The single nucleotide polymorphisms of solute like carrier 1B1 (SLCO1B1) c.521 T > C (rs4149056 p. Val174Ala) and ATP binding cassette G2 (ABCG2) c.421C > A (rs2231142 Gln141Lys), have generated a lot of interest owing to their consistent association with statin induced myopathy 1, 2. Studies show that these two polymorphisms not only affect interindividual variations in rosuvastatin pharmacokinetics 1, 2 but also affect interethnic variations as well 3, 4. The identification of these interethnic differences led to the issue of a warning for rosuvastatin dose in patients of Asian ancestry 5. Consequently, genetic tests that screen for these two polymorphisms have been developed for routine clinical use of rosuvastatin in the treatment of hypercholesterolaemia and mixed dyslipidaemias.
Our aim was to try to develop pharmacogenomic diagnostic tests that can be used in most populations motivated by the growing use of rosuvastatin in Sub‐Saharan patients. We set out to investigate the utility of the polymorphisms SLCO1B1 rs4149056 and ABCG2 rs2231142 as genetic markers for statin induced myopathy in individuals of African ancestry. A pharmacokinetic study was conducted with an open label single oral dose of rosuvastatin in 30 healthy males (age 18–30 years). This study was approved by the Medicines Research Council of Zimbabwe (Ref: MRCZ/A/1793), the University of Cape Town Faculty of Health Sciences Research Ethics Committee (Ref: 197/2014) and the Chitungwiza Central Hospital Institutional Review Board. Exclusion criteria included use of statins or fibrates within 6 weeks of the study, the use of investigational or over the counter drugs known to interfere with rosuvastatin therapy, active alcohol or drug dependence, hereditary muscle disease or a family history of the disease. Eligible participants gave written consent prior to the study. Thirty adult males aged 18–30 years of self‐reported Bantu African ancestry took part in the study. After 48 h refraining from alcohol and a 12 h overnight fast, each participant was administered 20 mg of rosuvastatin calcium with 450 ml of water. Participants fasted for another 4 h post‐drug administration. Venous blood samples (5 ml) were collected for drug concentrations at 0, 0.5, 1, 2, 3, 4, 5, 6, 8, 10, 24 and 30 h post‐drug administration. Whole blood samples were collected into EDTA anticoagulant tubes and the separated plasma was stored at −80 °C until assay. An additional 1.5 ml of blood was collected from each participant to use in the extraction of DNA for genetic analysis. Medical examination, clinical laboratory data, clinical observation and adverse drug reports were used to assess safety and tolerability. Plasma concentrations of rosuvastatin were analyzed using ultra performance liquid chromatography (UPLC) with mass spectrometric detection according to a method developed and validated by Astra Zeneca (Sweden). The limit of quantification for rosuvastatin in plasma was 0.47 ng ml−1. Allelic discrimination was used to genotype for SLCO1B1 rs4149056 and ABCG2 rs2231142 using validated Applied Biosystems Taqman assay (Applied Biosystems, USA) genotyping kits SLCO1B1 (rs4149056, C_30633906_10) and ABCG2 (rs2231142, C_15854163_70).
Of the 30 individuals enrolled in the study, 90 % (n = 27) were self‐reported as Shona and 3.3 % each (n = 1) as either Ndebele, Sotho or Kalanga. These four ethnic groups are all of African Bantu ancestry and reside in Zimbabwe. The mean age of the participants was 24 (2.7) years, their mean height was 1.7 (0.7) m, with a mean weight of 64.2 (10.7) kg, making their mean body mass index 21.0 (3.2) kg m−2. Rosuvastatin mean plasma C max was 12.22 ng ml– 1 ranging from 2.81 ng ml– 1 to 31 ng ml– 1 (Figure 1 and Table 1). The mean AUC(0,t) was 105.94 ng ml– 1 h ranging from 31.66 ng ml– 1 h to 242.98 ng ml– 1 h. The 20 mg orally administered rosuvastatin dose was well tolerated in this group of healthy male volunteers as no serious adverse events were reported.
Figure 1.
