Abstract
Different methods are available for measuring medication adherence. In this paper, we conducted a scoping review to identify and summarize evidence of all studies comparing the Medication Event Monitoring System (MEMS) with alternative methods for measuring medication adherence. A literature search was performed using the open database www.iAdherence.org that includes all original studies reporting findings from the MEMS. Papers comparing methods for measuring adherence to solid oral formulations were included. Data was extracted using a standardized extraction table. A total of 117 articles fulfilled the inclusion criteria, including 251 comparisons. Most frequent comparisons were against self‐report (n = 119) and pill count (n = 59). Similar outcome measures were used in 210 comparisons (84%), among which 78 used dichotomous variables (adherent or not) and 132 used continuous measures (adherence expressed as percentage). Furthermore, 32% of all comparisons did not estimate adherence over the same coverage period and 44% of all comparisons did not use a statistical method or used a suboptimal one. Only eighty‐seven (35%) comparisons had similar coverage periods, similar outcome measures and optimal statistical methods. Compared to MEMS, median adherence was grossly overestimated by 17% using self‐report, by 8% using pill count and by 6% using rating. In conclusion, among all comparisons of MEMS versus alternative methods for measuring adherence, only a few used adequate comparisons in terms of outcome measures, coverage periods and statistical method. Researchers should therefore use stronger methodological frameworks when comparing measurement methods and be aware that non‐electronic measures could lead to overestimation of medication adherence.
Keywords: measurement methods, medication adherence, medication event monitoring system, methodology, pill count, self‐report
Introduction
Poor medication adherence has grown as a major health concern over the last decade. It is of rising concern to clinicians, healthcare systems and other stakeholders because of the increasing evidence that poor medication adherence is prevalent and associated with adverse outcomes and higher costs of care 1. The ABC taxonomy 2 defines medication adherence as the process by which patients take their medication as prescribed. This process of taking medication begins with the initiation of therapy, when patients take their first dose of a prescribed medicine. Initiation precedes the implementation phase, defined as the extent to which patients' actual dosing corresponds with their prescribed dosing regimen. Finally, discontinuation eventually occurs, which refers to the end of therapy.
Unbiased and precise measurement of the three elements of medication adherence (initiation, implementation and persistence) is the cornerstone for sound interpretation of clinical trials 3 as well as for the implementation of successful interventions to enhance medication adherence in medical practice 4. Measurement methods that rely on patients' recall and/or allow patients to censor information on their dosing histories (e.g. pill counts or self‐report) have repeatedly been discredited, as they are strongly biased toward overestimation of drug actual exposure 5.
A Medication Event Monitoring System (MEMS) consists in electronic detection of package entry by incorporating micro‐circuitry into pharmaceutical packages of various design, which detects, time‐stamps and stores the manoeuvres needed to remove a dose of the drug. This automatic compilation of times of medication intake (dosing history) provides a thorough characterization of medication adherence, with clear distinctions between initiation, implementation and discontinuation 3. It is, of course, an indirect method of estimating when and how much drug is administered, but it has been shown to predict well drug concentration in plasma 3. Electronic monitoring of package entry is the current gold standard for automatically compiling drug dosing history in trial settings 3, as illustrated by more than 700 peer‐reviewed publications (www.iAdherence.org).
Numerous studies have compared alternative methods against electronic monitoring. However, studies comparing methods of measuring adherence against each other often oversimplify the comparison and do not consider the richness of the dosing history data derived from electronic monitoring. Vrijens and Urquhart 6 introduced two attributes, reliability and richness of the measurement method. Reliability refers to the degree to which a method is biased, and richness refers to the precision of assessments over time. The less biased and the more precise the assessment, the better the method of measurement is able to distinguish and characterize the three elements of medication adherence 6. A comparison along these two attributes can offer a good understanding of the strengths and limitations of different methods for measuring adherence.
Given the numerous studies comparing multiple methods of measuring adherence, this review was designed to scope and synthesize the evidence of studies comparing MEMS with alternative non‐electronic methods, in order to see whether the comparisons made are optimal in terms of their outcome measure, precision and statistical method.
Method
Literature search
The iAdherence database (www.iadherence.org) is a repository, which contains all peer‐reviewed papers reporting studies where dosing histories are electronically compiled using MEMS. The database is constructed from a monthly search of company records and MEDLINE using a systematic approach described in the Appendix. Identified papers are then screened to confirm the use of MEMS and re‐confirmed using company records. The MEMS‐based papers are systematically filed in Reference Manager® and potential duplicates are automatically removed.
As the objective of iAdherence is to provide a comprehensive list of all MEMS‐based peer‐reviewed papers, this database was used to identify all studies comparing MEMS with non‐electronic methods.
The literature search was conducted by one researcher (JD), according to a prespecified research protocol and using the iAdherence database. All abstracts available in the iAdherence database were systematically searched for the following terms: ‘self‐report OR self report OR questionnaire OR pill count OR refill OR diar* OR claims OR concentration OR compar* OR pharmacokinet* OR measur* OR valid*’. All articles published in the English language from January 1989 to 31 March 2015 were searched.
Selection of studies
All original studies that compared, within a same sample, MEMS with at least one other non‐electronic method for measuring adherence to solid oral formulations were included. The selection of studies took place in three stages. The first selection of studies based on title and abstract was performed by one researcher (ME). Some articles were discussed further during a meeting with three co‐authors (MH, BV, JD), which resulted in a second selection of studies based on title and abstract. In a third step, assessment of the full text of articles was performed by at least two independent reviewers (ME, MH or JD). In case of disagreement, consensus was reached after discussion during a face‐to‐face meeting (ME, MH, JD, BV).
Data extraction
Different characteristics were extracted from each publication, such as geographical area, year of publication and journal. Furthermore, study design, length of patient follow‐up period, sample size, therapeutic area and dosing regimen were recorded. For categorization of the therapeutic area, the classification of diseases according to the British National Formulary 7 was used. Dosing regimen was reported as once daily (OD), twice daily (BID), three times daily (TID), combined (multiple medications with different dosing regimens) and other (more than three times daily).
The measurement methods compared against MEMS were categorized as self‐report, pill count, rating by healthcare provider/caregiver/parent, chemical markers (pharmacokinetic or pharmacodynamics), pharmacy records, diaries and composite adherence score. All types of questionnaires or interviews were characterized as self‐report. The composite adherence score is a method that combines values of MEMS, pill count and interview 8.
For comparison of measurement methods and to check for reliability and precision of the measurement methods, five types of data were extracted:
The type of outcome derived from the measurement method of adherence could be expressed as a continuous outcome (i.e. a percentage), a dichotomous outcome (i.e. adherent or not adherent), or another type of outcome, which was reported as other.
The precision of each measure was extracted as the coverage period used to estimate the adherence outcome. This coverage period could be different for every method used. For instance, the coverage period for chemical markers is typically subject to the half‐life of the drug (e.g. 6 hours) while pill count could be performed over different periods of time (e.g. 30, 60 or 90 days).
The adherence estimate of each adherence assessment method was extracted. In studies where adherence was considered as a continuous outcome (i.e. the proportion of prescribed doses taken or the proportion of days with correct intake), the overall average percentage of adherence was extracted. In studies where adherence was considered as a dichotomous outcome (adherent vs. non‐adherent patients based on an arbitrary threshold), the proportion of adherent patients was retrieved. In the latter situation, the most frequent threshold used to dichotomize medication adherence was 80%, but other thresholds could be used.
The statistical methods used to compare the outcomes were extracted.
