Abstract
The prospects for effectively treating well-established dementia, such as Alzheimer’s disease (AD), are slim, due to the destruction of key brain pathways that underlie higher cognitive function. There has been a substantial shift in the field towards detecting conditions such as AD in their earliest stages, which would allow preventative or therapeutic approaches to substantially reduce risk and/or slow the progression of disease. AD is characterized by hallmark pathological changes such as extracellular Aβ plaques and intracellular neurofibrillary pathology, which selectively affect specific subclasses of neurons and brain circuits. Current evidence indicates that Aβ plaques begin to form many years before overt dementia, a gradual and progressive pathology which offers a potential target for early intervention. Early Aβ changes in the brain result in localized damage to dendrites, axonal processes and synapses, to which excitatory synapses and the processes of projection neurons are highly vulnerable. Aβ pathology is replicated in a range of transgenic models overexpressing mutant human familial AD genes (eg APP and presenilin 1). Studying the development of aberrant regenerative and degenerative changes in neuritic processes associated with Aβ plaques may represent the best opportunity to understand the relationship between the pathological hallmarks of AD and neuronal damage, and to develop early interventions to prevent, slow down or mitigate against Aβ pathology and/or the neuronal alterations that leads to cognitive impairment.
Keywords: Alzheimer’s disease, amyloid precursor protein, Aß, plaque, dystrophic neurite, selective vulnerability, transgenic mice
INTRODUCTION
Alzheimer’s disease (AD) is characterized by the presence in the brain of ‘hallmark’ lesions such as Aβ plaques, abnormal ‘dystrophic’ neurites associated with plaques, neurofibrillary tangles (NFTs) and neuropil threads. These lesions follow a specific pattern of regional and cellular vulnerability, with characteristic distribution patterns of Aβ deposits and subtype-specific neuronal susceptibility to NFT pathology and degeneration. Cytoskeletal changes in dystrophic neurites near plaques resemble the filamentous changes seen in cell body NFTs, suggesting that Aβ plaques may induce cytoskeletal alterations [1]. However, there is also significant heterogeneity among dystrophic neurites at different stages of AD, which may provide further insight into the relationship between Aβ plaque and neuronal cytoskeletal pathology. Furthermore, while individual plaques may represent ‘focal’ lesions in the brain, how this may result in wider patterns of neuronal degeneration requires elucidation. It is also clear that neurons may have some capacity to react or adapt to such lesions, making the relationship between overt pathology and functional disruption dynamic and complex. This review focuses on the effects of Aβ plaque formation on neurons at different stages of AD, and
explores the capacity of commonly used transgenic mouse models expressing familial AD (FAD) mutated human genes to recapitulate such pathology, as well as to provide further insight into mechanisms and therapy.
Aβ PATHOLOGY DURING THE PROGRESSION OF AD
To date, there are a number of strong indications that Aβ abnormalities and plaque accumulation are an early and potentially necessary event in the sequence of brain changes that lead to AD. These include familial forms of AD involving mutations in the amyloid precursor protein (APP), Down syndrome in which the presence of three copies of the APP gene leads to an AD-like syndrome; studies of the staging of brain pathology; and in vivo human brain imaging for Aβ. However, there is no clear consensus on whether the critical damage is caused by abnormal Aβ species as intracellular accumulations, or as extracellular monomers, aggregates or plaques. The neocortex is an early site of Aβ accumulation, where it tends to localize in particular layers [2], indicating that misprocessing of Aβ leading to extracellular deposits may be specific to synaptic pathways terminating in these layers. However, the origin of abnormal Aβ and the formation of oligomers or plaques are still controversial, having been variably attributed to blood vessels, glial secretion, neuronal secretion from terminals and cell bodies, lysis of neurons or dystrophic neurites, and fibrillation by microglia (reviewed in [3]).
While it is commonly assumed that extracellular Aβ deposits ‘mature’ from diffuse forms to more dense, fibrillar plaques, staging studies in human cases indicate that these plaque subtypes develop separately, with a higher proportion of fibrillar Aβ deposits in later stages [4]. In vivo imaging studies of FAD transgenic mice show that Aβ plaques can form rapidly and are then relatively stable in morphology [5] and that smaller plaque deposits can occur in clusters and then merge into larger plaques [6]. It is not well understood why some amyloid protein deposits remain diffuse, whereas others densely aggregate into highly fibrillar and dense forms. Analysis by confocal microscopy also indicates that the more fibrillar Aβ plaques are spheres with a complex internal geometry, often around a dense amyloid core [4]; the factors that influence the morphology and size of these deposits are also unknown. Furthermore, human FAD involving PS1 mutations produces a larger and morphologically distinct plaque form (‘cotton wool’ plaques [7]), indicating that the means of Aβ production can influence plaque formation and morphology.
Human pathology staging [8] and recent human in vivo imaging using radio ligands for Aβ deposits [9], strongly indicate that Aβ deposition in the brain occurs early in AD, perhaps even decades before overt symptoms. However, it is also clear that there is substantial individual heterogeneity in the damaging effects of such deposits, since Aβ ‘load’ by itself does not correlate well with cognitive deficits in established AD [10]. Low Aβ load can accompany overt dementia, whereas some individuals show few cognitive and behavioural alterations despite relatively high Aβ deposition.
