Abstract
Aims
The aim of the study was to evaluate the effect of an e‐learning educational program meant to foster the quality of drug prescription in hospitalized elderly patients.
Methods
Twenty geriatric and internal medicine wards were randomized to intervention (e‐learning educational program) or control (basic geriatric pharmacology notions). Logistic regression analysis was used in order to assess the effect of the intervention on the use of potentially inappropriate medication (PIM, primary outcome) at hospital discharge. Secondary outcomes were a reduced prevalence of at least one potential drug–drug interaction (DDI) and potentially severe DDI at discharge. Mortality rate and incidence of re‐hospitalizations were other secondary outcomes assessed at the 12‐month follow‐up.
Results
A total of 697 patients (347 in the intervention and 350 in the control arms) were enrolled. No difference in the prevalence of PIM at discharge was found between arms (OR 1.29 95%CI 0.87–1.91). We also found no decrease in the prevalence of DDI (OR 0.67 95%CI 0.34–1.28) and potentially severe DDI (OR 0.86 95%CI 0.63–1.15) at discharge, nor in mortality rates and incidence of re‐hospitalization at 12‐month follow‐up.
Conclusions
This e‐learning educational program had no clear effect on the quality of drug prescription and clinical outcomes in hospitalized elderly patients. Given the high prevalence of PIMs and potential DDIs recorded in the frame of this study, other approaches should be developed in order to improve the quality of drug prescription in this population.
Keywords: comprehensive geriatric assessment, drug prescription, drug–drug interaction, elderly people, E‐learning, potentially inappropriate medication
What is Already Known about this Subject
Older people usually have multiple chronic diseases, so that they are often exposed to chronic polypharmacy and to a high risk of prescription of potentially inappropriate medications and drug–drug interactions.
E‐learning is a educational technology with several advantages, never used before for improvement of drug prescription in hospitalized older people.
What this Study Adds
This e‐learning educational program failed to improve the quality of drug prescription in older patients acutely hospitalized in internal medicine and geriatrics wards.
But this randomized‐controlled study confirms the high prevalence of PIM, DDI and severe DDI in hospitalized older people and confirms that drug‐related problems and drug–drug interactions are often underestimated issues among clinicians.
Introduction
In Western countries, people aged 75 years or older are the fastest growing segment of the population, and will account for more than 20% of the total population by 2060 1. This group is characterized by the occurrence of multiple chronic diseases, almost always accompanied by the use of multiple drugs (polypharmacy) 1, 2, 3. Polypharmacy has been associated with negative outcomes, including adverse drug reactions (ADRs) and increased risks of morbidity, mortality and multiple hospital admissions 2, 4. Older people are usually frail and more susceptible to ADRs owing to changes in pharmacokinetics and pharmacodynamics 5, so that ADR prevalence has been estimated to range from 10% to more than 60% in hospitalized older persons 6. Although many ADRs are unpredictable, others can be foreseen and prevented, such as those due to well‐established drug–drug interactions (DDIs) 6, 7. This picture is further complicated by the high risk of prescription of potentially inappropriate medications (PIMs), with a prevalence between 20% and 77% in hospital settings 8, 9, 10. All in all, because the management of drugs in the elderly represents a relevant challenge for prescribing clinicians, geriatricians developed the Comprehensive Geriatric Assessment (CGA) 11, 12, 13, 14, a tool demonstrated to help to prioritize health problems and needs, simplify drug prescription and reduce the risk of drug‐related problems 12. Despite its widespread use, CGA is usually taught in formal courses, while new technologies and teaching methods have scarcely been implemented for this purpose. E‐learning is a technology based upon the use of the internet to deliver an array of educational materials that enhance knowledge and performance in comparison with more traditional educational methods 15, 16. Other advantages are easy access and reduced costs 17, 18.
With this background, an investigator‐driven, randomized, controlled pragmatic study (Project no. FARM87SA2B) was designed, within the framework of the Italian Program for Independent Research, in order to evaluate whether or not an e‐learning program teaching CGA and basic geriatric pharmacological notions was able to improve the quality of drug prescription in older patients hospitalized in internal medicine and geriatric wards. Another aim was to assess the impact of such an intervention on such clinical outcomes as length of hospital stay, overall mortality and re‐hospitalization during a 12‐month follow‐up. A pilot study was undertaken before starting the full project in order to demonstrate its feasibility and to estimate the study sample size 19.
Methods
Study design
This is a cluster randomized, single‐blind controlled study (where the cluster was the hospital wards) performed in 20 Italian internal medicine and geriatric wards.
Study population
All patients aged 75 years or over consecutively admitted to the participating wards were eligible. Exclusion criteria were consent refusal or estimated life expectancy of less than 6 months.
Ward selection
Ten internal medicine and ten geriatric wards of Italian hospitals were selected. Among these, ten were academic and ten were not academic wards. The wards participating in the pilot phase remained in the same randomization arm in the full study.
Intervention
All physicians in the participating wards were involved in the study. Every clinician had to finish his/her e‐learning program within 1 month from the start of the study in his/her ward. The enrolment of patients began soon after the completion of training.
E‐learning platform
E‐learning was delivered through an interactive web‐based platform accessed by a personal identification code and password. A system meant to assess the implementation and completion by clinicians of the e‐learning training was also set up. An automatic electronic system recorded and rated for each participating clinician the number of access occasions, the time spent on each e‐learning module, the right/wrong answers and the number of attempts needed to complete each module correctly.