-
The results of each statistical method including their corresponding P‐values were extracted, if reported.
Data were extracted using a standardized extraction table. The process of data extraction was carried out by one researcher (ME). A random selection of 10% of the articles was used to confirm the quality of data extraction by JD.
Analysis
In this phase of the review, compatible and optimal comparisons of measurement methods were identified. A comparison is defined as compatible if MEMS and the other non‐electronic methods used similar adherence outcomes and similar coverage periods, and if an optimal statistical method was used to assess the agreement between the two measurement methods. First, comparisons between MEMS and other non‐electronic methods were checked to determine whether a similar outcome was reached. Comparisons using continuous variables vs. continuous variables (%‐%) and comparisons using dichotomous variables versus dichotomous variables (y/n‐y/n) were considered similar outcomes. Other comparisons with different outcomes were reported as other. Second, each comparison was checked to see whether the adherence outcome was assessed over the same coverage period. Third, the comparisons that used similar outcomes and the same coverage periods were checked to see whether or not a statistical method was used, and, if one was used, to see whether the method was optimal or not to assess the agreement between measurement methods. Statistical methods used to assess the agreement between continuous outcomes were considered to be optimal when they evaluated the absolute difference between each pair of the two measurements 9 or when a formal measure of consistency was used 10. In those approaches, the null hypothesis that is tested is that there is an agreement between the two measures (difference = 0). Therefore, a P‐value below 0.05 indicates a significant inconsistency between the two methods being compared. Pearson and Spearman correlation coefficients were considered to be suboptimal statistical approaches, as they consider only relative position and the null hypothesis tested is the absence of linear association (rho = 0). A rejection of the null (P < 0.05) is an indication of some form of linear association between the two measures, but is less relevant to the question of agreement between the two methods 11.
Statistical methods that were considered optimal for evaluating the consistency between measurement methods when assessed as a dichotomous variable were Kappa/McNemar's test, as well as an approach based on sensitivity and specificity of the measurement methods. The null hypothesis tested with a Kappa or a McNemar's test is that there is an agreement between the two measures. A rejection of the null hypothesis (P < 0.05) will thus conclude that there is a significant disagreement between the methods. In calculating sensitivity and specificity, the optimal cut point for dichotomization is often identified using receiver operating characteristic (ROC) curves 12 and no formal hypothesis is tested (no P‐value is reported).
This screening resulted in a number of comparisons that were compatible in terms of similar outcomes and coverage periods and had optimal statistical methods. Compatible and optimal comparisons were further checked on significance and were classified as significant (P < 0.05), not significant or not reported (no P‐value). This classification was performed for both continuous outcomes and dichotomous outcomes.
Finally, the median adherence outcome was estimated for MEMS and each alternative non‐electronic method separately. To illustrate its reliability in comparison with MEMS, the difference between the most frequent methods was estimated. This estimation was performed based on compatible and optimal comparisons only.
Results
Study selection process
Figure 1 shows the flow chart of the studies' identification and selection, listing explicit reasons for exclusion. After the first screening of abstract and title, 136 potential eligible papers were identified.
Figure 1.
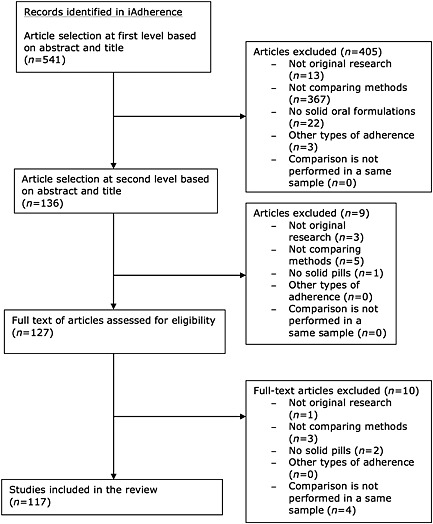
Flowchart describing the literature search
Uncertainties among the 136 papers were discussed during the second selection among three co‐authors, and nine additional studies were excluded. Of the 127 studies eligible for full text screening, ten additional articles were excluded, resulting in 117 articles included in the analysis.
Overview of included studies
The characteristics of the studies included are reported in Table 1. Sixty‐three (54%) studies were conducted in North America 8, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74 and 35 (30%) in Europe 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94, 95, 96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109. A few studies were conducted in Africa (n = 10) 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, Asia (n = 6) 5, 120, 121, 122, 123, 124, South America (n = 2) 125, 126 or Australia (n = 1) 127. Most of the studies compared one non‐electronic measurement method with the MEMS (n = 47), while 34 studies compared two measurement methods with the MEMS, 19 studies included three comparisons and 17 studies four or more comparisons.
Table 1.
Study characteristics
| Variable | n (%) |
|---|---|
| Geographical area | |
| North America | 63 (54) |
| Europe | 35 (30) |
| Africa | 10 (9) |
| Asia | 6 (5) |
| South America | 2 (1) |
| Australia | 1 (1) |
| Therapeutic area | |
| Infections | 44 (38) |
| Cardio‐vascular system | 23 (20) |
| Central nervous system | 18 (15) |
| Nutrition and blood | 13 (11) |
| Malignant diseases and immunosuppression | 7 (6) |
| Other | 12 (10) |
| Publication year | |
| 1989–1994 | 8 (7) |
| 1995–1999 | 17 (14) |
| 2000–2004 | 25 (21) |
| 2005–2009 | 37 (32) |
| >2010 | 30 (26) |
| Study design | |
| Cohort | 91 (78) |
| Randomized controlled trial from which: | 26 (22) |
| Intervention | 11 (9) |
| Treatment | 15 (13) |
| Dosing regimen | |
| Combined | 80 (68) |
| OD | 17 (15) |
| BID | 12 (10) |
| TID | 5 (4) |
| Other | 3 (3) |
| Sample size | |
| 0–50 | 48 (41) |
| 51–100 | 30 (26) |
| 101–200 | 22 (19) |
| >201 | 17 (14) |
| Follow‐up period | |
| <1 month | 6 (5) |
| 1–5 months | 62 (53) |
| 6–11 months | 25 (21) |
| >12 months | 21 (18) |
| Not reported | 3 (3) |
| Comparisons | |
| 1 | 47 (40) |
| 2 | 34 (29) |
| 3 | 19 (16) |
| >4 | 17 (15) |
OD, once daily; BID, twice daily; TID, three times daily.
Analysis of extracted data
The 117 articles included resulted in 251 comparisons between MEMS and alternative non‐electronic measurement methods. Table 2 presents the outcome measures and the corresponding statistical method used to assess the comparison. A total of 44% (n = 110) of all comparisons did not specify a statistical method at all or used a suboptimal method.
Table 2.
Statistical methods used to compare adherence methods
| Outcome | Statistical method | n (%) |
|---|---|---|
| % ‐ % (132) | Absolute difference | 46 (35) |
| Consistency | 2 (2) | |
| Correlation | 53 (40) | |
| No | 31 (23) | |
| Y/n ‐ y/n (78) | Kappa/Mcnemar's test | 38 (48) |
| Sensitivity & specificity | 27 (35) | |
| Correlation | 4 (5) | |
| Absolute difference | 3 (4) | |
| No | 6 (8) | |
| Other (41) | Yes | 28 (68) |
| No | 13 (32) |
Statistical method in bold are considered as optimal for continuous and dichotomous comparisons
Figure 2 presents a scatter plot of the coverage periods when adherence outcome was summarized from the MEMS or from an alternative non‐electronic method. The median of the coverage period for both MEMS and other non‐electronic methods was 30 days. Figure 2 highlights that 68% (n = 170) of the comparisons are on the diagonal line of the scatter plot, indicating that they used a similar coverage period.