SPECIFIC PATTERNS OF DYSTROPHIC NEURITE FORMATION IN THE HUMAN BRAIN
While it is possible that Aβ oligomers have a more distributed role in neurotoxicity or compromising neuronal function, it is also clear that the more dense and fibrillar Aβ plaques act as discrete lesions, causing local damage to axons, dendrites and synapses and focal stimulation of astrocytes and microglia. In this regard, an accumulating burden of more dense, damaging, plaques concentrated in association areas of the cerebral cortex would likely have substantial effects on higher level processing capacity.
Interestingly, plaque formation does not appear to be directly related to cell death of adjacent neurons, although neurons can be deflected to the margins of such deposits [11]. Dendrites of pyramidal neurons within and proximal to Aβ plaques demonstrate deflection around the plaque, as well as withering and dendritic spine loss [12, 13]. However, dendrites intersecting with Aβ plaques rarely show ‘reactive’ changes resembling the dystrophy that characterizes ‘neuritic’ plaques. Those dystrophic neurites appear to derive principally from axons undergoing reactive, aberrant regenerative and/or frank degenerative changes near plaques [14-16].
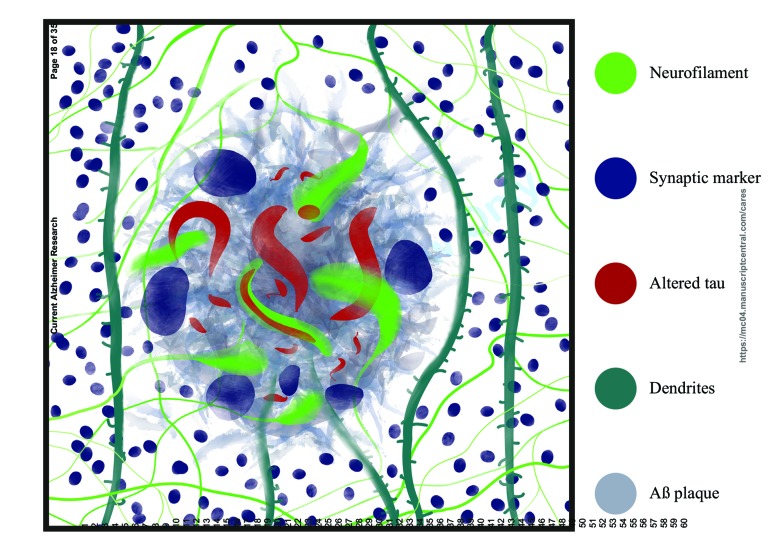
In human cases, dystrophic neurites can be classified by morphology, neurochemistry and association with different stages of AD (Fig. 1). In ‘end-stage’ AD, the major subtypes are distinguished by their complement of specific cytoskeletal proteins and synaptic markers. Angular dystrophic neurites commonly labelled with antibodies directed to tau (including abnormally phosphorylated tau isoforms, closely resembling the cytoskeletal pathology of neurofibrillary tangles) are usually seen within and very near plaques, and are probably a degenerative form of dystrophic neurite. These tau-immunolabelled dystrophic neurites likely correspond to the plaque-associated abnormal neurites seen in thioflavine S staining [17], further reinforcing their identity as end-stage pathology involving a substantially transformed cytoskeleton.
Fig. (1).
Diagram indicating the range of alterations associated with neuritic plaque formation. These include clipping and deflection of dendrites, loss of spines, axons and synapses. Four dystrophic neurite isoforms are present – those predominantly containing either NFs or altered tau, and a further group containing NFs with a core of pathological tau. Another group of dystrophic neurites are characterized by the presence of synaptic markers.
Another dystrophic neurite subtype is immunolabelled for neurofilament (NF) proteins, the NF triplet and alpha-internexin. These neurites are typically larger than the tau-labelled structures, and appear as swellings or torturous tubular structures localized within the pores of plaques or around the corona [15, 16, 18]. Interestingly, a subset of these larger NF labeled dystrophic neurites has a core of abnormal tau [16] which stains with thioflavine S [19], which we hypothesized may represent a transition from abnormally reactive and regenerative axons responding to Aβ plaques, into tau-abundant degenerative forms [20]. The NF abundant dystrophic neurites also colocalise with growth-related proteins (eg GAP43 [15]) and often have a long neurite ‘tail’ that can be traced out to the neuropil [15, 16]. In this regard, they closely resemble the sprouting axons near plaques that have been described with neurofibrillar silver staining (eg Cajal, 1928, in [21]) and by Golgi staining [22].
Another type of dystrophic neurite observed in human cases with Aβ plaques has a swollen globular morphology, and predominantly contains synaptic markers such as synaptophysin, chromogranin A, and, potentially, APP [16, 23-25]. These appear to form largely independently of those containing neurofilaments or altered tau, although some NF labeled dystrophic neurites show co-labelling for synaptic markers [16]. Numerous other proteins have been implicated in dystrophic neurite formation, including GAP-43, ubiquitin, ubiquilin, prion protein, cytochrome C, C9 or f72, reticulon-3 and BACE-1 [26-32]. In this regard, many of these markers correspond to proteins implicated in amyloidogenic and neuropathological pathways, potentially supporting the view that dystrophic neurite formation may lead to, for example, abnormal Aβ processing, release of abnormal Aβ oligomers and subsequent Aβ fibrillization into plaques. Notably, BACE1, an enzyme critical for generating Aβ fragments, may have a role in axon and synapse development, and also accumulates in axons and terminals in association with Aβ plaques, and there is interest in potentially reducing this axonal pathology and subsequent amyloid pathology by BACE1 inhibition (see [33] for a review).