Contents of e‐learning for the intervention arm
The program delivered to clinicians on the wards randomly assigned to the intervention arm included notions of CGA and geriatric pharmacology, together with training for the use of a third generation assessment instrument (InterRAI Acute Care) 20, 21. The course on geriatric pharmacology was structured in three main areas and five modules as follows: Area 1: main concepts of CGA (Module A). Area 2: general geriatric pharmacology notions (Module B). Area 3: prescription appropriateness and related issues in older adults: (a) assessment and management of patients exposed to polypharmacy (Module C); (b) criteria and tools for the revision and evaluation of prescription appropriateness in older people, such as Beers Criteria, Screening Tool of Older Person's Prescriptions (STOPP), Assessing Care of the Vulnerable Elderly (ACOVE), Inappropriate Prescribing in the Elderly Tool (IPET) and the Medication Appropriateness Index (MAI) (Module D); (c) criteria and tools to evaluate potential drug–drug interactions (Module E). The access to and utilization of each teaching module was linked to a self‐evaluation test and to specific centralized controls. Each module was divided in four sub‐modules that each participant completed with specific case reports and questions. The INTERcheck® software, a computerized prescription support system 22, was made available to clinicians in the intervention arm through the interactive web‐based platform, separately from the electronic clinical report form.
Contents of e‐learning for the control arm
The e‐learning program for clinicians of the control arm consisted only of a refresher on the basic notions of geriatric pharmacology using Module B as a weapon.
Primary and secondary study outcomes
The primary outcome of the study was whether or not there was a reduction in the prescriptions at hospital discharge of PIMs, as defined by the updated (2012) Beers Criteria 23, 24. Patients were considered exposed to PIM if at least one of the Beers Criteria was met. Secondary outcomes were whether or not at discharge there was a reduction of prescription of potential DDIs (PDDIs) or potentially severe DDIs, and to evaluate the clinical impact of the integrated e‐learning intervention on the length of hospital stay, mortality and incidence of any re‐hospitalization during the 12‐month follow‐up period. The presence of potential DDIs regarding the 20 most frequently prescribed combinations was defined by the presence at discharge of at least one combination of potentially interacting drugs. The INTERcheck® software was used to detect PIMs and DDIs 22.
Sample size
We assumed that the baseline rate of PIM in both groups would be 25% 8. The criterion for statistical significance (alpha) was set at 0.05 and the test was two‐tailed. With a proposed sample size of 250 cases and 250 controls, the study has a power of 80% to yield a statistically significant result in the presence of a 10% difference in PIM rate (specifically, 25% vs. 15%). As the unit of randomization was the medical ward, a correction factor was introduced based upon an estimated 0.01 intraclass correlation coefficient, yielding the total number of 350 cases and 350 controls to be enrolled in the study. This sample size was confirmed by the feasibility pilot study results 19.
Randomization
The 20 hospital wards were centrally randomized to the intervention (n = 10) or control arm (n = 10). All physicians received a personal user identification code and a password to access the e‐learning platform, which provided access only to the ward assigned by randomization to e‐learning. The randomization of wards was balanced taking into account clinical specialty (geriatric or internal medicine), academic setting or not and the allocation group assigned in the feasibility pilot study 19.
Blinding
The individual patient codes and the codes of participating wards and physicians were anonymized. All investigators involved in data collection were blinded to arm allocation.
Ethical aspects
The study was conducted in accordance with Good Clinical Practices and was regulated by the latest revision of the Helsinki Declaration. All the data were managed according to the Italian law on privacy, and all enrolled patients were appropriately informed about the study aims and were requested to sign a written consent. No experimental procedure was performed on patients outside best routine clinical practice. The study was first approved by the Ethical Committee of the coordinating clinical unit (IRCCS Cà Granda Maggiore Hospital Foundation, Milan, Italy) and then also by the Ethical Committees of all other participating hospitals.
Statistical analysis
To analyze the primary outcome, we assessed the primary endpoint (difference between intervention and control arms in the prevalence of subjects with at least one PIM at discharge) by means of a logistic regression analysis without covariates in the intention‐to‐treat population (697 patients). For patients who died in hospital (nine and six in the treatment and control arms, respectively), the last available in‐hospital drug regimen was used to define PIM and DDI. Since the mean number of diagnoses at baseline was different between the two study arms, a further analysis was performed using as covariate the number of diagnoses. The same analyses were repeated in the subpopulation of patients on polypharmacy. We also tested whether or not the prevalence of patients who increased their number of PIMs was different between the two arms, using logistic regression. Per‐protocol analyses were also performed on patients discharged alive. Clustered robust standard errors were employed in order to correct for non‐independence among subjects, both for primary and secondary outcomes. We also explored whether or not an association existed between mean number of errors made by clinicians during the training phase and the presence of at least a PIM. To this end we used a multilevel logistic regression analysis among the ten centres randomized to intervention and separately among the control centres. The number of errors is defined as the number of wrong attempts made by the doctors of each ward before getting the right answer to the test questions.
To analyze the secondary outcomes, differences in prevalence regarding the presence of at least one potential DDI or potentially severe DDI at discharge were treated in the same way as PIM. Survival analysis was used to explore the effects on mortality and re‐hospitalization rates of the intervention arm at 12 months of follow‐up using a log‐rank test. Data were analysed using JMP Pro v. 11 (SAS Institute Inc.) and Stata IC 13.1 (Stata Inc.).
Results
Figure 1 provides the flow chart of the study. Ninety per cent of the clinicians involved in the study completed the e‐learning program. Between January and June 2013, 1715 patients (814 in the intervention and 901 in the control arms) were admitted to the 20 hospital wards and were assessed for eligibility. Among them, a total of 697 patients (347 intervention and 350 control) were ultimately included in the study.
Figure 1.