Figure 2.
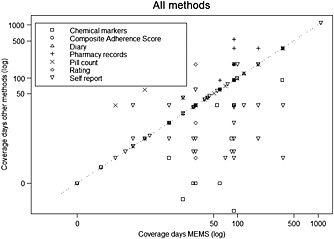
Scatter plot of coverage periods for all measurement methods; 68% (n = 170) of comparisons are on diagonal. Points below the diagonal, 21% (n = 53), are comparisons in which MEMS has wider coverage period than other measurement methods. Points above the diagonal, 7% (n = 18), are comparisons in which other measurement methods have a wider coverage period than MEMS
Among the 251 comparisons, 132 (52%) summarized medication adherence as a continuous outcome (%‐%) for both the MEMS and its comparator (see Figure 3). Of those 132 comparisons, only 41 (31%) used an optimal statistical method and a compatible coverage period, of which 37 reported a P‐value. The null hypothesis of agreement between the measurement methods was rejected (P < 0.05) in 28 cases (76%) 15, 16, 26, 44, 45, 46, 48, 52, 56, 57, 60, 66, 71, 89, 93, 101, 102, 103, 115, 119, leading to the conclusion that there is a significant disagreement between the adherence measurement methods.
Figure 3.
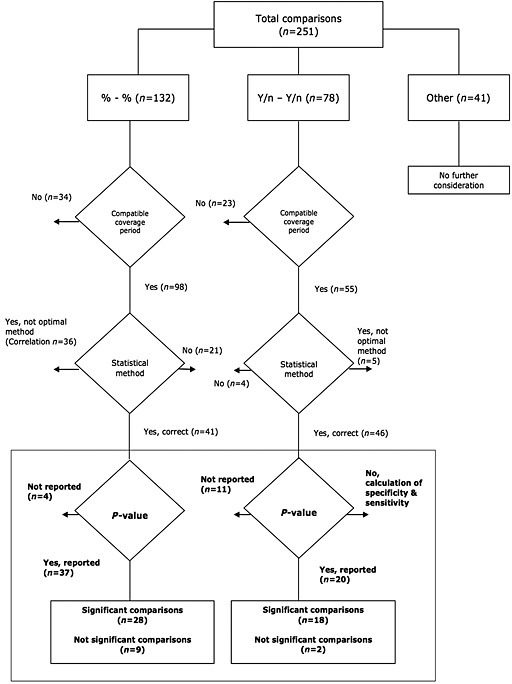
Flowchart of the evaluation of methodology of comparisons. Comparisons using compatible coverage periods and optimal statistical methods are shown in bold
Of note, when a continuous outcome was used, a correlation approach was considered in 36 comparisons to assess agreement between measurement methods leading to an average correlation of 0.35, ranging from −0.67 to 0.76 5, 8, 16, 19, 24, 29, 33, 34, 41, 43, 55, 62, 63, 65, 67, 69, 86, 88, 90, 91. For 31 of those correlations, the null hypothesis of no correlation was formally tested and rejected (P < 0.05) in 24 cases (77%) leading to the conclusion that there is a significant linear association between the measurement methods. This conclusion, based on correlation, is, however, less relevant to the question of agreement.
Among the 251 comparisons, 78 (31%) summarized medication adherence as a binary outcome (y/n‐y/n) for both the MEMS and its comparator (see Figure 3). Of those 78 comparisons, 46 (59%) used an optimal statistical method and a compatible coverage period. Fifteen of those comparisons used a statistical approach based on sensitivity and specificity 39, 92, 94, 107, 113, 117, 122. Taking MEMS as the reference method, the mean specificity was 65% and the mean sensitivity was 55%, leading to a mean positive predictive value of 35% and a mean negative predictive value of 78%.
Among the 46 comparisons that used an optimal statistical method over a compatible coverage period, 20 formally tested the null hypothesis of agreement between the measurement methods. The null was rejected (P < 0.05) in 18 cases (90%) 17, 25, 32, 64, 68, 76, 77, 89, 104, 118, leading to the conclusion that there is a significant disagreement between the measurement methods.
Taking together the optimal comparisons from both groups, continuous and dichotomous, the most frequently used methods were self‐report (n = 38), pill count (n = 27) and rating (n = 12). Sample sizes for these comparisons ranged from 7 to 669 with an average of 92 participants.
Besides assessing the appropriateness of the comparison and formally testing for agreement between the measurement methods, Figure 4 highlights the distribution of the difference in adherence estimates between MEMS and the comparison measurement method for the three most frequent methods that used compatible coverage periods and optimal statistical methods. When compared to MEMS, the median adherence per method was overestimated by 17% [range: −21%, 75%] for self‐report, 8% [−25%, 50%] for pill count and 6% [−15%, 50%] for rating.
Figure 4.
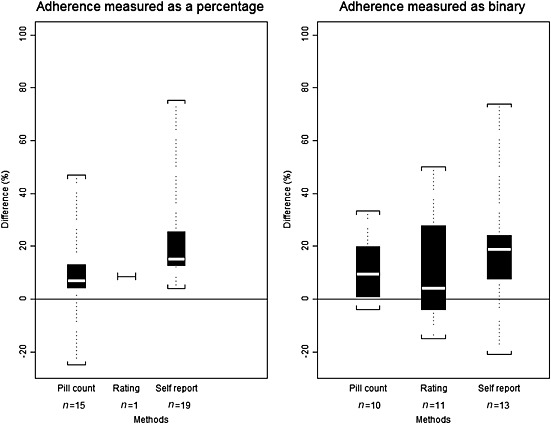
The extent to which other methods overestimate adherence compared with MEMS. The white line in the middle of the box is the median. The lower and upper bounds represent the 25th and 75th percentile of the distribution. The ends of the whiskers represent the minimum and maximum (n = number of comparisons)
Discussion
Of the 117 studies that reported 251 comparisons between MEMS and non‐electronic measurement methods for medication adherence, only a small percentage of comparisons (35%) used similar outcomes, similar coverage periods and optimal statistical methods. Of these compatible and optimal comparisons, 57 comparisons formally tested the null hypothesis of agreement between the measurement methods. The null hypothesis was rejected (P < 0.05) in 46 comparisons (81%), leading to the conclusion that there is a significant disagreement between adherence measures when assessed using MEMS vs. a non‐electronic method. Self‐report, rating by others and pill count tend to overestimate adherence compared to MEMS. For other methods, chemical markers, pharmacy refill, diaries or composite adherence score, there were too few comparisons to derive a formal conclusion.
When a specificity/sensitivity approach was used to evaluate how well an alternative measurement predicts the binary MEMS‐based classification as adherent versus non‐adherent, the specificity and sensitivity of such a test are respectively 65% and 55%, leading to poor positive predictive value (35%) and negative predictive value (78%).
Finally, it is interesting to see that a correlation, which is a measure of linear association rather than a measure of agreement, was used in 36 of the comparisons.