Ultrastructural studies have also indicated that dystrophic neurites can contain filamentous structures including NFs and paired helical filaments (the latter only in human cases), abundant organelles such as mitochondria and lysosomes, abnormal swollen vesicles, and multilamellar and dense bodies [14, 34-36]. Accumulation of proteins and organelles within dystrophic neuritis suggests an interruption of normal axonal transport in damaged axons. In addition, the abnormal structures may accumulate because of disruption in normal autophagic-lysosomal pathways within dystrophic neurites [36].
Broadly, therefore, there appear to be two processes of dystrophic neurite formation. The major form arises from long axons reacting and/or aberrantly regenerating around Aβ plaques [15], causing progressive changes to the neuronal cytoskeleton. The second subtype resembles swollen axon terminals, which do not show regenerative features or substantial cytoskeletal pathology. It may be that the Aβ plaques have a differential effect on axonal or terminal compartments; or, given the localization of APP and potential autophagic-lysosomal dysfunction, these swollen terminals may be abnormally processing Aβ, leading to plaque formation.
With respect to neuronal susceptibility to dystrophic neurite formation, there may also be differences between subsets of neurons responding to Aβ plaque formation. As noted above, many dystrophic neurites may correspond to long axons, including corticocortical axons (see also [37]), as well as from neighbouring pyramidal neurons [22, 38] and from specific subcortical brain regions. We have also shown that axons are demyelinated around and within plaques [39]. NF abundant axons may also correspond to neurons that preferentially express, for example, NF triplet proteins in the cerebral cortex. A subset of pyramidal neurons show high levels of NF triplet proteins across mammalian species (reviewed in [40]) and are likely to contribute to long corticocortical projections, as seen in studies of non-human primate species [41]. These NF triplet containing neurons are particularly susceptible to NFT formation [42, 43] and degeneration [44] in AD. Conversely, non-pyramidal neurons, generally lacking NF triplet proteins, show very little propensity to NFT formation and overt cell loss in AD [43], and also very little reactive changes or dystrophic neurite formation around Aβ plaques [44]. This indicates that NF content may be a predisposing factor for axons to undergo a substantial reactive and regenerative response to plaque-related injury.
STAGING OF DYSTROPHIC NEURITE FORMATION
Aβ deposits probably accumulate in the brain for many years before cognitive deficits and behavioural changes are discernible. In this regard, pathological staging studies indicate that the clinical features of AD are closely associated with the presence of Aβ plaques throughout the neocortex and the spread of neurofibrillary pathology from medial temporal regions into other neocortical association areas. Increasing plaque density has also been associated with cognitive impairments in prodromal AD [46]. In vivo Aβ imaging studies also indicate that Aβ accumulation in the brain may represent a key early brain change, significantly increasing the risk of developing AD [9]. However, it is not yet clear whether all AD cases follow a lengthy period of Aβ accumulation, or whether all people who accumulate Aβ will necessarily transform into fulminant disease. We have also demonstrated that overall Aβ deposition load may be less critical than a change in the proportion of plaque types from predominantly diffuse forms to more compact, dense structures able to induce neuronal pathology [4]. In this regard, we have hypothesized that fibrillar plaques precipitate aberrant regenerative changes preceding classic neurofibrillary pathology, in neurons whose axons and terminal fields are impinged by plaque formation [20].
The dystrophic neurite profile of Aβ plaques also differs in prodromal disease compared to end-stage AD. For example, abnormal tau in dystrophic neurites is rarely seen near fibrillar Aβ plaques of preclinical, which are instead surrounded by abundant NF immunoreactive dystrophic neurites, including large spherical structures with axons that can be traced out into the neuropil, and smaller ring-like neurofilament structures [16, 47]. Both of these resemble the reactive and regenerative changes in axons subjected to structural injury in vivo [48] and in vitro [48, 49]. The accumulation of NFs, but not abnormal tau, in these dystrophic neurites supports the proposal that they are a relatively early form of abnormality, and that the comprehensive cytoskeletal changes of tau pathology take a relatively long time to develop.
Aβ PLAQUE-ASSOCIATE DYSTROPHIC NEURITE FORMATION IN EXPERIMENTAL MODELS
The full complement of AD pathological hallmarks have only been observed in human brains. Aβ plaques and associated dystrophic neurites have been described in a small number of non-human species, such as aged primates and dogs (e.g., [50, 51]), whereas transgenic mice expressing human FAD mutated APP (often in combination with mutated human PS1) typically develop Aβ deposits and plaques without substantial neuronal degenerationor neurofibrillary pathology such as paired helical filaments and highly modified tau. Some models combine human APP and PS1 mutations with a tau mutation (P301L) implicated in a subset of frontotemporal dementia cases [52]. Although the latter shows an augmentation of the tau pathology, it does not develop paired helical filament pathology, or significant cell loss and atrophy resembling human AD.