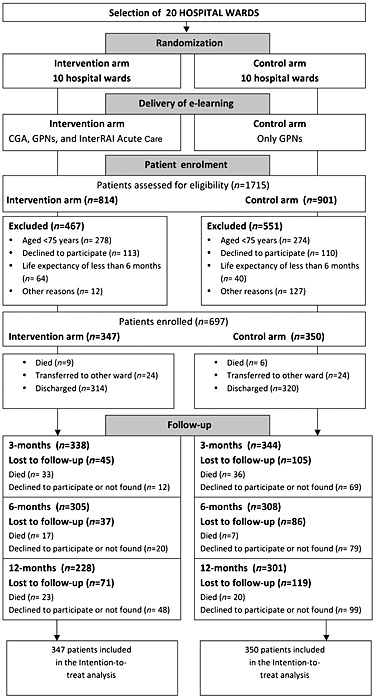
Study flow chart. CGA: Comprehensive Geriatric Assesment; GPNs: Geriatric pharmacological notions
Sample characteristics
Baseline characteristics at hospital admission and discharge of the 697 patients randomized to intervention or control are shown in Table 1. There were no between‐arm statistically significant differences pertaining to socio‐demographic variables, risk factor and clinical variables, except for the number of diagnoses (P < 0.0001), CIRS comorbidity index (P < 0.0001) and CIRS severity index (P < 0.0001) both at admission and discharge. Both in the intervention and control arms the most frequent comorbidities were hypertension (274/347, 79% vs. 281/350, 80%), chronic kidney disease (92/347, 26% vs. 81/350, 23%) and ischemic heart disease (73/347, 21% vs. 69/350, 20%).
Table 1.
Patient characteristics at hospital admission and discharge in relation to the allocation arm
| Intervention arm n = 347 | Control arm n = 350 | ||||
|---|---|---|---|---|---|
| Socio‐demographic variables | |||||
| Females, n (%) | 205 (59.1) | 197 (56.3) | |||
| Age (years), mean (±SD) | 83.7 (±5.9) | 83.8 (±5.6) | |||
| Age groups, n (%) | |||||
| <85 | 208 (60,0) | 207 (59,1) | |||
| <85 | 139 (40.0) | 143 (40.9) | |||
| Education (years), mean (±SD) | 7.2 (±4.2) | 6.5 (±3.9) | |||
| Marital status, n (%) | |||||
| Married | 137 (39.5) | 137 (39.1) | |||
| Widow/er | 171 (49.3) | 181 (51.7) | |||
| Single (alone,separated, divorced) | 28 (8.1) | 24 (6.9) | |||
| Missing | 8 (2.3) | 8 (2.3) | |||
| With whom lives, n (%) | |||||
| Alone | 85 (24.5) | 83 (23.7) | |||
| With partner o children | 170 (48.6) | 217 (62.0) | |||
| Other | 40 (11.5) | 20 (5.7) | |||
| Missing | 52 (15.0) | 30 (8.6) | |||
| Caregiver, n (%) | |||||
| Yes | 173 (49.9) | 172 (49.1) | |||
| No | 174 (50.1) | 162 (46.3) | |||
| Missing | 0 | 16 (4.6) | |||
| Risk factors | |||||
| Smoke, n (%) | |||||
| Never smoker | 176 (50.7) | 183 (52.3) | |||
| Past smoker | 118 (34.0) | 116 (33.1) | |||
| Smoker | 32 (9.2) | 23 (6.6) | |||
| Missing | 21 (6.1) | 28 (8) | |||
| Consumer of alcohol, n (%) | |||||
| Never drinker | 230 (66.3) | 241 (68.9) | |||
| Past drinker | 16 (4.6) | 25 (7.1) | |||
| Drinker | 78 (22.5) | 54 (15.4) | |||
| Missing | 23 (6.6) | 30 (8.6) | |||
| Clinical variables | |||||
| Previous 6‐month hospital admission, n (%) | 98 (28.2) | 84 (24.0) | |||
| Barthel Index, mean (±SD) | 71.6 (±30.9) | 71.3 (±29.8) | |||
| Barthel Index, n (%) | |||||
| 0–24 | 31 (11.2) | 34 (9.7) | |||
| 25–49 | 29 (8.4) | 41 (11.7) | |||
| 50–74 | 60 (17.3) | 51 (14.6) | |||
| 75–90 | 63 (18.1) | 69 (19.7) | |||
| 91–100 | 133 (38.3) | 118 (33.7) | |||
| Missing | 31 (6.7) | 37 (10.6) | |||
| Geriatric Depression Scale (GDS), mean (±SD) | 1.32 (±1.2) | 1.29 (±1.2) | |||
| Mini Mental State Examination (MMSE), mean (±SD) | 23.5 (±5.9) | 22.6 (±6.0) | |||
| Mini Mental State Examination (MMSE), n (%) | |||||
| 0–17 | 44 (7.9) | 54 (15.4) | |||
| 18–23 | 76 (21.9) | 84 (24.0) | |||
| 24–30 | 183 (52.7) | 156 (44.6) | |||
| Missing | 44 (7.9) | 56 (16.0) | |||
| Admission | Discharge | ||||
|---|---|---|---|---|---|
| Intervention arm n = 347 | Control arm n = 350 | Intervention arm n = 347 | Control arm n = 350 | ||
| Number of diagnoses, mean (±SD) | 7.5 (±3.4) | 6.0 (±2.8) | 8.2 (±3.4) | 6.5 (±2.9) | |
| 5 or more diagnoses, n (%) | 310 (89.3) | 292 (83.4) | 298 (85.9) | 254 (72.6) | |
| CIRS comorbidity index, mean (±SD) | 3.7 (±1.9) | 3.1 (±1.7) | 3.8 (±2.0) | 3.2 (±1.9) | |
| CIRS comorbidity index < 3, n (%) | 173 (49.9) | 221 (63.1) | 158 (45.5) | 206 (58.9) | |
| CIRS comorbidity index > 3, n (%) | 174 (50.4) | 129 (36.9) | 189 (54.5) | 144 (41.1) | |
| CIRS severity index, mean (±SD) | 1.7 (±0.3) | 1.6 (±0.3) | 1.8 (±0.3) | 1.7 (±0.3) | |
| Number of drugs, median (interquartile range) | 7 (4–9) | 7 (4–9) | 7 (5–10) | 7(5–9) | |
| Number of drugs, mean (±SD) | 6.3 (±3.3) | 5.7 (±3.1) | 7.4(±3.7) | 7.6(±3.5) | |
| Number of drugs, n (%) | |||||
| 0 | 3 (0.9) | 10 (2.8) | 2 (0.6) | 1 (0.3) | |
| 1–4 | 110 (31.7) | 104 (29.7) | 72 (20.7) | 46 (13.1) | |
| ≥5 | 234 (67.4) | 236 (67.4) | 273 (78.7) | 303 (86.6) | |
CIRS, Cumulative Illness Rating Scale.
