up for Scopus/Elsevier as follows: TITLE‐ABS‐KEY ((“keppra” OR “levetiracetam”) AND (surgery OR craniotomy OR operation OR “brain tumour” OR “supratentorial tumour” OR “seizure prophylaxis”)).
Searching strategy, using combination of terms was set up for EMBASE as follows: SUBJECT HEADING: ((“keppra” OR “levetiracetam”), USED FOR (surgery OR craniotomy OR operation OR brain tumour OR supratentorial tumour OR seizure prophylaxis)).
Searching strategy, using combination of terms was set up for The International Web of Science as follows: TOPIC: ((“keppra” OR “levetiracetam”) AND (surgery OR craniotomy OR operation OR brain tumour OR supratentorial tumour OR seizure prophylaxis)).
Searching strategy, using combination of terms was set up for The Cochrane Central Register of Controlled Trials (CENTRAL) as follows: Levetiracetam, Levetiracetam AND surgery.
Pourzitaki, C. , Tsaousi, G. , Apostolidou, E. , Karakoulas, K. , Kouvelas, D. , and Amaniti, E. (2016) Efficacy and safety of prophylactic levetiracetam in supratentorial brain tumour surgery: a systematic review and meta‐analysis. Br J Clin Pharmacol, 82: 315–325. doi: 10.1111/bcp.12926.
References
- 1. Shaw MD, Foy PM. Epilepsy after craniotomy and the place of prophylactic anticonvulsant drugs: discussion paper. J R Soc Med 1991; 84: 221–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herman ST. Epilepsy after brain insult: targeting epileptogenesis. Neurology 2002; 59: S21–6. [DOI] [PubMed] [Google Scholar]
- 3. Manaka S, Ishijima B, Mayanagi Y. Postoperative seizures: epidemiology, pathology, and prophylaxis. Neurol Med Chir (Tokyo) 2003; 43: 589–600. [DOI] [PubMed] [Google Scholar]
- 4. Bartolini E, Lenzi B, Vannozzi R, Parenti GF, Iudice A. Incidence and management of late postsurgical seizures in clinical practice. Turk Neurosurg 2012; 22: 651–5. [DOI] [PubMed] [Google Scholar]
- 5. Glantz MJ, Cole BF, Forsyth PA, Recht LD, Wen PY, Chamberlain MC, Grossman SA, Cairncross JG. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2000; 54: 1886–93. [DOI] [PubMed] [Google Scholar]
- 6. Sperling MR, Ko J. Seizures and brain tumors. Semin Oncol 2006; 33: 333–41. [DOI] [PubMed] [Google Scholar]
- 7. Mintzer S, Mattson RT. Should enzyme‐inducing antiepileptic drugs be considered first‐line agents? Epilepsia 2009; 50: 42–50. [DOI] [PubMed] [Google Scholar]
- 8. Nasreddine W, Beydoun A. Valproate‐induced thrombocytopenia: a prospective monotherapy study. Epilepsia 2008; 49: 438–45. [DOI] [PubMed] [Google Scholar]
- 9. Szupera Z, Mezei Z, Kis B, Gecse A, Vecsei L, Telegdy G. The effects of valproate on the arachidonic acid metabolism of rat brain microvessels and of platelets. Eur J Pharmacol 2000; 387: 205–10. [DOI] [PubMed] [Google Scholar]
- 10. Lee YJ, Kim T, Bae SH, Lee YJ, Kim T, Bae SH, Kim YH, Han JH, Yun CH, Kim CY. Levetiracetam compared with valproic acid for the prevention of postoperative seizures after supratentorial tumor surgery: a retrospective chart review. CNS Drugs 2013; 27: 753–9. [DOI] [PubMed] [Google Scholar]
- 11. Zafar SN, Khan AA, Ghauri AA, Shamim MS. Phenytoin versus leviteracetam for seizure prophylaxis after brain injury – a meta analysis. BMC Neurol 2012; 12: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lloyd P, Flesch G, Dieterle W. Clinical pharmacology and pharmacokinetics of oxcarbazepine. Epilepsia 1994; 35: S10–3. [DOI] [PubMed] [Google Scholar]
- 13. Crepeau AZ, Treiman DM. Levetiracetam: a comprehensive review. Expert Rev Neurother 2010; 10: 159–71. [DOI] [PubMed] [Google Scholar]
- 14. Lynch BA, Lambeng N, Nocka K, Kensel‐Hammes P, Bajjalieh SM, Matagne A, Fuks B. The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam. Proc Natl Acad Sci U S A 2004; 101: 9861–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. French J, Edrich P, Cramer JA. A systematic review of the safety profile of levetiracetam: a new antiepileptic drug. Epilepsy Res 2001; 47: 77–90. [DOI] [PubMed] [Google Scholar]