Notwithstanding these shortcomings, FAD transgenic models have been very useful in modeling early AD pathology. We have demonstrated that commonly used transgenic AD models such as the APPSwe/PS1dE9, Tg2576 and CRND8 lines, develop a pattern of pathology that is most reminiscent of early or preclinical AD. As they age, these lines develop dense, fibrillar plaques surrounded by NF labeled dystrophic neurites that appear identical to those in preclinical human cases [45, 53]. Hence, such transgenic mice can model the earliest pathogenic events of AD, and will be useful for suggesting and exploring potential disease-modifying strategies.
Aβ PLAQUES CAUSE DAMAGE TO SYNAPSES AND CORTICAL MICROCIRCUITRY
The mammalian cerebral cortex has a highly conserved, repetitive organization including columnar arrangements of neurons which dynamically group into units to enable sensory processing, integration of information and intentional behavior. In the human neocortex, Aβ plaques cluster in layers involved in corticocortical connectivity, and are more abundant in association areas relative to primary motor and sensory regions [2]. Cortical Aβ plaques are comparatively sparse in preclinical AD, but during disease progression, their spread throughout cortical layers may damage and disrupt most of the neuron groups in association areas, reducing the capacity for compensation in disrupted information processing.
AD is also associated with the degeneration and death of specifically susceptible neurons, as well as a generalized loss of brain ‘substance’ leading to atrophy. There has also been substantial interest in how damage to particular cortical circuits may represent a critical degenerative change that triggers progressive deterioration in cognitive function and alterations in behavior. Earlier work by Terry and associates emphasized the critical role played by synaptic loss in AD, correlating more closely than either plaques or neurofibrillary tangles with indices of cognitive decline [54]. The extent of synapse loss in higher association neocortex has been reported to vary between 30-45% [14] with a predilection for layers involved in corticocortical connectivity. The particular vulnerability of connection-related neurons and synapses likely gives rise to a pattern of disconnection between higher cortical areas.
With respect to cortical microcircuitry, as noted above, densely fibrillar plaques cause dendritic withering, spine loss, loss of normal axons, demyelination, reactive axonal changes and swelling of synaptic terminals (Fig. 1). In addition, these plaques also represent a focus of synapse loss [54, 55].The greatest degree of synapse degeneration occurs in the central region of the plaque, with a substantial loss of both excitatory and inhibitory synapses [54]. However, in the periphery of plaques and throughout the neuropil, there is a selective loss of excitatory synapses, as demonstrated with the presynaptic marker,VGlut-1, whereas, inhibitory synapses appear unaffected, in the cortical neuropil between plaques in both established human AD and in transgenic models [55]. However, inhibitory synapses are reduced on cortical neuron cell body surfaces and initial axon segments near plaques [56, 57]. In preclinical AD cases, synapse loss is restricted to the plaques, with no discernible decreased density in the wider neuropil [55].
Collectively, this indicates that plaques can cause substantial localized damage primarily to excitatory cortical connections in preclinical stages, but that further disease progression, perhaps associated with neuronal degeneration and increased Aβ plaque load, is necessary for more widespread synapse loss. In end-stage AD cases, remaining VGlut-1 labelled puncta in and around plaquesare relatively reduced in size, whereas VGAT labeled inhibitory boutons are larger [55]. Interestingly, in preclinical AD cases, both VGlut-1 and VGAT labeled boutons were larger in the neuropil and at the periphery of plaques [55]. These specific and stage-specific alterations in bouton size may reflect type-specific adaptive changes in response to the disruption in cortical circuitry.
Aside from synaptic changes, recent studies have suggested that substantial reactive changes may also occur in glial GABAergic systems. Increased glutamate decarboxylase (GAD) activity was detected in glial membrane fraction preparations from 12 month old APP/PS1 mice, but not from neural synaptosomes [55]. More recent studies show increased GABA labeling in reactive astrocytes in human AD brains, as well as release of GABA by astrocytes in transgenic AD models [58, 59]. This may be linked with increased, and synchronized, activity of glial cells as shown by in vivo calcium imaging of transgenic models [60].
In human AD, the combination of specific synapse vulnerability, synaptic remodeling and altered inhibitory glio transmission, may contribute to local processing abnormalities in the vicinity of Aβ plaques. In vivo calcium imaging of AD transgenic models has demonstrated abnormal hypo- and hyperactivity in subsets of neurons, the latter specifically in neurons and neurites near Aβ plaques [61, 62]. While there is limited evidence of nerve cell body degeneration around plaques [27], increased calcium in neurites adjacent to plaques could contribute to degeneration, since calcineurin inhibition in experimental models reduces peri-plaque neurite beading [62]. In this regard, the relative preservation of inhibitory synaptic structures, including increased bouton size and GABA production and release by reactive astrocytes may partly compensate for abnormal excitation and hyperactivity around plaques. The large spatial extent of astrocytes compared to neurons could explain the wider spread of abnormally ‘silent’ or hypoactive neurons between plaques in transgenic models [61]. In vivo calcium imaging of transgenic models has shown hypoactive neurons in the visual cortex which were unresponsive to visual stimuli, whereas visual processing alterations were associated with abnormally hyperactive subsets of neurons [63]. Increased epileptiform activity in AD and transgenic models has been well described, and likely represents an altered balance between excitatory and inhibitory transmission, for which the system may compensate by means such as sprouting of inhibitory processes and increased miniature inhibitory postsynaptic currents in the hippocampus [64]. Furthermore, GABAA antagonism is capable of restoring a degree of activity in previously abnormally hypoactive neurons, further supporting the proposition that this pathological milieu involves excessive inhibition [63]. Plaques also locally reduce experience-induced expression of the immediate early gene Arc following visual stimulation in transgenic models [65], indicating that excitatory plasticity is also disrupted.