At discharge the prevalence of patients on polypharmacy (defined as five or more drugs) increased in both groups in comparison with hospital admission (79% and 87%, respectively). The most frequently prescribed active substances were furosemide (163/347, 47.0% vs. 190/350, 54.3% in the intervention and control arms, respectively), acetylsalicylic acid (122/347, 35.1% vs. 134/350, 38.3%) and bisoprolol (77/347, 22.2% vs. 82/350, 23.4%).
Primary and secondary outcomes
Overall, 292 (42%) participants were discharged with at least one PIM, 617 (88%) with at least one potential DDI and 391(56%) with at least one potentially severe DDI. Table 2 shows that there was no difference between the intervention and control arms regarding the prevalence of these events. In the intention‐to‐treat analysis, the great majority of patients with PIMs had only one PIM (79% and 82%, respectively for intervention and control), 18% and 17% had two, while a very small number (3% and 1%) had three or more PIMs. A very similar situation was found for per‐protocol (PP) analysis. The PIM most frequently prescribed at discharge in the intervention and control arms were acetylsalicylic acid (49/347, 14.1% vs. 49/350, 14%), benzodiazepines (41/347, 11.8% vs. 26/350, 7.4%), antiarrhytmic drugs (22/347, 6.3% vs. 21/350, 6%) and ticlopidine (20/347, 5.8% vs. 18/350, 5.1%). Table 3 shows the prevalence of the first ten potentially severe DDIs prescribed at hospital discharge. The combination of a statin (simvastatin or atorvastatin) with a calcium antagonist (amlodipine, or verapamil or diltiazem) were the most frequent DDIs in both groups (Table 3).
Table 2.
Results of primary and secondary outcomes: prevalence of at least one PIM, DDI and severe DDI at discharge
| Intention‐to‐treat analysis | Per protocol analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Intervention arm n (%) | Control arm n (%) | Odds ratio * (95%CI) | P‐value | Intervention arm n (%) | Control arm n (%) | Odds ratio * (95%CI) | P‐value | |
| Primary outcome | ||||||||
| At least one PIM | 155 (44.7) | 137 (39.1) | 1.29 (0.87–1.91) | 0.2 | 136 (43.3) | 126 (39.4) | 1.20 (0.80–1.80) | 0.3 |
| Secondary outcomes | ||||||||
| At least one potential DDI | 297 (85.6) | 320 (91.4) | 0.67 (0.34–1.28) | 0.2 | 268 (85.4) | 290 (90.1) | 0.72 (0.36–1.42) | 0.7 |
| At least one potentially severe DDI | 187 (53.9) | 204 (58.3) | 0.86 (0.63–1.15) | 0.3 | 151 (48.1) | 180 (56.3) | 0.85 (0.63–1.16) | 0.3 |
DDI, drug–drug interaction; PIM, potential inappropriate medication.
Adjusted for wards and number of drugs.
Table 3.
First ten potentially severe DDIs at hospital discharge among the intervention (347 patients) and control (350 patients) arms
| Drug combination | Potential adverse events | Patients, n | |
|---|---|---|---|
| Intervention arm | Control arm | ||
| Hydrochlorothiazide + proton pump inhibitor | Increased risk of hypomagnesemia | 17 | 11 |
| Statin * + calcium antagonist † | Increased risk of myopathy including rhabdomyolysis | 26 | 25 |
| Digoxin + furosemide | Increased risk of digoxin toxicity | 8 | 17 |
| Digoxin + proton pump inhibitor | Increased risk of digoxin toxicity | 9 | 16 |
| Potassium‐sparing diuretics + ACEIs or ARB | Increased risk of hyperkalemia | 17 | 24 |
| Clopidogrel + atorvastatin | Reduction in clinical efficacy of clopidogrel and increased risk for thrombosis | 6 | 12 |
| Clopidogrel + proton pump inhibitor ‡ | Reduction in clinical efficacy of clopidogrel and increased risk for thrombosis | 10 | 16 |
| ASA + SSRI | Increased risk of hemorrhage | 12 | 13 |
| Simvastatin + warfarin | Increased risk of hemorrhage | 7 | 9 |
| Allopurinol + warfarin | Increased risk of hemorrhage | 2 | 8 |
| Amiodarone + warfarin | Increased risk of hemorrhage | 4 | 6 |
Simvastatin, atrovastatin.
Amlodipine, verapamil or diltiazem.
Excluding pantoprazole.
ACEIs, angiotensin converting enzymes inhibitors; ARB, angiotensin II receptor blockers; ASA, acetylsalicylic acid; SSRI, selective serotonin re‐uptake inhibitors.
When those patients taking a higher number of PIM at discharge than on admission were analysed, there was no difference between intervention and control arms (OR 0.98 95%CI 0.57–1.68; P = 0.98). In the subgroup of patients treated with polypharmacy, there was no difference between the two arms for the prevalence of at least one PIM (OR 1.11 95%CI 0.70–1.76; P = 0.47), one potential DDI (OR 0.51 95%CI 0.23–1.13; P = 0.10) and one potentially severe DDI (OR 0.97 95%CI 0.66–1.43; P = 0.88).