During the process of conducting this scoping review, some limitations could be identified. First, the analysis focused only on continuous and dichotomous outcomes (84% of all comparisons). Other outcomes (e.g. longitudinal) were not considered, as more advanced and unique statistical analysis was used. Those more advanced methods, while rarely used, are, however, required to better capture the dynamic and complex behaviour of medication adherence and should become the focus of further investigations. Second, the iAdherence database (www.iAdherence.org) includes only studies using MEMS and therefore other electronic methods for measuring medication adherence were not considered. In addition, studies comparing methods for measuring medication adherence using inhalation drugs, eye drops or topical medication were not considered in this review. Solid oral formulations (tablets and capsules) remain, however, the most widely used formulations of drugs used in ambulatory medical care. Third, as the main focus of this study was to evaluate the methodology used to compare MEMS against non‐electronic methods, the quality of the studies was not evaluated. Finally, the iAdherence database includes only peer‐reviewed journals, which could result in publication bias, as the grey literature was not searched.
While there are some limitations, this scoping review gives a comprehensive overview of the methods used in the peer‐reviewed literature to compare adherence measurement methods. The findings highlight major limitations and pitfalls in the methodological approaches used to compare and validate adherence measurement methods.
None of the comparisons addressed the three fundamental elements of medication adherence: initiation, implementation and persistence. Therefore, the average adherence values reported in those papers may be misleading as they may include treatment discontinuation, which is a time to event outcome.
Further research should use adequate approaches (including optimal statistical methods) in order to adequately compare methods for measuring medication adherence. Furthermore, researchers should be aware of the possible overestimation of medication adherence using certain non‐electronic methods (self‐report, rating and pill count), and should better define the conceptual framework to compare measurement methods. The different measurement methods need to be evaluated against their ability to measure the different elements of medication adherence 128.
Conclusion
Results from this review showed that among comparisons between MEMS and non‐electronic methods, only a few used similar outcomes, similar coverage periods and optimal statistical methods. From the comparisons of measurement methods that were compatible and optimal, about 80% showed a significant difference between MEMS and non‐electronic methods. Compared to MEMS, median adherence was overestimated by 17% using self‐report, by 8% using pill count and by 6% using rating. Those differences suggest that non‐electronic measures could lead to overestimation of medication adherence.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; BV and JD had a specified relationship with WestRock Healthcare in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
Contributors
ME: study rationale and design, literature selection, data extraction, interpretation and reflection, writing the manuscript. BV: study rationale and design, literature selection, interpretation and reflection, reviewing the manuscript. JD: literature search, literature selection, interpretation and reflection, reviewing the manuscript. SE: interpretation and reflection, reviewing the manuscript. MH: study rationale and design, literature selection, interpretation and reflection, reviewing the manuscript.
Systematic approach of literature search
((electronic OR microelectronic or eDEM) AND monitoring) OR ((mems AND (electronic OR medication)) AND (“patient compliance”[MESH] OR treatment refusal “[MESH] OR “patient drop outs mesh))
(compliance OR execution OR persistence) AND ((electronic OR microelectronic) monitoring)
(“Patient compliance”[MESH] OR “treatment refusal”[MESH] OR “Patient Dropouts”[Mesh]) OR ((mems OR electronic OR microelectronic) AND monitoring)
(“Patient Compliance”[Mesh]) AND “electronic”
(“Patient Compliance”[Mesh]) OR (Treatment refusal[Mesh])
“Patient Compliance”[Mesh] AND MEMS
El Alili, M. , Vrijens, B. , Demonceau, J. , Evers, S. M. , and Hiligsmann, M. (2016) A scoping review of studies comparing the medication event monitoring system (MEMS) with alternative methods for measuring medication adherence. Br J Clin Pharmacol, 82: 268–279. doi: 10.1111/bcp.12942.
References
- 1. Blaschke TF, Osterberg L, Vrijens B, Urquhart J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu Rev Pharmacol Toxicol 2012; 52: 275–301. [DOI] [PubMed] [Google Scholar]
- 2. Vrijens B, De Geest S, Hughes DA, Przemyslaw K, Demonceau J, Ruppar T, et al. A new taxonomy for describing and defining adherence to medications. Br J Clin Pharmacol 2012; 73: 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vrijens B, Urquhart J. Methods for measuring, enhancing, and accounting for medication adherence in clinical trials. Clin Pharmacol Ther 2014; 95: 617–26. [DOI] [PubMed] [Google Scholar]
- 4. Demonceau J, Ruppar T, Kristanto P, Hughes DA, Fargher E, Kardas P, et al. Identification and assessment of adherence‐enhancing interventions in studies assessing medication adherence through electronically compiled drug dosing histories: a systematic literature review and meta‐analysis. Drugs 2013; 73: 545–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng CW, Woo KS, Chan JC, Tomlinson B, You JH. Assessing adherence to statin therapy using patient report, pill count, and an electronic monitoring device. Am J Health Syst Pharm 2005; 62: 411–5. [DOI] [PubMed] [Google Scholar]
- 6. Vrijens B, Urquhart J. Patient adherence to prescribed antimicrobial drug dosing regimens. J Antimicrob Chemother 2005; 55: 616–27. [DOI] [PubMed] [Google Scholar]
- 7. Britain BMAatRPSoG . British National Formulary, 58th edn. London: BMJ Publishing Group, 2014. [Google Scholar]
- 8. Liu H, Golin CE, Miller LG, Hays RD, Beck CK, Sanandaji S, et al. A comparison study of multiple measures of adherence to HIV protease inhibitors. Ann Intern Med 2001; 134: 968–77. [DOI] [PubMed] [Google Scholar]
- 9. Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res 1999; 8: 135–60. [DOI] [PubMed] [Google Scholar]
- 10. Chen C, Barnhart HX. Assessing agreement with intraclass correlation coefficient and concordance correlation coefficient for data with repeated measures. Comput Stat Data Anal 2013; 60: 132–45. [Google Scholar]
- 11. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1: 307–10. [PubMed] [Google Scholar]
- 12. Karve S, Cleves MA, Helm M, Hudson TJ, West DS, Martin BC. Good and poor adherence: optimal cut‐point for adherence measures using administrative claims data. Curr Med Res Opin 2009; 25: 2303–10. [DOI] [PubMed] [Google Scholar]