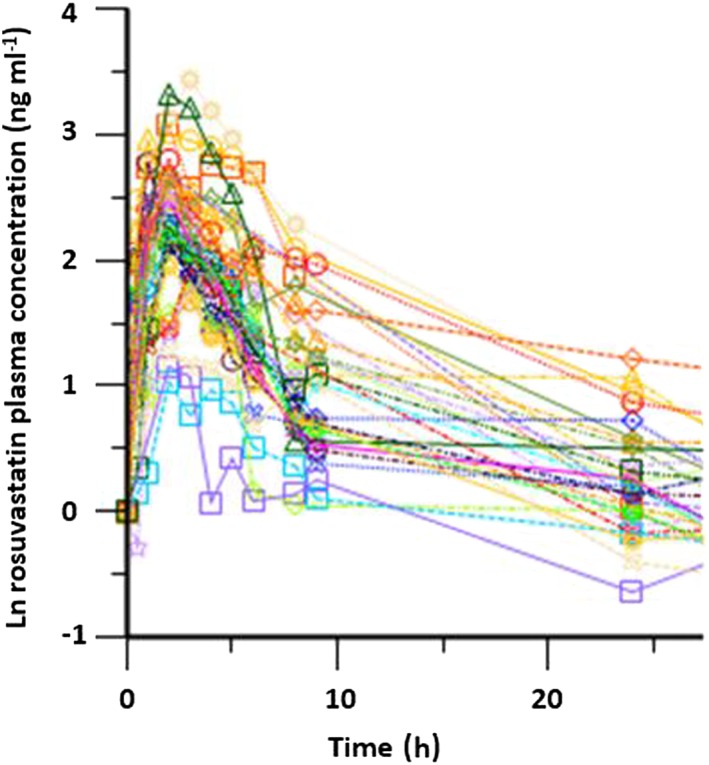
Rosuvastatin plasma natural log concentration–time profiles in 30 unrelated volunteers of African ancestry
Table 1.
Summary of the non‐compartmental analysis of the pharmacokinetics of rosuvastatin in the 30 healthy individuals
| Parameter | Zimbabwean (this study) | Caucasian *(SLCO1B1 c.521C) 4 | Asian *(ABCG2 c.421 A) 4 |
|---|---|---|---|
| Genotype frequencies | n = 30 | n = 26 | n = 131 |
| SLCO1B1 T521 > C[Val174Ala] | 0.00 % | 68.0 % | 78 % |
| ABCG2 C421 > A[Gln141Lys] | 0.00 % | 68.0 % | 49 % |
| C max (ng ml −1 ) | |||
| Geometric mean | 12.22 | 11.9 | 22.0 |
| 95% CI | 9.75, 14.69 | 9.69, 14.6 | 20.1, 24.1 |
| 90% CI | 10.17, 14.27 | — | 1.54, 2.23 |
| AUC(0,t) (ng ml −1 h) | |||
| Geometric mean | 131.44 | 116 | 202 |
| 95% CI | 103.24, 159.64 | 116 | 186, 218 |
| 90% CI | 108.01, 154.87 | 97.4, 138 | 1.48, 2.04 |
| t 1/2 (ng ml −1 ) | |||
| Geometric mean (SD) | 2.27 (0.81) | 4.35 (1.24) | 3.86 (1.36) |
| Range | 1.00–5.00 | 2.00–16.00 | 0.50–6.00 |
Most PK parameters in the current rosuvastatin study among 30 healthy male Zimbabwean volunteers (Figure 1) are similar to those observed among Caucasian wild type (Table 1). In this study, we reported a shorter average time to reach C max (2 h vs. 3 to 5 h) compared with Caucasian groups 3, 4, 6. The shorter time to C max is indicative of a much faster rate of absorption of the rosuvastatin from the enterocytes. Preliminary genetic analysis for both SLCO1B1 c.521C (rs4149056) and ABCG2 c.421 A (rs 2 231 142) variants showed that none of the participants had either variant. The observed lack of both the SLCO1B1 c.521 T > C (rs4149056) and ABCG2 c.421C > A (rs2231142) variants amongst these participants in the presence of high inter‐individual variation (see Figure 1) is a strong suggestion for the involvement of other genetic variants in the same genes or in other genes that could be playing a significant role in this particular population. There is vast genetic heterogeneity within African populations. For example, high frequencies of SLCO1B1 c.521C of 6 % and 21 % have been reported among Ethiopians 7 and Tanzanians 7, 8, respectively. As such, these SNPs may not account for the observed subject variations in rosuvastatin pharmacokinetics in Zimbabwean populations but they may still be relevant in other African populations. Thus, further investigations are needed in African populations to identify specific genetic markers that explain observed pharmacokinetic variability in different ethnic groups. Future studies using whole genome or exome sequencing should be used to identify possible associations between observed pharmacokinetic interindividual variations amongst patients of Sub‐Saharan ancestry and underlying genetic variants. A pathway approach to investigating the relevant variants which affect rosuvastatin pharmacokinetics in these genetically diverse populations could be useful.