The multilevel association in the intervention arm between number of errors and presence of PIMs was statistically significant (P = 0.01): however, this result was mainly due to a single centre, as removing it made the association statistically nonsignificant. Centres in the control arm did not show a statistically significant association (P = 0.09).
No differences between arms were also observed for mortality rate and incidence of re‐hospitalization at the 12‐month follow‐up (Figure 2 A,B). The mean length of hospital stay was even higher in the intervention than in the control group (12.6 vs. 11.3, P = 0.038). No clinician allocated to the intervention arm downloaded and used the available and recommended INTERcheck® software.
Figure 2.
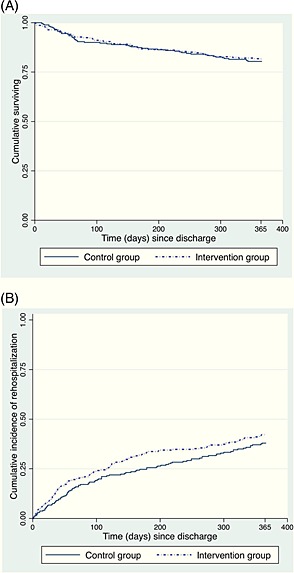
Secondary outcome results: (A) survival and (B) incident re‐hospitalization rates at 12‐month follow‐up
Discussion
E‐learning tools continue to proliferate as methods for medical education in several contexts, that usually emphasize their validity. Nevertheless, this randomized controlled study showed that an e‐learning educational program based on the concepts of CGA and reinforced knowledge of geriatric pharmacology notions failed to improve the quality of drug prescription in older patients acutely hospitalized in internal medicine and geriatrics wards.
Why was this study conceived?
The high prevalence of PIM found in the framework of large‐scale European epidemiological studies 3, 7 establishes the need for clinicians to improve their drug prescription capacity. Accordingly, in the framework of our own study, 42% of older people were discharged from hospital wards with at least one PIM, 88% with at least one potential DDI and 56% with at least one potentially severe DDI. Furthermore, only very few ADRs were reported, suggesting under‐recognition of these important events. Notwithstanding the high experience of clinicians from these wards in the management of older people with multimorbidity, the drug‐related problems and drug–drug interactions are still often underestimated issues. Moreover, some studies have shown that junior doctors are more likely to make a prescribing error in their first 2 years of practice 25, 26, 27.
Another issue to be considered is that, although several methods (such as medication reviews and reconciliation) 28, 29, educational programs (e‐learning or traditional) or computerized prescription support system (CPSS, e.g. INTERcheck®) 22, 30 are available in different settings to help clinicians optimize drug prescription, surprisingly no clinical ward in the intervention arm of this study used the software made available (INTERcheck®). This occurred in spite of the fact that the software was previously demonstrated to be associated with a significant reduction of PIMs and of new onset of potentially severe DDIs in older patients hospitalized in a geriatric ward 22. This is perhaps due to the fact that few medical wards in Italy have the habit of using electronic medical records associated with CPSS, leading to an underestimation of drug‐related problems and to medication errors by clinicians. On the other hand, it must be emphasized that usually hospital pharmacists are not involved in drug prescription in the framework of clinical practice, notwithstanding the fact that the presence of an expert pharmacist in a multidisciplinary team dealing with prescription review is an efficient method to optimize prescription and improve patient outcomes 31, 32, 33, 34. Furthermore, lack of interest in drug‐related problems could also be concluded from our results. In fact, there was an association between a low number of errors made by clinicians in the e‐learning test and a low prevalence of PIM at discharge, thus demonstrating that the active involvement of clinicians may be an important factor in positive outcomes.
Why was our e‐learning approach unsuccessful?
E‐learning strategies meant to support prescription may include different approaches: passive (fixed text, lectures), facilitated learning (direct link to on‐line resources), interactive learner‐system (partially simulated reality, where the learners interact with a system that provides feedback based upon inputs), learner/teacher (close communication between learner and teacher), and virtual reality learner‐systems in which the reality, i.e., the scenario of a hospital ward and the decisions taken with the related consequences, are simulated virtually 18. Our approach was a mix between passive and interactive learner systems, because static texts and pictures were delivered together with self‐assessment exercises with feedback, including multiple‐choice questions on a case report simulating a prescription scenario. Because we attempted to meet all the prerequisites for developing a successful e‐learning program (academic expertise of teachers, easy and acceptable platform, a check system of clinical performance, planned and logical contents) 18, a possible explanation for the poor efficacy of this e‐learning program is its low level of interactivity, which failed to enhance learning retention. Indeed, interactive approaches have given more successful results. For instance, using an animate feature function to move images and text within slides or using spoken words and videos to describe a figure, Gordon et al. demonstrated an improvement in prescribing skills in paediatric trainees undergoing an e‐learning intervention 35. Wong et al. concluded that interactivity is highly valued by learners because they are keen to enter into a dialogue with the course tutor, fellow students and/or a virtual tutor and thus obtain ongoing feedback on their understanding and performance 36. Moreover, education and training using simulation techniques and reproducing reliable clinical scenarios are validated methods to empower expertise in healthcare through improvement of decision making and clinical judgment processes in ordinary and complex clinical situations 37. In addition, many factors could contribute to the poor quality of prescribing and prescription of PIMs. Poor knowledge of clinicians is just one of these. For instance, the study of Reeve et al. 38 showed that the decision to stop an inappropriate medication is influenced by multiple patient barriers and enablers. Accordingly only a multifaceted intervention involving different strategies and stakeholders (not only physicians but also patients, nurses, caregivers, etc.) would be required in order to truly obtain an improvement of prescription quality.