- 13. Adler LA, Lynch LR, Shaw DM, Wallace SP, Ciranni MA, Briggie AM, et al. Medication adherence and symptom reduction in adults treated with mixed amphetamine salts in a randomized crossover study. Postgrad Med 2011; 123: 71–9. [DOI] [PubMed] [Google Scholar]
- 14. Ailinger RL, Black PL, Lima‐Garcia N. Use of electronic monitoring in clinical nursing research. Clin Nurs Res 2008; 17: 89–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Applebaum AJ, Reilly LC, Gonzalez JS, Richardson MA, Leveroni CL, Safren SA. The impact of neuropsychological functioning on adherence to HAART in HIV‐infected substance abuse patients. AIDS Patient Care STDS 2009; 23: 455–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, Chang CJ, et al. Antiretroviral therapy adherence and viral suppression in HIV‐infected drug users: comparison of self‐report and electronic monitoring. Clin Infect Dis 2001; 33: 1417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bachmann LH, Stephens J, Richey CM, Hook EW 3rd. Measured versus self‐reported compliance with doxycycline therapy for chlamydia‐associated syndromes: high therapeutic success rates despite poor compliance. Sex Transm Dis 1999; 26: 272–8. [DOI] [PubMed] [Google Scholar]
- 18. Badiee J, Riggs PK, Rooney AS, Vaida F, Grant I, Atkinson JH, et al. Approaches to identifying appropriate medication adherence assessments for HIV infected individuals with comorbid bipolar disorder. AIDS Patient Care STDS 2012; 26: 388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy M, Sheiner L, et al. Adherence to protease inhibitors, HIV‐1 viral load, and development of drug resistance in an indigent population. AIDS 2000; 14: 357–66. [DOI] [PubMed] [Google Scholar]
- 20. Baros AM, Latham PK, Moak DH, Voronin K, Anton RF. What role does measuring medication compliance play in evaluating the efficacy of naltrexone? Alcohol Clin Exp Res 2007; 31: 596–603. [DOI] [PubMed] [Google Scholar]
- 21. Burney KD, Krishnan K, Ruffin MT, Zhang D, Brenner DE. Adherence to single daily dose of aspirin in a chemoprevention trial: an evaluation of self‐report and microelectronic monitoring. Arch Fam Med 1996; 5: 297–300. [DOI] [PubMed] [Google Scholar]
- 22. Buscher A, Hartman C, Kallen MA, Giordano TP. Validity of self‐report measures in assessing antiretroviral adherence of newly diagnosed, HAART‐naive, HIV patients. HIV Clin Trials 2011; 12: 244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Byerly M, Fisher R, Whatley K, Holland R, Varghese F, Carmody T, et al. A comparison of electronic monitoring vs. clinician rating of antipsychotic adherence in outpatients with schizophrenia. Psychiatry Res 2005; 133: 129–33. [DOI] [PubMed] [Google Scholar]
- 24. Byerly MJ, Nakonezny PA, Rush AJ. The Brief Adherence Rating Scale (BARS) validated against electronic monitoring in assessing the antipsychotic medication adherence of outpatients with schizophrenia and schizoaffective disorder. Schizophr Res 2008; 100: 60–9. [DOI] [PubMed] [Google Scholar]
- 25. Byerly MJ, Thompson A, Carmody T, Bugno R, Erwin T, Kashner M, et al. Validity of electronically monitored medication adherence and conventional adherence measures in schizophrenia. Psychiatr Serv 2007; 58: 844–7. [DOI] [PubMed] [Google Scholar]
- 26. Charach A, Gajaria A, Skyba A, Chen S. Documenting adherence to psychostimulants in children with ADHD. J Can Acad Child Adolesc Psychiatry 2008; 17: 131–6. [PMC free article] [PubMed] [Google Scholar]
- 27. Choo PW, Rand CS, Inui TS, Lee ML, Cain E, Cordeiro‐Breault M, et al. Validation of patient reports, automated pharmacy records, and pill counts with electronic monitoring of adherence to antihypertensive therapy. Med Care 1999; 37: 846–57. [DOI] [PubMed] [Google Scholar]
- 28. Choo PW, Rand CS, Inui TS, Lee ML, Canning C, Platt R. A cohort study of possible risk factors for over‐reporting of antihypertensive adherence. BMC Cardiovasc Disord 2001; 1: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cramer JA, Mattson RH, Prevey ML, Scheyer RD, Ouellette VL. How often is medication taken as prescribed? A novel assessment technique. JAMA 1989; 261: 3273–7. [PubMed] [Google Scholar]
- 30. Curtin RB, Svarstad BL, Keller TH. Hemodialysis patients' noncompliance with oral medications. ANNA J 1999; 26: 307–16 .discussion 317, 335 [PubMed] [Google Scholar]
- 31. Das M, Santos D, Matheson T, Santos GM, Chu P, Vittinghoff E, et al. Feasibility and acceptability of a phase II randomized pharmacologic intervention for methamphetamine dependence in high‐risk men who have sex with men. AIDS 2010; 24: 991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Farley J, Hines S, Musk A, Ferrus S, Tepper V. Assessment of adherence to antiviral therapy in HIV‐infected children using the Medication Event Monitoring System, pharmacy refill, provider assessment, caregiver self‐report, and appointment keeping. J Acquir Immune Defic Syndr 2003; 33: 211–8. [DOI] [PubMed] [Google Scholar]
- 33. Feinn R, Tennen H, Cramer J, Kranzler HR. Measurement and prediction of medication compliance in problem drinkers. Alcohol Clin Exp Res 2003; 27: 1286–92. [DOI] [PubMed] [Google Scholar]
- 34. Fletcher CV, Testa MA, Brundage RC, Chesney MA, Haubrich R, Acosta EP, et al. Four measures of antiretroviral medication adherence and virologic response in AIDS clinical trials group study 359. J Acquir Immune Defic Syndr 2005; 40: 301–6. [DOI] [PubMed] [Google Scholar]
- 35. Frick PA, Gal P, Lane TW, Sewell PC. Antiretroviral medication compliance in patients with AIDS. AIDS Patient Care STDS 1998; 12: 463–70. [DOI] [PubMed] [Google Scholar]
- 36. Galloway GP, Coyle JR, Guillen JE, Flower K, Mendelson JE. A simple, novel method for assessing medication adherence: capsule photographs taken with cellular telephones. J Addiction Med 2011; 5: 170–4. [DOI] [PubMed] [Google Scholar]
- 37. Gonzalez JS, Schneider HE, Wexler DJ, Psaros C, Delahanty LM, Cagliero E, et al. Validity of medication adherence self‐reports in adults with type 2 diabetes. Diabetes Care 2013; 36: 831–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Greenley RN, Kunz JH, Biank V, Martinez A, Miranda A, Noe J, et al. Identifying youth nonadherence in clinical settings: data‐based recommendations for children and adolescents with inflammatory bowel disease. Inflamm Bowel Dis 2012; 18: 1254–9. [DOI] [PubMed] [Google Scholar]
- 39. Guerrero D, Rudd P, Bryant‐Kosling C, Middleton B, Middleton BF. Antihypertensive medication‐taking: investigation of a simple regimen. Am J Hypertens 1993; 6: 586–92. [DOI] [PubMed] [Google Scholar]
- 40. Hansen RA, Kim MM, Song L, Tu W, Wu J, Murray MD. Comparison of methods to assess medication adherence and classify nonadherence. Ann Pharmacother 2009; 43: 413–22. [DOI] [PubMed] [Google Scholar]
- 41. Holzemer WL, Bakken S, Portillo CJ, Grimes R, Welch J, Wantland D, et al. Testing a nurse‐tailored HIV medication adherence intervention. Nurs Res 2006; 55: 189–97. [DOI] [PubMed] [Google Scholar]