- 16. Zelano J, Kumlien E. Levetiracetam as alternative stage two antiepileptic drug in status epilepticus: a systematic review. Seizure 2012; 21: 233–6. [DOI] [PubMed] [Google Scholar]
- 17. Bauer J, Ben‐Menachem E, Krämer G, Fryze W, Da SS, Kasteleijn‐Nolst Trenité DG. Levetiracetam: a long‐term follow‐up study of efficacy and safety. Acta Neurol Scand 2006; 114: 169–76. [DOI] [PubMed] [Google Scholar]
- 18. Lim DA, Tarapore P, Chang E, Burt M, Chakalian L, Barbaro N, Chang S, Lamborn KR, McDermott MW. Safety and feasibility of switching from phenytoin to levetiracetam monotherapy for glioma‐related seizure control following craniotomy: a randomized phase II pilot study. J Neurooncol 2009; 93: 349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wagner GL, Wilms EB, Van Donselaar CA, Vecht C. Levetiracetam: preliminary experience in patients with primary brain tumours. Seizure 2003; 12: 585–6. [DOI] [PubMed] [Google Scholar]
- 20. Usery JB, Michael LM, Sills AK, Finch CK. A prospective evaluation and literature review of levetiracetam use in patients with brain tumors and seizures. J Neurooncol 2010; 99: 251–60. [DOI] [PubMed] [Google Scholar]
- 21. Thorlund K, Wetterslev J, Awad T, Thabane L, Gluud C. Comparison of statistical inferences from the DerSimonian‐Laird and alternative random‐effects model meta‐analyses – an empirical assessment of 920 Cochrane primary outcome meta‐analyses. Res Synth Methods 2011; 2: 238–53. [DOI] [PubMed] [Google Scholar]
- 22. Takada M, Sozu T, Sato T. Practical approaches for design and analysis of clinical trials of infertility treatments: crossover designs and the Mantel–Haenszel method are recommended. Pharm Stat 2015; 14: 198–204. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. London: The Cochrane Collaboration, 2011. . Available from www.cochrane‐handbook.org.Version 5.1.0 [updated March 2011] [Google Scholar]
- 24. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Iuchi T, Kuwabara K, Matsumoto M, Kawasaki K, Hasegawa Y, Sakaida T. Levetiracetam versus phenytoin for seizure prophylaxis during and early after craniotomy for brain tumours: a phase II prospective, randomised study. J Neurol Neurosurg Psychiatry 2015; 86: 1158–62. [DOI] [PubMed] [Google Scholar]
- 26. Fuller KL, Wang YY, Cook MJ, Murphy MA, D'Souza WJ. Tolerability, safety, and side effects of levetiracetam versus phenytoin in intravenous and total prophylactic regimen among craniotomy patients: a prospective randomized study. Epilepsia 2013; 54: 45–57. [DOI] [PubMed] [Google Scholar]
- 27. Kazerooni R, Bounthavong M. Cost‐effectiveness analysis of intravenous levetiracetam versus intravenous phenytoin for early onset seizure prophylaxis after neurosurgery and traumatic brain injury. Clinicoecon Outcomes Res 2010; 2: 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Komotar RJ, Raper DM, Starke RM, Iorgulescu JB, Gutin PH. Prophylactic antiepileptic drug therapy in patients undergoing supratentorial meningioma resection: a systematic analysis of efficacy. J Neurosurg 2011; 115: 483–90. [DOI] [PubMed] [Google Scholar]
- 29. Sughrue ME, Rutkowski MJ, Chang EF, Shangari G, Kane AJ, McDermott MW, Berger MS, Parsa AT. Postoperative seizures following the resection of convexity meningiomas: are prophylactic anticonvulsants indicated? Clinical article. J Neurosurg 2011; 114: 705–9. [DOI] [PubMed] [Google Scholar]
- 30. Dinapoli L, Maschio M, Jandolo B, Fabi A, Pace A, Sperati F, Muti P. Quality of life and seizure control in patients with brain tumor‐related epilepsy treated with levetiracetam monotherapy: preliminary data of an open‐label study. Neurol Sci 2009; 30: 353–9. [DOI] [PubMed] [Google Scholar]
- 31. Maschio M, Dinapoli L, Sperati F, Pace A, Fabi A, Vidiri A, Muti P. Levetiracetam monotherapy in patients with brain tumor‐related epilepsy: seizure control, safety, and quality of life. J Neurooncol 2011; 104: 205–14. [DOI] [PubMed] [Google Scholar]