Besides the aforementioned possible reasons for the negative results of this e‐learning effort, a number of limitations of our study design must be mentioned. For instance, participating clinicians were not blind to study aims and treatment allocation, so that those randomized to the control arm may have increased their attention toward the issue of prescription quality, flattening the differences with the intervention arm. Furthermore, the study did not introduce limitations in the characteristics of the wards included, sometimes making the collection of data difficult, for example during the follow‐up phase if a phone was not present in the ward. In addition, we did not collect any data on clinicians' characteristics. However, the randomization and the intervention were done at the ward level, thus we did not expect differences among the mean values of these characteristics. Moreover, not having scores before the exposure to the e‐learning process, and not having a direct link between a physician and a patient, we cannot evaluate the improvement in theoretical knowledge or the effect on the single physician, as was done in other studies 39. Furthermore, we calculated the time taken to complete the e‐learning modules and the errors made by physicians, by arm and by wards, but we had only one time point in which the measures were taken. Therefore, during e‐learning we could not assess any possible improvement. We were also unable to separate time effectively devoted to e‐learning from time during which the program was left unattended, so even if clear outliers were removed we had an extremely dispersed distribution of these durations. Also, as the patients were cared for collectively by physicians on the same ward, it was impossible to relate the data of a single physician to the outcome on a single patient (PIMs). Finally, it must be pointed out that not all DDIs are to be considered inappropriate (e.g. the combination of potassium‐sparing diuretics with ACEIs or ARB may be prescribed under strict monitoring of potassium levels).
In conclusion, this e‐learning educational program, teaching CGA and geriatric pharmacological notions, failed to improve clinician drug prescription for hospitalized older patients. On the other hand, the data obtained confirm once more the high degree of inadequacy of hospital clinicians to deal with the issue of drug prescription appropriateness. Hence, other approaches and different educational programs should be devised and evaluated in order to improve clinicians' prescription in hospitalized older people with multimorbidity.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
The ELICADHE Study was approved and financially supported by the Italian Medicines Agency (AIFA) according to the 2008 Italian Program for Independent Research (Project no. FARM87SA2B).
Investigators and co‐authors of the ELICADHE Study (E‐Learning Intervention focused on Comprehensive geriatric Assessement to improve the quality of Drug prescribing in Hospitalized Elderly) are as follows:
Steering Committee: Alessandro Nobili, Carlotta Franchi, Mauro Tettamanti (IRCCS – Istituto di Ricerche Farmacologiche ‘Mario Negri’, Milan); Pier Mannuccio Mannucci (Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan); Graziano Onder, Roberto Bernabei (Università Cattolica del Sacro Cuore – Policlinico ‘Agostino Gemelli’, Rome); Gualberto Gussoni (Centro Studi FADOI, Milan); Stefano Bonassi (IRCCS – San Raffaele Pisana, Rome).
Study coordinating group: Carlotta Franchi, Alessandro Nobili (IRCCS – Istituto di Ricerche Farmacologiche ‘Mario Negri’, Milan).
Clinical data monitoring and revision: Carlotta Franchi (IRCCS – Istituto di Ricerche Farmacologiche ‘Mario Negri’, Milan); Raffaella Rossio (Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan; Medicina Interna); Antonella Valerio (Centro Studi FADOI, Milan); Federica Mammarella (Università cattolica del Sacro Cuore – Policlinico Universitario ‘Agostino Gemelli’, Rome); Moira Ceci (Ospedale Israelitico, Rome).
Database Management and Statistics: Mauro Tettamanti, Codjo Djignefa Djade (IRCCS – Istituto di Ricerche Farmacologiche ‘Mario Negri’, Milan).
Investigators: Raffaella Rossio, Barbara Ferrari (Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan; Ematologia non tumorale e coagulopatie); Daniela Mari, Marco Ferretti (Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan; Geriatria); Francesco Salerno, Alessio Conca (Medicina Interna, Policlinico IRCCS San Donato, San Donato Milanese, Università di Milano); Antonino Tuttolomondo, Anna Cirrincione, Antonio Pinto (U.O.C. di Medicina Interna e Cardioangiologia, AOUP ‘P. Giaccone’, Palermo); Antonio Cherubini, Giuseppina Dell'Aquila (Istituto Nazionale Ricovero e Cura Anziani‐INRCA, Ancona; Geriatria); Roberto Bernabei, Graziano Onder, Michele Ciaburri (Università cattolica del Sacro Cuore – Policlinico Universitario ‘Agostino Gemelli’, Rome; Geriatria); Dario Manfellotto, Irene Caridi (Ospedale Fatebenefratelli – AFaR, Isola Tiberina, Rome; Medicina Interna); Enzo Lancia, Alessandra Forgione, Concetta Donato (IRCCS San Raffaele Pisana, Rome; Medicina Interna); Serra Maria Grazia (Azienda Ospedaliera ‘Cardinale G. Panico’ – Tricase (LE); Medicina Interna); Luigi Lusiani, Bullo Cristina, Brocco Stefano (Ospedale San Giacomo Apostolo, Castelfranco Veneto; Medicina Interna); Pedretti Giovanni, Pattacini Corrado (Ospedale di Fidenza – San Secondo Parmense; Medicina Interna); Francesco Violi, Ludovica Perri (Policlinico Umberto I, Rome; Medicina Interna); Luigi Di Cioccio, Carlo Di Meo, Laura Minchella (Ospedale Santa Scolastica, Cassino; Geriatria); Moira Ceci (Ospedale Israelitico, Rome; Geriatria); Alberto Ferrari, Salvatore Foderaro (Arcispedale Santa Maria Nuova – I.R.C.C.S, Reggio Emilia; Medicina Interna), Antonio Brucato, Anna Valenti, Silvia Ghidoni (Azienda Ospedaliera Papa Giovanni XXIII, Bergamo; Medicina Interna); Renzo Rozzini, Lina Falanga (Istituto Ospedaliero, Fondazione Poliambulanza, Brescia; Geriatria); Alessandra Marengoni, Simona Ghibelli (Dipartimento di Scienze Cliniche e Sperimentale, Università degli Studi di Brescia); Chiara Mussi, Maria Alice Ferri (Nuovo Ospedale S. Agostino‐Estense, Modena; Geriatria).