Taken together, studies of early human AD and corresponding transgenic models indicate a disruption in normal cortical processing in the vicinity of Aβ plaques, consisting of altered and degenerating synapses, cytoskeletal and aberrant regenerative changes in axons, but little overt neuronal degeneration. AD progression is linked to an increased density of fibrillar Aβ plaques, a wider loss of synapses throughout affected cortical regions and the substantial transformation of the normal neuronal cytoskeleton in subsets of vulnerable neurons. The evolution of these initial neuronal changes and network disruption into frank degeneration of associative cortical pathways suggests multiple points of potential intervention, from pharmacological manipulation of synaptic activity through to inhibition of cytoskeletal pathology that results in disconnection. Common animal models involving familial AD mutant transgenes broadly reflect initial stages of AD, and may provide insights into how Aβ pathology results in structural and physiological changes in neurons and network dynamics, as well as informing potential new therapeutic approaches for early intervention and/or prevention of fulminant disease.
ACKNOWLEDGEMENTS
We would like to acknowledge the funding of related research by the JO and JR Wicking Trust (Equity Trustees) as well as the Australian National Health and Medical Research Council.
LIST OF ABBREVIATIONS
- AD
Alzheimer’s disease
- GAD
glutamate decarboxylase
- NF
neurofilament
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Vickers J.C., King A.E., Woodhouse A., Kirkcaldie M.T., Staal J.A., McCormack G.H., et al. Axonopathy and cytoskeletal disruption in degenerative diseases of the central nervous system. Brain Res. Bull. 2010;80:217–223. doi: 10.1016/j.brainresbull.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Rogers J., Morrison J.H. Quantitative morphology and regional and laminar distributions of senile plaques in Alzheimer’s disease. J. Neurosci. 1985;5:2801–2808. doi: 10.1523/JNEUROSCI.05-10-02801.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fiala J.C. Mechanisms of amyloid plaque pathogenesis. Acta Neuropathol. 2007;114:551–571. doi: 10.1007/s00401-007-0284-8. [DOI] [PubMed] [Google Scholar]
- 4.Dickson T.C., Vickers J.C. The morphological phenotype of β-amyloid plaques and associated neuritic changes in Alzheimer’s disease. Neuroscience. 2001;105:99–107. doi: 10.1016/s0306-4522(01)00169-5. [DOI] [PubMed] [Google Scholar]
- 5.Meyer-Luehmann M., Spires-Jones T.L., Prada C., Garcia-Alloza M., de Calignon A., Rozkaine A., et al. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarter J.F., Liebscher S., Bachhuber T., Abou-Ajram C., Hübener M., Hyman B.T., et al. Clustering of plaques contributes to plaque growth in a mouse model of Alzheimer’s disease. Acta Neuropathol. 2013;126:179–188. doi: 10.1007/s00401-013-1137-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shepherd C., McCann H., Halliday G.M. Variations in the neuropathology of Alzheimer’s disease. Acta Neuropathol. 2009;118:37–52. doi: 10.1007/s00401-009-0521-4. [DOI] [PubMed] [Google Scholar]
- 8.Braak H., Thal D.R., Ghebremedhin E., Del Tredici K. Stages of the pathological process in Alzheimer’s disease: Age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011;70:960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 9.Villemagne V.L., Burnham S., Bourgeat P., Brown B., Ellis K.A., Salvado O., et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 10.Giannakopolous P., Herrman F.R., Bussiére T., Bouras C., Kövari E., Perl D.P., et al. Tangle and neuron numbers, but not amyloid load predict cognitive status in Alzheimer’s disease. Neurology. 2003;60:1495–1500. doi: 10.1212/01.wnl.0000063311.58879.01. [DOI] [PubMed] [Google Scholar]
- 11.Woodhouse A., Dickson T.C., West A.K., McLean C.A., Vickers J.C. No difference in expression of apoptosis-related proteins and apoptotic morphology in control, pathologically aged and Alzheimer’s disease cases. Neurobiol. Dis. 2006;22:323–333. doi: 10.1016/j.nbd.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Adlard P.A., Vickers J.C. Morphologically distinct plaque-types differentially affect dendritic structure and organisation in the early and late stages of Alzheimer’s disease. Acta Neuropathol. 2002;103:377–383. doi: 10.1007/s00401-001-0476-6. [DOI] [PubMed] [Google Scholar]