- 42. Jin Y, Pollock BG, Frank E, Florian J, Kirshner M, Fagiolini A, et al. The effect of reporting methods for dosing times on the estimation of pharmacokinetic parameters of escitalopram. J Clin Pharmacol 2009; 49: 176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kimmerling M, Wagner G, Ghosh‐Dastidar B. Factors associated with accurate self‐reported adherence to HIV antiretrovirals. Int J STD AIDS 2003; 14: 281–4. [DOI] [PubMed] [Google Scholar]
- 44. Lee JY, Kusek JW, Greene PG, Bernhard S, Norris K, Smith D, et al. Assessing medication adherence by pill count and electronic monitoring in the African American Study of Kidney Disease and Hypertension (AASK) Pilot Study. Am J Hypertens 1996; 9: 719–25. [DOI] [PubMed] [Google Scholar]
- 45. Leopold NA, Polansky M, Hurka MR. Drug adherence in Parkinson's disease. Mov Disord 2004; 19: 513–7. [DOI] [PubMed] [Google Scholar]
- 46. Levine AJ, Hinkin CH, Marion S, Keuning A, Castellon SA, Lam MM, et al. Adherence to antiretroviral medications in HIV: differences in data collected via self‐report and electronic monitoring. Health Psychol 2006; 25: 329–35. [DOI] [PubMed] [Google Scholar]
- 47. Llabre MM, Weaver KE, Duran RE, Antoni MH, McPherson‐Baker S, Schneiderman N. A measurement model of medication adherence to highly active antiretroviral therapy and its relation to viral load in HIV‐positive adults. AIDS Patient Care STDS 2006; 20: 701–11. [DOI] [PubMed] [Google Scholar]
- 48. Lu M, Safren SA, Skolnik PR, Rogers WH, Coady W, Hardy H, et al. Optimal recall period and response task for self‐reported HIV medication adherence. AIDS Behav 2008; 12: 86–94. [DOI] [PubMed] [Google Scholar]
- 49. Martin S, Elliott‐DeSorbo DK, Calabrese S, Wolters PL, Roby G, Brennan T, et al. A comparison of adherence assessment methods utilized in the United States: perspectives of researchers, HIV‐infected children, and their caregivers. AIDS Patient Care STDS 2009; 23: 593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mason BJ, Matsuyama JR, Jue SG. Assessment of sulfonylurea adherence and metabolic control. Diabetes Educ 1995; 21: 52–7. [DOI] [PubMed] [Google Scholar]
- 51. Mathews WC, Mar‐Tang M, Ballard C, Colwell B, Abulhosn K, Noonan C, et al. Prevalence, predictors, and outcomes of early adherence after starting or changing antiretroviral therapy. AIDS Patient Care STDS 2002; 16: 157–72. [DOI] [PubMed] [Google Scholar]
- 52. Matsui D, Hermann C, Klein J, Berkovitch M, Olivieri N, Koren G. Critical comparison of novel and existing methods of compliance assessment during a clinical trial of an oral iron chelator. J Clin Pharmacol 1994; 34: 944–9. [DOI] [PubMed] [Google Scholar]
- 53. Matsuyama JR, Mason BJ, Jue SG. Pharmacists' interventions using an electronic medication‐event monitoring device's adherence data versus pill counts. Ann Pharmacother 1993; 27: 851–5. [DOI] [PubMed] [Google Scholar]
- 54. Melbourne KM, Geletko SM, Brown SL, Willey‐Lessne C, Chase S, Fisher A. Medication adherence in patients with HIV infection: a comparison of two measurement methods. AIDS Read 1999; 9: 329–38. [PubMed] [Google Scholar]
- 55. Modi AC, Guilfoyle SM, Morita DA, Glauser TA. Development and reliability of a correction factor for parent‐reported adherence to pediatric antiepileptic drug therapy. Epilepsia 2011; 52: 370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Modi AC, Lim CS, Yu N, Geller D, Wagner MH, Quittner AL. A multi‐method assessment of treatment adherence for children with cystic fibrosis. J Cyst Fibros 2006; 5: 177–85. [DOI] [PubMed] [Google Scholar]
- 57. Namkoong K, Farren CK, O'Connor PG, O'Malley SS. Measurement of compliance with naltrexone in the treatment of alcohol dependence: research and clinical implications. J Clin Psychiatry 1999; 60: 449–53. [DOI] [PubMed] [Google Scholar]
- 58. Olivieri NF, Matsui D, Hermann C, Koren G. Compliance assessed by the Medication Event Monitoring System. Arch Dis Child 1991; 66: 1399–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Parker CS, Chen Z, Price M, Gross R, Metlay JP, Christie JD, et al. Adherence to warfarin assessed by electronic pill caps, clinician assessment, and patient reports: results from the IN‐RANGE study. J Gen Intern Med 2007; 22: 1254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pearson CR, Simoni JM, Hoff P, Kurth AE, Martin DP. Assessing antiretroviral adherence via electronic drug monitoring and self‐report: an examination of key methodological issues. AIDS Behav 2007; 11: 161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Potter L, Oakley D, de Leon‐Wong E, Canamar R. Measuring compliance among oral contraceptive users. Fam Plann Perspect 1996; 28: 154–8. [PubMed] [Google Scholar]
- 62. Remington G, Kwon J, Collins A, Laporte D, Mann S, Christensen B. The use of electronic monitoring (MEMS) to evaluate antipsychotic compliance in outpatients with schizophrenia. Schizophr Res 2007; 90: 229–37. [DOI] [PubMed] [Google Scholar]
- 63. Rudd P, Ahmed S, Zachary V, Barton C, Bonduelle D. Improved compliance measures: applications in an ambulatory hypertensive drug trial. Clin Pharmacol Ther 1990; 48: 676–85. [DOI] [PubMed] [Google Scholar]
- 64. Smith SR, Wahed AS, Kelley SS, Conjeevaram HS, Robuck PR, Fried MW, et al. Assessing the validity of self‐reported medication adherence in hepatitis C treatment. Ann Pharmacother 2007; 41: 1116–23. [DOI] [PubMed] [Google Scholar]
- 65. Steele RG, Anderson B, Rindel B, Dreyer ML, Perrin K, Christensen R, et al. Adherence to antiretroviral therapy among HIV‐positive children: examination of the role of caregiver health beliefs. AIDS Care 2001; 13: 617–29. [DOI] [PubMed] [Google Scholar]
- 66. Straka RJ, Fish JT, Benson SR, Suh JT. Patient self‐reporting of compliance does not correspond with electronic monitoring: an evaluation using isosorbide dinitrate as a model drug. Pharmacotherapy 1997; 17: 126–32. [PubMed] [Google Scholar]
- 67. Svarstad BL, Chewning BA, Sleath BL, Claesson C. The Brief Medication Questionnaire: a tool for screening patient adherence and barriers to adherence. Patient Educ Couns 1999; 37: 113–24. [DOI] [PubMed] [Google Scholar]
- 68. Velligan DI, Wang M, Diamond P, Glahn DC, Castillo D, Bendle S, et al. Relationships among subjective and objective measures of adherence to oral antipsychotic medications. Psychiatr Serv 2007; 58: 1187–92. [DOI] [PubMed] [Google Scholar]
- 69. Wagner GJ, Rabkin JG. Measuring medication adherence: are missed doses reported more accurately than perfect adherence? AIDS Care 2000; 12: 405–8. [DOI] [PubMed] [Google Scholar]
- 70. Walter T, Wang L, Chuk K, Ng P, Tannock IF, Krzyzanowska MK. Assessing adherence to oral chemotherapy using different measurement methods: lessons learned from capecitabine. J Oncol Pharm Pract 2013; 20: 249–56. [DOI] [PubMed] [Google Scholar]
- 71. Waterhouse DM, Calzone KA, Mele C, Brenner DE. Adherence to oral tamoxifen: a comparison of patient self‐report, pill counts, and microelectronic monitoring. J Clin Oncol 1993; 11: 1189–97. [DOI] [PubMed] [Google Scholar]
- 72. Wohl DA, Stephenson BL, Golin CE, Kiziah CN, Rosen D, Ngo B, et al. Adherence to directly observed antiretroviral therapy among human immunodeficiency virus‐infected prison inmates. Clin Infect Dis 2003; 36: 1572–6. [DOI] [PubMed] [Google Scholar]