Franchi, C. , Tettamanti, M. , Djade, C. D. , Pasina, L. , Mannucci, P. M. , Onder, G. , Gussoni, G. , Manfellotto, D. , Bonassi, S. , Salerno, F. , Nobili, A. , and on behalf of ELICADHE Investigators (2016) E‐learning in order to improve drug prescription for hospitalized older patients: a cluster‐randomized controlled study. Br J Clin Pharmacol, 82: 53–63. doi: 10.1111/bcp.12922.
Clinicaltrials.gov Identifier: NCT02339792
References
- 1. Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A, Meinow B, Fratiglioni L. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev 2011; 10: 430–9. [DOI] [PubMed] [Google Scholar]
- 2. Mannucci PM, Nobili A, REPOSI Investigators . Multimorbidity and polypharmacy in the elderly: lessons from REPOSI. Intern Emerg Med 2014; 9: 723–34. [DOI] [PubMed] [Google Scholar]
- 3. Onder G, Bonassi S, Abbatecola AM, Folino‐Gallo P, Lapi F, Marchionni N, Pani L, Pecorelli S, Sancarlo D, Scuteri A, Trifirò G, Vitale C, Zuccaro SM, Bernabei R, Fini M, Geriatrics Working Group of the Italian Medicines Agency . High prevalence of poor quality drug prescribing in older individuals: a nationwide report from the Italian Medicines Agency (AIFA). J Gerontol A Biol Sci Med Sci 2014; 69: 430–7. [DOI] [PubMed] [Google Scholar]
- 4. Onder G, van der Cammen TJ, Petrovic M, Somers A, Rajkumar C. Strategies to reduce the risk of iatrogenic illness in complex older adults. Age Ageing 2013; 42: 284–91. [DOI] [PubMed] [Google Scholar]
- 5. Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev 2009; 41: 67–76. [DOI] [PubMed] [Google Scholar]
- 6. Mallet L, Spinewine A, Huang A. The challenge of managing drug interactions in elderly people. Lancet 2007; 370: 185–91. [DOI] [PubMed] [Google Scholar]
- 7. Johnell K, Klarin I. The relationship between number of drugs and potential drug–drug interactions in the elderly. A survey of over 600 000 elderly patients from the Swedish Prescribed Drug Register. Drug Saf 2007; 30: 911–8. [DOI] [PubMed] [Google Scholar]
- 8. Onder G, Landi F, Liperoti R, Fialova D, Gambassi G, Bernabei R. Impact of inappropriate drug use among elderly hospitalized older adults. Eur J Clin Pharmacol 2005; 61: 453–9. [DOI] [PubMed] [Google Scholar]
- 9. Gallagher P, Lang PO, Cherubini A, Topinková E, Cruz‐Jentoft A, Montero Errasquín B, Mádlová P, Gasperini B, Baeyens H, Baeyens JP, Michel JP, O'Mahony D. Prevalence of potentially inappropriate prescribing in an acutely ill population of older patients admitted to six European hospitals. Eur J Clin Pharmacol 2011; 67: 1175–88. [DOI] [PubMed] [Google Scholar]
- 10. Pasina L, Djade CD, Tettamanti M, Franchi C, Salerno F, Corrao S, Marengoni A, Marcucci M, Mannucci PM, Nobili A, REPOSI Investigators . Prevalence of potentially inappropriate medications and risk of adverse clinical outcome in a cohort of hospitalized elderly patients: results from the REPOSI study. J Clin Pharm Ther 2014; 39: 511–5. [DOI] [PubMed] [Google Scholar]
- 11. Rubenstein LZ, Wieland D, Bernabei R. Geriatric Assessment Technology: The State of the Art. Milan: Kurtis, 1995. [DOI] [PubMed] [Google Scholar]
- 12. Schmader KE, Hanlon JT, Pieper CF, Sloane R, Ruby CM, Twersky J, Francis SD, Branch LG, Lindblad CI, Artz M, Weinberger M, Feussner JR, Cohen HJ. Effects of geriatric evaluation and management on adverse drug reactions and suboptimal prescribing in the frail elderly. Am J Med 2004; 116: 394–401. [DOI] [PubMed] [Google Scholar]
- 13. Stuck AE, Aronow HU, Steiner A, Alessi CA, Büla CJ, Gold MN, Yuhas KE, Nisenbaum R, Rubenstein LZ, Beck JC. A trial of annual in‐home comprehensive geriatric assessments for elderly people living in the community. N Engl J Med 1995; 333: 1184–9. [DOI] [PubMed] [Google Scholar]
- 14. Reuben DB, Borok GM, Wolde‐Tsadik G, Ershoff DH, Fishman LK, Ambrosini VL, Liu Y, Rubenstein LZ, Beck JC. A randomized trial of comprehensive geriatric assessment in the care of hospitalized patients. N Engl J Med 1995; 332: 1345–50. [DOI] [PubMed] [Google Scholar]
- 15. Curran V, Lockyer J, Sargeant J, Fleet L. Evaluation of learning outcomes in Web‐based continuing medical education. Acad Med 2006; 81: S30–4. [DOI] [PubMed] [Google Scholar]
- 16. Cook DA, Levinson AJ, Garside S, Dupras DM, Erwin PJ, Montori VM. Internet‐based learning in the health professions: a meta‐analysis. JAMA 2008; 300: 1181–96. [DOI] [PubMed] [Google Scholar]
- 17. Walsh K. E‐learning: controlling costs and increasing value. J Coll Physicians Surg Pak 2015; 25: 292–3. [PubMed] [Google Scholar]
- 18. Maxwell S, Mucklow J. e‐Learning initiatives to support prescribing. Br J Clin Pharmacol 2012; 74: 621–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Franchi C, Mari D, Tettamanti M, Pasina L, Djade CD, Mannucci PM, Onder G, Bernabei R, Gussoni G, Bonassi S, Nobili A, ELICADHE Investigators . E‐learning to improve the drug prescribing in the hospitalized elderly patients: the ELICADHE feasibility pilot study. Aging Clin Exp Res 2014; 26: 435–43. [DOI] [PubMed] [Google Scholar]