- 13.Spires T.L., Meyer-Luehmann M., Stern E.A., McLean P.J., Skoch J., Nguyen P.T., et al. Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J. Neurosci. 2005;25:7278–7287. doi: 10.1523/JNEUROSCI.1879-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masliah E., Mallory M., Hansen L., Alford M., Albright T., DeTeresa R., et al. Patterns of aberrant sprouting in Alzheimer's disease. Neuron. 1991;6:729–739. doi: 10.1016/0896-6273(91)90170-5. [DOI] [PubMed] [Google Scholar]
- 15.Masliah E., Mallory M., Hansen L., Alford M., DeTeresa R., Terry R. An antibody against phosphorylated neurofilaments identifies a subset of damaged association axons in Alzheimer’s disease. Am. J. Pathol. 1993;142:871–882. [PMC free article] [PubMed] [Google Scholar]
- 16.Dickson T.C., King C.E., McCormack G.H., Vickers J.C. Neurochemical diversity of dystrophic neurites in the early and late stages of Alzheimer’s disease. Exp. Neurol. 1999;156:100–110. doi: 10.1006/exnr.1998.7010. [DOI] [PubMed] [Google Scholar]
- 17.Dickson D.W., Farlo J., Davies P., Crystal H., Fuld P., Yen S-H. Alzheimer’s disease. A double-labelling immunohistochemical study of senile plaques. Am. J. Pathol. 1988;132:86–101. [PMC free article] [PubMed] [Google Scholar]
- 18.Su J.H., Cummings B.J., Cotman C.W. Plaque biogenesis in brain aging and Alzheimer’s disease. II. Progressive transformation and developmental sequence of dystrophic neurites. Acta Neuropathol. 1998;96:463–471. doi: 10.1007/s004010050920. [DOI] [PubMed] [Google Scholar]
- 19.Vickers J.C., Riederer B.M., Marugg R.A., Buée-Scherrer V., Buée L., Delacourte A., et al. Alterations in neurofilament protein immunoreactivity in human hippocampal neurons related to normal aging and Alzheimer’s disease. Neuroscience. 1994;62:1–13. doi: 10.1016/0306-4522(94)90310-7. [DOI] [PubMed] [Google Scholar]
- 20.Vickers J.C., Dickson T.C., Adlard P.A., Saunders H.L., King C.E., McCormack G. The cause of neuronal degeneration in Alzheimer’s disease. Prog. Neurobiol. 2000;60:139–165. doi: 10.1016/s0301-0082(99)00023-4. [DOI] [PubMed] [Google Scholar]
- 21.DeFelipe J., Jones E.G. Cajal's Degeneration and Regeneration of the Nervous System. New York: Oxford University Press; 1991. [Google Scholar]
- 22.Probst A., Basler V., Bron B., Ulrich J. Neuritic plaques in senile dementia of Alzheimer type: A Golgi analysis in the hippocampal region. Brain Res. 1983;268:249–254. doi: 10.1016/0006-8993(83)90490-0. [DOI] [PubMed] [Google Scholar]
- 23.Adams L.A., Munoz D.G. Differential incorporation of processes derived from different classes of neurons into senile plaques in Alzheimer’s disease. Acta Neuropathol. 1993;86:365–370. doi: 10.1007/BF00369449. [DOI] [PubMed] [Google Scholar]
- 24.Yasuhara O., Kawamata T., Aimi Y., McGeer E.G., McGeer P.L. Two types of dystrophic neuritis in senile plaques of Alzheimer’s disease and elderly non-demented cases. Neurosci. Lett. 1994;171:73–76. doi: 10.1016/0304-3940(94)90608-4. [DOI] [PubMed] [Google Scholar]
- 25.Guevera J., Dilhuydy J., Espinosa B., Delacourte A., Quirion R., Mena R., et al. Coexistence of reactive plasticity and neurodegeneration in Alzheimer’s diseased brains. Histol. Histopathol. 2004;19:1075–1084. doi: 10.14670/HH-19.1075. [DOI] [PubMed] [Google Scholar]
- 26.Zhan S-S., Kamphorst W., Van Nostrand W.E., Eikelenboom P. Distribution of neuronal growth-promoting factors and cytoskeletal proteins in altered neuritis in Alzheimer’s disease and non-demented elderly. Acta Neuropathol. 1995;89:356–362. doi: 10.1007/BF00309629. [DOI] [PubMed] [Google Scholar]
- 27.Woodhouse A., Vickers J.C., Dickson T.C. Cytoplasmic cytochrome c immunolabelling in dystrophic neurites in Alzheimer’s disease. Acta Neuropathol. 2006;112:429–437. doi: 10.1007/s00401-006-0107-3. [DOI] [PubMed] [Google Scholar]
- 28.Prior M., Shi Q., Hu X., He W., Levey A., Yan R. RTN/Nogo in forming Alzheimer’s neuritic plaques. Neurosci. Biobehav. Rev. 2010;34:1201–1206. doi: 10.1016/j.neubiorev.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi R.H., Tobiume M., Sato Y., Sata T., Gouras G.K., Takahashi H. Accumulation of cellular prion protein within dystrophic neurites of amyloid plaques in the Alzheimer's disease brain. Neuropathology. 2011;31:208–214. doi: 10.1111/j.1440-1789.2010.01158.x. [DOI] [PubMed] [Google Scholar]