- 73. Bangsberg DR, Hecht FM, Charlebois ED, Chesney M, Moss A. Comparing objective measures of adherence to HIV antiretroviral therapy: electronic medication monitors and unannounced pill counts. AIDS Behav 2001; 5: 6. [Google Scholar]
- 74. Mooney ME, Sayre S, Green C, Rhoades H, Schmitz J. Comparing measures of medication taking in a pharmacotherapy trial for cocaine dependence. Addict Disorders Their Treat 2004; 3: 165–73. [Google Scholar]
- 75. Arnet I, Haefeli WE. Overconsumption detected by electronic drug monitoring requires subtle interpretation. Clin Pharmacol Ther 2000; 67: 44–7. [DOI] [PubMed] [Google Scholar]
- 76. Bosman J, Ter Horst PG, Smit JP, Dijkstra JR, Beekhuis HR, Slingersland RJ, et al. Adherence of antidepressants during pregnancy: MEMS compared with three other methods. Ther Adv Psychopharmacol 2014; 4: 61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Brain C, Sameby B, Allerby K, Lindstrom E, Eberhard J, Burns T, et al. Twelve months of electronic monitoring (MEMS(R)) in the Swedish COAST‐study: a comparison of methods for the measurement of adherence in schizophrenia. Eur Neuropsychopharmacol 2014; 24: 215–22. [DOI] [PubMed] [Google Scholar]
- 78. Butler JA, Peveler RC, Roderick P, Horne R, Mason JC. Measuring compliance with drug regimens after renal transplantation: comparison of self‐report and clinician rating with electronic monitoring. Transplantation 2004; 77: 786–9. [DOI] [PubMed] [Google Scholar]
- 79. de Klerk E, van der Heijde D, Landewe R, van der Tempel H, van der Linden S. The compliance‐questionnaire‐rheumatology compared with electronic medication event monitoring: a validation study. J Rheumatol 2003; 30: 2469–75. [PubMed] [Google Scholar]
- 80. Denhaerynck K, Schafer‐Keller P, Young J, Steiger J, Bock A, De Geest S. Examining assumptions regarding valid electronic monitoring of medication therapy: development of a validation framework and its application on a European sample of kidney transplant patients. BMC Med Res Methodol 2008; 8: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Deschamps AE, De Geest S, Vandamme AM, Bobbaers H, Peetermans WE, Van Wijngaerden E. Diagnostic value of different adherence measures using electronic monitoring and virologic failure as reference standards. AIDS Patient Care STDS 2008; 22: 735–43. [DOI] [PubMed] [Google Scholar]
- 82. Deschamps AE, Graeve VD, van Wijngaerden E, De Saar V, Vandamme AM, van Vaerenbergh K, et al. Prevalence and correlates of nonadherence to antiretroviral therapy in a population of HIV patients using Medication Event Monitoring System. AIDS Patient Care STDS 2004; 18: 644–57. [DOI] [PubMed] [Google Scholar]
- 83. Doro P, Benko R, Czako A, Matuz M, Thurzo F, Soos G. Optimal recall period in assessing the adherence to antihypertensive therapy: a pilot study. Int J Clin Pharm 2011; 33: 690–5. [DOI] [PubMed] [Google Scholar]
- 84. Dutrey‐Dupagne C, Vaur L, Genes N, Mallion JM, Meredith P, Elkik F. Errors in trough:peak ratio determinations induced by patient behaviour. Blood Press Monit 1996; 1: 273–7. [PubMed] [Google Scholar]
- 85. Fallab‐Stubi CL, Zellweger JP, Sauty A, Uldry C, Iorillo D, Burnier M. Electronic monitoring of adherence to treatment in the preventive chemotherapy of tuberculosis. Int J Tubercul Lung Dis 1998; 2: 525–30. [PubMed] [Google Scholar]
- 86. Finigan J, Naylor K, Paggiosi MA, Peel NF, Eastell R. Adherence to raloxifene therapy: assessment methods and relationship with efficacy. Osteoporos Int 2013; 24: 2879–86. [DOI] [PubMed] [Google Scholar]
- 87. George CF, Peveler RC, Heiliger S, Thompson C. Compliance with tricyclic antidepressants: the value of four different methods of assessment. Br J Clin Pharmacol 2000; 50: 166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Gillespie D, Hood K, Farewell D, Stenson R, Probert C, Hawthorne AB. Electronic monitoring of medication adherence in a 1‐year clinical study of 2 dosing regimens of mesalazine for adults in remission with ulcerative colitis. Inflamm Bowel Dis 2014; 20: 82–91. [DOI] [PubMed] [Google Scholar]
- 89. Grosset KA, Bone I, Reid JL, Grosset D. Measuring therapy adherence in Parkinson's disease: a comparison of methods. J Neurol Neurosurg Psychiatry 2006; 77: 249–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hamilton GA. Measuring adherence in a hypertension clinical trial. Eur J Cardiovasc Nurs 2003; 2: 219–28. [DOI] [PubMed] [Google Scholar]
- 91. Hugen PW, Langebeek N, Burger DM, Zomer B, van Leusen R, Schuurman R, et al. Assessment of adherence to HIV protease inhibitors: comparison and combination of various methods, including MEMS (electronic monitoring), patient and nurse report, and therapeutic drug monitoring. J Acquir Immune Defic Syndr 2002; 30: 324–34. [DOI] [PubMed] [Google Scholar]
- 92. Knobel H, Alonso J, Casado JL, Collazos J, Gonzalez J, Ruiz I, et al. Validation of a simplified medication adherence questionnaire in a large cohort of HIV‐infected patients: the GEEMA Study. AIDS 2002; 16: 605–13. [DOI] [PubMed] [Google Scholar]
- 93. Kruse W, Nikolaus T, Rampmaier J, Weber E, Schlierf G. Actual versus prescribed timing of lovastatin doses assessed by electronic compliance monitoring. Eur J Clin Pharmacol 1993; 45: 211–5. [DOI] [PubMed] [Google Scholar]
- 94. Llor C, Hernandez S, Bayona C, Moragas A, Sierra N, Hernandez M, et al. A study of adherence to antibiotic treatment in ambulatory respiratory infections. Int J Infect Dis 2013; 17: e168–72. [DOI] [PubMed] [Google Scholar]
- 95. Nieuwenhuis MM, Jaarsma T, van Veldhuisen DJ, van der Wal MH. Self‐reported versus ‘true’ adherence in heart failure patients: a study using the Medication Event Monitoring System. Neth Heart J 2012; 20: 313–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Oldenmenger WH, Echteld MA, de Wit R, Sillevis Smitt PA, Stronks DL, Stoter G, et al. Analgesic adherence measurement in cancer patients: comparison between electronic monitoring and diary. J Pain Symptom Manage 2007; 34: 639–47. [DOI] [PubMed] [Google Scholar]
- 97. Paes AH, Bakker A, Soe‐Agnie CJ. Measurement of patient compliance. Pharm World Sci 1998; 20: 73–7. [DOI] [PubMed] [Google Scholar]
- 98. Parienti JJ, Barrail‐Tran A, Duval X, Nembot G, Descamps D, Vigan M, et al. Adherence profiles and therapeutic responses of treatment‐naive HIV‐infected patients starting boosted atazanavir‐based therapy in the ANRS 134‐COPHAR 3 trial. Antimicrob Agents Chemother 2013; 57: 2265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Schafer‐Keller P, Steiger J, Bock A, Denhaerynck K, De Geest S. Diagnostic accuracy of measurement methods to assess non‐adherence to immunosuppressive drugs in kidney transplant recipients. Am J Transplant 2008; 8: 616–26. [DOI] [PubMed] [Google Scholar]
- 100. Schroeder K, Fahey T, Hay AD, Montgomery A, Peters TJ. Adherence to antihypertensive medication assessed by self‐report was associated with electronic monitoring compliance. J Clin Epidemiol 2006; 59: 650–1. [DOI] [PubMed] [Google Scholar]
- 101. Schwed A, Fallab CL, Burnier M, Waeber B, Kappenberger L, Burnand B, et al. Electronic monitoring of compliance to lipid‐lowering therapy in clinical practice. J Clin Pharmacol 1999; 39: 402–9. [DOI] [PubMed] [Google Scholar]