- 32. van Breemen MS, Rijsman RM, Taphoorn MJ, Walchenbach R, Zwinkels H, Vecht CJ. Efficacy of anti‐epileptic drugs in patients with gliomas and seizures. J Neurol 2009; 256: 1519–26. [DOI] [PubMed] [Google Scholar]
- 33. Rossetti AO, Jeckelmann S, Novy J, Roth P, Weller M, Stupp R. Levetiracetam and pregabalin for antiepileptic monotherapy in patients with primary brain tumors: a phase II randomized study. Neuro Oncol 2014; 16: 584–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maschio M, Dinapoli L, Gomellini S, Ferraresi V, Sperati F, Vidiri A, Muti P, Jandolo B. Antiepileptics in brain metastases: safety, efficacy and impact on life expectancy. J Neurooncol 2010; 98: 109–16. [DOI] [PubMed] [Google Scholar]
- 35. Rosati A, Buttolo L, Stefini R, Todeschini A, Cenzato M, Padovani A. Efficacy and safety of levetiracetam in patients with glioma: a clinical prospective study. Arch Neurol 2010; 67: 343–6. [DOI] [PubMed] [Google Scholar]
- 36. Szaflarski JP, Sangha KS, Lindsell CJ, Shutter LA. Prospective, randomized, single‐blinded comparative trial of intravenous levetiracetam versus phenytoin for seizure prophylaxis. Neurocrit Care 2010; 12: 165–72. [DOI] [PubMed] [Google Scholar]
- 37. Maschio M, Dinapoli L, Vidiri A, Pace A, Fabi A, Pompili A, Carapella MC, Jandolo B. The role side effects play in the choice of antiepileptic therapy in brain tumor‐related epilepsy: a comparative study on traditional antiepileptic drugs versus oxcarbazepine. J Exp Clin Cancer Res 2009; 28: 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Swisher CB, Doreswamy M, Gingrich KJ, Vredenburgh JJ, Kolls BJ. Phenytoin, levetiracetam, and pregabalin in the acute management of refractory status epilepticus in patients with brain tumors. Neurocrit Care 2012; 16: 109–13. [DOI] [PubMed] [Google Scholar]
- 39. Kern K, Schebesc KM, Schlaier J, Hansen E, Feigl GC, Brawanski AT, Lange M. Levetiracetam compared to phenytoin for the prevention of postoperative seizures after craniotomy for intracranial tumours in patients without epilepsy. J Clin Neurosci 2012; 19: 99–100. [DOI] [PubMed] [Google Scholar]
- 40. Bernett A, Phenis R, Fonkem E, Aceves J, Kirmani B, Cruz‐Laureano D. Neurobehavioral effects of levetiracetam in brain tumor related epilepsy. Front Neurol 2013; 4: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dominguez‐Paez M, Herranz‐Fernandez JL, Villanueva‐Haba V, Sanchez‐Alvarez JC, Olivares‐Granados G, Sola RG, Albisua‐Sanchez J, Arraez‐Sanchez MA, Mosqueira‐Centurion B, Amaro‐Cendon S, Bollar‐Zabala A, Carceller‐Benito F, Salazar‐Hernandez J, Fernandez‐Carballal C, Garcia‐Allut A, Garcia‐Navarrete E, Gutierrez‐Martin A, Lara‐Cantalejo JL, Marquez‐Rivas J, Oliver‐Abadal B, Pomposo‐Gaztelu IN, Prieto‐Gonzalez A, Rumia‐Arboix J, Urculo‐Bareno E. [Primary prophylaxis of early seizures after surgery of cerebral supratentorial tumors: Group for the Study of Functional‐Sterotactic Neurosurgery of The Spain Society of Neurosurgery recommendations]. Neurocirugia (Astur) 2012; 23: 29–35. [DOI] [PubMed] [Google Scholar]
- 42. Engrand N, Osinski D. Antiepileptic prophylaxis for elective neurosurgery. Ann Fr Anesth Reanim 2012; 31: e235–46. [DOI] [PubMed] [Google Scholar]
- 43. Fonkem E, Bricker P, Mungall D, Aceves J, Ebwe E, Tang W, Kirmani B. The role of levetiracetam in treatment of seizures in brain tumor patients. Front Neurol 2013; 4: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kerrigan S, Grant R. Antiepileptic drugs for treating seizures in adults with brain tumours. Cochrane Database Syst Rev 2011; CD008586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Klimek M, Dammers R. Antiepileptic drug therapy in the perioperative course of neurosurgical patients. Curr Opin Anaesthesiol 2010; 23: 564–7. [DOI] [PubMed] [Google Scholar]