- 20. Gray LC, Bernabei R, Berg K, Finne‐Soveri H, Fries BE, Hirdes JP, Jónsson PV, Morris JN, Steel K, Ariño‐Blasco S. Standardizing assessment of elderly people in acute care: the interRAI Acute Care instrument. J Am Geriatr Soc 2008; 56: 536–41. [DOI] [PubMed] [Google Scholar]
- 21. Bernabei R, Landi F, Onder G, Liperoti R, Gambassi G. Second and third generation assessment instruments: the birth of standardization in geriatric care. J Gerontol A Biol Sci Med Sci 2008; 63: 308–13. [DOI] [PubMed] [Google Scholar]
- 22. Ghibelli S, Marengoni A, Djade CD, Nobili A, Tettamanti M, Franchi C, Caccia S, Giovarruscio F, Remuzzi A, Pasina L. Prevention of inappropriate prescribing in hospitalized older patients using a computerized prescription support system (INTERcheck®). Drugs Aging 2013; 30: 821–8. [DOI] [PubMed] [Google Scholar]
- 23. American Geriatrics Society 2012 Beers Criteria Update Expert Panel . American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012; 60: 616–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fick DM, Semla TP. 2012 American Geriatrics Society Beers Criteria: new year, new criteria, new perspective. J Am Geriatr Soc 2012; 60: 614–5. [DOI] [PubMed] [Google Scholar]
- 25. Lewis PJ, Ashcroft DM, Dornan T, Taylor D, Wass V, Tully MP. Exploring the causes of junior doctors' prescribing mistakes: a qualitative study. Br J Clin Pharmacol 2014; 78: 310–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ryan C, Ross S, Davey P, Duncan EM, Francis JJ, Fielding S, Johnston M, Ker J, Lee AJ, MacLeod MJ, Maxwell S, McKay GA, McLay JS, Webb DJ, Bond C. Prevalence and causes of prescribing errors: the PRescribing Outcomes for Trainee Doctors Engaged in Clinical Training (PROTECT) study. PLoS One 2014; 9: e79802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ross S, Ryan C, Duncan EM, Francis JJ, Johnston M, Ker JS, Lee AJ, MacLeod MJ, Maxwell S, McKay G, McLay J, Webb DJ, Bond C. Perceived causes of prescribing errors by junior doctors in hospital inpatients: a study from the PROTECT programme. BMJ Qual Saf 2013; 22: 97–102. [DOI] [PubMed] [Google Scholar]
- 28. Geurts MM, Talsma J, Brouwers JR, de Gier JJ. Medication review and reconciliation with cooperation between pharmacist and general practitioner and the benefit for the patient: a systematic review. Br J Clin Pharmacol 2012; 74: 16–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Graabaek T, Kjeldsen LJ. Medication reviews by clinical pharmacists at hospitals lead to improved patient outcomes: a systematic review. Basic Clin Pharmacol Toxicol 2013; 112: 359–73. [DOI] [PubMed] [Google Scholar]
- 30. O'Connor MN, Gallagher P, O'Mahony D. Inappropriate prescribing: criteria, detection and prevention. Drugs Aging 2012; 29: 437–52. [DOI] [PubMed] [Google Scholar]
- 31. O'Sullivan D, O'Mahony D, O'Connor MN, Gallagher P, Cullinan S, O'Sullivan R, Gallagher J, Eustace J, Byrne S. The impact of a structured pharmacist intervention on the appropriateness of prescribing in older hospitalized patients. Drugs Aging 2014; 31: 471–81. [DOI] [PubMed] [Google Scholar]
- 32. Holland R, Desborough J, Goodyer L, Hall S, Wright D, Loke YK. Does pharmacist‐led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta‐analysis. Br J Clin Pharmacol 2008; 65: 303–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hohl CM, Wickham ME, Sobolev B, Perry JJ, Sivilotti ML, Garrison S, Lang E, Brasher P, Doyle‐Waters MM, Brar B, Rowe BH, Lexchin J, Holland R. The effect of early in‐hospital medication review on health outcomes: a systematic review. Br J Clin Pharmacol 2015; 80: 51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Christensen M, Lundh A. Medication review in hospitalised patients to reduce morbidity and mortality. Cochrane Database Syst Rev 2013; 2: CD008986. [DOI] [PubMed] [Google Scholar]
- 35. Gordon M, Chandratilake M, Baker P. Low fidelity, high quality: a model for e‐learning. Clin Teach 2013; 10: 258–63. [DOI] [PubMed] [Google Scholar]
- 36. Wong G, Greenhalgh T, Pawson R. Internet‐based medical education: a realist review of what works, for whom and in what circumstances. BMC Med Educ 2010; 10: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gaba DM. The future vision of simulation in healthcare. Simul Healthc 2007; 2: 126–35. [DOI] [PubMed] [Google Scholar]
- 38. Reeve E, To J, Hendrix I, Shakib S, Roberts MS, Wiese MD. Patient barriers to and enablers of deprescribing: a systematic review. Drugs Aging 2013; 30: 793–807. [DOI] [PubMed] [Google Scholar]
- 39. Franson KL, Dubois EA, de Kam ML, Cohen AF. Measuring learning from the TRC pharmacology E‐Learning program. Br J Clin Pharmacol 2008; 66: 135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]