- 102. Stubi CL, Landry PR, Petignat C, Bille J, Genton B, Darioli R, et al. Compliance to live oral Ty21a typhoid vaccine, and its effect on viability. J Travel Med 2000; 7: 133–7. [DOI] [PubMed] [Google Scholar]
- 103. van Onzenoort HA, Verberk WJ, Kessels AG, Kroon AA, Neef C, van der Kuy PH, et al. Assessing medication adherence simultaneously by electronic monitoring and pill count in patients with mild‐to‐moderate hypertension. Am J Hypertens 2010; 23: 149–54. [DOI] [PubMed] [Google Scholar]
- 104. Walsh JC, Mandalia S, Gazzard BG. Responses to a 1 month self‐report on adherence to antiretroviral therapy are consistent with electronic data and virological treatment outcome. AIDS 2002; 16: 269–77. [DOI] [PubMed] [Google Scholar]
- 105. Wetzels GE, Nelemans PJ, Schouten JS, van Wijk BL, Prins MH. All that glisters is not gold: a comparison of electronic monitoring versus filled prescriptions – an observational study. BMC Health Serv Res 2006; 6: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Winkler A, Teuscher AU, Mueller B, Diem P. Monitoring adherence to prescribed medication in type 2 diabetic patients treated with sulfonylureas. Swiss Med Wkly 2002; 132: 379–85. [DOI] [PubMed] [Google Scholar]
- 107. Zeller A, Ramseier E, Teagtmeyer A, Battegay E. Patients' self‐reported adherence to cardiovascular medication using electronic monitors as comparators. Hypertens Res 2008; 31: 2037–43. [DOI] [PubMed] [Google Scholar]
- 108. Zeller A, Schroeder K, Peters TJ. An adherence self‐report questionnaire facilitated the differentiation between nonadherence and nonresponse to antihypertensive treatment. J Clin Epidemiol 2008; 61: 282–8. [DOI] [PubMed] [Google Scholar]
- 109. Zeller A, Taegtmeyer A, Martina B, Battegay E, Tschudi P. Physicians' ability to predict patients' adherence to antihypertensive medication in primary care. Hypertens Res 2008; 31: 1765–71. [DOI] [PubMed] [Google Scholar]
- 110. Baxi SM, Liu A, Bacchetti P, Mutua G, Sanders EJ, Kibengo FM, et al. Comparing the novel method of assessing PrEP adherence/exposure using hair samples to other pharmacologic and traditional measures. J Acquir Immune Defic Syndr 2015; 68: 13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Bell DJ, Wootton D, Mukaka M, Montgomery J, Kayange N, Chimpeni P, et al. Measurement of adherence, drug concentrations and the effectiveness of artemether‐lumefantrine, chlorproguanil‐dapsone or sulphadoxine‐pyrimethamine in the treatment of uncomplicated malaria in Malawi. Malar J 2009; 8: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Haberer JE, Kahane J, Kigozi I, Emenyonu N, Hunt P, Martin J, et al. Real‐time adherence monitoring for HIV antiretroviral therapy. AIDS Behav 2010; 14: 1340–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Muller AD, Bode S, Myer L, Roux P, von Steinbuchel N. Electronic measurement of adherence to pediatric antiretroviral therapy in South Africa. Pediatr Infect Dis J 2008; 27: 257–62. [DOI] [PubMed] [Google Scholar]
- 114. Olds PK, Kiwanuka JP, Nansera D, Huang Y, Bacchetti P, Jin C, et al. Assessment of HIV antiretroviral therapy adherence by measuring drug concentrations in hair among children in rural Uganda. AIDS Care 2015; 27: 327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Thirumurthy H, Siripong N, Vreeman RC, Pop‐Eleches C, Habyarimana JP, Sidle JE, et al. Differences between self‐reported and electronically monitored adherence among patients receiving antiretroviral therapy in a resource‐limited setting. AIDS 2012; 26: 2399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Twagirumukiza M, Kayumba PC, Kips JG, Vrijens B, Stichele RV, Vervaet C, et al. Evaluation of medication adherence methods in the treatment of malaria in Rwandan infants. Malar J 2010; 9: 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. van den Boogaard J, Lyimo RA, Boeree MJ, Kibiki GS, Aarnoutse RE. Electronic monitoring of treatment adherence and validation of alternative adherence measures in tuberculosis patients: a pilot study. Bull World Health Organ 2011; 89: 632–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Vreeman RC, Nyandiko WM, Liu H, Tu W, Scanlon ML, Slaven JE, et al. Measuring adherence to antiretroviral therapy in children and adolescents in western Kenya. J Int AIDS Soc 2014; 17: 19227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Vriesendorp R, Cohen A, Kristanto P, Vrijens B, Rakesh P, Anand B, et al. Adherence to HAART therapy measured by electronic monitoring in newly diagnosed HIV patients in Botswana. Eur J Clin Pharmacol 2007; 63: 1115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kheir N, Greer W, Yousif A, Al‐Geed H, Okkah RA, Zirie M, et al. The utility of an electronic adherence assessment device in type 2 diabetes mellitus: a pilot study of single medication. Patient Prefer Adher 2010; 4: 247–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Lee MS, Lee HY, Kang SG, Yang J, Ahn H, Rhee M, et al. Variables influencing antidepressant medication adherence for treating outpatients with depressive disorders. J Affect Disord 2010; 123: 216–21. [DOI] [PubMed] [Google Scholar]
- 122. Ruslami R, van Crevel R, van de Berge E, Alisjahbana B, Aarnoutse RE. A step‐wise approach to find a valid and feasible method to detect non‐adherence to tuberculosis drugs. Southeast Asian J Trop Med Public Health 2008; 39: 1083–7. [PubMed] [Google Scholar]
- 123. Yang J, Ko YH, Paik JW, Lee MS, Han C, Joe SH, et al. Symptom severity and attitudes toward medication: impacts on adherence in outpatients with schizophrenia. Schizophr Res 2012; 134: 226–31. [DOI] [PubMed] [Google Scholar]
- 124. Yang J, Yoon BM, Lee MS, Joe SH, Jung IK, Kim SH. Adherence with electronic monitoring and symptoms in children with attention deficit hyperactivity disorder. Psychiatry Investig 2012; 9: 263–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. da Costa TM, Barbosa BJ, Gomes e Costa DA, Sigulem D, de Fatima Marin H, Filho AC, et al. Results of a randomized controlled trial to assess the effects of a mobile SMS‐based intervention on treatment adherence in HIV/AIDS‐infected Brazilian women and impressions and satisfaction with respect to incoming messages. Int J Med Inform 2012; 81: 257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Tornheim JA, Lozano Beltran DF, Gilman RH, Castellon M, Solano Mercado MA, Sullca W, et al. Improved completion rates and characterization of drug reactions with an intensive Chagas disease treatment program in rural Bolivia. PLoS Negl Trop Dis 2013; 7: e2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Starr M, Sawyer S, Carlin J, Powell C, Newman R, Johnson P. A novel approach to monitoring adherence to preventive therapy for tuberculosis in adolescence. J Paediatr Child Health 1999; 35: 350–4. [PubMed] [Google Scholar]
- 128. Vrijens B, Heidbuchel H. Non‐vitamin K antagonist oral anticoagulants: considerations on once‐ vs. twice‐daily regimens and their potential impact on medication adherence. Europace 2015; 17: 514–23. [DOI] [PubMed] [Google Scholar]