- 46. Maschio M, Dinapoli L, Zarabia A, Jandolo B. Issues related to the pharmacological management of patients with brain tumours and epilepsy. Funct Neurol 2006; 21: 15–9. [PubMed] [Google Scholar]
- 47. Rossetti AO, Stupp R. Epilepsy in brain tumor patients. Curr Opin Neurol 2010; 23: 603–9. [DOI] [PubMed] [Google Scholar]
- 48. Ruda R, Bello L, Duffau H, Soffietti R. Seizures in low‐grade gliomas: natural history, pathogenesis, and outcome after treatments. Neuro Oncol 2012; 14: iv55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rowe AS, Goodwin H, Brophy GM, Bushwitz J, Castle A, Deen D, Johnson D, Lesch C, Liang N, Potter E, Roels C, Samaan K, Rhoney DH. Seizure prophylaxis in neurocritical care: a review of evidence‐based support. Pharmacotherapy 2014; 34: 396–409. [DOI] [PubMed] [Google Scholar]
- 50. Shershever AS, Bentsion DL, Lavrova SA, Sorokova EV. [Use of keppra during the radiotherapy in patients with brain tumors and epileptic seizures after surgical treatment]. Zh Nevrol Psikhiatr Im S S Korsakova 2008; 108: 31–6. [PubMed] [Google Scholar]
- 51. van Breemen MS, Vecht CJ. Optimal seizure management in brain tumor patients. Curr Neurol Neurosci Rep 2005; 5: 207–13. [DOI] [PubMed] [Google Scholar]
- 52. van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol 2007; 6: 421–30. [DOI] [PubMed] [Google Scholar]
- 53. Vecht CJ, van Breemen M. Optimizing therapy of seizures in patients with brain tumors. Neurology 2006; 67: S10–3. [DOI] [PubMed] [Google Scholar]
- 54. Vecht CJ, Wilms EB. Seizures in low‐ and high‐grade gliomas: current management and future outlook. Expert Rev Anticancer Ther 2010; 10: 663–9. [DOI] [PubMed] [Google Scholar]
- 55. van Breemen MS, Wilms EB, Vecht CJ. Seizure control in brain tumors. Handb Clin Neurol 2012; 104: 381–9. [DOI] [PubMed] [Google Scholar]
- 56. Bahr O, Hermisson M, Rona S, Rieger J, Nussbaum S, Kortvelyessy P, Franz K, Tatagiba M, Seifert V, Weller M, Steinbach JP. Intravenous and oral levetiracetam in patients with a suspected primary brain tumor and symptomatic seizures undergoing neurosurgery: the HELLO trial. Acta Neurochir 2012; 154: 229–35. [DOI] [PubMed] [Google Scholar]
- 57. Zachenhofer I, Donat M, Oberndorfer S, Roessler K. Perioperative levetiracetam for prevention of seizures in supratentorial brain tumor surgery. J Neurooncol 2011; 101: 101–6. [DOI] [PubMed] [Google Scholar]
- 58. Gokhale S, Khan SA, Agrawal A, Friedman AH, McDonagh DL. Levetiracetam seizure prophylaxis in craniotomy patients at high risk for postoperative seizures. Asian J Neurosurg 2013; 8: 169–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Garbossa D, Panciani PP, Angeleri R, Battaglia L, Tartara F, Ajello M, Agnoletti A, Versari P, Ducati A, Fontanella M, Spena G. A retrospective two‐center study of antiepileptic prophylaxis in patients with surgically treated high‐grade gliomas. Neurol India 2013; 61: 131–7. [DOI] [PubMed] [Google Scholar]
- 60. Kerkhof M, Dielemans JC, van Breemen MS, Zwinkels H, Walchenbach R, Taphoorn MJ, Vecht CJ. Effect of valproic acid on seizure control and on survival in patients with glioblastoma multiforme. Neuro Oncol 2013; 15: 961–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Milligan TA, Hurwitz S, Bromfield EB. Efficacy and tolerability of levetiracetam versus phenytoin after supratentorial neurosurgery. Neurology 2008; 71: 665–9. [DOI] [PubMed] [Google Scholar]
- 62. Merrell RT, Anderson SK, Meyer FB, Lachance DH. Seizures in patients with glioma treated with phenytoin and levetiracetam. J Neurosurg 2010; 113: 1176–81. [DOI] [PubMed] [Google Scholar]
- 63. Weston J, Greenhalgh J, Marson AG. Antiepileptic drugs as prophylaxis for post‐craniotomy seizures. Cochrane Database Syst Rev 2015; 3: CD007286. [DOI] [PubMed] [Google Scholar]
- 64. Yuan Y, Peizhi Z, Maling G, Wu L, Yunhe M, Xiang W, Qing M, Yanhui L, Ruofei L, Jiewen L. The efficacy of levetiracetam for patients with supratentorial brain tumors. J Clin Neurosci 2015; 22: 1227–31. [DOI] [PubMed] [Google Scholar]