Competing Interests
All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare CM had support from Astra Zeneca for the submitted work and there are no other financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years.
Contributors
Nyarai Soko: designed the project, conducted the study, wrote and revised the manuscript.
Collet Dandara: designed the study, supervised certain aspects of the project, wrote and corrected the manuscript.
Raj Ramesar: supervised certain aspects of the project, wrote and corrected the manuscript.
Gerard Kadzirange: was the clinician who conducted and supervised the pharmacokinetic trial in the healthy participants.
Collen Masimirembwa: was the principal investigator of the study, designed the study, supervised the project, wrote and corrected the manuscript.
Soko, N. , Dandara, C. , Ramesar, R. , Kadzirange, G. , and Masimirembwa, C. (2016) Pharmacokinetics of rosuvastatin in 30 healthy Zimbabwean individuals of African ancestry. Br J Clin Pharmacol, 82: 326–328. doi: 10.1111/bcp.12915.
References
- 1. Pasanen MK, Neuvonen M, Neuvonen PJ, Niemi M. SLCO1B1 polymorphism markedly affects the pharmacokinetics of simvastatin acid. Pharmacogenet Genomics 2006; 16: 873–9. [DOI] [PubMed] [Google Scholar]
- 2. Zhang W, Yu B‐N, He Y‐J, Fan L, Li Q, Liu Z‐Q, Rong TZ. Fen‐Jiang, Huang Y. F and Zhou H.H. Role of BCRP 421C > A polymorphism on rosuvastatin pharmacokinetics in healthy Chinese males. Clin Chim Acta 2006; 373: 99–103. [DOI] [PubMed] [Google Scholar]
- 3. Lee E, Ryan S, Birmingham B, Zalikowski J, March R, Ambrose H, Moore R, Lee C, Chen Y, Schneck D. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin Pharmacol Ther 2005; 78: 330–41. [DOI] [PubMed] [Google Scholar]
- 4. Birmingham BK, Bujac SR, Elsby R, Azumaya CT, Zalikowski J, Chen Y, Kim K, Ambrose HJ. Rosuvastatin pharmacokinetics and pharmacogenetics in Caucasian and Asian subjects residing in the United States. Eur J Clin Pharmacol 2015; 71: 329–40. [DOI] [PubMed] [Google Scholar]
- 5. Zeneca A. Crestor (rosuvastatin calcium) tablets. Prescr Inf 2015; 1–43; ID: 3771849. [Google Scholar]
- 6. Martin PD, Warwick MJ, Dane AL, Hill SJ, Giles PB, Phillips PJ, Lenz E. Metabolism, excretion, and pharmacokinetics of rosuvastatin in healthy adult male volunteers. Clin Ther 2003 Nov; 25: 2822–35. [DOI] [PubMed] [Google Scholar]
- 7. Ngaimisi E, Habtewold A, Minzi O, Makonnen E, Mugusi S, Amogne W, Yimer G, Riedel KD, Janabi M, Aderaye G, Mugusi F, Bertilsson L, Aklillu E, Burhenne J. Importance of ethnicity, CYP2B6 and ABCB1 genotype for efavirenz pharmacokinetics and treatment outcomes: a parallel‐group prospective cohort study in two sub‐Saharan Africa populations. PLoS One 2013; 8: e67946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Aklillu E, Mugusi S, Ngaimisi E, Hoffmann MM, König S, Ziesenitz V, Mikus G, Haefeli W, Weiss J. Frequency of the SLCO1B1 388 A > G and the 521 T > C polymorphism in Tanzania genotyped by a new LightCycler®‐based method. Eur J Clin Pharmacol 2011; 67: 1139–45. [DOI] [PubMed] [Google Scholar]