- 30.Satoh J-I., Tabunoki H., Ishida T., Saito Y., Arima K. Dystrophic neurites express C9orf72 in Alzheimer’s disease brains. Alzheimers Res. Ther. 2012;4:33. doi: 10.1186/alzrt136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kandalepas P.C., Sadleir K.R., Eimer W.A., Zhao J., Nicholson D.A., Vassar R. The Alzheimer’s β-secretase BACE1 localizes to normal presynaptic terminals and to dystrophic presynaptic terminals surrounding amyloid plaques. Acta Neuropathol. 2013;126:329–352. doi: 10.1007/s00401-013-1152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satoh J., Tabunoki H., Ishida T., Saito Y., Arima K. Ubiquilin-1 immunoreactivity is concentrated on Hirano bodies and dystrophic neurites in Alzheimer's disease brains. Neuropathol. Appl. Neurobiol. 2013;39:817–830. doi: 10.1111/nan.12036. [DOI] [PubMed] [Google Scholar]
- 33.Yan X-X., Ma C., Gai W-P., Cai H., Luo G. CANs? BACE1 inhibition mitigate early axonal pathology in neurological disease? J. Alzheimers Dis. 2014;38:705–718. doi: 10.3233/JAD-131400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paprotnick D., Smith M.A., Richey P.L., Vinters H.V., Perry G. Filament heterogeneity within the dystrophic neurites of senile plaques suggests blockage of fast axonal transport in Alzheimer’s disease. Acta Neuropathol. 1997;91:226–235. doi: 10.1007/s004010050420. [DOI] [PubMed] [Google Scholar]
- 35.Fiala J.C., Feinberg M., Peters A., Barbas H. Mitochondrial degeneration in dystrophic neuritis of senile plaques may lead to extracellular deposition of fine filaments. Brain Struct. Funct. 2007;212:195–207. doi: 10.1007/s00429-007-0153-1. [DOI] [PubMed] [Google Scholar]
- 36.Nixon R.A., Yang D-S. Autophagy failure in Alzheimer’s disease – locating the primary defect. Neurobiol. Dis. 2011;43:38–45. doi: 10.1016/j.nbd.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delatour B., Blanchard V., Pradier L., Duyckaerts C. Alzheimer pathology disorganizes cortico-cortical circuitry: direct evidence from a transgenic animal model. Neurobiol. Dis. 2004;16:41–47. doi: 10.1016/j.nbd.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 38.Adalbert R., Nogradi A., Babetto E., Janeckova L., Walker S.A., Kerschensteiner M., Misgeld M., Coleman M.P. Severely dystrophic axons at amyloid plaques remain continuous and connected to viable cell bodies. Brain. 2009;132:402–416. doi: 10.1093/brain/awn312. [DOI] [PubMed] [Google Scholar]
- 39.Mitew S., Kirkcaldie M.T., Halliday G.M., Shepherd C.E., Vickers J.C., Dickson T.C. Focal demyelination in Alzheimer’s disease and transgenic mouse models. Acta Neuropathol. 2010;119:567–577. doi: 10.1007/s00401-010-0657-2. [DOI] [PubMed] [Google Scholar]
- 40.Vickers J.C., Dickson T.C., Adlard P.A., Saunders H.L., King C.E., McCormack G. The cause of neuronal degeneration in Alzheimer’s disease. Prog. Neurobiol. 2000;60:139–165. doi: 10.1016/s0301-0082(99)00023-4. [DOI] [PubMed] [Google Scholar]
- 41.Hof P.R., Ungerleider L.G., Webster M.J., Gattass R., Adams M.F., Sailstad C.A., et al. Neurofilament protein is differentially distributed in subpopulations of corticocortical projection neurons in the macaque monkey visual pathways. J. Comp. Neurol. 1996;376:112–127. doi: 10.1002/(SICI)1096-9861(19961202)376:1<112::AID-CNE7>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 42.Vickers J.C., Delacourte A., Morrison J.H. Progressive transformation of the cytoskeleton associated with normal aging and Alzheimer’s disease. Brain Res. 1992;594:273–278. doi: 10.1016/0006-8993(92)91134-z. [DOI] [PubMed] [Google Scholar]
- 43.Sampson V.L., Morrison J.H., Vickers J.C. The cellular basis for the relative resistance of parvalbumin and calretinin immunoreactive neocortical neurons to the pathology of Alzheimer’s disease. Exp. Neurol. 1997;145:295–302. doi: 10.1006/exnr.1997.6433. [DOI] [PubMed] [Google Scholar]
- 44.Hof P.R., Cox K., Morrison J.H. Quantitative analysis of a vulnerable subset of pyramidal neurons in Alzheimer’s disease: I. Superior frontal and inferior temporal cortex. J. Comp. Neurol. 1990;301:44–54. doi: 10.1002/cne.903010105. [DOI] [PubMed] [Google Scholar]
- 45.Mitew S., Kirkcaldie M.T., Dickson T.C., Vickers J.C. Neurites containing the neurofilament-triplet proteins are selectively vulnerable to cytoskeletal pathology in Alzheimer's disease and transgenic mouse models. Front. Neuroanat. 2013;7:30. doi: 10.3389/fnana.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morris J.C., Storandt M., McKeel D.W., Rubin E.H., Price J.L., Grant E.A., et al. Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presymptomatic and very mild Alzheimer’s disease. Neurology. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- 47.Vickers J.C., Chin D., Edwards A.M., Sampson V., Harper C., Morrison J. Dystrophic neurite formation associated with age-related beta amyloid deposition in the neocortex: clues to the genesis of neurofibrillary pathology. Exp. Neurol. 1996;141:1–11. doi: 10.1006/exnr.1996.0133. [DOI] [PubMed] [Google Scholar]
- 48.Dickson T.C., Chuckowree J.A., Chuah M.I., West A.K., Vickers J.C. Alpha-internexin immunoreactivity reflects variable neuronal vulnerability in Alzheimer’s disease and supports the role of the beta-amyloid plaques in inducing neuronal injury. Neurobiol. Dis. 2005;18:286–295. doi: 10.1016/j.nbd.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Dickson T.C., Adlard P.A., Vickers J.C. Sequence of cellular changes following localized axotomy to cortical neurons in glia-free culture. J. Neurotrauma. 2000;17:1095–1103. doi: 10.1089/neu.2000.17.1095. [DOI] [PubMed] [Google Scholar]
- 50.Miyawaki K., Nakayama H., Nakamura S-I., Uchida K., Doi K. Three-dimensional structures of canine senile plaques. Acta Neuropathol. 2001;102:321–328. doi: 10.1007/s004010000354. [DOI] [PubMed] [Google Scholar]
- 51.Perez S.E., Raghati M.A., Hof P.R., Kramer L., Ikonomovoc M.D., Lacor P.N., et al. Alzheimer’s disease pathology in the neocortex and hippocampus of the Western lowland gorilla (Gorilla gorilla gorilla). J. Comp. Neurol. 2013;521:4318–4338. doi: 10.1002/cne.23428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oddo S., Caccamo A., Shepherd J.D., Murphy M.P., Golde T.E., Kayed R., et al. Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 53.Woodhouse A., Vickers J.C., Adlard P.A., Dickson T.C. Dystrophic neurites in TgCRND8 and Tg2576 mice mimic human pathological brain aging. Neurobiol. Aging. 2009;30:864–874. doi: 10.1016/j.neurobiolaging.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 54.Terry R.D., Masliah E., Salmon D.P., Butters N., DeTeresa R., Hill R., et al. Physical basis of cognitive alterations in Alzheimer’s disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 55.Mitew S., Kirkcaldie M.T., Dickson T.C., Vickers J.C. Altered synapses and gliotransmission in Alzheimer’s disease and AD model mice. Neurobiol. Aging. 2013;34:2341–2351. doi: 10.1016/j.neurobiolaging.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 56.Garcia-Marin V., Blazquez-Llorca L., Rodriguez J-R., Biluda S., Muntane G., Ferrer I., et al. Diminished perisomatic GABAergic terminals on cortical neurons adjacent to amyloid plaques. Front. Neuroanat. 2009;3:28. doi: 10.3389/neuro.05.028.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Léon-Espinosa G., DeFelipe J., Munoz A. Effects of amyloid-β plaque proximity on the axon initial segment of pyramidal cells. J. Alzheimers Dis. 2012;29:841–852. doi: 10.3233/JAD-2012-112036. [DOI] [PubMed] [Google Scholar]
- 58.Jo S., Yarishkin O., Hwang Y.J., Chun Y.E., Park M., Woo D.H., et al. GABA from reactive astrocytes impairs memory in mouse models of Alzheimer’s disease. Nat. Med. 2014;20:886–896. doi: 10.1038/nm.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu Z., Guo Z., Gearing M., Chen G. Tonic inhibition in dentate gyrus inhibits long term potentiation and memory in an Alzheimer’s disease model. Nat. Commun. 2014;5:4159. doi: 10.1038/ncomms5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuchibotla K.V., Lattarulo C.R., Hyman B.T., Bacskai B.J. Synchronous hyperactivity and calcium waves in astrocytes in Alzheimer mice. Science. 2009;323:1211–1215. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Busche M.A., Eichoff G., Adelsberger H., Abramowski D., Wiederhold K.H., Haass C., et al. Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science. 2008;321:1686–1689. doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- 62.Kuchibhotla K.V., Goldman S.T., Lattarulo C.R., Wu H.Y., Hyman B.T., Backsai B.J. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grienberger C., Rochefort N.K., Adelsberger H., Henning H.A., Hill D.N., Reichwald J., et al. Staged decline of neuronal function in vivo in an animal model of Alzheimer’s disease. Nat. Commun. 2012;3:774. doi: 10.1038/ncomms1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Palop J.J., Chin J., Roberson E.D., Wang J., Thwin M.T., Bien-Ly N., et al. Aberrant excitatory neuronal activity and compensatory remodelling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rudinskiy N., Hawkes J.M., Betensky R.A., Eguchi M., Yamaguchi S., Spires-Jones T.L., et al. Orchestrated experience-driven Arc responses are disrupted in a mouse model of Alzheimer’s disease. Nat. Neurosci. 2012;15:1422–1429. doi: 10.1038/nn.3199. [DOI] [PMC free article] [PubMed] [Google Scholar]